Introduction
Inflammation is a typical physiological response of
an organism against various injurious insults including invading
microorganisms, dust particles, altered self-cells, and transformed
cancer cells (1). This response is
usually classified into acute and chronic reactions according to
the inflammatory process and cellular mechanisms (2). During an inflammatory response, a
series of processes including dilation of venules and arterioles,
enhanced blood vessel permeability, and blood flow with percolation
of leukocytes into tissues have been detected in specific cells and
tissues (3). Furthermore,
inflammation could be associated with various chronic diseases
including cancer, diabetes, obesity, autoimmune disease,
inflammatory bowel disease, asthma, rheumatoid arthritis, and
cardiovascular disease (2,4).
Meanwhile, several drug types including
corticosteroids, NSAIDs, and biologics have been used to treat
inflammatory diseases in humans, even though these drugs may have
side effects and high cost. In particular, corticosteroids have
long been used to treat rheumatoid arthritis, but they induce some
serious side effects such as hypertension, Cushing's habitus,
hyperglycemia, osteoporosis, and growth arrest (5). Therefore, natural products with
anti-inflammatory activity have received a lot of attention as
novel materials to overcome side effect problems (6). In particular, some natural products
including Actinidia argute (7), Ampelopsis grossedentata
(8), Artemisia annua Herba
(9), Cheilanthes
albomarginata (10) and
Grateloupia lanceolate (11)
have been shown to significantly reduce the expression of key
inflammatory mediators.
Among these natural products, the anti-inflammatory
activity of an A. cochinchinesis extract has been reported.
The A. cochinchinesis extract significantly inhibited
secretion of the pro-inflammatory cytokine, tumor necrosis factor
(TNF)-α in LPS- and substance P-stimulated mouse astrocytes
(12). In addition, aspacochinosides
N, O, and P extracted from ethanol-treated A. cochinchinesis
decreased NO concentration in LPS-stimulated BV-2 microglial cells.
Moreover, an ethanol extract from A. cochinchinesis greatly
decreased ectopic edema degree, ear thickness, cytokine secretion,
and myeloperoxidase activity, which are considered indicators of
skin inflammation progression, in a skin inflammation-induced mouse
model treated with 12-O-tetradecanoyl-phorbol-13-acetate (13). A crude aqueous extract of A.
cochinchinensis effectively inhibits TNF-α-induced cytotoxicity
(14), as well as increases the
spleen index and SOD activity and decreases malondialdehyde in mice
(15). A recent study reported
inhibitory effects of A. cochinchinensis in allergic
asthma-associated airway remodeling. The standardized herbal
formula PM014, which includes the roots of A.
cochinchinensis, efficiently inhibited the number of total
cells, eosinophils, neutrophils, macrophages, and lymphocytes in
the bronchoalveolar lavage fluid of cockroach allergen-induced mice
(16). However, the underlying
mechanism by which the fermented roots of A. cochinchinensis
exert their anti-inflammatory effects in macrophages has not yet
been clearly identified, even though the effects of fermented
extracts were investigated in antigen-stimulated macrophages.
In this study, we investigated the fundamental
mechanisms responsible for anti-inflammatory activities of BAW in
LPS-induced RAW264.7 microphage cells. The results provide novel
data indicating that BAW may be associated with suppression of
various chronic inflammation-related diseases through the
regulation of the iNOS-mediated COX-2 induction pathway and
cytokine expression.
Materials and methods
Preparation of BAW and butanol extract
from A
cochinchinensis roots fermented with Lactobacillus
plantarum (BAL). The roots of A. cochinchinensis used in
this study were collected from plantations in the Go-Chang area of
Korea and dried in a drying machine (Ilshinbiobase, Dongducheon,
Korea) at 60°C. Voucher specimens of A. cochinchinensis
roots (WPC-14-003) were deposited in the functional materials bank
of the PNU-Wellbeing RIS Center at Pusan National University. These
samples were also identified by Dr. Shin Woo Cha at the Herbal Crop
Research Division, National Institute of Horticultural & Herbal
Science.
Firstly, to prepare aqueous fractions of unfermented
A. cochinchinensis (UnF), 20 g of freeze-dried A.
cochinchinensis roots were ground to a powder and a hot water
extract prepared with 1.2 l of deionized distilled water
(dH20) for 2.5 h in a hot water extractor (DW-290,
Daewoong, Kyunggi, Korea). After finishing aqueous extraction,
samples were filtered through filter paper (Whatman No. 2, Whatman,
Brentford, UK) and then evaporated by using a rotary vacuum
evaporator (EYELA, N-1100 series, Tokyo, Japan). Finally, UnF was
used for fermentation after freeze-drying. Yield from the hot water
extraction process was 60.7%.
The two bacterial strains (W. cibaria and
Lactobacillus plantarum) used in the fermentation process
were provided by Professor Hong Joo Son, Department of Life Science
and Environmental Biochemistry, Pusan National University. To
prepare fermented products, UnF powder was dissolved with 1% (w/v)
in dH2O (pH 5.3), and the mixture sterilized at 121°C
for 15 min. After cooling at room temperature, W. cibaria or
L. plantarum precultivated in lactobacilli MRS broth (Difco
Laboratories, Detroit, MI) until the final cell density approached
107 CFU/ml (OD600=0.1) were inoculated [5% (v/v)] to the
UnF mixture. The mixture was then incubated in a shaking incubator
(VS-8480; Vision Scientific, Bucheon, Korea) at 37°C at 200 rpm/min
for 4.3 d. Fermented A. cochinchinensis products of W.
cibaria (FPW) or L. plantarum (FPL) were obtained from
the mixtures solution fermented with W. cibaria or L.
plantarum by using centrifugation at 12,000 × g for 10 min.
To obtain the n-butanol fractions of UnF (BUnF), FPW
(BAW) and FPL (BAL), an equal volume of butanol was firstly added
to UnF, FPW or FPL. After vigorous mixing and incubating, the
butanol phase was collected from each mixture by centrifugation at
12,000 × g for 10 min. Butanol extraction was repeated three times,
after which all butanol phases were combined, evaporated under a
rotary vacuum evaporator, freeze-dried, and stored at −20°C until
use.
Hyaluronidase inhibition assay
The inhibition activity of hyaluronidase against
BUnF, BAW, and BAL was investigated by using a colorimetric assay
based on the modified Morgan-Elson method (17–19)
which measures the amount of N-acetyl-D-glucosamine produced
from sodium hyaluronate. Briefly, 12 µl of BUnF, BAW, or BAL were
mixed with 12 µl of hyaluronidase (10 mg/ml) in 0.1 M sodium
acetate buffer (pH 3.5), then incubated in a water bath at 37°C.
After pre-incubation for 20 min, the sample and hyaluronidase
solution mixture was added to 12 µl of 12.5 mM calcium chloride to
activate the hyaluronidase and then incubated for 20 min. To the
activated hyaluronidase solution, 24 µl of sodium hyaluronate (6
mg/ml) dissolved in 0.1 M sodium acetate buffer (pH 3.5) was added
and incubated for an additional 40 min. Next, the solution was
amended with 12 µl of 0.4 N NaOH and 0.4 M potassium tetraborate,
then vortexed, boiled for 3 min, and placed on ice to terminate the
hyaluronidase activity. Subsequently, 360 µl of DMAB solution
(p-dimethylaminobenzaldehyde 4 g in a solution of acetic
acid 350 ml mixed with 50 ml of 10 N HCl) was added, and the sample
placed in a 37°C water bath for 20 min. The absorbance of each test
tube at 540 nm was measured by using a microplate reader (Tecan
Sunrise, Tecan, Hombrechtikon, Switzerland). Hyaluronidase
inhibition activity was estimated by determining differences in the
absorbance values of the sample solutions.
Analysis of bioactive compounds in
BUnF and BAW
The concentration of three bioactive compounds
including total phenols and crude saponins in BUnF and BAW were
measured as previously described. The amount of total phenols in
BAW was determined according to the Folin-Ciocalteu method
(20). Briefly, BUnF and BAW (20 µl)
was mixed with 100 µl of 0.2 N Folin-Ciocalteu reagent for 5 min,
after which 300 µl of 20% sodium carbonate was added. Following
incubation at room temperature for 2 h, the absorbance of the
reaction mixture was measured at 765 nm. A calibration curve was
generated with Gallic acid as the standard. Total phenolic content
was expressed in milligrams of gallic acid equivalents per gram of
BAW.
The total crude saponins amount was detected as
described previously (21). Briefly,
BUnF and BAW dissolved in 30 ml dH2O was repeatedly
extracted with ethyl ether (50 ml) to remove lipid soluble
substances. After collection of the aqueous layer, samples were
further extracted with n-butanol (30 ml) three times. This layer
was then concentrated by vacuum evaporation and lyophilization by
using circulation extraction equipment (IKA Labortechnik, Seoul,
Korea). Finally, total crude saponins was calculated by using the
equation: crude saponin (mg/g)=A-B/S, where, A is the dry weight of
the n-butanol layer (mg), B is the weight of the flask (mg), and S
is the weight of the solid volume of the sample (g).
High performance liquid chromatography
(HPLC) analysis
Protodioscin, a saponin, was identified in BUnF and
BAW by using an ILC 3000 HPLC system (Interface Engineering Co.
Ltd., Seoul, Korea) equipped with a Corona®
CAD® detector (ESA Biosciences, Inc., Chelmsford, MA,
USA). Chromatographic separation was performed on a CapCell PAK MG
C18 (4.6×250 mm, particle size 5 µm; Shiseido Co., Ltd., Tokyo,
Japan). The mobile phase consisted of solvent A (dH2O)
and solvent B (acetonitrile) prepared by using a gradient elution
program: 0–25 min with 30–90% solvent B and 25–40 min with 90%
solvent B. A flow rate of 1.0 ml/min was used for sample analysis.
The nebulizer gas was compressed nitrogen. Gas flow rate and gas
pressure were maintained at 1.53 l/min and 35±2 psi, respectively.
The output signal of the detector was recorded by using Clarity™
chromatography software (DataApex, Prague, Czech Republic).
Free radical scavenging activity
The scavenging activity of the
2,2-diphenyl-1-picrylhydrazyl (DPPH) radical against BAW was
measured as previously described (22). Briefly, each sample (100 µl) in eight
different concentrations of BAW (7.8 to 1,000 µg/ml) was mixed with
100 µl of 0.1 mM DPPH (Sigma-Aldrich Co., St. Louis, MO, USA) in
95% ethanol solution or 100 µl of 95% ethanol solution, then
incubated for 30 min at room temperature. Next, the absorbance of
the reaction mixture was measured at 517 nm by using a Versa-max
plate reader (Molecular Devices, Sunnyvale, CA, USA). The DPPH
radical scavenging activity of the BAW was expressed as the percent
decrease in absorbance relative to that in the control. The
IC50 value is defined as the concentration of substrate
that produces a 50% loss in DPPH activity.
Cell viability assay
The RAW264.7 cell line used in this study consists
of macrophage cells originating from the Abelson murine leukemia
virus-induced tumor provided by the Korean Cell Line Bank (Seoul,
Korea). The RAW264.7 cells were grown with Dulbecco's Modified
Eagle's Medium (DMEM, Thermo Scientific, Waltham, MA, USA)
containing 10% fetal bovine serum (FBS, S001-01, Welgene,
Gyeongsan-si, Korea), L-glutamine, penicillin, and streptomycin
(Thermo Scientific) in a humidified incubator at 37°C under 5%
CO2 and 95% air.
Cell viability was determined by using tetrazolium
compound 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium
bromide (MTT) (Sigma-Aldrich Co.). To determine cell viability,
RAW264.7 cells were seeded at a density of 5×104
cells/0.2 ml and grown for 24 h in a 37°C incubator. When the cells
attained 70–80% confluence, they were either untreated (No) or
treated with DMSO (Vehicle), or pretreated with 100 µg/ml (BAWLo)
or 200 µg/ml (BAWHi) of BAW dissolved in DMSO. Following incubation
for 24 h, the supernatants were discarded, after which 0.2 ml of
fresh MEM media and 50 µl of MTT solution [2 mg/ml in phosphate
buffered saline (PBS)] were added to each well. The cells were then
incubated at 37°C for 4 h. Formazan precipitate was dissolved in
DMSO, after which the absorbance at 570 nm was read directly in the
wells by using a Molecular Devices VERSA max Plate reader
(Sunnyvale, CA, USA). The morphological features of RAW264.7 cells
in each treated group were observed by using a microscope (Leica
Microsystems, Switzerland).
NO concentration analysis
NO concentration in the culture supernatant of
RAW264.7 cells was measured by using Griess reagent [1%
sulfanilamide, 5% phosphoric acid, 0.1% N-(1-naphthyl)
ethylenediamine dihydrochloride; Sigma-Aldrich Co.] as described
previously (23). Briefly, RAW264.7
cells were treated with Vehicle or BAW (100 or 200 µg/ml) for 2 h
followed by LPS (1 µg/ml) for 24 h. Following collection of the
supernatant, each sample (100 µl) was mixed with the same volume of
Griess reagent and incubated at room temperature for 10 min. The
absorbance was read at 540 nm by using a VersaMax microplate reader
(Molecular Devices).
Analysis of intracellular ROS
level
Intracellular ROS levels in RAW264.7 cells were
measured by staining with 2′,7′-dichlorofluorescein diacetate
(DCFH-DA; Sigma-Aldrich Co.), which is a cell permeable,
nonfluorescent agent that can be deacetylated by intracellular
esterases to form nonfluorescent DCFH. In the presence of ROS, DCFH
is converted to highly fluorescent DCF intracellularly. Briefly,
RAW364.7 cells were seeded at 5×105 cells/2 ml in 6-well
plates, then grown with two different concentrations of BAW for 2 h
in a 37°C incubator. After washing once with 1× PBS, the cells were
incubated with 1 µg/ml of LPS for another 24 h. Next, cells were
incubated with 25 µM DCFH-DA for 30 min at 37°C. Finally, the cells
were washed twice with PBS, after which the green fluorescence was
observed at 200× magnification via fluorescence microscopy (Eclipse
TX100, Nikon, Tokyo, Japan).
Western blot analysis
Total homogenate of RAW264.7 cells obtained by using
Pro-Prep Protein Extraction Solution (iNtRON Biotechnology,
Seongnam, Korea) was centrifuged at 13,000 rpm/min for 5 min, then
the concentration of total protein was quantified by using a
SMARTTM BCA protein assay kit (Thermo Scientific) for western
blotting. Briefly, the proteins were separated by 4–20% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for 2
h, after which resolved proteins were transferred to nitrocellulose
membranes for 2 h at 40 V. Each membrane was then incubated
overnight separately at 4°C with the following primary antibodies:
SAPK/JNK antibody (Cell Signaling Technology, Danvers, MA, USA),
p-SAPK/JNK (Thr183/Tyr185) antibody (Cell Signaling Technology),
ERK1 (K-23) antibody (Santa Cruz Biotechnology, Inc. Santa Cruz,
CA, USA), p-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) antibody (Cell
Signaling Technology), p38 MAPK antibody (Cell Signaling
Technology), p-p38 MAP Kinase (Thr180/Tyr182) antibody (Cell
Signaling Technology), and anti-actin antibody (Sigma-Aldrich Co.).
Next, the membranes were washed with washing buffer (137 mM NaCl,
2.7 mM KCl, 10 mM Na2HPO4, and 0.05% Tween
20) and incubated with 1:1,000 diluted horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG (Invitrogen, Carlsbad, CA,
USA) at room temperature for 1 h. Finally, membrane blots were
developed by using Amersham ECL Select western blotting detection
reagent (GE Healthcare, Little Chalfont, UK). The chemiluminescence
signals that originated from specific bands were detected by using
FluorChemi®FC2 (Alpha Innotech, San Leandro, CA,
USA).
Enzyme-linked immunosorbent assay
(ELISA) for IL-6 cytokine
The concentration of cytokine IL-6 in the culture
supernatant of RAW264.7 cells treated with BAW was determined by
using an IL-6 ELISA kit (Biolegend, San Diego, CA, USA) according
to the manufacturer's instructions. Briefly, RAW264.7 cells were
treated with two different concentrations of BAW (100 or 200 µg/ml)
for 2 h, followed by 1 µg/ml of LPS for 24 h. After collection of
the supernatant, 100 ml of serial dilutions of the culture
supernatant were added to a 96-well plate coated with anti-IL-6
antibody and then incubated for 2 h at room temperature. After five
washes with wash solution (PBS, 0.05% and Tween-20, pH 7.4), 100 µl
of avidin-horseradish peroxidase solution was added to each well,
and the plates were allowed to develop at 37°C for 2 h. After five
washes with wash solution, the plate was maintained at 37°C for 30
min to react with 100 µl of substrate solution. The reaction was
then stopped by the addition of 100 µl of blocking solution, after
which the absorbance at 450 nm was read with a VersaMax microplate
reader (Molecular Devices).
RT-PCR analysis for cytokine gene
expression
The mRNA levels of iNOS, COX-2, TNF-α, IL-1β, and
IL-6 were measured by RT-PCR as previously described (24). First, total RNA molecules were
purified by removing media from a subset group of cells and then
homogenizing the cells in RNAzol CS104 (Tel-Test Inc., Friendswood,
USA). The isolated RNA was then quantified by using UV
spectroscopy. Expressions of the target genes were assessed by
using RT-PCR with 5 µg of total RNA from cells of each group. Next,
500 ng of oligo-dT primer (Invitrogen, Carlsbad, CA, USA) were
annealed at 70°C for 10 min. The complementary DNA (cDNA), which
was used as the template for further amplification, was synthesized
by the addition of deoxyadenosine triphosphate (dATP),
deoxycytidine triphosphate (dCTP), deoxyguanosine triphosphate
(dGTP), and deoxythymidine triphosphate (dTTP) with 200 units of
reverse transcriptase (Superscript II, Invitrogen, 200 U/µl). Next,
10 pmol of the sense and antisense primers were added, and the
reaction mixture was subjected to 28–32 cycles of amplification.
Amplification was conducted in a Perkin-Elmer Thermal Cycler and
used the following cycle: 30 sec at 94°C, 30 sec at 62°C, and 45
sec at 72°C. The primer sequences for target gene expression
identification were as follows: iNOS, sense primer:
5′-CACTTGGAGTTCACCCAGT-3′, anti-sense primer:
5′-ACCACTCGTACTTGGGATGC-3′; COX-2, sense primer:
5′-CAGGTCATTGGTGGAGAGGTGTATC-3′, anti-sense primer:
5′-CCAGGAGGATGGAGTTGTTGTAGAG-3′; TNF-α, sense primer:
5′-CCTGTAGCCCACGTCGTAGC-3′, anti-sense primer:
5′-TTGACCTCAGCGCTGACTTG-3′; IL-1β, sense primer:
5′-GCACATCAACAAGAGCTTCAGGCAG-3′, anti-sense primer:
5′-GCTGCTTGTGAGGTGCTGATGTAC-3′; and IL-6, sense primer:
5′-TTGGGACTGATGTTGTTGACA-3′, anti-sense primer:
5′-TCATCGCTGTTGATACAATCAGA-3′. The experiment was repeated three
times, and all samples were analyzed in triplicate. The final
RT-PCR products were separated on 1–2% agarose gel and then
visualized by ethidium bromide staining. The densities of specific
bands were quantified by using the Kodak Electrophoresis
Documentation and Analysis System 120 (Eastman Kodak, Rochester,
NY).
Statistical analysis
One-way ANOVA was used to identify significant
differences between No-treated and LPS-treated groups (SPSS for
Windows, Release 10.10, Standard Version, Chicago, IL, USA).
Differences in the responses of the Vehicle+LPS-treated group and
the BAW+LPS-treated groups were evaluated by using a post hoc test
(SPSS for Windows, Release 10.10, Standard Version). All values are
reported as the mean ± standard deviation (SD) and a P-value of
<0.05 was considered significant.
Results
Selection of bacterial strain for
optimal fermentation
To enhance the anti-inflammatory activity of A.
cochinchinensis roots, fermentation technique was firstly
applied to these roots using two bacterial strains (W.
cibaria and L. plantarum). Anti-inflammatory activity
and toxicity were measured in the two final products (BAW and BAL)
obtained from fermented A. cochinchinensis roots using
butanol extraction. As shown Table
I, a significance alternation was detected in hyaluronidase
inhibition rate and suppression rate of NO concentration.
Hyaluronidase inhibition rate was dramatically enhanced after
fermentation although this level was slightly higher with 18–21% in
BAW than BAL. A similar pattern to that observed for the
hyaluronidase inhibition rate was also observed in the suppression
rate of NO concentration. After fermentation, the suppression rate
of NO concentration was increased regardless of their
concentration. But, the increase of these level was greater with
57–64% in BAW than BAL. Meanwhile, any significance differences
were not detected in cell viability of group treated with BUnF, BAW
and BAL. Therefore, the present results suggest that the
fermentation with W. cibaria can successfully change from
the roots of A. cochinchinensis to the fermented products
with high anti-inflammatory activity and non-toxicity. Also, above
results showed that BAW can be considered as the better candidate
for suppressing the inflammatory response.
 | Table I.Hyaluronidase inhibition rate,
suppression rate of NO concentration and cell viability of butanol
extracts of A. cochinchinensis fermented with W.
cibaria and L. platarum. |
Table I.
Hyaluronidase inhibition rate,
suppression rate of NO concentration and cell viability of butanol
extracts of A. cochinchinensis fermented with W.
cibaria and L. platarum.
| Category | Type of A.
cochinchinensis | Dose | BAW (Weissella
cibaria) |
| BAL
(Lactobacillus plantarum) |
|---|
| Hyaluronidase
inhibition rate (%) | BUnF | Low |
| ND |
|
|
|
| High |
| 4.14±0.3 |
|
|
| BF | Low |
26.95±3.5a,b |
|
22.43±2.7a |
|
|
| High |
28.35±2.9a,b |
|
23.96±3.4a |
| Suppression rate of
NO concentration (%) | BUnF | Low |
| 7.7±1.1 |
|
|
|
| High |
| 13.7±1.6 |
|
|
| BF | Low |
22.7±2.8a,b |
| 14.2±1.6 |
|
|
| High |
23.1±3.1a,b | 14.3±1.8 |
|
| Cell viability
(%) | BUnF | Low |
| 92±11 |
|
|
|
| High |
| 97±12 |
|
|
| BF | Low | 103±11 |
| 100±9 |
|
|
| High | 98±8 |
|
98±10 |
Change of key components in A.
cochinchinensis after fermentation with W. cibaria
To identify the key components altered by
fermentation, the concentration of several key components were
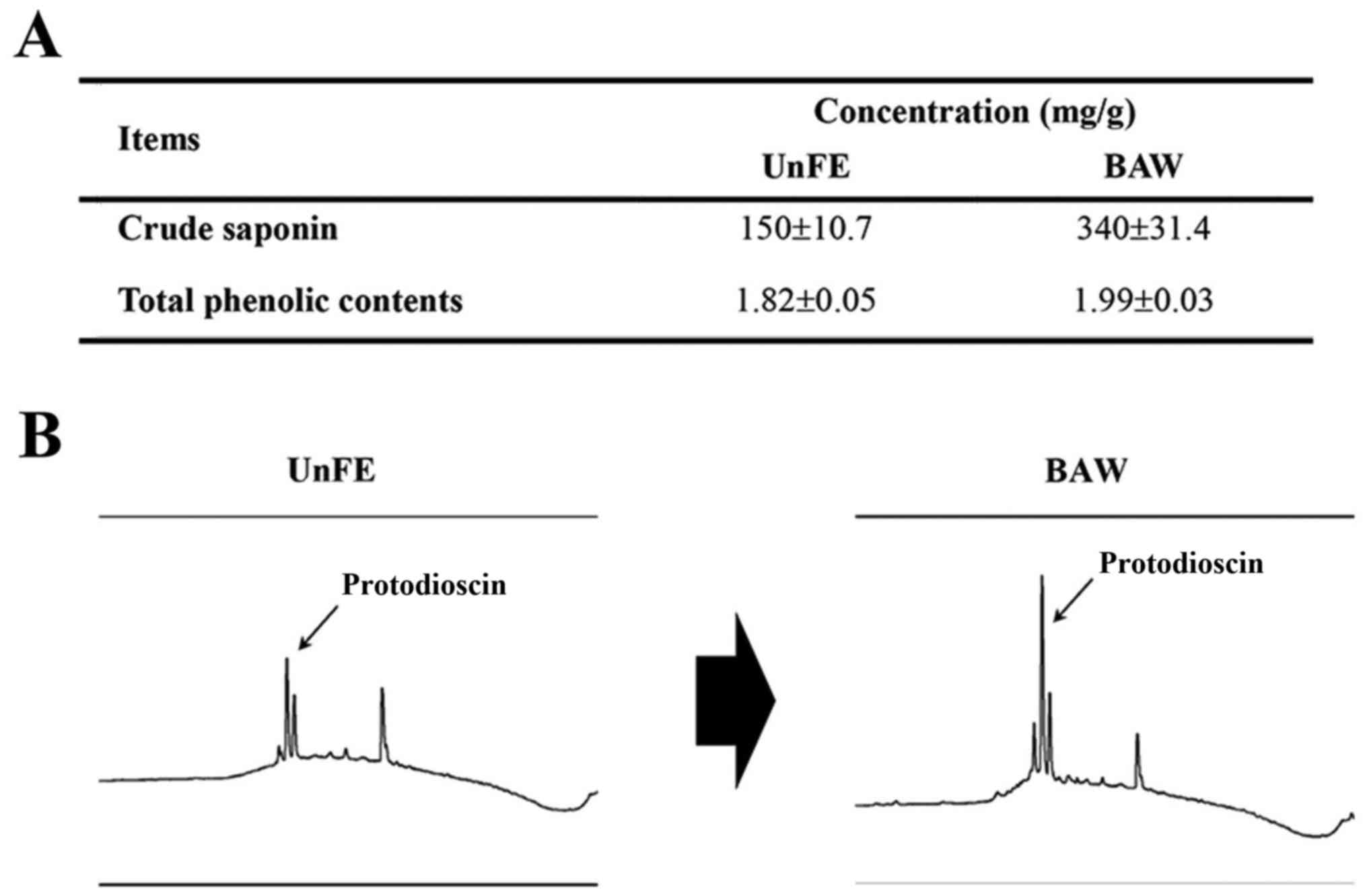
analyzed in BUnF and BAW. As shown in Fig. 1A, BUnF contained significantly
different concentrations of total phenolic compounds (1.82 mg/g)
and total crude saponins (150 mg/g). After fermentation, the total
phenolic and crude saponin concentrations in BAW were significantly
increased with 9.3, 126.6%, respectively. Furthermore, the
protodioscin concentration, detected as a sharp specific peak in
the HPLC curve, was also higher in BAW (Fig. 1B). These results demonstrate that
concentrations of several key components that associated with high
anti-oxidative capacity can be increased in the extracts of roots
of A. cochinchinensis after fermentation with W.
cibaria.
Antioxidative capacity of BAW
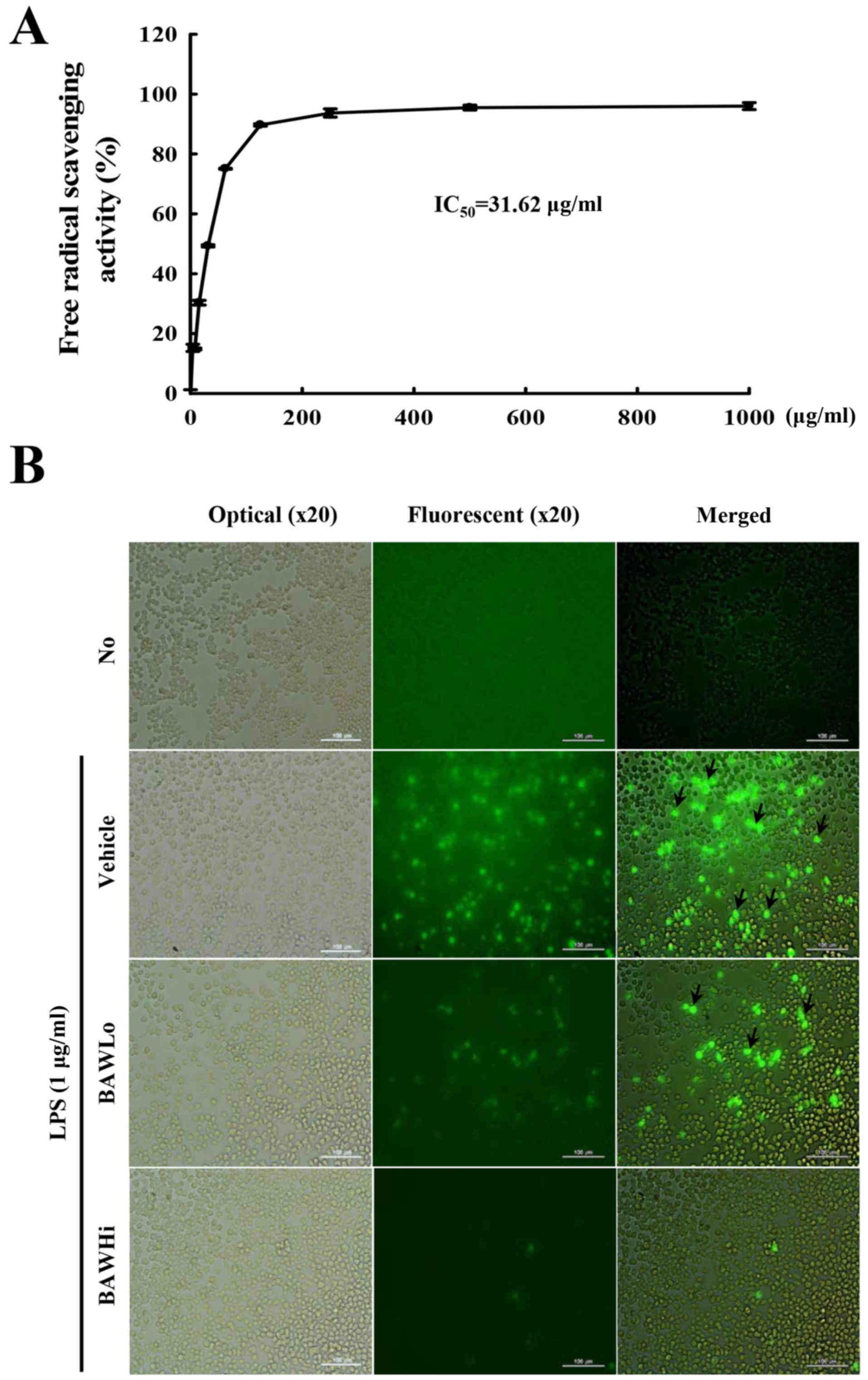
To investigate the antioxidative capacity of BAW,
alterations in DPPH scavenging activity and the inhibitory activity
of intracellular ROS production were measured in RAW264.7 cells
treated with BAW. The scavenging activity against the DPPH radical
was gradually enhanced with the increase in BAW concentration (7.8
and 1,000 µg/ml), and the IC50 value for BAW was 31.62
µg/ml (Fig. 2A). In addition, the
intracellular level of ROS was notably enhanced after LPS
treatment. However, the ROS level markedly decreased with depending
on BAW concentration increase in LPS+BAWLo and LPS+BAWHi treated
group (Fig. 2B). These results
indicate that BAW may have a high anti-oxidative capacity that is
obtained via suppression of intracellular ROS production and DPPH
radical scavenging activity.
Suppression effects on the
iNOS-mediated COX-2 induction pathway
The signaling pathway for iNOS-mediated COX-2
induction has a key role during the inflammation process (25). Thus, we investigated the suppressive
effects of BAW on the potential iNOS-mediated COX-2 induction
pathway in LPS-activated Raw264.7 cells after BAW pretreatment.
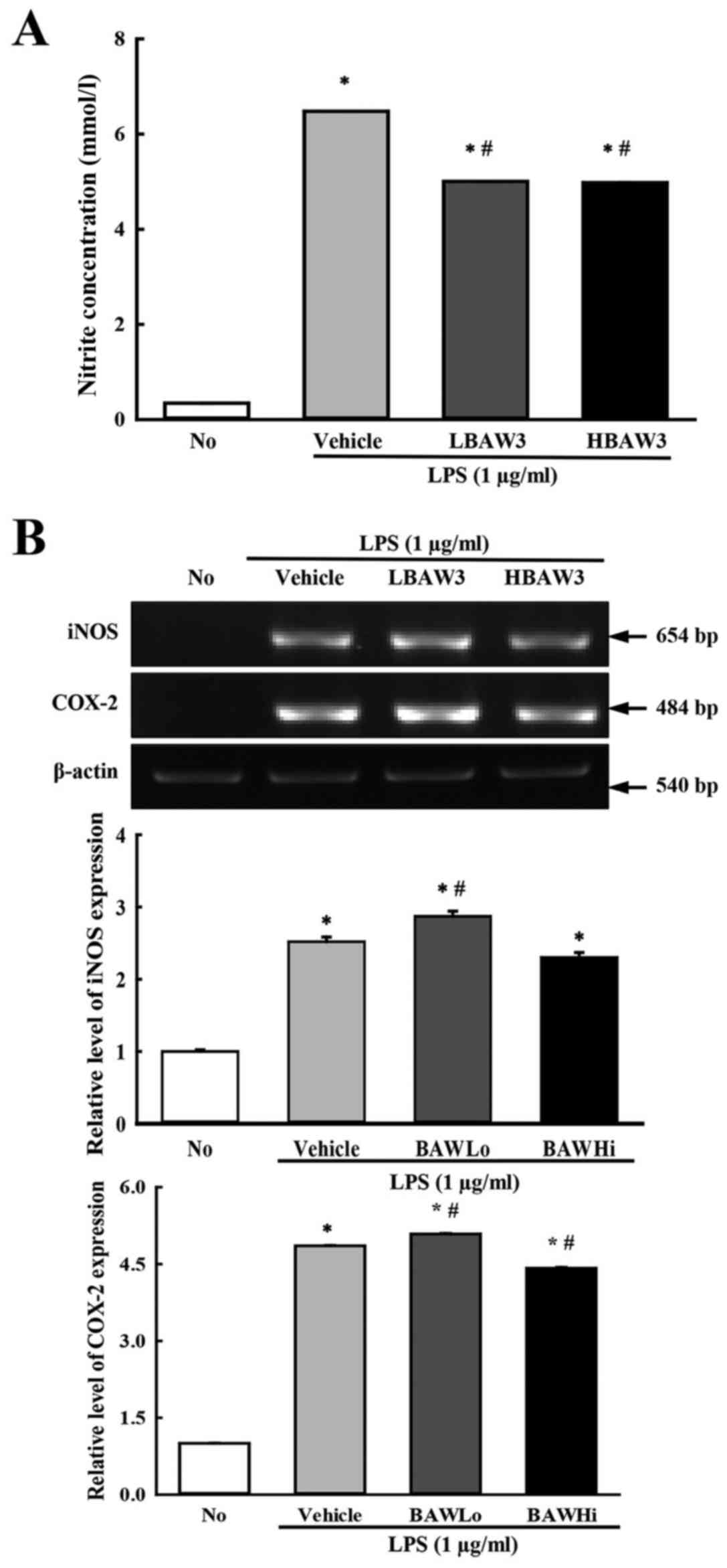
Firstly, we observed that the enhanced NO concentration after LPS
treatment was dramatically decreased in the BAW-treated group
(Fig. 3A). Also, the levels of the
iNOS and COX-2 gene transcripts were higher in the
Vehicle+LPS-treated group than that in the No-treated group.
However, these levels were slightly decreased in BAWHi+LPS-treated
Raw264.7 cells (Fig. 3B). These
results suggest that BAW pretreatment can recover the signaling
pathway for iNOS-mediated COX-2 induction during the inflammatory
process.
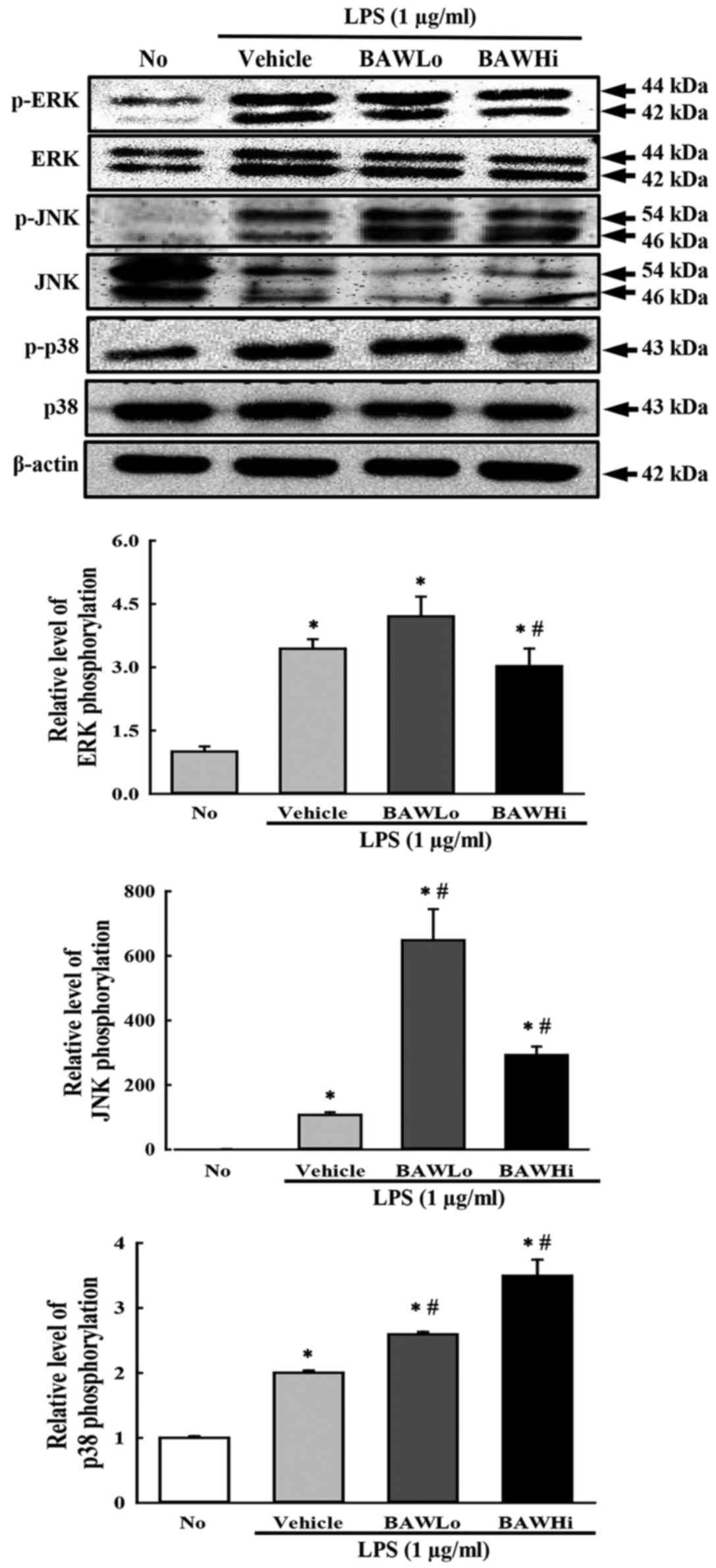
To investigate whether alteration of iNOS and NO
level accompanies a change in the MAP kinase pathway, the
phosphorylation levels of ERK, JNK and p38 were measured in
LPS-stimulated RAW 264.7 cells treated with BAW. As shown in
Fig. 4, the phosphorylation levels
of the three members were higher after LPS activation with various
ratios than they were after No treatment. However, the levels of
only ERK and JNK were significantly decreased after BAW
pretreatment, while the level of p38 was continuously increased
with depending on BAW concentration (Fig. 4). Therefore, the present data suggest
that the suppression of iNOS and NO induced by BAW treatment may
exert it regulatory activity by controlling the ERK and JNK in MAP
kinase pathway.
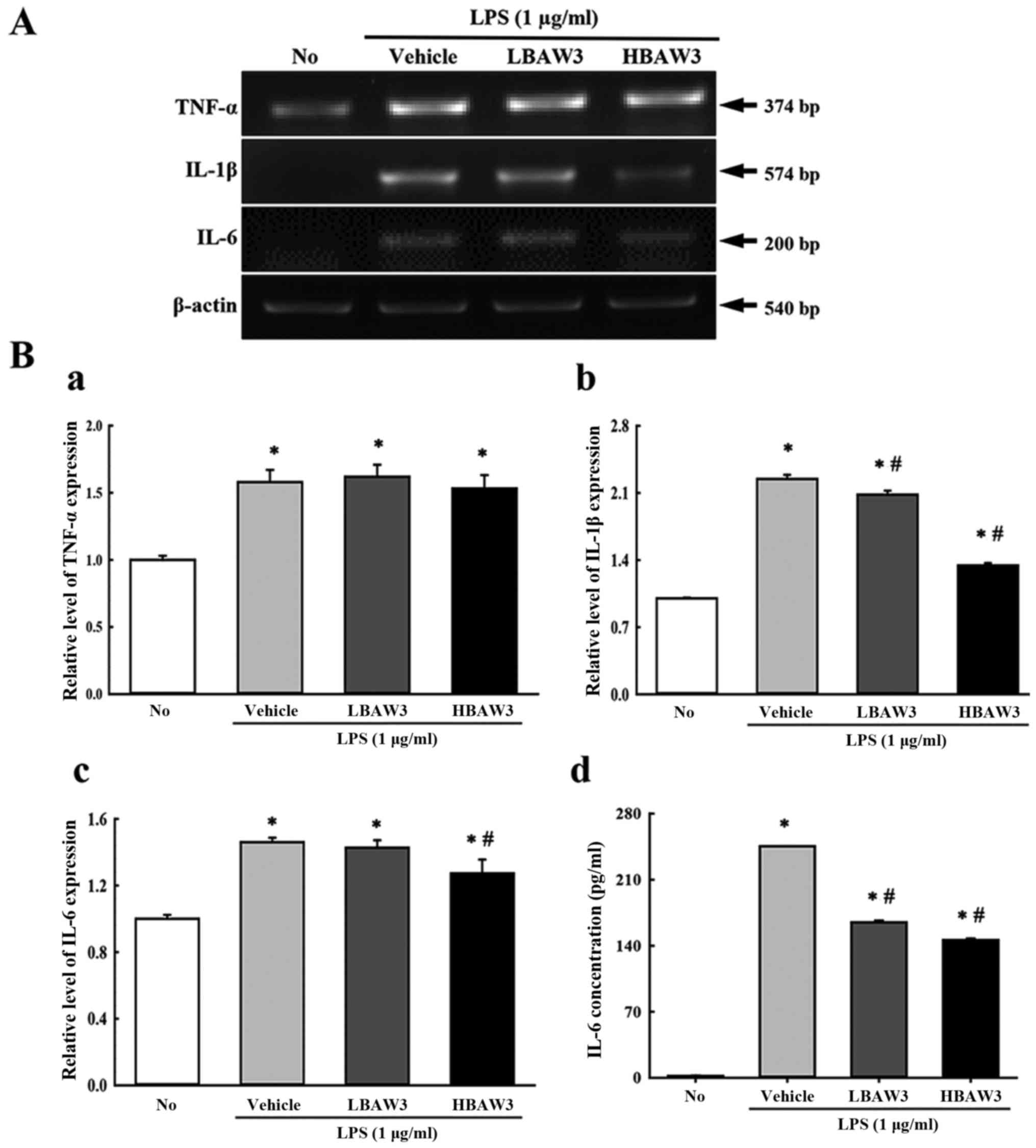
Suppression of the expression of
inflammatory cytokines
To investigate alteration of the expression of
pro-inflammatory and anti-inflammatory cytokines during an
anti-inflammatory response induced with BAW treatment, the
transcript levels of TNF-α, IL-1β, and IL-6 were measured by
performing RT-PCR of LPS-activated Raw264.7 cells. Among the three
cytokines, IL-1β and IL-6 showed a suppression pattern in the
BAW+LPS-treated group. In contrast, the TNF-α transcript maintained
a constant level (Fig. 5A and B).
Furthermore, the concentration of IL-6 in the culture supernatant
of LPS-activated Raw264.7 cells was measured by ELISA in order to
confirm the RT-PCR results. The IL-6 concentrations in the culture
supernatant completely reflected the results for the IL-6
transcripts, although a few differences were observed in the
BAWLo+LPS-treated group (Fig. 5B).
Therefore, these results indicate that BAW pretreatment suppresses
the enhancement of pro-inflammatory cytokine expressions that were
induced by LPS treatment in RAW264.7 cells.
Discussion
Fermentation techniques have been widely applied in
the production of various high value substances because
fermentation can provide economic and environmental advantages to
individuals and industry (26).
Moreover, many products produced by fermentation show therapeutic
effects against several inflammatory diseases (27–29). In
an effort to identify novel fermentation-derived candidates for the
treatment of inflammatory disease, we investigated the
anti-inflammatory effects of BAW in LPS-activated RAW264.7 cells.
Our results demonstrated that BAW with its high anti-oxidative
activity could significantly suppress the inflammatory response in
LPS-activated RAW 264.7 macrophages through regulation of
iNOS-mediated Cox-2 induction pathway and inflammatory cytokines
expression. Thus, BAW may be considered a potential
anti-inflammatory drug for use in the treatment of various chronic
inflammatory diseases.
The roots of A. cochinchinesis have long been
considered a therapeutic drug for various disease because their
anti-inflammatory, diuretic, antiseptic, antitussive,
antibacterial, nervine, sialogoue, antipyretic, and stomachic
effects. In addition, they are used in combination with other
natural products to treat the lungs, spleen, immune system, and
aging (15,30). Furthermore, the above functions of
A. cochinchinesis can be associated with some functional
compounds including β-sitosterol (31), daucosterol (32), n-ethatriacontanoic acid (33), palmitic acid (34), 9-heptacosylene (35), smilagenin (36), diosgenin (37), sarsasapogenin-3-O-β-D-glucoside
feeding grapes imidacloprid (38),
5-methoxy methyl furfural, yame sapogenin, diosgenin-3-O-β-D
imidacloprid feeding glucose glycosides (39,40),
aspacochioside D (41),
iso-agatharesinoside (42) and seven
steroidal saponins (43).
Concentration enhancement of the above compounds has been an
important issue in several recent studies. However, a fermentation
technique has not been applied previously to A.
cochinchinesis roots as a method to increase the concentration
of functional compounds and produce novel beneficial compounds. In
this study, the roots of A. cochinchinesis were fermented
with W. cibaria and L. plantarum for 4.3 days. After
fermentation, the hyaluronidase inhibition rate and NO suppression
rate enhancement was greater in the fermentation product from W.
cibaria than it was in that fermented with L. plantarum.
Furthermore, the concentration of total phenols and crude saponins
were significantly increased by 9.3 and 126.6% in BAW, and an
increased protodioscin peak was detected. The results, therefore,
indicate that the functional compounds in the roots of A.
cochinchinesis may be successfully enhanced by fermenting the
roots with W. cibaria.
Extracts from many fermented natural products can
effectively suppress an LPS-stimulated inflammatory response. The
production of pro-inflammatory mediators such as NO, iNOS, COX-2,
TNF-α, and IL-6 is inhibited by the treatment of Oyaksungisan (OY)
and Sipjeondaebotang (SJ) fermented by Lactobacillus
(28,44), fermented Rhizoma coptidis
(29), and three fermented herbs
(Rhizome Atractylodes macrocephalae, Massa Medicata
Fermentata, and Dolichoris Semen) (45) in LPS-stimulated RAW264.7 cells. Also,
a fermented barley extract can effectively suppress oxidative
stress in LPS-induced inflammation, while fermented red ginseng
extract and fermented soybean extract can inhibit the production of
NO in the same cells (30,46,47).
Similar inhibitory effects on the production of pro-inflammatory
mediators were observed in our BAW-based study, although the
inhibition rate and solvent used for extraction were different.
Furthermore, BAW pretreatment suppressed the level of NO, iNOS,
COX-2, IL-6, IL-1β, and TNF-α in LPS-activated macrophage cells.
However, additional research is needed to elucidate the key factors
within BAW that control the regulation of pro-inflammatory
mediators.
The iNOS and COX-2 proteins can be considered key
factors when investigating the therapeutic effects of
anti-inflammatory drugs because their expression can be induced by
a variety of pro-inflammatory stimuli such as LPS and TNF-α in many
disease conditions (48,49). Furthermore, NO production induced by
iNOS expression is regulated via COX-2 expression through the MAPK
signaling pathway, which has a critical role in the regulation of
cell growth and differentiation, as well as in the control of
cellular responses to cytokines and stresses (50,51).
Some fermented natural products induce significant alteration of
the iNOS-mediated COX-2 induction pathway in RAW264.7 cells. After
the treatment of fermented OY extracts (27), fermented SJ extract (27), fermented soybean extract (47), and fermented RG extract (46), the levels of NO and COX-2 were
significantly decreased in RAW264.7 cells. In the present study,
our results for the iNOS-mediated COX-2 induction pathway obtained
from BAW-treated RAW264.7 cells were very similar to those of
previous studies, although some differences in the suppression of
MAP kinases were observed. These differences might be due to
factors such as the innate composition of, and the key molecules
within, the herbal medicine.
Taken together, the results of the present study
indicate that the antioxidant and anti-inflammatory activity of the
roots of A. cochinchinensis can be enhanced by fermentation
with W. cibaria. In addition, the results provide novel
evidence that BAW may suppress inflammatory responses through the
regulation of iNOS-mediated COX-2 induction pathway and
inflammatory cytokine expression.
Acknowledgements
The present study was supported by grants to Dr. Dae
Youn Hwang from the Korea Institute of Planning Evaluation for
Technology of Food, Agriculture, Forestry and Fisheries
(114034–03-1-HD030).
References
|
1
|
Krishnamoorthy S and Honn KV: Inflammation
and disease progression. Cancer Metastasis Rev. 25:481–491. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arulselvan P, Fard MT, Tan WS, Gothai S,
Fakurazi S, Norhaizan ME and Kumar SS: Role of antioxidants and
natural products in inflammation. Oxid Med Cell Longev.
2016:52761302016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Henson PM, Larsen GL, Henson JE, Newman
SL, Musson RA and Leslie CC: Resolution of pulmonary inflammation.
Fed Pro. 43:2799–2806. 1984.
|
|
4
|
Gautam R and Jachak SM: Recent
developments in anti-inflammatory natural products. Med Res Rev.
29:767–820. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parker KL and Schimmer BP: Genetics of the
development and function of the adrenal cortex. Rev Endocr Metab
Disord. 2:245–252. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ravipati AS, Zhang L, Koyyalamudi SR,
Jeong SC, Reddy N, Bartlett J, Smith PT, Shanmugam K, Münch G, Wu
MJ, et al: Antioxidant and anti-inflammatory activities of selected
Chinese medicinal plants and their relation with antioxidant
content. BMC Complement Altern Med. 12:1732012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim HY, Hwang KW and Park SY: Extracts of
Actinidia arguta stems inhibited LPS-induced inflammatory responses
through nuclear factor-κB pathway in Raw 264.7 cells. Nutr Res.
34:1008–1016. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hou XL, Tong Q, Wang WQ, Shi CY, Xiong W,
Chen J, Liu X and Fang JG: Suppression of inflammatory responses by
dihydromyricetin, a flavonoid from Ampelopsis grossedentata, via
inhibiting the activation of NF-κB and MAPK signaling pathways. J
Nat Prod. 78:1689–1696. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oh YC, Jeong YH, Kim T, Cho WK and Ma JY:
Anti-inflammatory effect of Artemisiae annuae herba in
lipopolysaccharide-stimulated RAW 264.7 cells. Pharmacogn Mag. 10
Suppl 3:S588–S595. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lamichhane R, Kim SG, Poudel A, Sharma D,
Lee KH and Jung HJ: Evaluation of in vitro and in vivo biological
activities of Cheilanthes albomarginata Clarke. BMC Complement
Altern Med. 14:3422014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim DH, Kim ME and Lee JS: Inhibitory
effects of extract from G. lanceolata on LPS-induced production of
nitric oxide and IL-1β via down-regulation of MAPK in macrophages.
Appl Biochem Biotechnol. 175:657–665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim H, Lee E, Lim T, Jung J and Lyu Y:
Inhibitory effect of Asparagus cochinchinensis on tumor necrosis
factor-alpha secretion from astrocytes. Int J Immunopharmacol.
20:153–162. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee DY, Choo BK, Yoon T, Cheon MS, Lee HW,
Lee YA and Kim HK: Anti-inflammatory effects of Asparagus
cochinchinensis extract in acute and chronic cutaneous
inflammation. J Ethnopharmacol. 121:28–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koo HN, Jeong HJ, Choi JY, Choi SD, Choi
TJ, Cheon YS, Kim KS, Kang BK, Park ST, Chang CH, et al: Inhibition
of tumor necrosis factor-alpha-induced apoptosis by Asparagus
cochlnchinensis in HepG2 cells. J Ethnopharmacol. 73:137–143. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiong D, Yu LX, Yan X, Guo C and Xiong Y:
Effects of root and stem extracts of Asparagus cochinchinensis on
biochemical indicators related to aging in the brain and liver of
mice. Am J Chinese Med. 39:719–726. 2011. View Article : Google Scholar
|
|
16
|
Jung KH, Choi HL, Park S, Lee G, Kim M,
Min JK, Min BI and Bae H: The effects of the standardized herbal
formula PM014 on pulmonary inflammation and airway responsiveness
in a murine model of cockroach allergen-induced asthma. J
Ethnopharmacol. 155:113–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Elson LA and Morgan WTJ: A colorimetric
method for the determination of glucosamine and chondrosamine.
Biochem J. 27:1824–1828. 1933. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaegawa H, Matsumoto H, Endo K, Satoh T,
Nonaka GI and Noshioka I: Inhibitory effects of tannins on
hyaluronidase activation and on the degranulation from rat
mesentery mast cells. Chem Pharm Bull. 33:5079–5082. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee KK, Kim JH, Cho JJ and Choi JD:
Inhibitory effects of 150 plant extracts on elastase activity and
their anti-inflammatory effects. Int J Cosmet Sci. 21:71–82. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singleton VL and Rossi JA: Colorimetry of
total phenolics with phosphomolybdic-phosphotungstic acid reagents.
Am J Enol Vitic. 16:144–158. 1965.
|
|
21
|
Hassan SM, Al Aqil AA and Attimarad M:
Determination of crude saponin and total flavonoids content in guar
meal. Adv Med Plant Res. 1:24–28. 2013.
|
|
22
|
Oh H, Ko EK, Kim DH, Jan KK, Park SE, Lee
HS and Kim YC: Secoiridoid glucosides with free radical scavenging
activity from the leaves of Syringa dilatata. Phytother Res.
17:417–419. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jie S, Xueji Z, Mark B and Harry F:
Measurement of nitric oxide production in biological systems by
using griess reaction assay. Sensors. 3:276–284. 2003. View Article : Google Scholar
|
|
24
|
Kim JE, Park SH, Kwak MH, Go J, Koh EK,
Song SH, Sung JE, Lee HS, Hong JT and Hwang DY: Characterization of
changes in global genes expression in the distal colon of
loperamide-induced constipation SD rats in response to the laxative
effects of Liriope platyphylla. PLoS One. 10:e01296642015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ishimura N, Bronk SF and Gores GJ:
Inducible nitric oxide synthase upregulates cyclooxygenase-2 in
mouse cholangiocytes promoting cell growth. Am J Physiol
Gastrointest Liver Physiol. 287:G88–G95. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rahman M: Medical application of
fermentation technology. Adv Mat Res. 810:127–157. 2013.
|
|
27
|
Oh YC, Cho WK, Jeong YH, Im GY, Yang MC
and Ma JY: Fermentation improves anti-inflammatory effect of
sipjeondaebotang on LPS-stimulated RAW 264.7 cells. Am J Chin Med.
40:813–831. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bose S, Jeon S, Eom T, Song MY and Kim H:
Evaluation of the in vitro and in vivo protective effects of
unfermented and fermented Rhizoma coptidis formulations against
lipopolysaccharide insult. Food Chem. 135:452–459. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Giriwono PE, Shirakawa H, Hokazono H, Goto
T and Komai M: Fermented barley extract supplementation maintained
antioxidative defense suppressing lipopolysaccharide-induced
inflammatory liver injury in rats. Biosci Biotechnol Biochem.
75:1971–1976. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xiao PG: Modern Chinese Materia Medica.
Chemical Industry Press; Beijing: pp. 1502002
|
|
31
|
Liu YZ, Qu FY and Zhang PX: EfBFct of
chloroform extract of Tiandong on the brain antioxidation of
D-galactose-induced senile mice. Heilongjiang Med Pharm. 24:7–8.
2001.
|
|
32
|
Ni JM, Zhao R and Wang R: Comparison on
amino acid content in prepared and unprepared Asparagus
cochinchinensis. Chin Tradit Herb Drugs. 23:182–183. 1992.
|
|
33
|
Tenji K and Junzo S: Studies on the
constituents of Asparagi Radix. I. On the structures of furostanol
oligosides of Asparagus cochinchinensis (Lour.) Merr. Chem Pharm
Bull. 27:3086–3094. 1979. View Article : Google Scholar
|
|
34
|
Liang ZZ, Aquino R, De Simone F, Dini A,
Schettino O and Pizza C: Oligofurostanosides from Asparagus
cochinchinensis. Planta Med. 54:344–346. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang YC, Huang SY and Shi JG: Two new
furostanol glycosides from Asparagus cochinchinensis. Chin Chem
Lett. 13:1185–1188. 2002.
|
|
36
|
Cong PZ and Keman S: Handbook of
analytical chemistry-mass volume. Chemical Industry Publishing
House; Beijing: pp. 296–298. 2000
|
|
37
|
Gong YH: 13C NMR chemical shifts of
natural organic compounds. Yunnan Science and Technology Publishing
House; Kunming: pp. 2521986
|
|
38
|
Yang MH: Steroidal sapogenins of
dioscorea. Chin Tradit Herb Drugs. 12:43–44. 1981.
|
|
39
|
Xu CL, Chen HS and Tan XQ: Studies on the
active constituents of Asparagi radix. Nat Prod Res Dev.
17:128–130. 2005.
|
|
40
|
Shen Y, Chen HS and Wang Q: Studies on
chemical constituents of Asparagus cochinchinensis. J Second Med
Univ. 28:1241–1244. 2007.
|
|
41
|
Shen Y, Xu CL, Xuan WD, Li HL, Liu RH, Xu
XK and Chen HS: A new furostanol saponin from Asparagus
cochinchinensis. Arch Pharm Res. 34:1587–1591. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li XN, Chu C, Cheng DP, Tong SQ and Yan
JZ: Norlignans from Asparagus cochinchinensis. Nat Prod Commun.
7:1357–1358. 2012.PubMed/NCBI
|
|
43
|
Zhu GL, Hao Q, Li RT and Li HZ: Steroidal
saponins from the roots of Asparagus cochinchinensis. Chin J Nat
Med. 12:213–217. 2014.PubMed/NCBI
|
|
44
|
Oh YC, Cho WK, Oh JH, Im GY, Jeong YH,
Yang MC and Ma JY: Fermentation by Lactobacillus enhances
anti-inflammatory effect of Oyaksungisan on LPS-stimulated RAW
264.7 mouse macrophage cells. BMC Complement Altern Med. 12:172012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bose S, Song MY, Nam JK, Lee MJ and Kim H:
In vitro and in vivo protective effects of fermented preparations
of dietary herbs against lipopolysaccharide insult. Food Chem.
134:758–765. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jung HJ, Choi H, Lim HW, Shin D, Kim H,
Kwon B, Lee JE, Park EH and Lim CJ: Enhancement of
anti-inflammatory and antinociceptive actions of red ginseng
extract by fermentation. J Pharm Pharmacol. 64:756–762. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lee WH, Wu HM, Lee CG, Sung DI, Song HJ,
Matsui T, Kim HB and Kim SG: Specific oligopeptides in fermented
soybean extract inhibit NF-κB-dependent iNOS and cytokine induction
by toll-like receptor ligands. J Med Food. 17:1239–1246. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Endo K, Yoon BI, Pairojkul C, Demetris AJ
and Sirica AE: ERBB-2 overexpression and cyclooxygenase-2
up-regulation in human cholangiocarcinoma and risk conditions.
Hepatology. 36:439–450. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jaiswal M, LaRusso NF, Burgart LJ and
Gores GJ: Inflammatory cytokines induce DNA damage and inhibit DNA
repair in cholangiocarcinoma cells by a nitric oxide-dependent
mechanism. Cancer Res. 60:184–190. 2000.PubMed/NCBI
|
|
50
|
Hemish J, Nakaya N, Mittal V and
Enikolopov G: Nitric oxide activates diverse signaling pathways to
regulate gene expression. J Biol Chem. 278:42321–42329. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Berghe W Vanden, Plaisance S, Boone E,
Debosscher K, Schmitz ML, Fiers W and Haegeman G: p38 and
extracellular signal-regulated kinase mitogen-activated protein
kinase pathways are required for nuclear factor-kappaB p65
transactivation mediated by tumor necrosis factor. J Biol Chem.
273:3285–3290. 1998. View Article : Google Scholar : PubMed/NCBI
|