Introduction
Oral squamous cell carcinoma (OSCC) is an aggressive
tumor type that occurs at several sites of the oral mucosa. It
accounts for 3% of all cancer cases (1,2), and is
the most common type of head and neck cancer worldwide (3). Despite advances in its treatment, the
5-year survival rate of OSCC is only ~50%, which is due to its late
diagnosis (3,4). Understanding the molecular basis for
the carcinogenesis of OSCC is important and has clinical
implications for the therapy of OSCC.
Annexin A1 (ANXA1) belongs to the annexin family and
takes part in numerous pathophysiological processes, including cell
proliferation, differentiation, motility and inflammatory responses
(5–8). A increasing number of studies suggested
that the expression and the function of ANXA1 in tumors are tissue-
and tumor-specific (9,10). Upregulation of ANXA1 was observed in
human breast cancer (11),
hepatocellular carcinoma (12) and
melanoma (13,14), whereas downregulation of ANXA1 was
observed in gastric cancer (15),
prostate cancer (16,17) and oral cancer (18,19). The
function of ANXA1 in tumors appears to be paradoxical. In colon and
gastric cancers, ANXA1 interacts with formyl peptide receptors to
promote cell migration and invasion (20,21). In
non-small cell lung cancer, ANXA1 knockdown suppressed cell
proliferation and metastasis (22).
However, ANXA1 acts as a tumor suppressor by reducing cell
proliferation and attenuating the metastatic potential in breast
cancer (23). Certain clinical
studies revealed that ANXA1 is downregulated in OSCC patients, and
its expression is associated with the pathologic differentiation
grade in OSCC patients (18,24–26).
However, the direct function of ANXA1 in OSCC progression has
remained to be fully elucidated.
The aim of the present study was to investigate the
role of ANXA1 in OSCC cell proliferation and invasion in
vitro. Furthermore, whether ANXA1 is involved in transforming
growth factor β1 (TGFβ1)/epidermal growth factor (EGF)-induced
epithelial-mesenchymal transition (EMT) in OSCC was explored.
Materials and methods
Cell culture and transfection
The Tca-8113 cell line was purchased from Boster
Biological Technology (Wuhan, China), and SCC-9 cells were obtained
from the American Type Culture Collection (Manassas, VA, USA). The
cells were grown in RPMI1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Hyclone; GE Healthcare, Little Chalfont, UK),
and they were maintained at 37°C in a humidified atmosphere with 5%
CO2. Human EGF was from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany), and human TGFβ1 was purchased from PeproTech
(Rocky Hill, NJ, USA). The cells were treated with 100 ng/ml EGF in
combination with 5 ng/ml TGFβ1 for 6 h. ANXA1-pcDNA3.1 plasmid and
empty vector pcDNA3.1 (Shenzhen Zhonghong Boyuan Biological
Technology Co., Ltd., Shenzhen, China) were transfected into the
Tca-8113 and SCC-9 cells using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions.
Western blot analysis
The cells were harvested and proteins were extracted
from the cells using a Total Protein Extraction kit (Applygen
Technologies, Inc., Beijing, China). The protein concentration was
quantified using a bicinchoninic acid protein assay kit (cat. no.
C503021; Sangon Biotech Co., Ltd., Shanghai, China). A total of 40
µg protein was separated by 10% SDS-PAGE, and the separated
proteins were transferred onto a nitrocellulose membrane (EMD
Millipore, Billerica, MA, USA). The membranes were blocked with 5%
non-fat milk at 4°C overnight. Subsequently, the membranes were
probed with primary antibodies specific for ANXA1 (cat. no.
ab33061; rabbit polyclonal; 1:800 dilution; Abcam, Cambridge, MA,
USA), epithelial (E)-cadherin (cat. no. BA0475; rabbit polyclonal;
1:400 dilution; Boster Biological Technology), neural (N)-cadherin
(cat. no. BA0673; rabbit polyclonal; 1:400 dilution; Boster
Biological Technology) or vimentin (cat. no. ab45939; rabbit
polyclonal; 1:800 dilution; Abcam). GAPDH antibody (cat. no.
ab9485; rabbit polyclonal; 1:2,000 dilution; Abcam) was used as an
internal control. The membranes were incubated with the primary
antibodies at 4°C overnight, followed by incubation with
horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G
(cat. no. BA1054; 1:1,000 dilution; Boster Biological Technology)
at 37°C for 2 h. Blots were visualized using enhanced
chemiluminescence (cat. no. 21050; Western Blot Signal Enhancer
kit; Pierce; Thermo Fisher Scientific, Inc.). Band densities were
measured using ImageJ software 1.48 (National Institutes of Health,
Bethesda, MD, USA).
Cell proliferation assay
An MTT assay was used to assess the proliferation of
Tca-8113 and SCC-9 cells in vitro. In brief,
2.5×104 cells/well were cultured in 96-well plates in
RPMI1640 medium supplemented with 10% FBS. Following 1, 2, 3 or 4
days of incubation, MTT solution (Sigma-Aldrich; Merck KGaA) was
added to each well, and the cells were incubated at 37°C for 4 h.
The formazan crystals were dissolved in dimethyl sulfoxide
(Invitrogen; Thermo Fisher Scientific, Inc.). Finally, the optical
density at 570 nm was measured using a microplate reader (iMark
Microplate Absorbance Reader; Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Cell invasion assay
Matrigel (BD Biosciences, San Jose, CA, USA) was
used to coat the Transwell inserts (Corning Costar, Lowell, MA,
USA) prior to the experiments. 2.5×104 cells in
serum-free medium were placed on the upper chambers for the cell
invasion assay. Complete medium (1 ml) was added into the lower
chambers. Following incubation at 37°C for 24 h, the non-invaded
cells were removed with cotton swabs. Cells in the lower chamber
were fixed in 95% ethanol for 30 min and subsequently stained with
hematoxylin (Beyotime Institute of Biotechnology, Haimen, China)
for 10 min. Invaded cells were counted in 10 randomly selected
fields of each insert under a light microscope (BX51; Olympus,
Tokyo, Japan).
Statistical analysis
Values are expressed as the mean ± standard
deviation of at least three independent experiments. The results
were analyzed by one-way analysis of variance followed by least
significant difference test using SPSS 19.0 software (International
Business Machines Corp., Armonk, NY, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
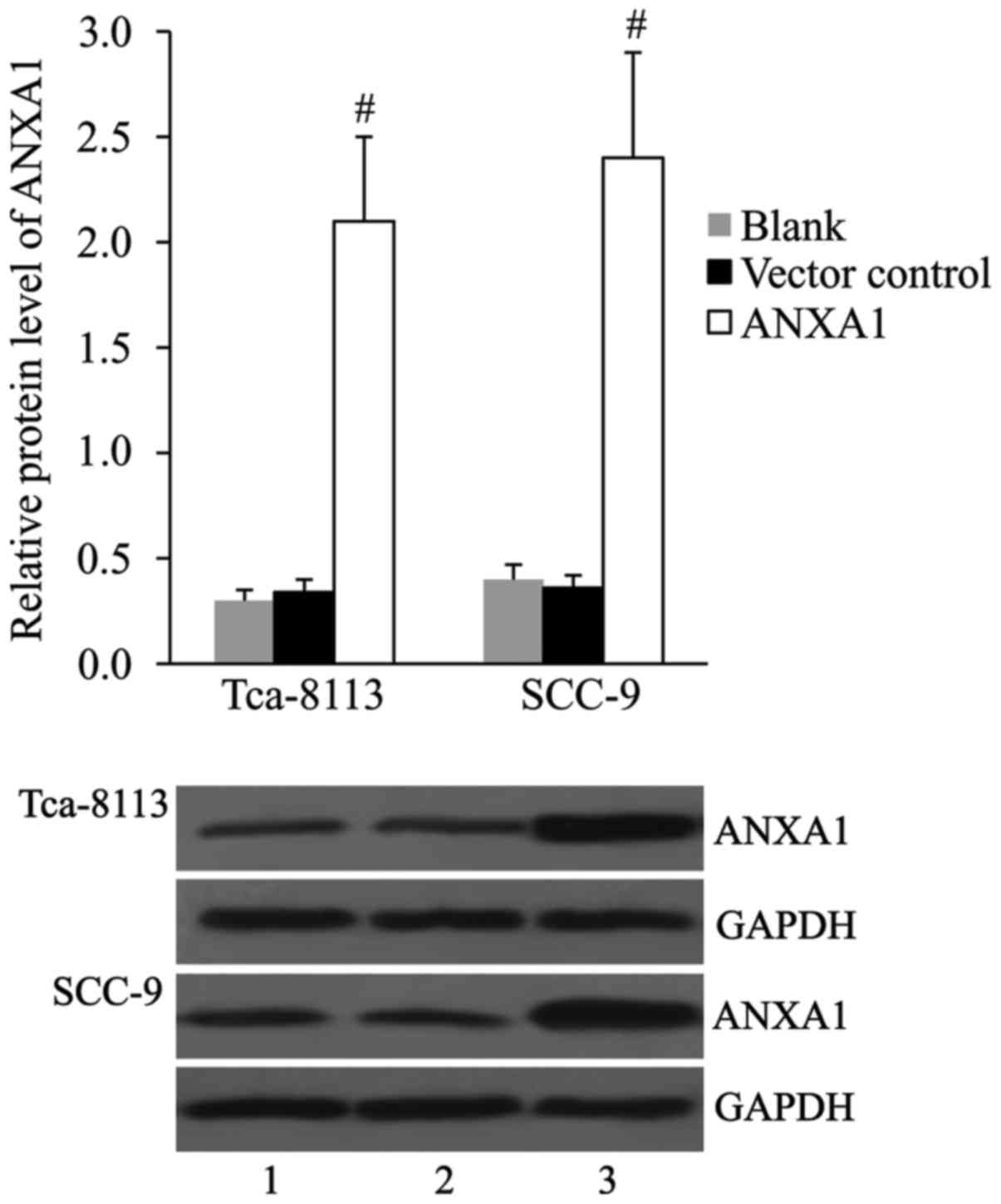
Overexpression of ANXA1 with
ANXA1-pcDNA3.1 plasmid in OSCC cell lines
The pcDNA3.1 vector control and ANXA1-pcDNA3.1
plasmid were transfected into Tca-8113 and SCC-9 cells, and the
expression of ANXA1 protein was detected by western blot at 48 h
post-transfection. The results indicated no significant difference
in ANXA1 expression between the vector control and blank groups.
Compared with those in the cells transfected with vector control,
the relative protein levels of ANXA1 were significantly increased
in the cells transfected with ANXA1-pcDNA3.1 (Fig. 1).
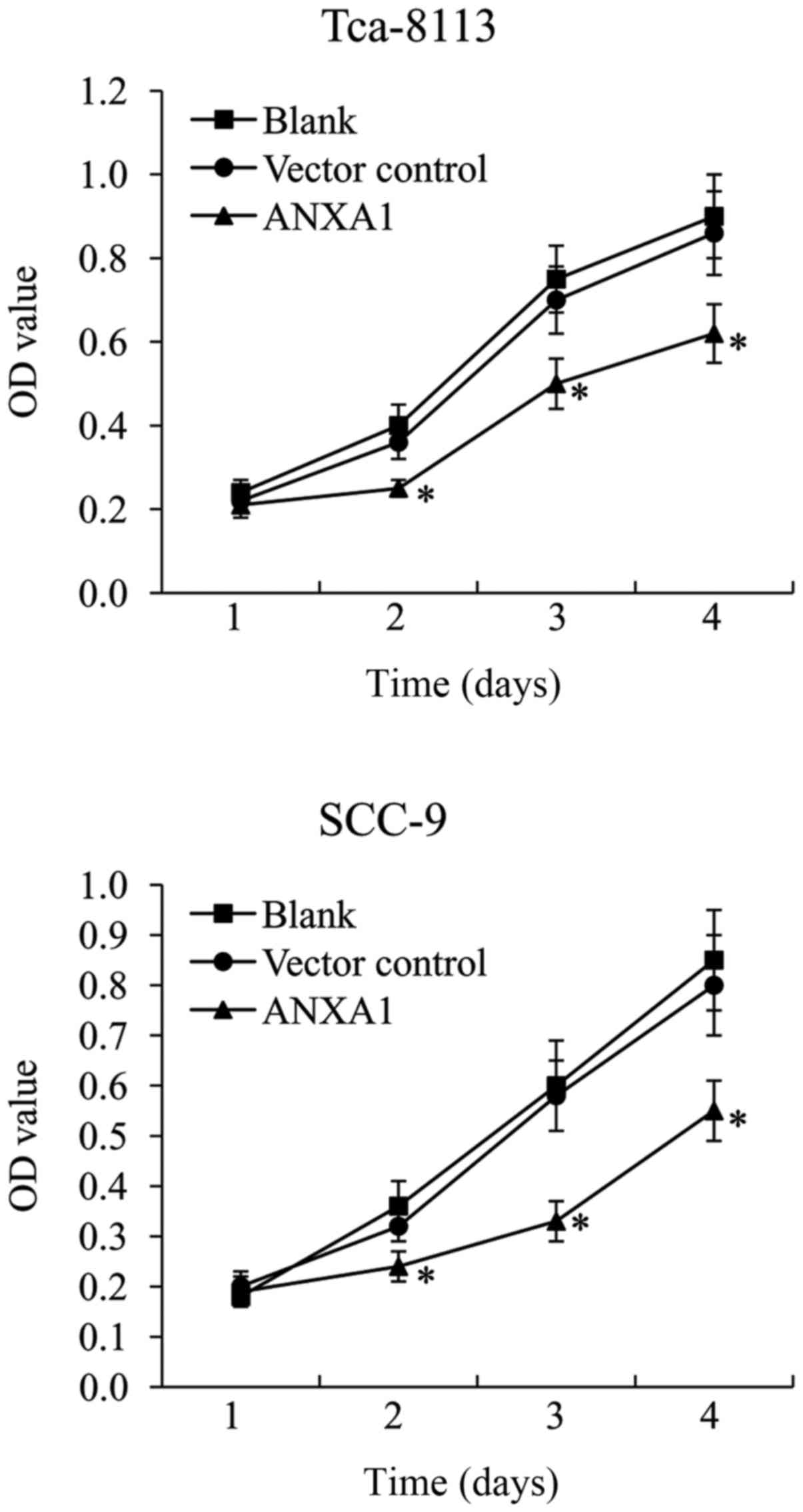
ANXA1 overexpression inhibits Tca-8113
and SCC-9 cell proliferation
An MTT assay was performed on ANXA1-overexpressing
Tca-8113 and SCC-9 cells to investigate the effect of ANXA1 on OSCC
cell proliferation. The results demonstrated that pcDNA3.1 vector
control did not affect Tca-8113 and SCC-9 cell growth. In
ANXA1-overexpressing Tca-8113 and SCC-9 cells, the cell survival
rate was significantly decreased compared with that in the vector
control-transfected cells (Fig.
2).
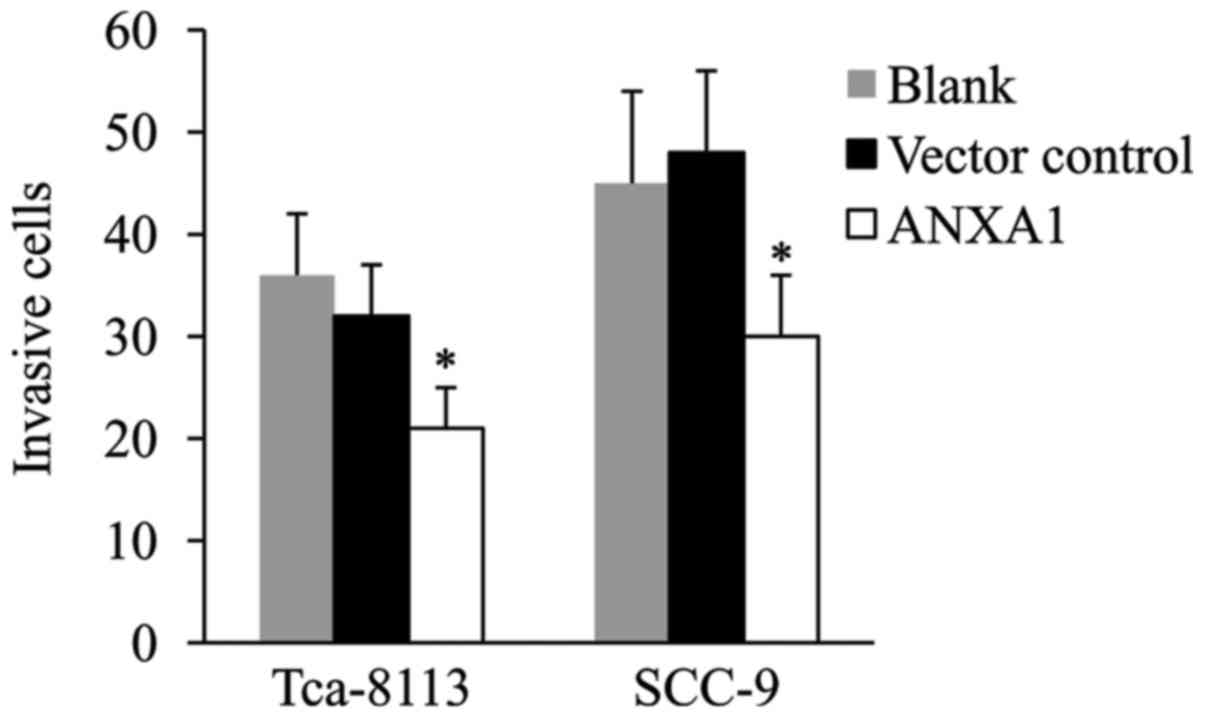
ANXA1 overexpression reduces Tca-8113
and SCC-9 cell invasion
The Transwell-Matrigel invasion assay was performed
using ANXA1-overexpressing Tca-8113 and SCC-9 cells to investigate
the effect of ANXA1 on OSCC cell invasion. As presented in Fig. 3, there was no significant difference
in the number of invasive cells between the vector control and
blank groups. Of note, ANXA1 overexpression significantly
suppressed the invasiveness of each of the two OSCC cell lines.
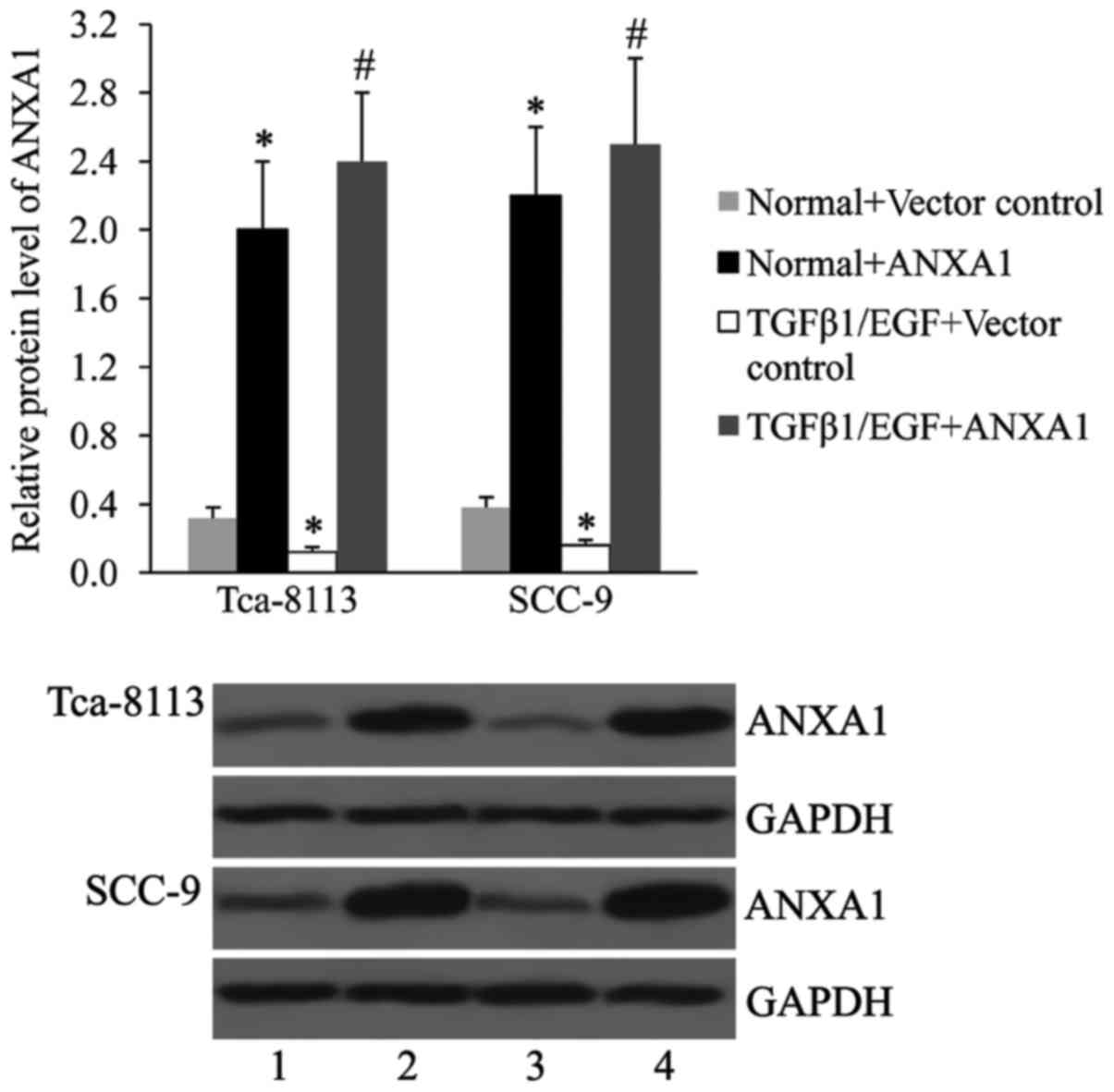
Effect of ANXA1 overexpression on
TGFβ1/EGF-induced EMT
The Tca-8113 and SCC-9 cells were treated with TGFβ1
and EGF for 6 h, and the ANXA1 protein levels was determined by
western blot analysis. As presented in Fig. 4, TGFβ1 and EGF treatment resulted in
a decreased expression of ANXA1 in Tca-8113 and SCC-9 cells.
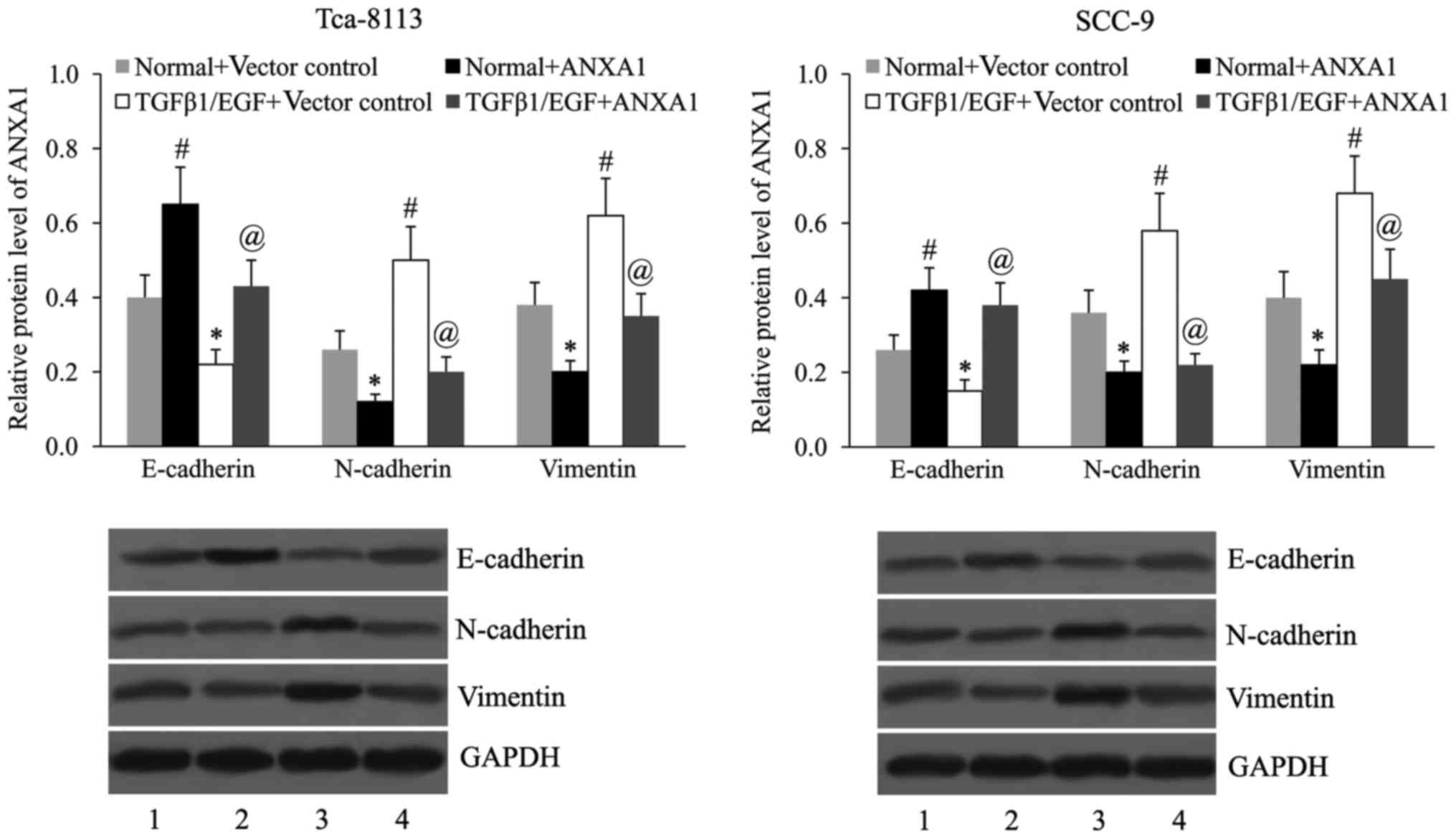
Subsequently, the effect of ANXA1 on the expression
of EMT markers in Tca-8113 and SCC-9 cells in the presence or
absence of TGFβ1/EGF was examined. As demonstrated in Fig. 5, TGFβ1 and EGF treatment induced EMT
in OSCC cells by downregulating E-cadherin expression, and
upregulating N-cadherin and vimentin expression. ANXA1
overexpression led to an upregulation of E-cadherin expression, and
a downregulation of N-cadherin and vimentin expression in untreated
as well as TGFβ1/EGF-treated Tca-8113 and SCC-9 cells.
Discussion
ANXA1 was suggested to be a prognostic biomarker for
OSCC in several clinical studies. Zhu et al (24,25) and
Zhang et al (26) reported
that ANXA1 expression is significantly correlated with the
pathological differentiation grade in OSCC patients. A lower ANXA1
expression in OSCC tissues correlates to a poorer pathologic
differentiation grade (25,26). ANXA1 mRNA is downregulated in
peripheral blood from OSCC patients compared with that in negative
control individuals (18). In
addition, patients with moderate or poorly differentiated OSCC as
well as low ANXA1 expression may benefit from induction
chemotherapy (24). All of these
clinical studies revealed that ANXA1 is associated with OSCC
development. In the present study, in vitro studies on
Tca-8113 and SCC-9 cells were performed to investigate the role of
ANXA1 in OSCC cell proliferation and invasion. The results
demonstrated that vector-mediated overexpression of ANXA1 in
Tca-8113 and SCC-9 cells induced a significant decrease in cell
growth and invasiveness, suggesting that ANXA1 inhibits OSCC cell
proliferation and invasion. Accumulating evidence has indicated
that the tumor-advancing effect of ANXA1 is tissue type-specific
(9,10). Through gain-of-function experiments,
the present study was the first to demonstrate that ANXA1 may act
as a tumor suppressor in OSCC, to the best of our knowledge. This
finding is consistent with those of previous clinical studies.
Further studies may be performed to investigate the effect of ANXA1
knockdown on OSCC progression.
EMT is a critical process in the development of
numerous types of tissue and organs (27). The importance of EMT in mediating
aggressiveness during carcinoma progression has attracted much
attention in recent years (28).
During EMT, epithelial cells lose their epithelial characteristics
and gain invasive properties to become mesenchymal-like cells
(29). ANXA1 has been demonstrated
to be important for epithelial differentiation in OSCC (19). Nomura et al (19) found that loss of ANXA1 occurs
frequently during oral carcinogenesis, and the loss of membranous
ANXA1 is correlated with a poor differentiation status of OSCC
cells. OSCC patients with nuclear localization of ANXA1 had poor
overall survival (30). TGFβ1 and
EGF are important regulators of the EMT. Previous studies have
demonstrated that EMT in OSCC is mediated by multiple growth
factors, and TGFβ1 and EGF co-stimulation induced phenotype
transition in OSCC cells (31,32). The
present study we further confirmed the involvement of ANXA1 in
TGFβ1/EGF-induced EMT. It was found that the expression of
E-cadherin, which is a key marker of the epithelial phenotype, was
increased in ANXA1-overexpressing Tca-8113 and SCC-9 cells. By
contrast, the expression of vimentin and N-cadherin, which are
mesenchymal cell markers, was decreased in ANXA1-overexpressing
Tca-8113 and SCC-9 cells. These results suggested that ANXA1
contributes to mesenchymal-to-epithelial transition, which is the
reverse process of EMT, in OSCC cells. TGFβ1/EGF treatment led to
downregulation of ANXA1 expression in OSCC cells. Furthermore, it
was found that TGFβ1/EGF-induced EMT in OSCC was attenuated by
ANXA1 overexpression. This finding is consistent with the role of
ANXA1 in EMT in breast cancer (23).
In conclusion, to the best of our knowledge, the
present study provided the first evidence that ANXA1 suppresses
cell proliferation and invasion of human OSCC cells in
vitro. Furthermore, it was demonstrated that TGFβ1/EGF-induced
EMT was reversed by ANXA1 in OSCC. ANXA1 was suggested to be a
potential marker for OSCC as well as a novel treatment. However,
further studies are required regarding the molecular mechanisms by
which ANXA1 exerts its role in OSCC.
References
|
1
|
Liu W, Wang YF, Zhou HW, Shi P, Zhou ZT
and Tang GY: Malignant transformation of oral leukoplakia: A
retrospective cohort study of 218 Chinese patients. BMC Cancer.
10:6852010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Camargo Cancela M, Voti L, Guerra-Yi M,
Chapuis F, Mazuir M and Curado MP: Oral cavity cancer in developed
and in developing countries: Population-based incidence. Head Neck.
32:357–367. 2010.PubMed/NCBI
|
|
3
|
Hunter KD, Parkinson EK and Harrison PR:
Profiling early head and neck cancer. Nat Rev Cancer. 5:127–135.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leemans CR, Braakhuis BJ and Brakenhoff
RH: The molecular biology of head and neck cancer. Nat Rev Cancer.
11:9–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gobbetti T and Cooray SN: Annexin A1 and
resolution of inflammation: Tissue repairing properties and
signalling signature. Biol Chem. 397:981–993. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Swa HL, Blackstock WP, Lim LH and
Gunaratne J: Quantitative proteomics profiling of murine mammary
gland cells unravels impact of annexin-1 on DNA damage response,
cell adhesion, and migration. Mol Cell Proteomics. 11:381–393.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guzmán-Aránguez A, Olmo N, Turnay J,
Lecona E, Pérez-Ramos P, de Silanes López I and Lizarbe MA:
Differentiation of human colon adenocarcinoma cells alters the
expression and intracellular localization of annexins A1, A2, and
A5. J Cell Biochem. 94:178–193. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rohwer N, Bindel F, Grimm C, Lin SJ,
Wappler J, Klinger B, Blüthgen N, Bois Du I, Schmeck B, Lehrach H,
et al: Annexin A1 sustains tumor metabolism and cellular
proliferation upon stable loss of HIF1A. Oncotarget. 7:6693–6710.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fatimathas L and Moss SE: Annexins as
disease modifiers. Histol Histopathol. 25:527–532. 2010.PubMed/NCBI
|
|
10
|
Guo C, Liu S and Sun MZ: Potential role of
Anxa1 in cancer. Future Oncol. 9:1773–1793. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang Y, Zhang C, Chen C, Sun S, Zheng H,
Wan S, Meng Q, Chen Y and Wei J: Investigation of circulating
antibodies to ANXA1 in breast cancer. Tumour Biol. 36:1233–1236.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin Y, Lin G, Fang W, Zhu H and Chu K:
Increased expression of annexin A1 predicts poor prognosis in human
hepatocellular carcinoma and enhances cell malignant phenotype. Med
Oncol. 31:3272014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boudhraa Z, Merle C, Mazzocut D, Chezal
JM, Chambon C, Miot-Noirault E, Theisen M, Bouchon B and Degoul F:
Characterization of pro-invasive mechanisms and N-terminal cleavage
of ANXA1 in melanoma. Arch Dermatol Res. 306:903–914. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boudhraa Z, Rondepierre F, Ouchchane L,
Kintossou R, Trzeciakiewicz A, Franck F, Kanitakis J, Labeille B,
Joubert-Zakeyh J, Bouchon B, et al: Annexin A1 in primary tumors
promotes melanoma dissemination. Clin Exp Metastasis. 31:749–760.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao Y, Chen Y, Xu D, Wang J and Yu G:
Differential expression of ANXA1 in benign human gastrointestinal
tissues and cancers. BMC Cancer. 14:5202014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Paweletz CP, Ornstein DK, Roth MJ, Bichsel
VE, Gillespie JW, Calvert VS, Vocke CD, Hewitt SM, Duray PH,
Herring J, et al: Loss of annexin 1 correlates with early onset of
tumorigenesis in esophageal and prostate carcinoma. Cancer Res.
60:6293–6297. 2000.PubMed/NCBI
|
|
17
|
Kang JS, Calvo BF, Maygarden SJ, Caskey
LS, Mohler JL and Ornstein DK: Dysregulation of annexin I protein
expression in high-grade prostatic intraepithelial neoplasia and
prostate cancer. Clin Cancer Res. 8:117–123. 2002.PubMed/NCBI
|
|
18
|
Faria PC, Sena AA, Nascimento R, Carvalho
WJ, Loyola AM, Silva SJ, Durighetto AF, Oliveira AD, Oliani SM and
Goulart LR: Expression of annexin A1 mRNA in peripheral blood from
oral squamous cell carcinoma patients. Oral Oncol. 46:25–30. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nomura H, Uzawa K, Yamano Y, Fushimi K,
Nakashima D, Kouzu Y, Kasamatsu A, Ogawara K, Shiiba M, Bukawa H,
et al: Down-regulation of plasma membranous Annexin A1 protein
expression in premalignant and malignant lesions of the oral
cavity: Correlation with epithelial differentiation. J Cancer Res
Clin Oncol. 135:943–949. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng TY, Wu MS, Lin JT, Lin MT, Shun CT,
Huang HY, Hua KT and Kuo ML: Annexin A1 is associated with gastric
cancer survival and promotes gastric cancer cell invasiveness
through the formyl peptide receptor/extracellular signal-regulated
kinase/integrin beta-1-binding protein 1 pathway. Cancer.
118:5757–5767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Babbin BA, Lee WY, Parkos CA, Winfree LM,
Akyildiz A, Perretti M and Nusrat A: Annexin I regulates SKCO-15
cell invasion by signaling through formyl peptide receptors. J Biol
Chem. 281:19588–19599. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fang Y, Guan X, Cai T, Long J, Wang H, Xie
X and Zhang Y: Knockdown of ANXA1 suppresses the biological
behavior of human NSCLC cells in vitro. Mol Med Rep. 13:3858–3866.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maschler S, Gebeshuber CA, Wiedemann EM,
Alacakaptan M, Schreiber M, Custic I and Beug H: Annexin A1
attenuates EMT and metastatic potential in breast cancer. EMBO Mol
Med. 2:401–414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu DW, Liu Y, Yang X, Yang CZ, Ma J, Yang
X, Qiao JK, Wang LZ, Li J, Zhang CP, et al: Low Annexin A1
expression predicts benefit from induction chemotherapy in oral
cancer patients with moderate or poor pathologic differentiation
grade. BMC Cancer. 13:3012013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu DW, Yang X, Yang CZ, Ma J, Liu Y, Yan
M, Wang LZ, Li J, Zhang CP, Zhang ZY and Zhong LP: Annexin A1
down-regulation in oral squamous cell carcinoma correlates to
pathological differentiation grade. Oral Oncol. 49:542–550. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang L, Yang X, Zhong LP, Zhou XJ, Pan
HY, Wei KJ, Li J, Chen WT and Zhang ZY: Decreased expression of
Annexin A1 correlates with pathologic differentiation grade in oral
squamous cell carcinoma. J Oral Pathol Med. 38:362–370. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Przybyla L, Muncie JM and Weaver VM:
Mechanical control of Epithelial-to-Mesenchymal transitions in
development and cancer. Annu Rev Cell Dev Biol. 32:527–554. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Diepenbruck M and Christofori G:
Epithelial-mesenchymal transition (EMT) and metastasis: Yes, no,
maybe?t. Curr Opin Cell Biol. 43:7–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin CY, Jeng YM, Chou HY, Hsu HC, Yuan RH,
Chiang CP and Kuo MY: Nuclear localization of annexin A1 is a
prognostic factor in oral squamous cell carcinoma. J Surg Oncol.
97:544–550. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Richter P, Umbreit C, Franz M and Berndt
A, Grimm S, Uecker A, Böhmer FD, Kosmehl H and Berndt A: EGF/TGFβ1
co-stimulation of oral squamous cell carcinoma cells causes an
epithelial-mesenchymal transition cell phenotype expressing laminin
332. J Oral Pathol Med. 40:46–54. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Diamond ME, Sun L, Ottaviano AJ, Joseph MJ
and Munshi HG: Differential growth factor regulation of N-cadherin
expression and motility in normal and malignant oral epithelium. J
Cell Sci. 121:2197–2207. 2008. View Article : Google Scholar : PubMed/NCBI
|