Introduction
Diabetic retinopathy (DR) is the most common
complication of diabetes and the main cause of new-onset blindness
in the developed world (1). This
complication is a result of multiple pathogenic processes caused by
hyperglycemia and the deregulation of insulin signalling pathways,
which in turn causes neuro-retinal dysfunction and retinal
microvascular degeneration (2,3). The
early clinical signs of DR include microaneurysms and hemorrhages,
while the later signs are narrowed, tortuous and irregular retinal
blood vessels, due to the formation of abnormal new blood vessels
at the back of the eye in proliferative DR (4). Although significant improvements have
been made, no effective prevention or treatment for DR exists.
Therefore, the development of novel and effective measures for the
treatment and prevention of DR is necessary.
During the new blood vessel formation process,
capillaries are formed when endothelial cells are stimulated to
migrate, proliferate and invade the surrounding tissues (5). Various pro- and anti-angiogenic
molecules regulate this process under normal conditions. When the
regulatory balance is adversely affected, dysfunction of
endothelial cells may occur. Among the angiogenic mediators,
vascular endothelial growth factor (VEGF) is considered to be the
main stimulating factor, which acts via promoting the migration and
proliferation of vascular endothelial cells, and thereby stimulates
the generation of new blood vessels (6). Endogenous inhibitors of angiogenesis
include thrombospondin 1 (TSP-1) and a disintegrin and
metalloproteinase with thrombospondin motifs 1 (ADAMTS-1), and
their aberrant expression may contribute to the diabetes-related
dysregulation of retinal vascular homeostasis and vasculopathies
(7,8).
Estrogen-related receptor α (ERRα) belongs to a
nuclear receptor superfamily characterized by their high levels of
sequence identity to estrogen receptors, and the primary role of
ERRα is in energy metabolism (9). A
study has demonstrated that activation of the peroxisome
proliferator-activated receptor g coactivator 1-α (PGC-1α)/ERRα
pathway increases VEGF expression and angiogenesis in endothelial
cells, which suggests that ERRα may be involved in the pathogenesis
of DR (10). Kaempferol
(3,4′,5,7-tetrahydoxyflavone), a commonly found dietary flavonoid,
has been isolated from grapefruit, tea, broccoli and other plant
sources (11). The anticancer
effects of kaempferol have been demonstrated in previous studies
(12,13), and kaempferol has been found to act
as an ERRα inverse agonist, which inhibits cell growth in different
cancer cell lines by antagonizing ERRα activity (14). Kaempferol has been shown to have an
anti-angiogenic effect in ovarian cancer (15); however, whether kaempferol inhibits
angiogenesis in DR is unclear. Therefore, the effect of kaempferol
on the angiogenesis of retinal endothelial cells under high glucose
conditions is worthy of investigation.
In the present study, it was demonstrated for the
first time that high glucose treatment increased the expression
level of ERRα mRNA and protein in human retinal endothelial cells
(HRECs). Luciferase reporter and in vitro functional assays
indicated that kaempferol inhibited ERRα activity and suppressed
high glucose-induced cell proliferation, migration and tube
formation. Further experiments revealed that kaempferol may exert
its anti-angiogenesis effect by reducing VEGF expression and
increasing TSP-1 and ADAMTS-1 expression.
Materials and methods
Cell culture
HRECs were purchased from ATCC (Manassas, VA, USA)
and were cultured in a human microvascular endothelial medium (Cell
Applications, Inc., San Diego, CA, USA) and were maintained at 37°C
in a humidified 5% CO2 incubator. Experiments were
performed using cells between passages 3 and 8.
Treatment of HRECs
For the time-dependence experiment, HRECs were
treated with 30 mM glucose for 12, 24 or 48 h, and HRECs incubated
in 5 mM normal glucose were used as a negative control. In the
kaempferol treatment experiments, HRECs were divided into 5 mM
normal glucose, 30 mM glucose, 30 mM glucose plus 10 µM kaempferol,
and 30 mM glucose plus 30 µM kaempferol groups. The HRECs were
split at 90% confluence and subcultured in 96-well plates or 6-well
plates according to the appropriate assay conditions.
Chemicals, plasmids and
transfection
Kaempferol and XTC-790 were purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany); pCMX-ERRα and
pcDNA-PGC-1α plasmids and the respective plasmids (pCMX and pCDNA)
used as negative controls were purchased from Generay Biotechnology
Co., Ltd. (Shanghai, China). All transfections were conducted using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer's instructions.
RNA preparation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was isolated from the cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). A TaqMan
Reverse Transcription kit (Takara Biotechnology Co., Ltd., Dalian,
China) was used to prepare cDNA from the ERRα, VEGF, TSP-1 and
ADAMTS-1 RNA. qPCR was performed using a SYBR Green PCR kit (Takara
Biotechnology Co., Ltd.) according to the manufacturer's
instructions. GAPDH was used as an internal control. The primers
for ERRα mRNA were: forward, 5′-TTCGGCGACTGCAAGCTC-3′ and reverse,
5′-CACAGCCTCAGCATCTTCAATG-3′; the primers for VEGF mRNA were:
forward, 5′-TGCCATCCAATCGAGACCCTG-3′ and reverse,
5′-GGTGATGTTGGACTCCTCAGTG-3′; the primers for TSP-1 mRNA were:
forward, 5′-GGTCGGCCTGCACTGTCACC-3′ and reverse,
5′-GGGGAAGCTGCTGCACTGGG-3′; the primers for ADAMTS-1 mRNA were:
forward, 5′-CTCCGCCTGCACGCCTTTGA-3′ and reverse,
5′-ATCGCCATTCACGGTGCCGG-3′. Data were expressed as fold changes
relative to GAPDH calculated based on the 2−∆∆Cq method
(16).
Luciferase reporter assay
HRECs were seeded in a 96-well plate
(1×104 cells/well), incubated for 24 h, and then
co-transfected with pGL3-ERRE-Luci (reporter; Promega Corporation,
Madison, WI, USA) and pMCX-ERRα with or without pcDNA-PGC-1α
plasmids. Renilla luciferase plasmid (Promega Corporation)
was used as an internal control. At 24 h after transfection, cells
were treated with kaempferol (10 or 30 µM) or XTC-790 (15 µM) for
24 h and then harvested for luciferase assay. Luciferase activity
was measured using a Dual-Luciferase Reporter Assay system (Promega
Corporation).
Cell proliferation assay
HRECs (3×103) were seeded into each well
of a 96-well plate and allowed to adhere for 24 h. When the cells
were adherent to the bottom of the plate, they were cultured in
serum-free medium for starvation for 24 h. The cells were then
treated with glucose alone (5 and 30 mM) or 30 mM glucose with
kaempferol (10 or 30 µM) for 24 h, and the proliferative activity
was determined by Cell Counting kit-8 (CCK-8) assay (Beyotime
Institute of Biotechnology, Haimen, China) according to the
manufacturer's instructions. In brief, 10 µl CCK-8 was added to
each well, and following incubation for 2 h, the absorbance at a
wavelength of 450 nm was detected.
Cell migration assay
When the HRECs had grown to 90% confluence in 6-well
plates, they were starved with serum-free medium for 12 h. When the
HRECs were over-confluent, a 200-µl pipette tip was used to create
a wound. The floating cells were removed by washing three times
with sterile 1X phosphate-buffered saline. After this, the cells
were incubated with serum-free media containing glucose alone (5
and 30 mM) or 30 mM glucose with kaempferol (10 or 30 µM) for 24 h,
and then cultured in a 6-well plate at 37°C in an incubator with 5%
CO2. The migration monolayer was photographed at 0, 12,
24 and 48 h. Photographic images of five fields were photographed
for each well, and the migration distance was measured.
Tube formation assay
After thawing in a refrigerator at 4°C overnight, 60
µl Matrigel was added to a pre-cooled 96-well plate and then
solidified by immediately placing the plate in a humidified
CO2 incubator at 37°C for 30 min. HRECs that had been
cultured with serum-free media containing glucose alone (5 and 30
mM) or 30 mM glucose with kaempferol (10 or 30 µM) for 24 h, were
seeded immediately on the solidified Matrigel at a density of
1.5×104 cells/well. The plates were placed in a
humidified atmosphere of 5% CO2 and 95% air at 37°C for
8 h. Images of the plates were captured, and the number of
capillaries formed was qualitatively assessed using Image-Pro Plus
6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
Western blot analysis
HRECs were lysed in SDS lysis buffer containing
protease inhibitor (Sigma-Aldrich; Merck KGaA), and the protein
concentration was measured using a Bio-Rad Protein Assay kit
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) according to the
manufacturer's instructions. Proteins were then separated by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred
to a polyvinylidene fluoride membrane. The membrane was incubated
with the following primary antibodies: Mouse anti-ERRα (1:1,500;
sc-65718; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), rabbit
anti-VEGF (1:2,000; no. 2463; Cell Signalling Technology, Inc.,
Boston, MA, USA), and mouse anti-β-actin (1:6,000; ab8226; Abcam,
Cambridge, MA, USA) at 4°C overnight. Membranes were then incubated
with the horseradish peroxidase-conjugated secondary antibodies
(1:4,000; ab6728; Abcam) at room temperature for 1 h. After further
incubation with the enhanced chemiluminescence substrate (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), the membranes were exposed
using a ChemoDoc XRS detection system (Bio-Rad Laboratories, Inc.).
The band intensities were analysed by the Image Lab v.6.0 software
(Bio-Rad Laboratories, Inc.).
Statistical analysis
All statistical analysis was performed using
GraphPad Prism 6 (GraphPad. Software, Inc., La Jolla, CA, USA).
Data are presented as the mean ± standard deviation; and
differences among the treatment groups were compared by one-way
analysis of variance followed by Dunnett's multiple comparison
test, or unpaired t-test as appropriate. P<0.05 was considered
to indicate a statistically significant difference.
Results
Effect of high glucose on ERRα mRNA
and protein expression in HRECs
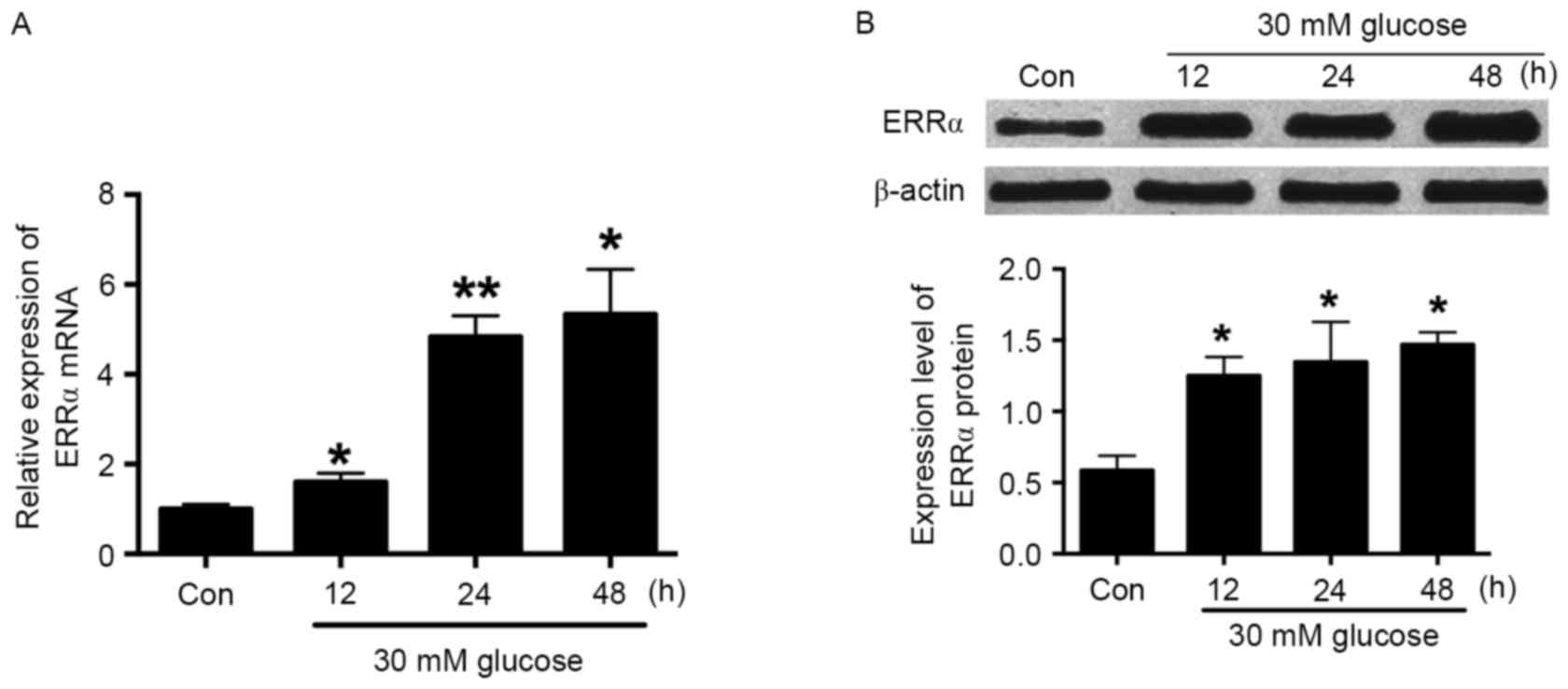
RT-qPCR was conducted to evaluate the ERRα mRNA
expression in HRECs following treatment with 30 mM glucose for 12,
24 and 48 h. The results showed that glucose (30 mM) significantly
increased ERRα mRNA expression in a time-dependent manner (Fig. 1A). The effect of 30 mM glucose on
ERRα protein expression was evaluated by western blot analysis, and
similar results were obtained; this concentration of glucose also
increased ERRα protein expression in a time-dependent manner
(Fig. 1B). As treatment with 30 mM
glucose for 24 h significantly increased ERRα expression at the
mRNA and protein levels, a 24-h treatment time was selected for
high glucose treatment in the following experiments.
Effects of kaempferol on the activity
of ERRα
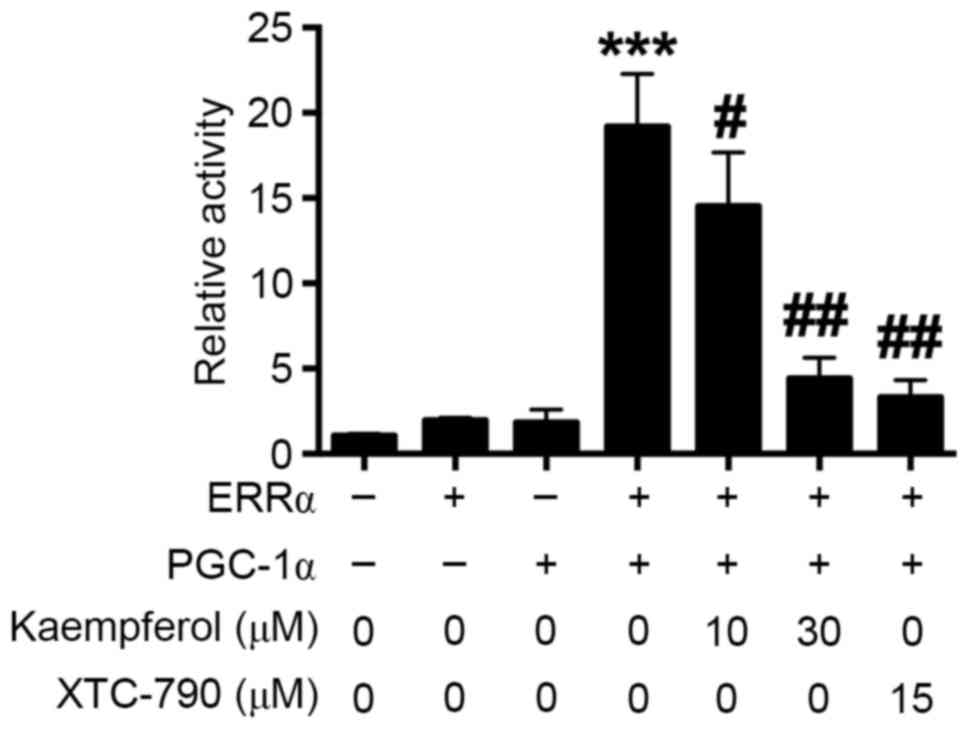
Kaempferol has been shown to inhibit ERRα activity
in cancer cells (12). In the
present study, whether kaepmferol is able to inhibit ERRα in HRECs
was investigated. The luciferase reporter assay showed that HREC
cells co-transfected with pCMX-ERRα and pcDNA-PGC-1α plasmids had a
higher luciferase activity comparing with that of the HRECs
transfected with only one plasmid (Fig.
2); kaempferol and XTC-790 treatment each significantly reduced
the luciferase activity in cells co-transfected with pCMX-ERRα and
pcDNA-PGC-1α plasmids compared with the untreated co-transfected
control, and kaempferol exhibited the inhibitory effect in a
concentration-dependent manner (Fig.
2). These results indicate that kaempferol inhibits ERRα
activity in HRECs.
Effects of kaempferol on
glucose-induced cell proliferation, migration and tube formation of
HRECs
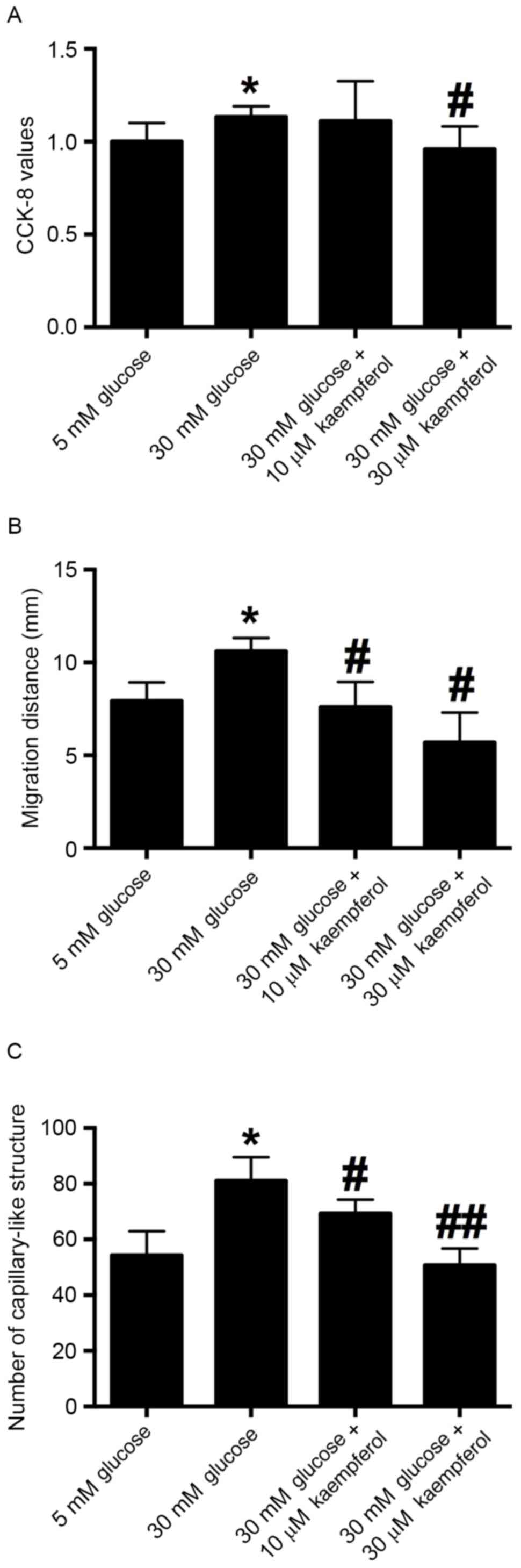
An in vitro CCK-8 assay was carried out to
examine the proliferative ability of HRECs after the cells had been
treated with glucose (30 mM) alone or in combination with 10 or 30
µM kaempferol for 24 h. The results showed that 30 mM glucose
treatment significantly increased the proliferation of HRECs
compared with 5 mM glucose treatment, and the proliferation of
HRECs induced by 30 mM glucose was inhibited by concurrent
treatment with 30 µM kaempferol (Fig.
3A).
A wound scratching assay was conducted to examine
whether kaempferol was able to modulate the glucose-induced
migration of HRECs. The results showed that 30 mM glucose
significantly accelerated the wound closure compared with that in
the 5 mM glucose treatment group. Treatment with kaempferol (10 or
30 µM) significantly inhibited the migration of HRECs induced by 30
mM glucose compared with that in the 30 mM glucose group (Fig. 3B). These results indicate that
kaempferol inhibits the high glucose-induced migration of
HRECs.
To examine the effect of glucose (30 mM) and
kaempferol (10 or 30 µM) on angiogenesis, the tube formation of
HRECs was evaluated by Matrigel assay. The results showed that 30
mM glucose significantly increased the number of capillary-like
structures compared with that in the 5 mM glucose treatment group.
HRECs treated with kaempferol (10 and 30 µM) had fewer
capillary-like structures compared with the high glucose-treatment
group (Fig. 3C). The results suggest
that kaempferol inhibits high glucose-induced tube formation.
Effects of high glucose and kaempferol
on VEGF, ADAMTS-1 and TSP-1 mRNA expression in HRECs
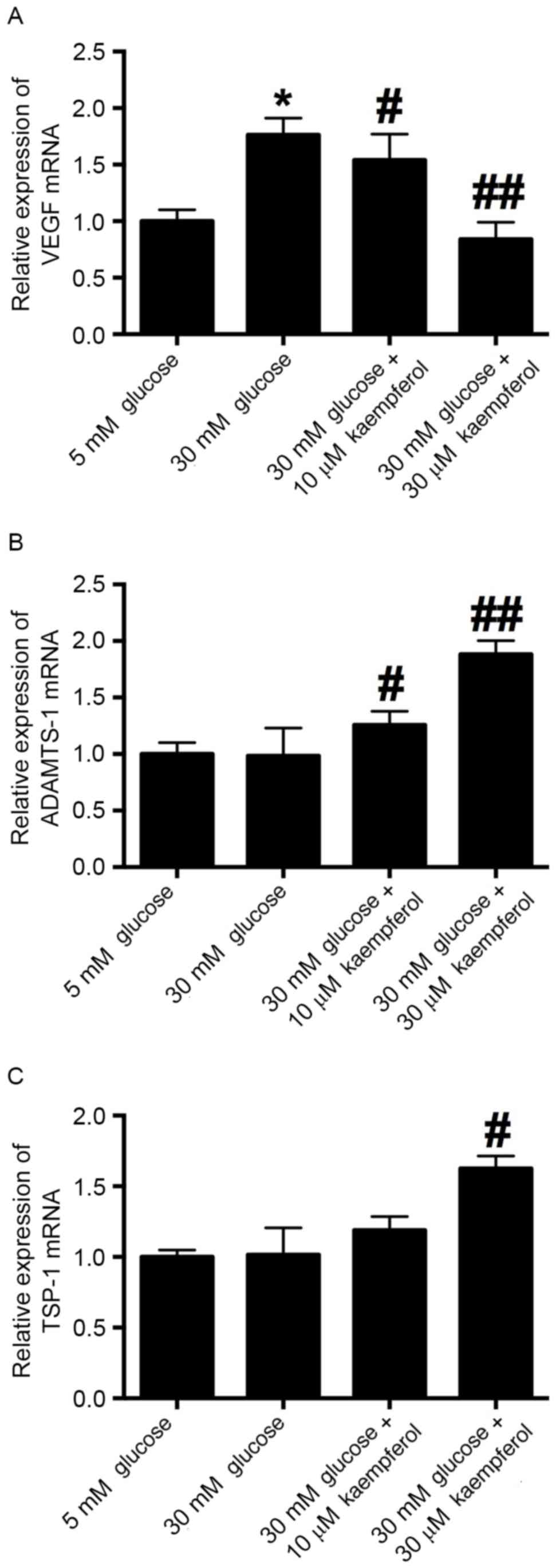
To examine whether high glucose and kaempferol
induce changes in VEGF, ADAMTS-1 and TSP-1 mRNA expression in
HRECs, RT-qPCR was conducted. The expression of VEGF mRNA was
significantly increased by treatment with 30 mM glucose compared
with that with 5 mM glucose, and kaempferol (10 and 30 µM)
significantly antagonized the glucose-induced increase in VEGF mRNA
expression (Fig. 4A). ADAMTS-1 and
TSP-1 mRNA levels did not differ significantly between the 5 and 30
mM glucose groups, while kaempferol treatment increased the
expression of ADAMTS-1 and TSP-1 mRNA in the HRECs (Fig. 4B and C).
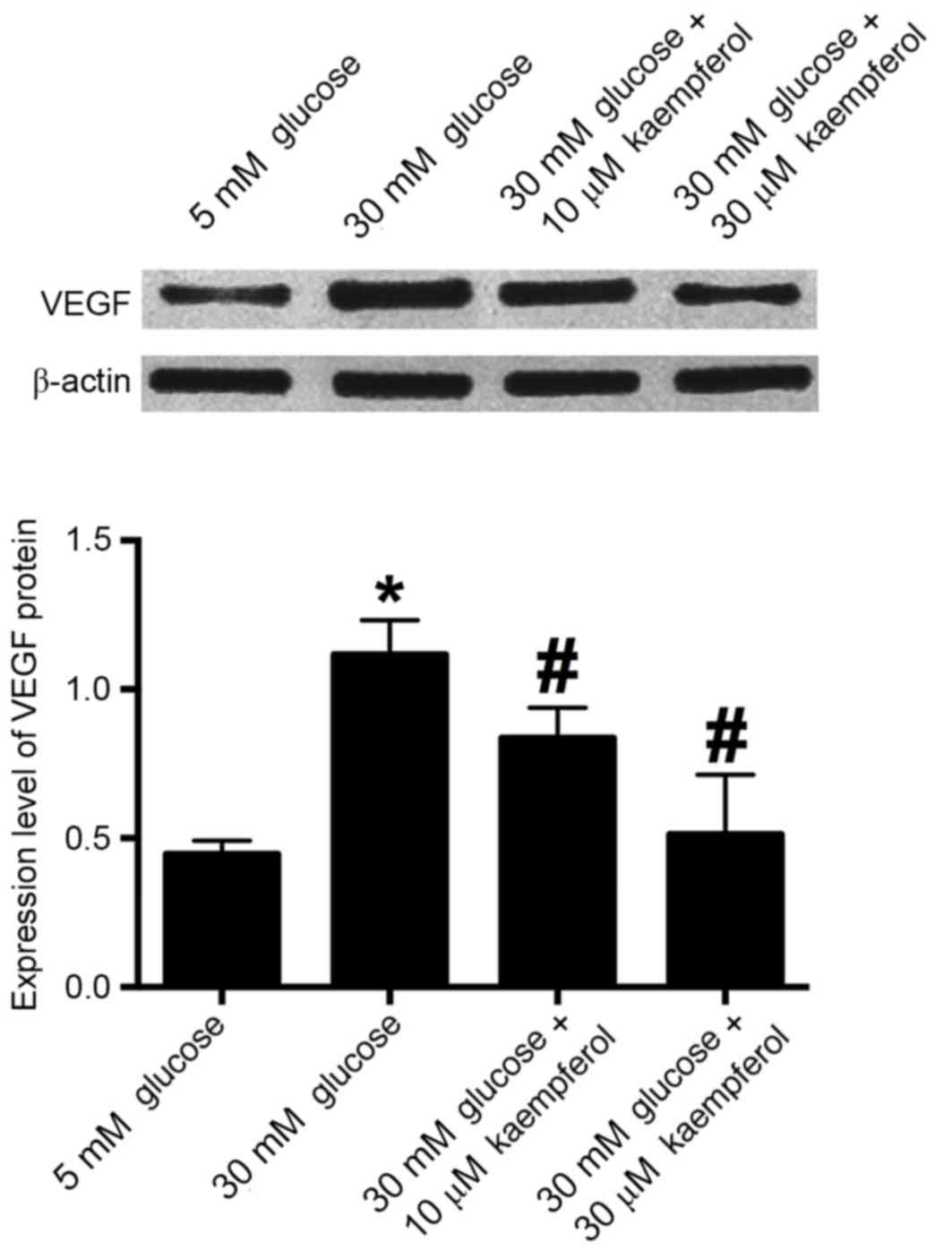
Effects of high glucose and keampferol
on VEGF protein in HRECs
Western blotting results demonstrated that 30 mM
glucose increased the expression of VEGF protein compared with that
in the 5 mM glucose group, and kaempferol (10 and 30 µM)
significantly antagonized the high glucose-induced increase in
expression (Fig. 5).
Discussion
The present study demonstrated for the first time
that high glucose treatment increases the expression of ERRα at the
mRNA and protein levels in HRECs. Luciferase reporter and in
vitro functional assays indicated that kaempferol was able to
inhibit ERRα activity and suppress the high glucose-induced cell
proliferation, migration and tube formation. Further experiments
suggested that kaempferol may exert its anti-angiogenic effect via
the downregulation of VEGF and upregulation of ADAMTS-1 and
TSP-1.
The development of DR is largely attributed to high
glucose levels, which generate cellular stress, cause injury to
vascular pericytes and endothelial cells, and induce the
development of abnormal capillaries (17). Endothelial cells play a key role in
regulating vascular tone, and dysfunction of these cells is crucial
for the development of DR (18). In
the present study, HRECs were used to simulate the pathogenesis of
DR under high glucose condition; and high glucose treatment for 24
h was shown to increase the proliferation, migration and tube
formation of HRECs. The high glucose-induced migration and tube
formation of HRECs have been well documented in previous reports
(19–21). However, the effect of high glucose on
the proliferation of HRECs found in these studies has varied. Yuan
et al demonstrated that treatment of HRECs with 30 mM
glucose for 48 h increased the proliferation of HRECs compared with
5 mM glucose treatment (18), while
two other studies indicated that treatment with 30 mM glucose for
24 and 72 h failed to increase the proliferation of HRECs (20,22).
Differences in cell lines and culture times have been suggested as
a cause of the discrepancy. The increase in the proliferation of
HRECs following treatment with 30 mM glucose for 24 h was only ~5%
in our study, and the effect was very marginal; thus, further
investigation may be required to elucidate the effect of high
glucose levels on these cells.
ERRα has roles in energy metabolism, and various
biosynthetic pathways, and is a key hypoxic growth regulator
(23). However, the role of ERRα in
the pathogenesis of DR is largely unknown. PGC-1α, a co-activator
of ERRα, has been shown to regulate VEGF and angiogenesis in a
HIF-independent manner (24).
Activation of the PGC-1α/ERRα pathway by baicalin has been shown to
increase VEGF expression and angiogenesis (10). More importantly, it has also been
found that PGC-1α affects both glucose metabolism and angiogenesis
in multiple myeloma cells by regulating VEGF and GLUT-4 (25). In the present study, high glucose
treatment was demonstrated to increase the expression of EERα at
the mRNA and protein levels. Collectively, these data may indicate
a potential role for EERα in DR. Kaempferol has been shown to be an
ERRα inverse agonist that is able to inhibit cell growth by
antagonizing ERRα (14), and its
anticancer effect has also been recognized (12,13).
However, its role in DR has not previously been reported. In the
present study, a luciferase reporter assay demonstrated that
kaempferol inhibited ERRα in HRECs, which suggests that the effects
of kaempferol may be mediated via the targeting of ERRα. To further
elucidate the effect of kaempferol on the pathogenesis of DR, in
vitro functional experiments were conducted, which showed that
kaempferol suppressed the high glucose-induced proliferation,
migration and tube formation of HRECs. Kaempferol has previously
been shown to inhibit angiogenesis and VEGF expression in human
ovarian cancer cells via an ERK-NFkB-cMyc-p21 pathway (15); Liang et al demonstrated that
kaempferol suppressed angiogenesis through inhibiting VEGF receptor
2 expression, and this effect was enhanced by fibroblast growth
factor (FGF) inhibition in a transgenic zebrafish model (26). On the basis of these findings, it may
be suggested that ERRα could be involved in the pathogenesis of DR,
and that kaempferol might be a useful tool for inhibiting
angiogenesis in DR.
Angiogenesis is a complex and multistep process, and
when the regulatory mechanism for angiogenesis is unbalanced,
dysfunction may occur. VEGF is considered to mediate the abnormal
angiogenesis that occurs in response to high glucose (6). Various studies have indicated that high
glucose stimulates VEGF secretion (27), and the findings of the present study
are consistent with this. The results of the present study
indicated that kaempferol significantly antagonized the high
glucose-induced increase in VEGF expression at the mRNA and protein
levels, which suggests that kaempferol exerts an anti-angiogenic
effect via the suppression of VEGF expression. Whether kaempferol
has an effect on the expression of the anti-angiogenic molecules,
TSP-1 and ADAMTS-1, which are constitutively present in HRECs, was
then investigated. These molecules can inhibit vascular development
(28,29). ADAMTS-1 acts on TSP-1, causing it to
release anti-angiogenic polypeptides (30). TSP-1 deficiency has been found to
exacerbate the pathogenesis of diabetic retinopathy (7). At high concentrations, ADAMTS-1 has
been shown to inhibit the endothelial migration induced by combined
treatment with VEGF and basic FGF (31). In the present study, it was found
that kaempferol increased the expression of TSP-1 and ADAMTS-1 mRNA
in HRECs under high glucose conditions. These results suggest that
kaempferol suppressed the high glucose-induced angiogenesis of
HRECs by regulating pro-angiogenic and anti-angiogenic factors.
In conclusion, the findings of the present study
suggest that kaempferol inhibits ERRα and reduces the high
glucose-induced proliferation, migration and tube formation of
HRECs via the regulation of pro- and anti-angiogenic factors. The
results suggest that kaempferol is potentially useful as a drug to
control the progression of DR. However, in vivo studies are
required to evaluate its efficacy and safety.
Acknowledgements
The present study was supported by the Innovative
Research Program of Shenzhen City (grant no. JYJ201304011).
References
|
1
|
Yu DY, Cringle SJ, Su EN, Yu PK, Jerums G
and Cooper ME: Pathogenesis and intervention strategies in diabetic
retinopathy. Clin Exp Ophthalmol. 29:164–166. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hendrick AM, Gibson MV and Kulshreshtha A:
Diabetic Retinopathy. Prim Care. 42:451–464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wan TT, Li XF, Sun YM, Li YB and Su Y:
Recent advances in understanding the biochemical and molecular
mechanism of diabetic retinopathy. Biomed Pharmacother. 74:145–147.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Osaadon P, Fagan XJ, Lifshitz T and Levy
J: A review of anti-VEGF agents for proliferative diabetic
retinopathy. Eye (Lond). 28:510–520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoo SY and Kwon SM: Angiogenesis and its
therapeutic opportunities. Mediators Inflamm. 2013:1271702013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Distler JH, Hirth A, Kurowska-Stolarska M,
Gay RE, Gay S and Distler O: Angiogenic and angiostatic factors in
the molecular control of angiogenesis. Q J Nucl Med. 47:149–161.
2003.PubMed/NCBI
|
|
7
|
Sorenson CM, Wang S, Gendron R, Paradis H
and Sheibani N: Thrombospondin-1 deficiency exacerbates the
pathogenesis of diabetic retinopathy. J Diabetes Metab. Suppl
12:2013.PubMed/NCBI
|
|
8
|
Basile DP, Fredrich K, Chelladurai B,
Leonard EC and Parrish AR: Renal ischemia reperfusion inhibits VEGF
expression and induces ADAMTS-1, a novel VEGF inhibitor. Am J
Physiol Renal Physiol. 294:F928–F936. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ranhotra HS: The estrogen-related receptor
alpha: The oldest, yet an energetic orphan with robust biological
functions. J Recept Signal Transduct Res. 30:193–205. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang K, Lu J, Mori T, Smith-Powell L,
Synold TW, Chen S and Wen W: Baicalin increases VEGF expression and
angiogenesis by activating the ERR{alpha}/PGC-1{alpha} pathway.
Cardiovasc Res. 89:426–435. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Olszewska MA: New validated
high-performance liquid chromatographic method for simultaneous
analysis of ten flavonoid aglycones in plant extracts using a C18
fused-core column and acetonitrile-tetrahydrofuran gradient. J Sep
Sci. 35:2174–2183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen AY and Chen YC: A review of the
dietary flavonoid, kaempferol on human health and cancer
chemoprevention. Food Chem. 138:2099–2107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim SH and Choi KC: Anti-cancer effect and
underlying mechanism (s) of kaempferol, a phytoestrogen, on the
regulation of apoptosis in diverse cancer cell models. Toxicol Res.
29:229–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang J, Fang F, Huang Z, Wang Y and Wong
C: Kaempferol is an estrogen-related receptor alpha and gamma
inverse agonist. FEBS Lett. 583:643–647. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luo H, Rankin GO, Juliano N, Jiang BH and
Chen YC: Kaempferol inhibits VEGF expression and in vitro
angiogenesis through a novel ERK-NFκB-cMyc-p21 pathway. Food Chem.
130:321–328. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dagher Z, Park YS, Asnaghi V, Hoehn T,
Gerhardinger C and Lorenzi M: Studies of rat and human retinas
predict a role for the polyol pathway in human diabetic
retinopathy. Diabetes. 53:2404–2411. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yuan L, Hu J, Luo Y, Liu Q, Li T, Parish
CR, Freeman C, Zhu X, Ma W, Hu X, et al: Upregulation of heparanase
in high-glucose-treated endothelial cells promotes endothelial cell
migration and proliferation and correlates with Akt and
extracellular-signal-regulated kinase phosphorylation. Mol Vis.
18:1684–1695. 2012.PubMed/NCBI
|
|
19
|
Chen X, Li J, Li M, Zeng M, Li T, Xiao W,
Li J, Wu Q, Ke X, Luo D, et al: KH902 suppresses high
glucose-induced migration and sprouting of human retinal
endothelial cells by blocking VEGF and PIGF. Diabetes Obes Metab.
15:224–233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen Z, Liu G, Xiao Y and Lu P:
Adrenomedullin22-52 suppresses high-glucose-induced migration,
proliferation, and tube formation of human retinal endothelial
cells. Mol Vis. 20:259–269. 2014.PubMed/NCBI
|
|
21
|
Hayashi JN, Ito H, Kanayasu T, Asuwa N,
Morita I, Ishii T and Murota S: Effects of glucose on migration,
proliferation and tube formation by vascular endothelial cells.
Virchows Arch B Cell Pathol Incl Mol Pathol. 60:245–252. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Premanand C, Rema M, Sameer MZ, Sujatha M
and Balasubramanyam M: Effect of curcumin on proliferation of human
retinal endothelial cells under in vitro conditions. Invest
Ophthalmol Vis Sci. 47:2179–2184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zou C, Yu S, Xu Z, Wu D, Ng CF, Yao X, Yew
DT, Vanacker JM and Chan FL: ERRalpha augments HIF-1 signalling by
directly interacting with HIF-1α in normoxic and hypoxic prostate
cancer cells. J Pathol. 233:61–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arany Z, Foo SY, Ma Y, Ruas JL,
Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J,
Rangwala SM, et al: HIF-independent regulation of VEGF and
angiogenesis by the transcriptional coactivator PGC-1alpha. Nature.
451:1008–1012. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cao D, Zhou H, Zhao J, Jin L, Yu W, Yan H,
Hu Y and Guo T: PGC-1α integrates glucose metabolism and
angiogenesis in multiple myeloma cells by regulating VEGF and
GLUT-4. Oncol Rep. 31:1205–1210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liang F, Han Y, Gao H, Xin S, Chen S, Wang
N, Qin W, Zhong H, Lin S, Yao X and Li S: Kaempferol identified by
zebrafish assay and fine fractionations strategy from dysosma
versipellis inhibits angiogenesis through VEGF and FGF pathways.
Sci Rep. 5:144682015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Betts-Obregon BS, Vellanki S, Buikema J,
Tsin AT and Wright K: Effect of glucose on retinal endothelial cell
viability and VEGF secretion. HSOA J Cell Biol Cell Metabol. 3:pii:
008. 2016.PubMed/NCBI
|
|
28
|
DiPietro LA, Nebgen DR and Polverini PJ:
Downregulation of endothelial cell thrombospondin 1 enhances in
vitro angiogenesis. J Vasc Res. 31:178–185. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun Y, Huang J and Yang Z: The roles of
ADAMTS in angiogenesis and cancer. Tumour Biol. 36:4039–4051. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee NV, Sato M, Annis DS, Loo JA, Wu L,
Mosher DF and Iruela-Arispe ML: ADAMTS1 mediates the release of
antiangiogenic polypeptides from TSP1 and 2. EMBO J. 25:5270–5283.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Krampert M, Kuenzle S, Thai SN, Lee N,
Iruela-Arispe ML and Werner S: ADAMTS1 proteinase is up-regulated
in wounded skin and regulates migration of fibroblasts and
endothelial cells. J Biol Chem. 280:23844–23852. 2005. View Article : Google Scholar : PubMed/NCBI
|