Introduction
Non-alcoholic fatty liver disease (NAFLD) is the
most common chronic liver disease (1). NAFLD is associated with the current
obesity pandemic, and ~20–33% of adults in developed countries
suffer from NAFLD (2). The term
NAFLD describes a spectrum of liver disease, which may develop from
fatty infiltration to steatohepatitis and hepatocellular carcinoma
(3) if effective intervention is
lacking. Hyperlipidemia-induced fatty infiltration and oxidative
injury are considered to be the major factors promoting the
occurrence and development of NAFLD (4–7).
Although controlling body weight with diet and exercise is
effective for NAFLD therapy (8),
drug treatment remains an important means of disease management.
Agents, including exenatide and statins (9), used for the treatment of diabetes and
hyperlipidemia are being tested as potential treatments for NAFLD
and non-alcoholic steatohepatitis (10). Considerable attention has been
focused on natural products as an alternative means of treating
NALFD; a numberof natural products are thought to have functions
that ameliorate the symptoms of NAFLD via the restoration of lipid
metabolism (11).
Polygoni Multiflori Radix, also known as Heshouwu
(HSW), a dried root of Polygonum multiflorum Thunb., is a
traditional Chinese medicine that has been used for supporting the
functions of the liver and kidney, and for regulating
hyperlipidemia for several decades (12). HSW is one of the most frequently used
crude drugs for the prevention and treatment of hyperlipidemia and
NAFLD (13,14), and a previous study revealed that HSW
exhibits a pronounced effect on lipid regulation in the treatment
of early-stage NAFLD (15).
Bioactive component analysis has revealed that HSW comprises
stilbenes, phenolic acid and flavonoids as potential lipase
inhibitors (16), and protocatechuic
acid and 2,3,5,4′-tetrahydroxy-stilbene-2-O-β-d-glucoside (TSG),
which exhibit antioxidant activity (17). Previous studies have demonstrated
that TSG has good hypolipidemic effects, particularly in the
reduction of low-density lipoprotein-cholesterol (LDL-C) via the
promotion of intracellular cholesterol 7α-hydroxylase (CYP7α)
expression (18–20), and is able to reverse NAFLD through
gut microbiota and toll-like receptor 4/nuclear factor-κB (NF-κB)
pathway modulation (21).
The present authors' research group has focused on
the bioactive component analysis, separation, pharmacodynamics and
toxicology evaluation of HSW for a number of years, with a
particular focus on TSG. In a previous study by the present group,
an extract containing >50% TSG was obtained using a macroporous
resin. A dose-dependent anti-hyperlipidemic effect was observed for
this extract in pharmacodynamic experiments, and a 9-month
long-term toxicity test of beagles revealed that a dosage of 1.0
g/kg/day is safe (data not published). Pharmacokinetic studies
revealed that TSG was rapidly absorbed and widely distributed
throughout the body with great efficiency, followed by rapid
elimination and clearance (22), and
indicated that the liver was the organ containing the highest
amount of TSG (23,24). All the aforementioned factors
indicate that TSG is a potential candidate for anti-NAFLD drug
development.
Previous studies concerning the anti-NAFLD related
effects of TSG have focused on the active component (25) and on a single effect, including lipid
regulation and anti-inflammatory functions (26,27), and
no comprehensive evaluation of the effect of TSG on anti-NAFLD
using multiple indices has been reported. Thus, the present study
used an NAFLD model induced by a high-fat diet (HFD) with fructose
drinking to systematically assess the effects of the TSG-rich
fraction (TSGP) of HSW in the prevention of NAFLD. This was
assessed with the aim of elucidating the main efficacy, indices and
the potential mechanisms of this composition.
Materials and methods
Reagents
TSGP was prepared through an adaptation of a
previous extraction process (28),
with several modifications. Briefly, Polygoni Multiflori Radix
(purchased from Zhejiang Chinese Medical University, Zhejiang,
China) was crushed and extracted with 60% (v/v) ethanol by a
refluxing method. Following concentration via evaporation, the
fluid ethanolic extract was subjected to open column chromatography
(1.5 m ×22 cm) with a macroporous resin (NKA II, The Chemical Plant
of Nankai University). The column was eluted stepwise with 10, 20
and 50% (v/v) ethanol solution. The 50% eluted fraction was
collected, concentrated and dried under vacuum conditions. The
content of TSG in this fraction was 54%, which was determined using
high-performance liquid chromatography with diode-array detection
(Agilent 1100 series; Agilent Technologies, Inc., Santa Clara, CA,
USA; Fig. 1). The separation was
achieved using an Ultimate XB-C18 column (150×4.6 mm ×5 µm; Welch
Materials, Inc., Austin, TX, USA) at 25°C with acetonitrile and
H2O2 (20:80 v/v) as mobile phase at flow rate
1.0 ml/min, and the sample injection volume was 5 µl. The HFD
consisted of standard fodder 76.5%, lard 12%, cholesterol 1%, yolk
powder 5%, whole milk powder 5% and cholate 0.5%, and was
formulated by the Animal Supply Centre of Zhejiang Academy of
Medical Science (Hangzhou, China).
Animals and treatments
A total of 38 male, 8-week-old Sprague-Dawley rats,
weighing between 180 and 200 g, were purchased from the animal
supply centre of Zhejiang Academy of Medical Science [certificate
no.: SCXK (Zhe)2014-0001, Hangzhou, China]. The animals were housed
at 25±1°C with humidity of 55±5%, and exposed to a 12-h light/dark
cycle for 1-week acclimatization prior to the experiment. All rats
were fed rodent laboratory chow with tap water ad libitum
and were fasted but had free access to water for 12 h prior to the
experiment. All procedures were conducted in strict accordance with
the Chinese legislation on the use and care of laboratory animals
and with the Animal Management Rules of the Health Ministry of PR
China (document no. 55, 2001). The study was approved by the Ethics
Committee of Zhejiang Chinese Medical University.
Animals were divided into the normal (n=10), model
control (n=10), positive control (polyene phosphatidylcholine, PPC;
n=10) and TSGP (n=8) groups according to their blood lipid levels,
which were measured prior to the experiment. Animals were provided
with free access to water and those in the normal group were fed a
control diet (CD), while those in the model, PPC and TSGP group
were fed the HFD with 10% fructose solution for 18 weeks. Distilled
water was provided to the rats in the normal and model control
groups, while the positive control and TSGP groups were
administered 136.8 mg/kg PPC preparation (Essentiale;
Sanofi-Aventis Beijing Pharmaceutical Co. Ltd., Beijing, China) and
160 mg/kg TSGP, respectively. All the water or test substances were
orally administrated once daily. Throughout the study, animals were
weighed once weekly, and the levels of total cholesterol (TC),
triglyceride (TG), high-density lipoprotein-cholesterol (HDL-C),
LDL-C, serum alanine aminotransferase (ALT) and aspartate
aminotransferase (AST) were measured at 4, 6, 10, 14 and 18 weeks.
At the end of the experiment all animals were anesthetized and
sacrificed. Blood was collected from the abdominal aorta and
centrifuged at 1,500 × g for 15 min at 4°C to separate the serum,
and liver tissue was harvested for histopathology, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analysis.
Biochemical assays and enzyme-linked
immune sorbent assay (ELISA)
ALT, AST, TC, TG, HDL-C, LDL-C, serum creatinine,
blood urea nitrogen, uric acid and glucose levels were measured
usinga fully automatic blood biochemistry analyzer (Toshiba
TBA-40FR; Toshiba Medical Systems Corporation, Otawara, Japan).
Serum apolipoprotein A-I (apoA1), apolipoprotein B (apoB), cholic
acid (CA), cholesterol ester, CYP7α1, lecithin-cholesterol
acyltransferase, insulin, adiponectin, leptin, nitric oxide (NO,
20150115), heme oxygenase-1,tumor necrosis factor α, interleukin-6,
endothelins, thromboxane, 6-keto-prostaglandinF1α (6-Keto-PGF1α),
liver free fatty acid (FFA), lower-density lipoprotein receptor
(LDL-R), superoxide dismutase (SOD, 20150101), malondialdehyde
(MDA, 20150112), glutathione (GSH), catalase (CAT, 20150115,
purchased from Nanjing Jiancheng Bioengineering Institute, Nanjing,
China), and β-hydroxy-β-methylglutaryl-coenzyme A (HMG-CoA), acyl
coenzyme A-cholesterol acyltransferase (ACAT), transforming growth
factor β1(TGF-β1) and NF-κB (20150101, obtained from Shanghai
Yuanye Biotechnology Co., Ltd., Shanghai, China) levels were
analyzed using ELISA kits according to the manufacturer's
instructions.
Liver histopathological
examination
The left lobe of the liver was fixed in 10% neutral
formalin for 48 h at 25–27°C, dehydrated in a 70–100% gradient of
ethyl alcohol, dealcoholized in xylene, embedded in paraffin and
sectioned (5-µm thickness). Tissue slides were deparaffinized in
xylene, rehydrated in a reverse-gradient series of ethyl alcohol
and stained with hematoxylin for 3 min and eosin for 1 min
(H&E; Merck KGaA, Darmstadt, Germany). Pathological changes
were observed under a light microscope with an advanced 3.2 image
analysis system (Motic China Group Co., Ltd., Xiamen, China).
RT-qPCR
The total RNA in liver tissue was extracted using
TRIzol (Thermo Fisher Scientific, Inc., Waltham, MA, USA). cDNA was
synthesized by reverse transcription (RT) using random hexamer
primers (Verso cDNA kit; Thermo Fisher Scientific, Inc.). The RT
system consisted of 1 µl M-mlv, 4 µl 5X RT Buffer, 1 µl Rnase A
inhibitor, 1 µl OligdT, 1 µl dNTP, and added Rnase-free water up to
20 µl. The reaction conditions were 42°C for 45 min and 70°C for 10
min. qPCR was performed using mRNA against the housekeeping gene
18s as an internal control. qPCR was performed by the TaqMan method
with RQ1 Rnase-Free Dnase (cat. no. M6101, Promega Corp, Madison,
WI, USA), and fluorescence biotin quantitation kit (cat. no.
PM10003, Hangzhou Biosci Biotech Co., Ltd., Hangzhou, China)
coupled with a Step One Plus Real-Time PCR System (Agilent
Stratagene Mx3005P; Agilent Technologies, Inc., Santa Clara, CA,
USA). The qPCR system consisted of 10 µl 2X qPCR mix, 0.4 µl
forward primer, 0.4 µl reverse primer, 0.4 µl cDNA, 8.8 µl
nuclease-free water (total volume 20 µl). Thermocycler conditions
were as follows: 94°C for 1 min, 95°C for 10 sec, 58°C for 10 sec,
72°C for 10 sec (40 cycles). A melting curve was also constructed
to ensure that only a single product was amplified. The sequences
of the primers used are provided in Table I. Furthermore, the relative mRNA
expression was calculated following normalization of values to that
of β-actin, and the relative amounts of the RNAs were calculated
using the comparative Cq method (29).
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Primer | Direction | Sequence
(5′-3′) |
|---|
| PPARα | Forward |
GCTTCATCACCCGAGAGTTC |
|
| Reverse |
GGGAAATGTCACTGTCATCCA |
| CYP2E1 | Forward |
TCTGCTCCTGTCTGCTATTCTG |
|
| Reverse |
ACTGCCAAAGCCAACTGTGA |
| β-actin | Forward |
GCTCTCTTCCAGCCTTCCTT |
|
| Reverse |
GGTCTTTACGGATGTCAACG |
Western blot analysis
100 mg liver tissues were ground with liquid
nitrogen and the total protein was extracted with a total protein
extraction kit (KGP250, Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China). In brief, the ground liver tissue was added with 0.5 ml
lysis buffer containing 10 mM Trise HCl (pH 7.5), 10 µl 0.25 M
sucrose and protease inhibitors, followed lysis for 10 min on ice
and centrifugation at 20,392 × g for 5 min at 4°C. The total
proteins were quantified by the Bradford method with a protein
quantitation kit (cat. no. MR04001, Hangzhou Biosci Biotech Co.,
Ltd.). Protein (60 µg/lane) was separated by 10% SDS-PAGE and
transferred onto polyvinylidene fluoride membranes (EMD Millipore;
Billerica, MA, USA). The membranes were blocked with 5% skimmed
milk in Tris-buffered saline containing 0.05% Tween-20 for 2 h at
room temperature. Following overnight incubation at 4°C with
primary antibodies PPRA-α (cat. no. SC-9000, Santa Cruz
Biotechnology, Inc., Dallas, TX, USA; dilution ratio, 1:500),
CYP2E1 (cat. no. SC-133491, Santa Cruz Biotechnology, Inc.;
dilution ratio, 1:500) and β-actin (cat. no. 4970, Cell Signaling
Technology, Inc., Danvers, MA, USA; dilution ratio, 1:1,000), the
membranes were incubated with HRP-conjugated rabbit anti-mouse IgG
(Cell Signaling Technology, Inc.) for 1 h at room temperature.
Immunodetection was performed with Amersham enhanced
chemiluminescence detection reagent (GE Healthcare, Chalfont St.
Giles, UK), with β-actin used as an internal control. The
expression levels were quantified by ImageJ 1.46r image analysis
software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
The obtained data were imported into SIMCA-P 11.5
(Umetrics AB, Umea, Sweden) for principal component analysis (PCA)
and orthogonal partial least squares discriminant analysis
(OPLS-DA). PCA was used to visualize whether the groups could be
differentiated on the basis of pharmacodynamic indices. PCA was
used to differentiate the characteristic indices and was conducted
using MATLAB 7.10 (The Math Works, Inc., Natick, MA, USA). Data
were auto-scaled prior to performing PCA. Variable importance for
projection (VIP) values produced during OPLS-DA were applied to
identify potential effective indices, and variables with VIP values
>1 were considered to be significant. All values were expressed
as the mean ± standard deviation. One-way analysis of selected
variance with least-significant difference post hoc analysis
multiple comparisons was applied to compare the differences amongst
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of TSGP on body weight (BW),
liver weight/BW ratio and liver histology
The effect of TSGP on the BW of the rats fed with a
HFD for 18 weeks was investigated, and a significant reduction in
the mean BW was observed in the TSGP group compared with the HFD
group after 3 weeks of feeding. The final mean BW of rats in the
TSGP group was significantly lower than that of the model group
(345.6±18.9 vs. 391.7±42.1 g; P<0.05; Fig. 2A). The liver weight/BW ratio was
significantly increased in the model group compared with the normal
control group (P<0.01), and the TSGP group presented a
significantly lower liver weight/BW ratio compared with the model
group (P<0.01; Fig. 2B).
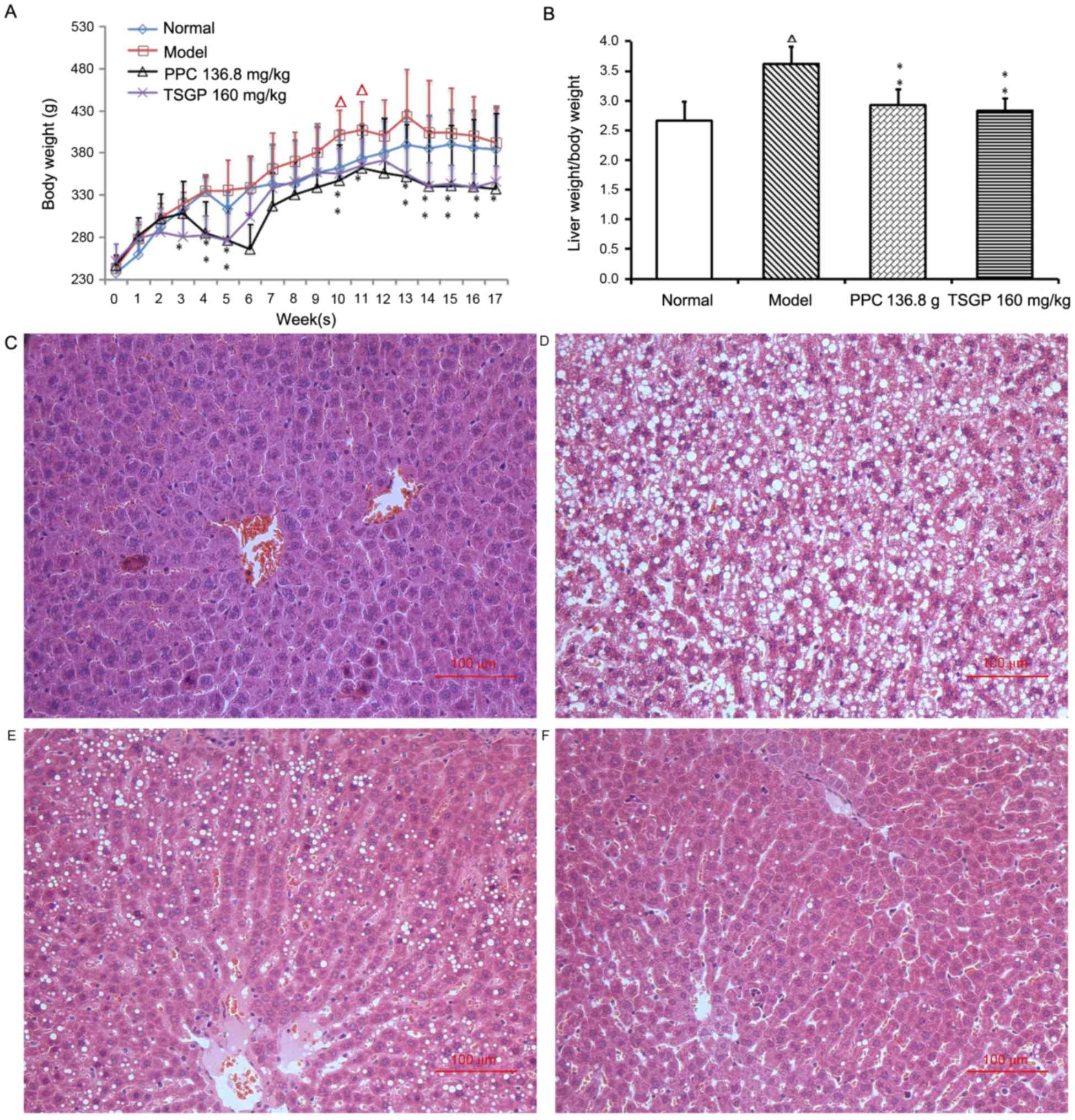 | Figure 2.Effects of TSGP on body weight, liver
weight/body weight ratio and liver histopathology. (A) Body weight
and (B) liver weight/body weight ratio. Data are presented as the
mean ± standard error of the mean. (C-F) Representative images of
liver histopathology (hematoxylin and eosin staining,
magnification, ×200) for the (C) normal, (D) model, (E) PPC 136.8
mg/kg and (F) TSGP 160 mg/kg groups. ΔP<0.05 vs. the
normal control group; *P<0.05 and **P<0.01 vs. the model
group. TSGP, 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside rich
fraction; PPC, polyene phosphatidylcholine. |
Representative images of liver histology are
depicted in Fig. 2C-F. The animals
in the normal group presented normal liver histology, hepatocytes
were observed with a common radial array encircling the central
veins and no hepatocyte lipid degeneration was observed (Fig. 2C). In the model group, the lobular
structures of hepatocytes were disrupted, and inflammatory cell
infiltration and evident lipid droplets were visible in the hepatic
plates (Fig. 2D). These
histopathological variations revealed that the NAFLD rat model was
established successfully. Compared with the model group, PPC and
TSGP markedly reduced the hepatic steatosis and vacuolar
degeneration and effectively alleviated the degree of NAFLD lesions
(Fig. 2E and F).
In vivo pharmacodynamic analysis
PCA and OPLS-DA, which are unsupervised and
supervised pattern recognition methods for the multivariate
statistics of mass data, were performed to explore the differences
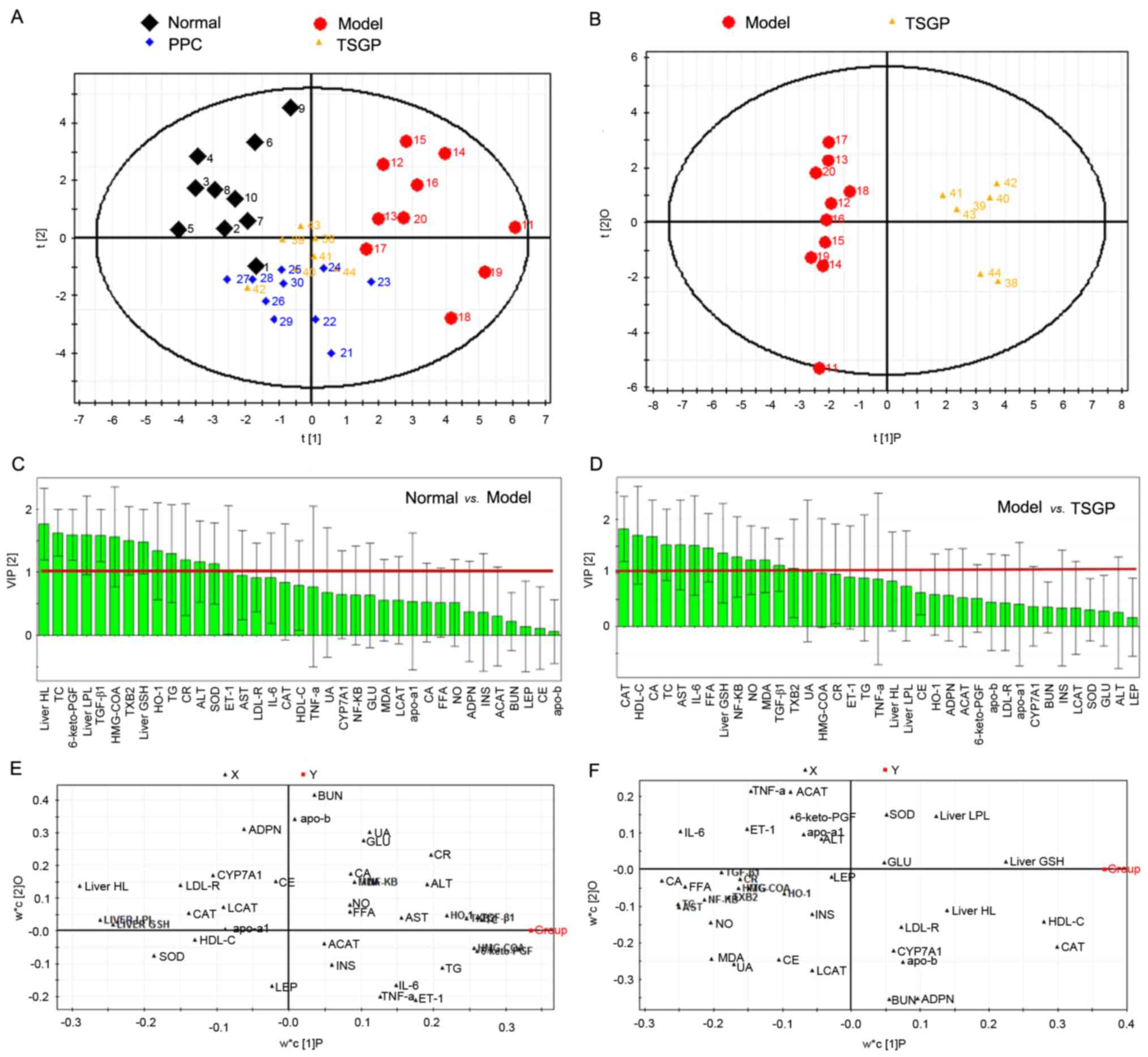
of indices among the groups. The PCA score plot (Fig. 3A) visibly demonstrated the
distribution for the four groups. The clear separation between the
normal control and model groups implied that the NAFLD model was
established successfully. The OPLS-DA score plot (Fig. 3B) confirmed that there was an evident
difference between the model and TSGP groups. In addition, the
small overlap of the normal and TSGP groups indicated amelioration
of the condition of rats treated with TSGP, indicating that TSGP
has an inhibitory effect on NAFLD development. VIPs of the OPLS-DA
results are presented in Fig. 3C and
D. CAT, HDL-C, CA, TC, FFA, liver GSH, NF-κB, NO and MDA, which
had VIP values for the OPLS-DA of >1, were selected as
significant indicators demonstrating a clear difference between the
model and TSGP groups. OPLC-DA loading plots (Fig. 3E and F) also revealed that these
indices were far from the origin. The differential abundance of
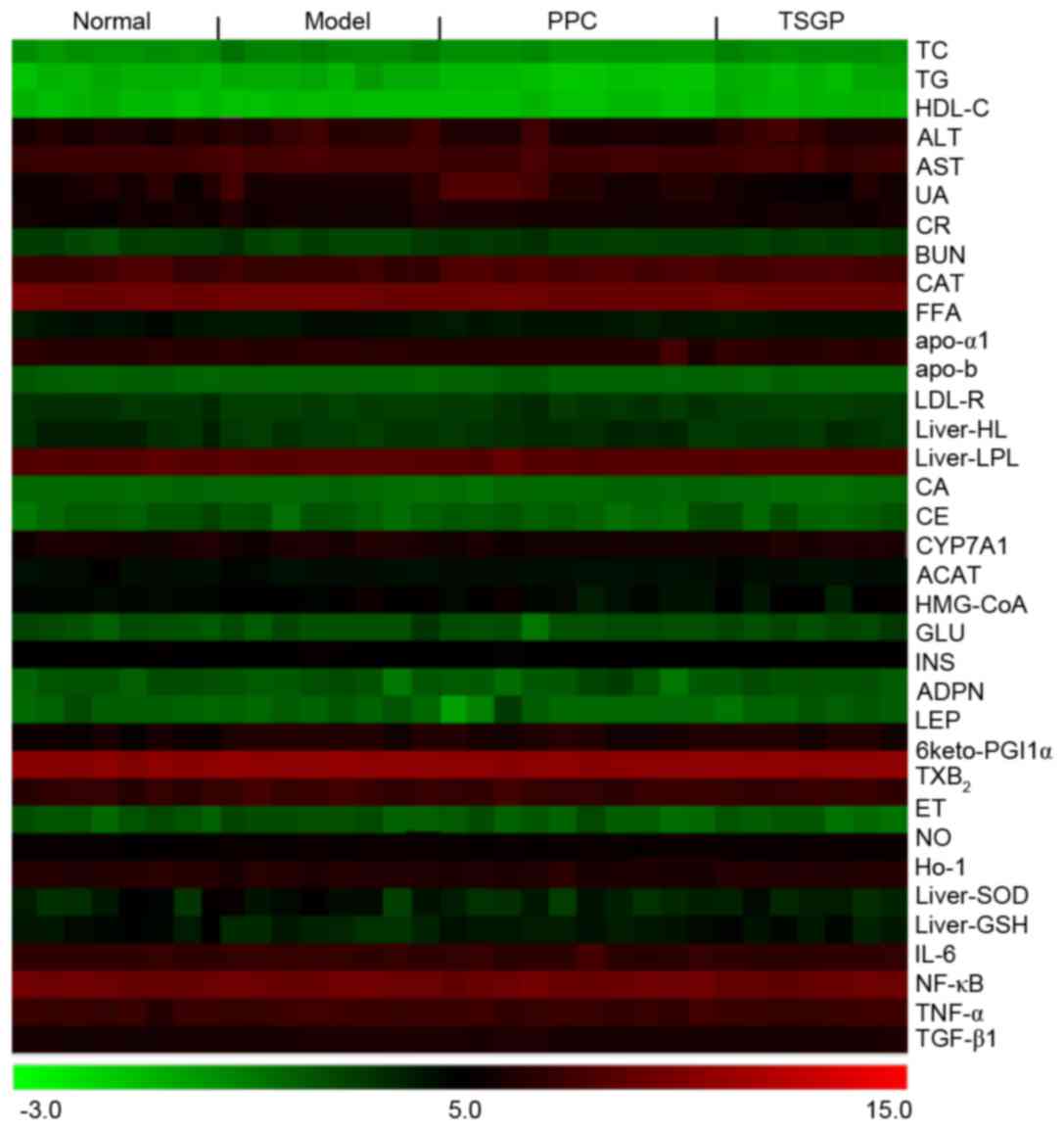
indices presented in a heat map (Fig.
4) confirmed that TC, HDL-C, CAT, SOD and MDA are the main
pharmacodynamic indicators of the anti-NAFLD effects of TSGP.
Therefore, the present study focused on the antioxidation
properties of TSGP in experimental NAFLD.
Effects of TSGP on serum TG, TC, HDL-C
and ALT levels
Serum TC and TG levels were significantly elevated
in the model group compared with the normal group from week 2 to 18
(P<0.05) and TSGP significantly lowered the levels of TC on
weeks 14 and 18 (P<0.01) and of TG (P<0.05) from week 10 to
18 (Fig. 5A and B). The serum HDL-C
levels in animals fed with a HFD were slightly lower compared with
those in normal rats, and no differences were observed between the
TSGP and model groups, with the exception of at the end of week 14
and week 18 (P<0.05 and P<0.01, respectively; Fig. 5C). Furthermore, the ALT levels in
model rats revealed a tendency to increase compared with those in
normal control rats, with a significant increase in week 14
(P<0.05), but no significant difference was observed between the
TSGP and model groups (Fig. 5D).
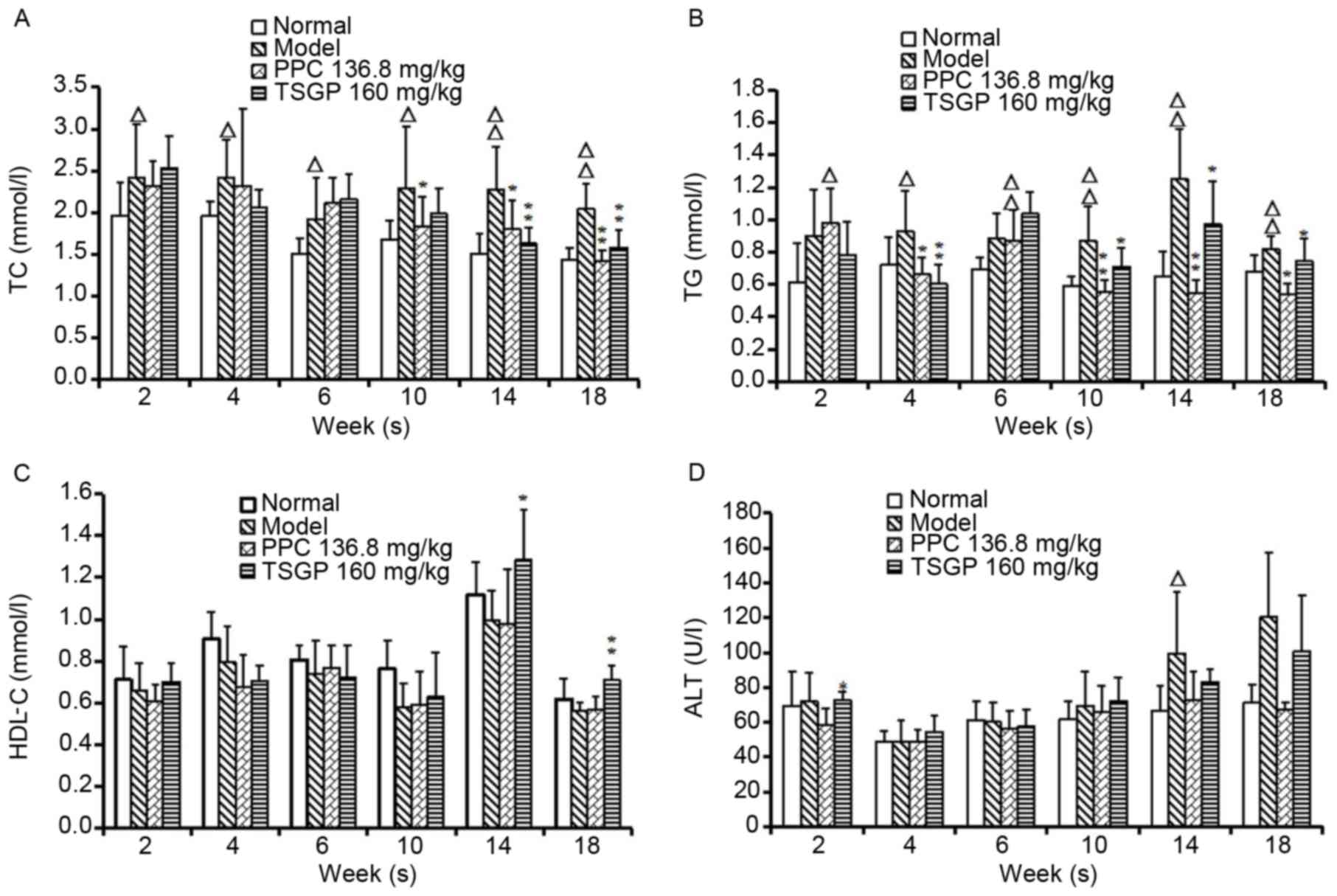 | Figure 5.Levels of serum lipids and ALT
measured at different time points throughout the study period.
Serum (A) TC, (B) TG, (C) HDL-C and (D) ALT levels.
ΔP<0.05 and ΔΔP<0.01 vs. the normal
control group; *P<0.05 and **P<0.01 vs. the model group. ALT,
alanine aminotransferase; TC, total cholesterol; TG, triglyceride;
HDL-C, high-density lipoprotein-cholesterol; PPC, polyene
phosphatidylcholine; TSGP,
2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside rich fraction. |
Effects of TSGP on hepatic MDA, FFA,
CAT, SOD and GSH levels
MDA and FFA were significantly elevated in model
rats compared with the normal group at the end of the experiment
(P<0.05), and TSGP significantly lowered the levels of MDA and
FFA (P<0.01; Fig. 6A and B). By
contrast, the levels of SOD, CAT and GSH in the liver of model rats
were lower than those in normal rats fed with a CD, although the
reduction was only significant for CAT (P<0.05; Fig. 6C-E). Finally, TSGP revealed a
significant elevation of CAT levels compared with the model group
(P<0.01; Fig. 6D), but no
significant differences in the SOD and GSH levels between the TSGP
and model groups were observed.
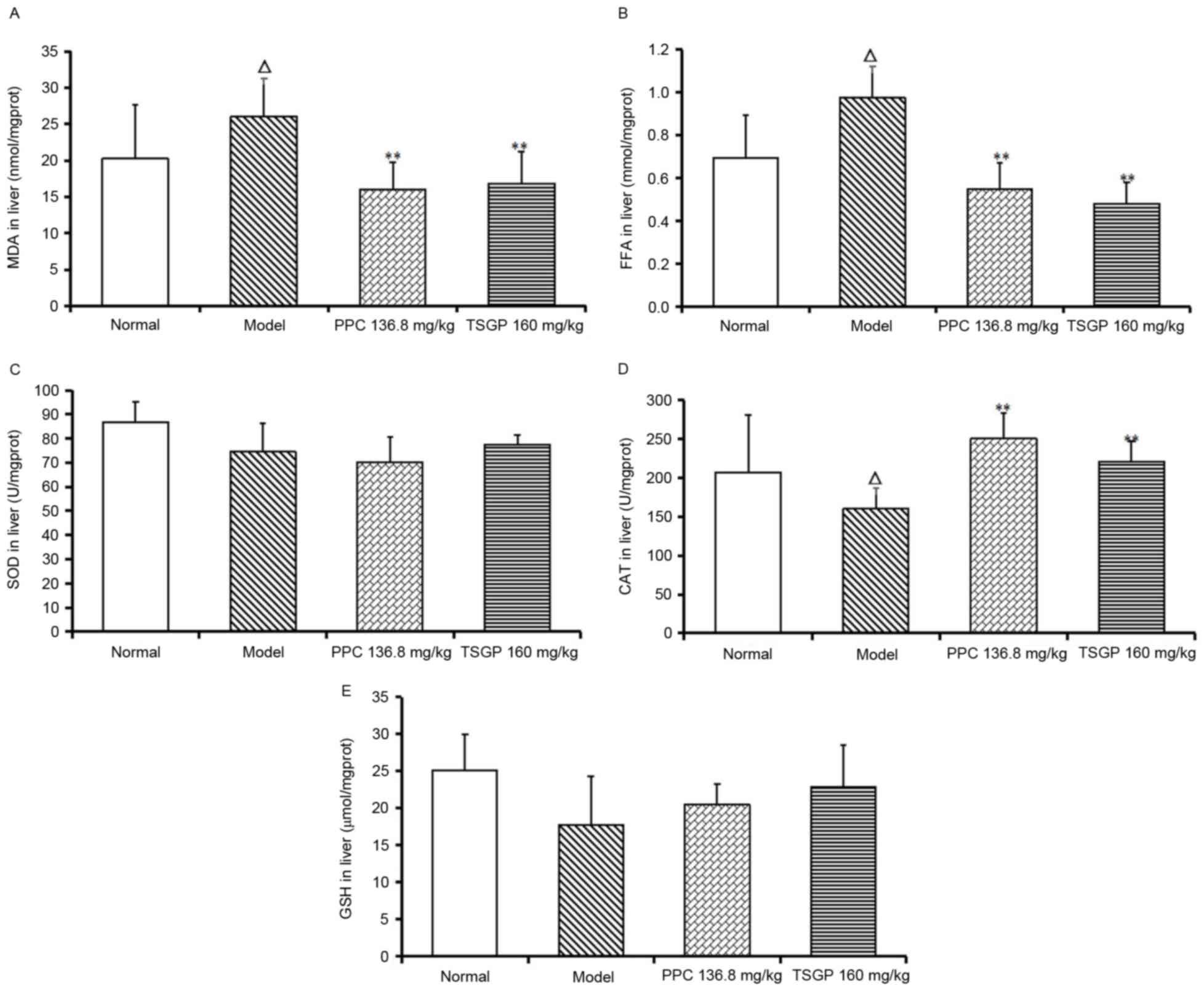 | Figure 6.Levels of MDA, FFA, CAT, SOD and GSH
in liver tissue at the end of the experiment. (A) MDA, (B) FFA, (C)
SOD, (D) CAT and (E) GSH levels in the liver. ΔP<0.05
vs. the normal control group; **P<0.01 vs. the model group. MDA,
malondialdehyde; FFA, free fatty acid; CAT, catalase; SOD,
superoxide dismutase; GSH, glutathione; PPC, polyene
phosphatidylcholine; TSGP,
2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside rich fraction. |
RT-qPCR and western blot analysis
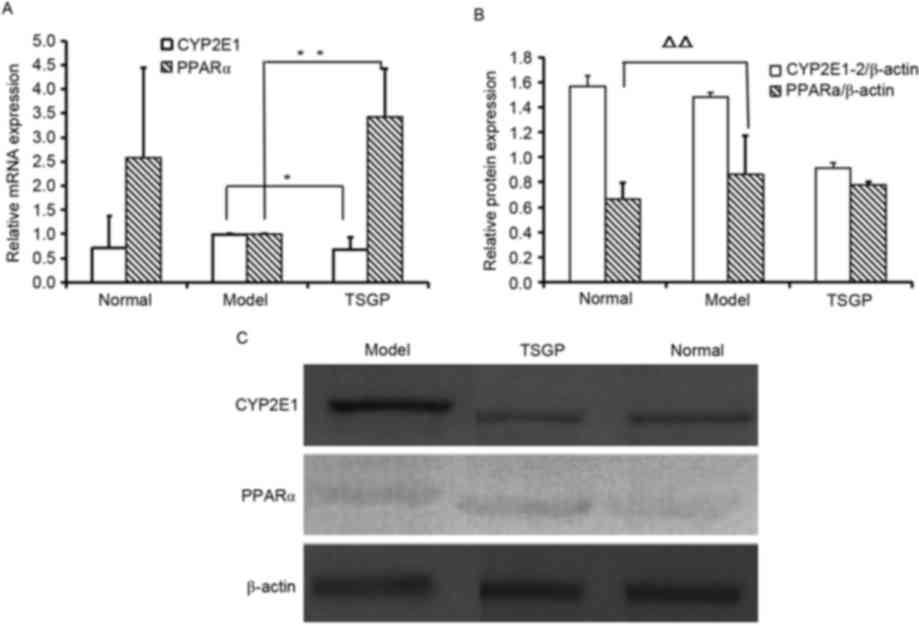
mRNA and protein expression of cytochrome P450 2E1
(CYP2E1) and peroxisome proliferator-activated receptor α (PPARα)
were tested. Compared with the control rats fed a CD, the mRNA
levels of CYP2E1 in model rats fed with a HFD were upregulated and
those of PPARα were downregulated, although neither change was
significant, and TSGP reversed the changes in expression of CYP2E1
and PPARα mRNA that were observed in the model rats (P<0.05 and
P<0.01, respectively; Fig. 7A).
Additionally, TSGP reduced CYP2E1 protein expression compared with
that in the model group, although the reduction was not
significant, and no significant difference in PPARα protein
expression was observed between the TSGP and model rats (Fig. 7B).
Discussion
The present study investigated the effects of TSGP
on an established experimental NAFLD model induced by a high-fat
high-cholesterol diet with fructose drinking. A single dataset
analysis may be limited and insufficient to provide a holistic
picture of the phenomenon being studied; therefore, the mode
recognition methods PCA and OPLS-DA were applied to analyze the
mass of data obtained in the present study. PCA is a powerful and
versatile method capable of providing an overview of complex
multivariate data and is used to reveal an association between
variables and relations between sample patterns (30). OPLS-DA is an improved partial least
squares method and is a powerful tool for distinguishing the
classes of observations and providing a meaningful interpretation
of the differences observed (31).
PCA and OPLS-DA have been widely used in
chemometrics and omics data analysis, and in the present study,
they were used to evaluate the overall efficacy of TSGP in NAFLD
models and to identify the main iconic pharmacodynamics indicators.
As the results demonstrate, a clear separation among the normal
control and model rats, and the model and TSG-treated rats was
observed in PCA and OPLS-DA analysis, indicating successful
construction of the NAFLD model and an efficient protective effect
of TSGP against NAFLD. VIP and loading plots for OPLS-DA enabled
the detection of characteristic indices, including serum TC and
liver GSH levels amongst rats in the normal, NAFLD and TSGP-treated
groups. The results of further analysis revealed that TSGP
significantly inhibited the elevation of serum TC and TG in the
later stages of NAFLD induction. TSGP treatment also mitigated
hepatic enlargement and alleviated liver steatosis. It also
exhibited the effects of a HFD on the levels of hepatic MDA, FFA,
CAT, and a significant reduction of CYP2E1 mRNA expression and
elevation in PPARα mRNA expression were observed. These data
indicate that TSGP in the context of the model of the present study
has a good effect in NAFLD prevention.
A previous study revealed that lipid metabolism and
oxidative stress are critical in the development of HFD-triggered
NAFLD (32). During metabolic
processing, FFAs released into the liver stimulate the expression
of the key factor controlling cholesterol synthesis
(3-hydroxy-3-methylglutaryl-coenzyme A reductase) as well as the
critical fatty acid synthesis factors, such as sterol regulatory
element binding protein 1c, which promote the synthesis of liver
cholesterol and TG (33,34). The inhibition of PPARα reduces the
metabolism of fatty acids and leads to the development of NAFLD
(35), while an excess of hepatic
FFA upregulates CYP2E1 expression and increases the production of
reactive oxygen species, subsequently inducing oxidative stress
(36), followed by intracellular
superoxide species production and hepatic injury. Elevated MDA and
reduced SOD, CAT and GSH-peroxidase (GSH-Px) are the common
biomarkers of oxidative stress in vivo (37–39).
High serum MDA levels are considered a key feature in liver injury
(40). SOD, CAT and GSH-Px are three
enzymes that act against oxidative stress by catalyzing the
dismutation, decomposition and reduction of superoxide anions,
H2O2 and hydroperoxides into non-toxic
products (41,42) to eliminate intracellular superoxide
species and thus prevent hepatic injury.
The current results revealed that hepatic FFA and
MDA levels, and the expression of CYP2E1 mRNA were elevated
significantly, while CAT, SOD and GSH-Px activities and the
expression of PPARα mRNA were decreased (although only the
reduction in CAT activity was statistically significant) in
response to HFD intake, indicating that oxidative damage existed in
the liver. A previous report has revealed that TSG exhibited
antioxidant activity on ROS (17),
and the experiments in the present study revealed that
administration of TSGP significantly decreased liver FFA and MDA
levels, inhibited CYP2E1 mRNA expression, promoted the activity of
CAT and the expression of PPARα mRNA in the liver of rats with
NAFLD. These changes reduced oxidative stress in NAFLD model rats
and improved the symptoms. Therefore, it is suggested that the
administration of TSGP effectively protects against HFD-induced
hepatic lipid peroxidation via hepatic antioxidant enzyme
regulation.
Overall, the present study demonstrated that PCA and
OPLS-DA were useful in the systematic assessment of the protective
effect of TSGP against experimental NAFLD using a multi-index
analysis. Blood lipid regulation and hepatic lipid blocking may be
the main mechanisms underlying the effects of TSGP in NAFLD
prevention. Therefore, the present study provides a basis for the
application of TSGP in the treatment of NAFLD and for novel
anti-NAFLD drug development.
Acknowledgements
The present study was supported by the China
National Natural Science Foundation (grant no. 81503328), the
National Science and Technology on New Drug Creation and
Development Projects (grant no. 2011ZX09101-002-07), Zhejiang
Provincial Natural Science Foundation of China (grant nos.
LY15H280007, LY14H270008 and LQ17H280004) and Science and
Technology Project of Zhejiang Province (grant no. 2016C33184).
References
|
1
|
Tiniakos DG, Vos MB and Brunt EM:
Nonalcoholic fatty liver disease: Pathology and pathogenesis. Annu
Rev Pathol. 5:145–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Preiss D and Sattar N: Non-alcoholic fatty
liver disease: An overview of prevalence, diagnosis, pathogenesis
and treatment considerations. Clin Sci (Lond). 115:141–150. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kopec KL and Burns D: Nonalcoholic fatty
liver disease: A review of the spectrum of disease, diagnosis, and
therapy. Nutr Clin Pract. 26:565–576. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chalasani N, Deeg MA and Crabb DW:
Systemic levels of lipid peroxidation and its metabolic and dietary
correlates in patients with nonalcoholic steatohepatitis. Am J
Gastroenterol. 99:1497–1502. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Day CP and James OF: Steatohepatitis: A
tale of two ‘hits’? Gastroenterology. 114:842–845. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Phung N, Pera N, Farrell G, Leclercq I,
Hou JY and George J: Pro-oxidant-mediated hepatic fibrosis and
effects of antioxidant intervention in murine dietary
steatohepatitis. Int J Mol Med. 24:171–180. 2009.PubMed/NCBI
|
|
7
|
Seki S, Kitada T, Yamada T, Sakaguchi H,
Nakatani K and Wakasa K: In situ detection of lipid peroxidation
and oxidative DNA damage in non-alcoholic fatty liver diseases. J
Hepatol. 37:56–62. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zivkovic AM, German JB and Sanyal AJ:
Comparative review of diets for the metabolic syndrome:
Implications for nonalcoholic fatty liver disease. Am J Clin Nutr.
86:285–300. 2007.PubMed/NCBI
|
|
9
|
Chalasani N, Younossi Z, Lavine JE, Diehl
AM, Brunt EM, Cusi K, Charlton M and Sanyal AJ; American
Gastroenterological Association; American Association for the Study
of Liver Diseases; American College of Gastroenterology, : The
diagnosis and management of non-alcoholic fatty liver disease:
Practice guideline by the American Gastroenterological Association,
American Association for the Study of Liver Diseases, and American
College of Gastroenterology. Gastroenterology. 142:1592–1609. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakajima K: Multidisciplinary
pharmacotherapeutic options for nonalcoholic Fatty liver disease.
Int J Hepatol. 2012:9506932012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu JY, Zhang L, Li ZP and Ji G: Natural
products on nonalcoholic fatty liver disease. Curr Drug Targets.
16:1347–1355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pharmacopoeia CoC: Pharmacopoeia of the
People's Republic of China. China Medical Science Press; Beijin:
2015
|
|
13
|
Chen CQ and Zhang TJ: Regulation of Blood
Lipid Research and Application of Modern Traditional Chinese
Medicine. People's Medical Publishing House; Beijing: 2007, (In
Chinese).
|
|
14
|
Jiang LD, He YS, Chen X, Tao O, Li GY and
Zhang YL: Study on anti-hyperlipidemia mechanism of high frequency
herb pairs by molecular docking method. Zhongguo Zhong Yao Za Zhi.
40:2413–2419. 2015.(In Chinese). PubMed/NCBI
|
|
15
|
Li N, Chen Z, Mao X, Yu J and Zhao R:
Effects of lipid regulation using raw and processed radix polygoni
multiflori in rats fed a high-fat diet. Evid Based Complement
Alternat Med. 2012:3291712012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang YX, Ge AH, Jiang Y, Azietaku J Teye,
Li J and Gao XM: A Bioactivity-based method for screening,
identification of lipase inhibitors, and clarifying the effects of
processing time on lipase inhibitory activity of Polygonum
multiflorum. Evid Based Complement Alternat Med. 2016:59650672016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen HF, Chen YH, Liu CH, Wang L, Chen X,
Yu BY and Qi J: Integrated chemometric fingerprints of antioxidant
activities and HPLC-DAD-CL for assessing the quality of the
processed roots of Polygonum multiflorum Thunb. (Heshouwu). Chin
Med. 11:182016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang M, Zhao R, Wang W, Mao X and Yu J:
Lipid regulation effects of Polygoni Multiflori Radix, its
processed products and its major substances on steatosis human
liver cell line L02. J Ethnopharmacol. 139:287–293. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu QL, Xiao JH, Ma R, Ban Y and Wang JL:
Effect of 2,3,5,4′-tetrahydroxystilbene-2-O-beta-D-glucoside on
lipoprotein oxidation and proliferation of coronary arterial smooth
cells. J Asian Nat Prod Res. 9:689–697. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang W, He Y, Lin P, Li Y, Sun R, Gu W, Yu
J and Zhao R: In vitro effects of active components of Polygonum
Multiflorum Radix on enzymes involved in the lipid metabolism. J
Ethnopharmacol. 153:763–770. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin P, Lu J, Wang Y, Gu W, Yu J and Zhao
R: Naturally occurring stilbenoid TSG reverses non-alcoholic fatty
liver diseases via gut-liver axis. PLoS One. 10:e01403462015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lv G, Gu H, Chen S, Lou Z and Shan L:
Pharmacokinetic profile of
2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside in mice after oral
administration of Polygonum multiflorum extract. Drug Dev Ind
Pharm. 38:248–255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lou ZH, Lu GY, Chen SH and Gu H:
Determination of 2,3,5,4′-tetrahydroxy-stilbene-2-O-β-D-glucoside
in Shouwugan preparation and its tissue distribution in mouse.
Zhongguo Xian Dai Ying Yong Yao Xue. 28:89–91. 2011.(In
Chinese).
|
|
24
|
Lv G, Lou Z, Chen S, Gu H and Shan L:
Pharmacokinetics and tissue distribution of
2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside from traditional
Chinese medicine Polygonum multiflorum following oral
administration to rats. J Ethnopharmacol. 137:449–456. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dong LH, Guo PP, Yan WY, Yang H and Wang
CY: Comparative study on hypolipidemic effect of
cis-2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside and
trans-2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside. Shenyang Yao
Ke Da Xue Xue Bao. 31:989–993. 2014.(In Chinese).
|
|
26
|
Wang CY, Zhang LT, Yuan ZF, Jin YB and
Zhang Z: Blood lipid regulation of ethyl acetate extracting fration
and stilbene glycoside from tuber of Polygonum multiflorum. Chin
Traditional Herbal Drugs. 39:78–83. 2008.(In Chinese).
|
|
27
|
Zhang W, Wang CH, Shen Y, Li F and Wang
YQ: Treatment of 2,3,4′,5-tetrahydroxystilbene-2-O-β-d-glucoside on
atherosclerosis in rats. Zhongguo Yao Ke Da Xue Xue Bao.
38:261–264. 2007.(In Chinese).
|
|
28
|
Lv L, Gu X, Tang J and Ho CT: Antioxidant
activity of stilbene glycoside from Polygonum multiflorum Thunb in
vivo. Food Chemistry. 104:1678–1681. 2007. View Article : Google Scholar
|
|
29
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bro R and Smilde AK: Principal component
analysis. Anal Methods. 6:2812–2831. 2014. View Article : Google Scholar
|
|
31
|
Boccard J and Rutledge DN: A consensus
orthogonal partial least squares discriminant analysis (OPLS-DA)
strategy for multiblock Omics data fusion. Anal Chim Acta.
769:30–39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nobili V, Donati B, Panera N,
Vongsakulyanon A, Alisi A, Dallapiccola B and Valenti L: A
4-polymorphism risk score predicts steatohepatitis in children with
nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr.
58:632–636. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu J, Han L, Zhu L and Yu Y: Free fatty
acids, not triglycerides, are associated with non-alcoholic liver
injury progression in high fat diet induced obese rats. Lipids
Health Dis. 15:272016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Papackova Z and Cahova M: Fatty acid
signaling: The new function of intracellular lipases. Int J Mol
Sci. 16:3831–3855. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Leamy AK, Egnatchik RA and Young JD:
Molecular mechanisms and the role of saturated fatty acids in the
progression of non-alcoholic fatty liver disease. Prog Lipid Res.
52:165–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Serviddio G, Bellanti F and Vendemiale G:
Free radical biology for medicine: Learning from nonalcoholic fatty
liver disease. Free Radic Biol Med. 65:952–968. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qiang M, Xu Y, Lu Y, He Y, Han C, Liu Y
and He R: Autofluorescence of MDA-modified proteins as an in vitro
and in vivo probe in oxidative stress analysis. Protein Cell.
5:484–487. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sayed AA: Ferulsinaic acid modulates SOD,
GSH, and antioxidant enzymes in diabetic kidney. Evid Based
Complement Alternat Med. 2012:5801042012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Suzuki M, Takeuchi H, Kakita T, Unno M,
Katayose Y and Matsuno S: The involvement of the intracellular
superoxide production system in hepatic ischemia-reperfusion
injury. In vivo and in vitro experiments using transgenic mice
manifesting excessive CuZn-SOD activity. Free Radic Biol Med.
29:756–763. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mateos R, Lecumberri E, Ramos S, Goya L
and Bravo L: Determination of malondialdehyde (MDA) by
high-performance liquid chromatography in serum and liver as a
biomarker for oxidative stress. Application to a rat model for
hypercholesterolemia and evaluation of the effect of diets rich in
phenolic antioxidants from fruits. J Chromatogr B Analyt Technol
Biomed Life Sci. 827:76–82. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cheng N, Ren N, Gao H, Lei X, Zheng J and
Cao W: Antioxidant and hepatoprotective effects of Schisandra
chinensis pollen extract on CCl4-induced acute liver damage in
mice. Food Chem Toxicol. 55:234–240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Heck DE, Shakarjian M, Kim HD, Laskin JD
and Vetrano AM: Mechanisms of oxidant generation by catalase. Ann N
Y Acad Sci. 1203:120–125. 2010. View Article : Google Scholar : PubMed/NCBI
|