Introduction
The geriatric population is rapidly increasing
worldwide. According to the World Health Organization, the number
of people aged 65 and older is expected to increase to 1.5 billion
by 2050 and to significantly outnumber the population of children 5
years old and younger (1). In 2012,
the per person health care spending of individuals 65 and older was
approximately 5 times higher than for children and 3 times higher
than for working-aged persons. Significant resources are devoted to
maintaining the health of this geriatric population, which is prone
to increased incidences of infections, cancers, and degenerative
diseases. For example, the estimated healthcare costs for
individuals with Alzheimer's and other dementias in 2017 is 259
billion USD (2), and the cost for
those with cancer is 87.8 billion USD (3).
Immunodeficiency in the geriatric population plays a
major role in their increased susceptibility to infections,
cancers, and neurodegeneration. Recent research has found evidence
suggesting that the nervous system and immune system are intimately
linked and engage in significant cross-talk to preserve
homeostasis. Several proteins that were originally thought to be
exclusive to the immune system have been detected in the healthy
nervous system (4), and the term
synaptoimmunology has been used recently to characterize the
interplay between immune modulators and synaptic function (5). The link between decreased immunological
vigor and increased infection and cancer has been substantiated in
many studies which show the reduction of leukocyte qualitative
functions during aging (6–9). Of particular interest to the study of
immunodeficiency in the geriatric population is the reported
age-dependent reduction of natural killer (NK) cells which
constitute the primary line of defense against malignant and
virally-infected cells (10–13). Enhancing NK cell activity is
therefore an essential therapeutic target in the geriatric
population.
Given the significant costs mentioned above,
attempts need to be made to find natural, safe, and inexpensive
agents to improve age-associated health and treatment of diseases.
In the current preliminary study, we hypothesized that the natural
dietary supplement arabinoxylan derived from rice bran,
Biobran/MGN-3, could counteract the age-induced decline of NK cell
activity in geriatric subjects over 56 years old. Biobran/MGN-3 is
manufactured by hydrolyzing rice bran with the enzymatic extract of
Shiitake mushrooms (14) and several
studies have demonstrated its potent immunomodulatory effect
(15–20). For example, Biobran/MGN-3 has been
shown to enhance NK cell activity by 2–3 fold in young adults
(human subjects aged 20–46 years) (21). The effect was dose and time dependent
and was associated with an increase in the binding capacity of NK
cells to tumor cell targets (21).
In addition, the positive effects of Biobran/MGN-3 on human T and B
cell mitogen response have also been examined in vivo
(14).
To test our hypothesis, we conducted a one-month,
randomized, double-blind, placebo-controlled clinical trial in
geriatric subjects attending Zagazig University Hospital in
Zagazig, Al Sharqia, Egypt. The aim of our study was to evaluate
the response of these geriatric subjects to the immune modulatory
effect of Biobran/MGN-3 and to determine whether the previously
reported positive effects of Biobran/MGN-3 supplementation on NK
cell activity could be extended to this population.
Subjects and methods
Biobran/MGN-3 is a denatured hemicellulose obtained
by reacting rice bran hemicellulose with multiple carbohydrate
hydrolyzing enzymes from Shiitake mushrooms. It is an arabinoxylane
with a xylose in its main chain and an arabinose polymer in its
side chain (14). 500 mg of
Biobran/MGN-3 was ingested once per day in the form of powder
enclosed in sachets. The control group ingested the same amount of
placebo powder, which was indistinguishable from Biobran/MGN-3 in
terms of color, smell, and consistency. The sachets of
Biobran/MGN-3 and placebo were indistinguishable except for a
letter code imprinted on the bottom of the sachets. Both
Biobran/MGN-3 and placebo sachets were kindly provided by Daiwa
Pharmaceuticals Co., Ltd., Tokyo, Japan.
Ingredients of the study
materials
Biobran/MGN-3 group sachets contained Biobran/MGN-3
(500 mg), Maltitol (1,000 mg), Dextrin (200 mg), Hydroxypropyl
Distarch Phosphate (280 mg), and Tricalcium Phosphate (20 mg).
Placebo group sachets contained Maltitol (1,000 mg), Dextrin (200
mg), Hydroxypropyl Distarch Phosphate (780 mg), and Tricalcium
Phosphate (20 mg).
Inclusion/exclusion criteria for
patients
Inclusion criteria
Included in the study were subjects 56 years or
older who were willing to provide written consent to participate
voluntarily in the study.
Exclusion criteria
We excluded subjects with current or a history of
infections or malignancies, auto-immune disorders, marked portal
hypertension and pancytopenia, or major psychological insult. We
also excluded those who were using vitamin or antibiotic
supplements, women who were lactating, and patients receiving other
anti-viral or anticancer therapies (radiation, chemotherapy).
Study subjects and design
Twelve apparently healthy geriatric subjects (≥56
years old) of both sexes (6 males and 6 females) were randomly
selected from visitors of outpatient clinics at Zagazig University
Hospital, Zagazig, Egypt. Subjects were randomly assigned to either
the Biobran/MGN-3 group (n=6, 500 mg/day orally) or the placebo
control group (n=6, 500 mg/day orally) using the random sample
selection function of SPSS. Only the Principal Investigator (PI)
had the code for Biobran/MGN-3 and placebo groups. Each participant
gave 2 blood samples (4 ml each) at the initiation and termination
of the study for laboratory and flow cytometry investigation.
During the study, enrolled subjects were asked not to take any
over-the-counter drugs, including vitamins, without consulting with
the study PI. All participants were asked to report any eruption of
abnormal symptoms or signs to the PI. The study protocol conformed
to the ethical guidelines of the 1975 Declaration of Helsinki and
was approved by Institutional Review Board (IRB approval no. 1507,
June 2015), Zagazig University Hospital, Faculty of Medicine.
Laboratory investigations
This included red blood cells (RBC), hematocrit
(HCT), hemoglobin (Hb), mean corpuscular hemoglobin (MCH), mean
corpuscular volume (MCV), platelet count, white blood cells (WBC),
neutrophils, and basophils + eosinophils. Laboratoy investigations
of liver and kidney function included alanine aminotransferase
(serum glutamic pyruvic transaminase) (ALT/SGPT), aspartate
aminotransferase (serum glutamic oxaloacetic transaminase)
(AST/SGOT), and uric acid (UA). ALT, AST, ALP, and UA levels were
measured by photometric methods using COBAS Integra 400 plus
Analyzer (Roche, Basel, Switzerland).
Degranulation assay
Blood was dispensed at a volume of 100 µl into each
of 4 tubes to serve as stimulated, unstimulated control, positive
control, or IgG isotypic negative control. Stimulation of NK/NKT
cells was conducted chemically by incubation (5 h, 37°C, under 5%
(v/v) CO2) with a combination of
Phorbol-12-myristate-13-acetate (PMA, 50 ng/ml; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) and Ca2+ Ionophore
(Ionomycin, 250 ng/ml, Sigma) in a final volume of 500 µl (adjusted
with RPMI-1640 medium). Unstimulated samples were incubated with
RPMI-1640 medium without PMA/Ionomycin stimulation, whereas in
positive control samples, cytochalasin (Sigma-Aldrich; Merck KGaA)
was added in conjunction with PMA/Ionomycin. FITC-labeled mouse
anti-human CD-107a clone H4A3 (BD Bioscience, San Jose, CA, USA)
was added during PMA/Ionomycin stimulation. Monensin (GolgiStop; BD
Bioscience, San Jose, CA, USA) was added to a final concentration
of 6 µg/ml 1 h after incubation to block internalization and
degradation of CD107a. In the IgG isotypic negative control tube,
mouse anti-human FITC-labeled IgG AB (clone G18-145; BD Bioscience)
was added instead of CD107a (22).
After incubation, RBCs were lysed with BD PharmLyse
(BD Bioscience) before cells were collected by spinning, washed
once with staining buffer, and re-suspended in 100 µl staining
buffer. Cells were stained by incubation with 5 µl of PE-labeled
mouse anti-human CD56 AB clone R19-760 (NCAM-1, BD Bioscience) and
5 µl of PerCP-labeled mouse anti-human CD3 clone SK7 (BD
Bioscience) for 15 min at 37°C. Cells were then collected by
spinning, washed once with FACS buffer, and then re-suspended in
FACS buffer. Analysis was carried out using BD FACSCalibur with
CellQuest software (BD Bioscience).
Sample size
A power analysis using a large effect size of 0.80
for a repeated measure analysis of variance with two measurements
in 2 groups was performed using G*Power software package (Version
3.1.9.2; Franz Faul, Germany). With the criterion of significance
(α) set at 0.05, a sample size of 6 subjects per group (total of 12
subjects) results in a power of 0.82 to yield a statistically
significant result.
Statistical analysis
Continuous variables were expressed as means ±
standard deviation. Two-tailed Student's t-test or Fisher exact
test were used to compare between groups, whereas two-tailed paired
t-test was used to examine the effect of treatment within a group
(i.e., compare post-treatment level with the pre-treatment level).
We used Shapiro-Wilks test to determine whether the data were
normally distributed. For non-normally distributed data, we used
non-parametric test, Wilcoxon Singed Rank test, and Mann-Whitney
test to examine the effect of treatment within and between groups,
respectively. P<0.05 was considered to indicate a statistically
significant difference. To produce graphs, the mean ± 95%
confidence interval was used. SPSS version 22 (IBM Corp., Armonk,
NY, USA) and GraphPad Prism 6 (GraphPad Software, Inc., La Jolla,
CA, USA) were used for analysis.
Results
Hematological and biochemical
profile
Different base-line blood parameters, liver enzymes,
and kidney function were examined in 12 geriatric subjects of both
Biobran/MGN-3 and placebo groups. Data in Tables I and II show that there was no statistically
significant difference between the two groups (P>0.05) in all
baseline variables except for AST level (P=0.041). Table I further shows that there was no
significant difference between baseline values and values after 1
month of supplementation for the measured characteristics in the
Biobran/MGN-3 group, except for levels of Hb, MCV, MCH and AST
(P<0.05). Table II shows that
there was no significant difference between baseline values and
values after 1 month of supplementation for the measured
characteristics in the Placebo group, except for levels of Hb and
MCH (P<0.05). This is further supported by lack of observation
of any side effects in the Biobran/MGN-3 group, indicating that its
treatment was safe at the used dosage and duration. Instead, some
beneficial effects were observed after one month treatment in the
Biobran/MGN-3 group, and the placebo group to a lesser extent.
These included improved levels of Hb, MCV, and MCH from
pre-treatment values. In addition, Biobran/MGN-3 supplementation
significantly down-regulated AST (SGOT) levels, suggesting improved
liver functions.
 | Table I.Hematological and biochemical
characteristics (blood parameters, liver enzymes, and kidney
function) of geriatric subjects in the Biobran/MGN-3 group. |
Table I.
Hematological and biochemical
characteristics (blood parameters, liver enzymes, and kidney
function) of geriatric subjects in the Biobran/MGN-3 group.
|
| Biobran/MGN-3
Group |
|
|---|
|
|
|
|
|---|
| Parameters | Baseline | 1 Month | P-value |
|---|
| RBC
(×103/µl) |
4.10±0.50 |
4.08±0.51 | 0.462 |
| Hb
(g/dl)c |
10.28±1.73 |
12.02±1.87 | 0.027a |
| HCT
(%)c |
32.30±4.62 |
32.47±4.86 | 0.892 |
| MCV
(fl)c |
78.63±4.14 |
79.65±4.57 | 0.027a |
| MCH
(pg/cell)c |
24.95±1.79 |
29.37±1.71 | 0.028a |
| Platelet
(×103/µl)c |
240.00±40.41 |
250.67±36.06 | 0.345 |
| WBC
(×103/µl)b |
5.50±2.51 |
5.65±2.32 | 0.651 |
| Neutrophils
(%)c |
58.78±9.63 |
61.47±8.90 | 0.173 |
|
Eosinophils+Basophils (%)b |
6.27±1.51 |
7.48±1.80 | 0.156 |
| ALT
(U/l)c |
19.17±9.87 |
18.50±8.14 | 0.461 |
| AST
(U/l)c |
30.00±8.10 |
27.83±6.47 | 0.027a |
| UA
(mg/dl)c |
5.71±2.70 |
5.88±2.65 | 0.046 |
 | Table II.Hematological and biochemical
characteristics (blood parameters, liver enzymes, and kidney
function) of geriatric subjects in the Placebo group. |
Table II.
Hematological and biochemical
characteristics (blood parameters, liver enzymes, and kidney
function) of geriatric subjects in the Placebo group.
|
| Placebo Group |
|
|---|
|
|
|
|
|---|
| Parameters | Baseline | 1 Month | P-value |
|---|
| RBC
(×103/µl)d |
4.50±0.47 |
4.59±0.45 | 0.575 |
| Hb
(g/dl)d | 11.93±1.67 | 13.82±1.44 |
<0.001a |
| HCT
(%)e | 36.20±4.53 | 36.68±3.52 | 0.463 |
| MCV (fl) | 80.65±5.76 | 80.20±4.62 | 0.885 |
| MCH
(pg/cell)e | 26.47±2.15 | 30.12±1.84 | 0.028a |
| Platelet
(×103/µl)d |
217.17±50.92 | 231.50±48.03 | 0.185 |
| WBC
(×103/µl)e |
5.37±1.47 |
6.05±1.24 | 0.043b |
| Neutrophils
(%)d |
53.43±17.26 |
57.57±11.62 | 0.321 |
|
Eosinophils+Basophils (%)e | 6.25
(5.85–8.2)c | 7.65
(6.68–22.95)c | 0.345 |
| ALT
(U/l)d |
23.67±12.80 | 20.33±8.36 | 0.147 |
| AST
(U/l)d | 20.17±6.55 | 18.33±5.82 | 0.069 |
| UA
(mg/dl)d |
8.24±3.74 |
8.17±3.81 | 0.508 |
Percentage of lymphocyte, NK, and NKT
cells
Data in Table III
show that basal values (before treatment) of percentage of
lymphocyte, NK, and NKT cells were not significantly different
between the Biobran/MGN-3 and placebo groups (P>0.05).
Furthermore, Table III shows that
Biobran/MGN-3 and placebo supplementation for 1 month did not
induce significant changes in the percentage of lymphocyte, NK, and
NKT cells when compared with their baseline values (P>0.05). The
observed increase in the % lymphocytes is within the normal range
in adults and aged humans (18–44%).
 | Table III.Effect of Biobran/MGN-3 and placebo
supplementation on percentages of lymphocyte, NK, and NKT cells
within each group before and after treatment. |
Table III.
Effect of Biobran/MGN-3 and placebo
supplementation on percentages of lymphocyte, NK, and NKT cells
within each group before and after treatment.
| Treatment | Parameters | Baseline | 1 Month | P-value |
|---|
| Biobran/MGN-3 | Lymph (%) |
32.25±6.05 |
36.58±7.77 | 0.157 |
|
| NK (%) |
4.73±1.69 |
7.02±3.94 | 0.102 |
|
| NKT (%) |
2.72±0.98 |
3.6±2.03 | 0.256 |
| Placebo | Lymph (%) |
33.33±10.19 |
39.52±9.82 | 0.014a |
|
| NK (%) |
4.83±2.18 |
10.97±5.5 | 0.013a |
|
| NKT (%) |
4.18±1.66 |
5.55±2.44 | 0.271 |
NK cells expressing CD107a
Flow cytometric analysis was carried out to examine
the effect of Biobran/MGN-3 on CD107a degranulation in
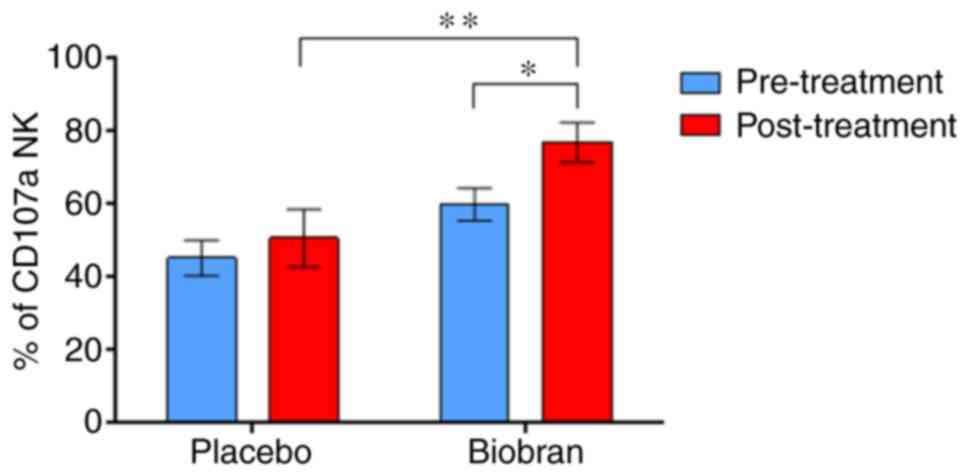
PMA/Ionomycin activated NK cells. Supplementation with
Biobran/MGN-3 significantly upregulated stimulated
PMA/Ionomycin-induced CD107a-expressing NK cells (Fig. 1 and Table
IV). Both groups showed similar changes in the levels of CD107a
expression in PMA/Ionomycin-stimulated NK cells before initiation
of treatment. It is important to note that the comparison of the
percentages of pre-treatment levels of PMA/Ionomycin-activated NK
cells that express CD107a in Biobran/MGN-3 group (60.5%) and
placebo group (40.9%) (Table IV)
was not significant using Mann-Whitney U test (results of test not
shown).
 | Table IV.Comparison of CD107a expression on
activated NK and NKT cells within each group before and after
treatment. |
Table IV.
Comparison of CD107a expression on
activated NK and NKT cells within each group before and after
treatment.
| Variable | Group | Pre-treatment
median | Post-treatment
median | Wilcoxon Singed
Rank test Z-value | Asym. Sig.
(2-tailed) P-value |
|---|
| PMA/Ionomycin
stimulated NK 107a | Biobran/MGN-3 | 60.5 | 83.0 | −1.992 | 0.046a |
|
| Placebo | 40.9 |
54.35 | −0.734 | 0.463 |
| PMA/Ionomycin
non-stimulated NKT 107a | Biobran/MGN-3 | 78.0 |
88.55 | −0.734 | 0.463 |
|
| Placebo | 69.0 | 84.0 | −0.943 | 0.345 |
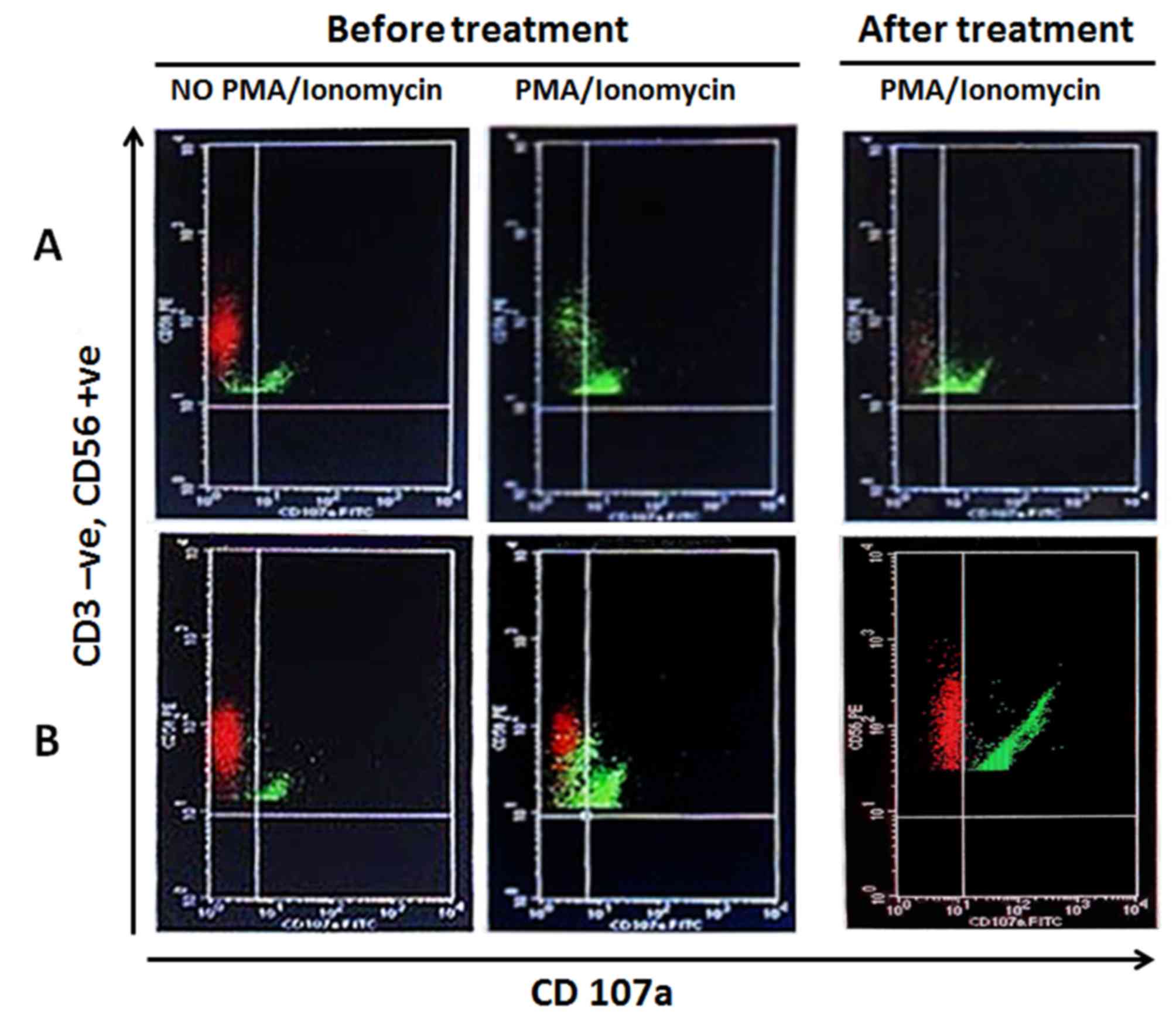
Furthermore, Fig. 2
shows the effect of Biobran/MGN-3 supplementation on upregulating
the percentage of stimulated CD107a expressing NK cells. The number
of PMA/Ionomycin-stimulated NK cells that express CD107a was
significantly higher than baseline values. On the other hand,
supplementation for the placebo group did not upregulate
PMA/Ionomycin-stimulated NK expressing CD107a. CD107a was positive
only in very few NK cells in the placebo subjects (Fig. 2). The data suggest that Biobran/MGN-3
supplementation induces NK cell expression of CD107a, which has
been reported to parallel an increase in the cytotoxic effect of NK
cells.
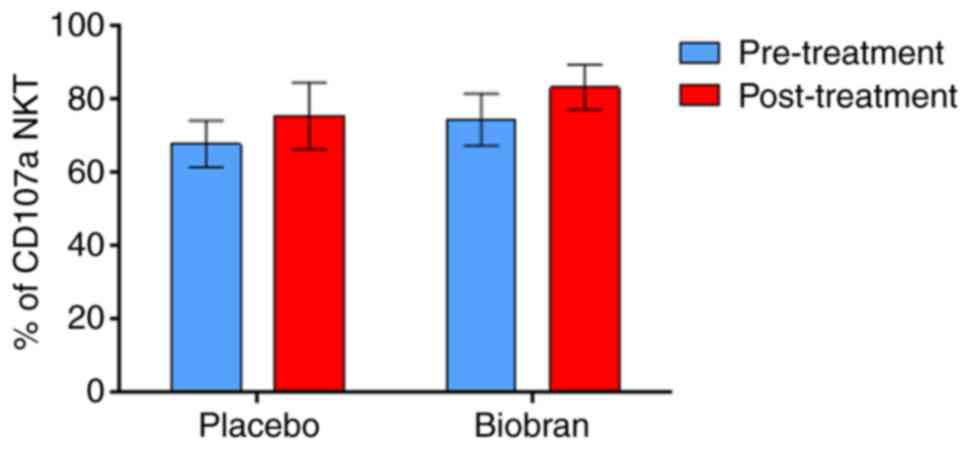
NKT cells expressing CD107a
Biobran/MGN-3 and placebo supplementation did not
significantly upregulate the percentages of
PMA/Ionomycin-stimulated NKT (CD3+ve, CD56+ve) cells expressing
CD107a as compared to their baseline values (Fig. 3 and Table
IV).
Discussion
Biobran/MGN-3 is an arabinoxylan derived from rice
bran. It has a xylose in its main chain and an arabinose polymer in
its side chain (14). Biobran/MGN-3
is a potent biological response modifier (BRM) as manifested by its
ability to increase NK cell activity and IFN-γ production by
peripheral blood lymphocytes in vitro (15) and to enhance NK activity in young
adult humans (21) and cancer
patients (19,23,24).
Biobran/MGN-3 also enhances NK activity in animals, including aged
mice (25) and animal bearing tumor
(17,26). This increase in activity was
associated with an increase in both the granular content of NK
cells and their binding capacity to tumor cell targets (21,25,27).
Further studies on NK function in geriatric subjects are needed to
ascertain the benefits of this BRM on aged humans.
NK cells represent the first line of defense against
cancer and virally infected cells, due to the fact that their
cytotoxic activity directly kills target cells. However, the
impairment of NK activity due to aging has been reported in both
experimental animals and humans, and renders the geriatric
population more susceptible to viral infection and malignancies
(9,28–32).
Age-related decline in NK activity is associated with decreased
lytic activity per cell (31,32) and
drops in the expression of granular perforins, which are primarily
responsible for destroying cancer and virally infected cells, a
characteristic of both NK cells and cytotoxic T lymphocytes
(32). The preliminary data in this
study shows that daily intake of Biobran/MGN-3 supplement at a dose
of 500 mg/day for 1 month increases NK cell activity in the
geriatric population. The underlying mechanism is not known but may
be attributed to an increase in NK granular perforins and
Granzyme-B (25,27), or due to the ability of Biobran/MGN-3
to influence the early signaling events leading to the triggering
of protein kinase C (30).
Aged subjects in both groups (Biobran/MGN-3 and
placebo) showed similar insignificant changes in the levels of
CD107a expression on non-PMA/Ionomycin-stimulated NK cells at
one-month post-treatment relative to baseline values. However,
Biobran/MGN-3 supplementation significantly upregulated the percent
of PMA/Ionomycin-stimulated NK cells expressing CD107a compared to
the baseline value and the placebo group (Fig. 1). Biobran/MGN-3 supplementation
upregulation of NK expressing CD107a is also well illustrated in
flow cytometry depicted in Fig. 2B.
Subjects in the placebo group did not show upregulated
PMA/Ionomycin-induced NK expressing CD107a; CD107a was positive
only in very few NK cells (Fig. 2A).
The increase in activity post-treatment with Biobran/MGN-3 was not
associated with the percent of NK cells. Data in Table III showed that there was no
significant change in % lymphocytes and % NK cells in the
Biobran/MGN-3 or placebo group. These data are in accordance with
the work of others, who show the normal range for lymphocytes from
healthy subjects is between 18–44% (33) and the normal range for NK cells is
between 7–28% (34,35). The NK cell expression of CD107a has
been reported to be parallel to the increase in the cytotoxic
effect of NK cells (22).
Recent research showed CD107a is not merely a marker
of degranulation but it plays a critical role in lysosome formation
and the degranulation process. RNAi-mediated silencing of CD107a
expression in NK cells resulted in failure to deliver Granzyme B to
target cells, impaired perforin trafficking, decreased perforin
content in lytic granules, and impaired NK cell cytotoxic activity
(36). On the other hand, increased
expression of CD107a has been shown to correlate with cytokine
secretion and cytotoxic activity of NK cells (37). Thus, our results suggest that
Biobran/MGN-3 supplement increases lytic granules trafficking,
degranulation capacity, and cytotoxicity of NK cells. NK cells have
been considered to be a bridge between the innate response and the
adaptive system (38), and their
activation will lead to modulating other immune cell subsets. This
conclusion has been demonstrated in our earlier studies, which
showed the ability of Biobran/MGN-3 to modulate human
CD8+ T cells, CD4+ T cells, and B cells
post-ingestion (14).
Natural killer T (NKT) cells are a subset of
regulatory lymphocytes that co-express cell surface receptors
characteristic of both T lymphocytes (e.g., CD3, α/β T-cell
receptor [TCR]) and NK cells (e.g., CD56, NK1.1) (39). The reason that Biobran/MGN-3 induces
PMA/Ionomycin-stimulated NK cells expressing CD107a and does not
induce NKT cells in geriatric subjects is not fully understood, but
could be attributed to differences in the cell receptors of NK
cells and NKT cells. Our earlier study showed that Biobran/MGN-3
supplementation in vitro for 16 h upregulates three key cell
surface receptors on NK cells: CD69, an early activation antigen;
CD25, an interleukin-2 receptor; and CD54 (ICAM-I), an adhesion
molecule (15). NKT cells are a
population of T cells that are activated by lipid antigens bound to
CD1d (cluster of differentiation 1d) molecules at the surface of
antigen-presenting cells. There are five different CD1 genes
(CD1a-e) that have been identified. CD1a-c proteins are recognized
by diverse conventional αβ T cells, while CD1d proteins are
recognized by a specialized subset of αβ T cells. The intracellular
metabolic pathways of lipid antigens are key in forming the
functional NKT cell repertoire (40,41). We
believe that more analysis of the lipid nature of these cells is
needed in order to assess their response to the immunomodulatory
effect of Biobran/MGN-3.
Results of this study showed that dietary supplement
Biobran/MGN-3 activates NK cell activity in geriatric subjects. A
direct relation between diet and changes in the brain structure and
activity has been reported in the guidelines of the National
Institute on Aging-Alzheimer's Association. Growing experimental
evidence suggests that synapses may be the locus for abnormalities
underlying diseases such as Alzheimer's, Parkinson's, and multiple
sclerosis. Indeed, perturbations in the induction, maintenance, or
reversal of long term potentiation (LTP) and long term depression
(LTD) are a common thread in the different brain disease models
(42,43), as well as in human pathologies
associated with inflammation (44).
Thus, it has been suggested that the etiopathogenesis of different
conditions is a result of the combination of abnormal expression of
immune mediators with other disease-specific features (5). It is of interest to note that the
dietary supplement L-Tryptophan prevents the age-induced decline of
hippocampal serotonin (5-HT) production and thus avoids cognitive
decline (45), and that treatment
with sildenafil, a phosphodiesterase-5 inhibitor, resulted in an
improvement in hippocampal synaptic dysfunction and memory deficits
in Alzheimer's disease mouse model (46). Results of our study and those of
others may lead to the design of immunoneurological-targeted
therapies.
It is of special interest to note that the NK
immunomodulatory effect of Biobran/MGN-3 in geriatric subjects was
achieved at a low dose of 500 mg/day. Aged subjects who
participated in this randomized, double-blind, clinical trial were
healthy, did not complain of any disease, and did not ingest other
immune modulators, suggesting the great response of healthy aged
subjects to the NK immunomodulatory effect of Biobran/MGN-3. Data
in the present study showed Biobran/MGN-3 to be safe and that
treatment did not induce any significant toxic changes in any of
the studied parameters. Instead, some beneficial effects were
observed after 1 month of treatment in the Biobran/MGN-3 group, and
the placebo group to a lesser extent. These effects included
improved levels of Hb, MCH, and ALP. In addition, Biobran/MGN-3
supplementation significantly down-regulated AST (SGOT) levels,
suggesting improved liver functions. This finding is consistent
with our recent study that shows the ability of Biobran/MGN-3 to
improve liver functions as indicated by a significant decrease of
viremia levels in Hepatitis C patients post-treatment with
Biobran/MGN-3 (47). Biobran/MGN-3
has been shown to be a safe and nontoxic agent in several toxicity
studies including: i) the subchronic toxicity study in beagle dogs,
the guinea pig antigenicity study, and mutagenicity testing
(48,49); ii) the LD50 (lethal dose, 50%) of
Biobran/MGN-3 is greater than 36 g/kg; and iii) the Ames test for
mutagenicity was negative. Furthermore, studies among cancer
patients treated with chemotherapy have shown a marked improvement
in appetite and other quality of life (QOL) parameters (50,51).
The placebo group in this study ingested dietary
fiber, hydroxy propyl distarch phosphate (HDP), which is currently
used as a food additive. Data depicted in Tables II and III demonstrate that subjects who ingested
HDP showed significantly improved levels of Hb and MCH relative to
their baseline values. Earlier studies have showed the beneficial
role of dietary fiber in overall health. For example, subjects who
ingested an HDP meal showed significantly lower postprandial
glucose, insulin, and GIP responses than subjects who ingested a
waxy maize starch meal (52).
Additionally, different types of starch have been shown to
influence the retention of 59Fe (53) and absorption of zinc and iron
(54). One limitation of our study
was the small number of our sample. Studies with larger sample
sizes are needed to validate our results. Another limitation was
our limited ability to study the full range of molecular markers
that are needed to identify potential mechanisms involved in
Biobran/MGN-3-induced activation of NK but not NKT cells.
In conclusion, the preliminary results of this study
strongly suggest that Biobran/MGN-3, a nutritional supplement from
rice bran, can counteract NK cell immunosenescence in geriatric
subjects, suggesting the potential of Biobran/MGN-3 for lowering
the incidence of cancer and viral infection that is reported by
older humans. To our knowledge, the current study represents the
first clinical trial that extends the biological response modifier
(BRM) effect of Biobran/MGN-3 to include enhancing NK cell
degranulation and cytotoxic activity in geriatric population.
Biobran/MGN-3 is a non-toxic, inexpensive supplement, and thus may
provide a means to reduce the significant healthcare costs incurred
by both healthy and unhealthy geriatric subjects. Our future
research directions include conducting multicenter prospective
clinical trials to assess the preventive capacity of Biobran/MGN-3
supplementation in the geriatric population to reduce the incidence
of infections and tumorigenesis.
Acknowledgements
The authors would like to acknowledge Daiwa
Pharmaceutical Co., Ltd., Tokyo, Japan, for supplying Biobran/MGN-3
for this study. This study was supported in part by NIH-NIMHD grant
nos. U54MD007598 and NIH/NCATS, and grant nos. UL1TR000124 and S21
MD000103. The authors would also like to thank Dr. B. J. Winjum at
UCLA for assistance in writing the manuscript and Dr. S. Gollapudi
at UCI for discussions relevant to experimental design and
analysis.
References
|
1
|
Garza A: The Aging Population: The
Increasing Effects on Health Care. http://www.pharmacytimes.com/publications/issue/2016/january2016/the-aging-population-the-increasing-effects-on-health-careJanuary
19–2016
|
|
2
|
Alzheimer's Association, . 2017
Alzheimer's Disease Facts and Figures. http://www.alz.org/facts/August 18–2017
|
|
3
|
American Cancer Society Cancer Action
Network, . The Costs of Cancer. Addressing Patient Costs.
https://www.acscan.org/sites/default/files/Costs%20of%20Cancer%20-%20Final%20Web.pdfAugust
18–2017
|
|
4
|
Boulanger LM: Immune proteins in brain
development and synaptic plasticity. Neuron. 64:93–109. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nisticò R, Salter E, Nicolas C, Feligioni
M, Mango D, Bortolotto ZA, Gressens P, Collingridge GL and Peineau
S: Synaptoimmunology-roles in health and disease. Mol Brain.
10:262017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gomez CR, Nomellini V, Faunce DE and
Kovacs EJ: Innate immunity and aging. Exp Gerontol. 43:718–728.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zanussi S, Serraino D, Dolcetti R,
Berretta M and De Paoli P: Cancer, aging and immune reconstitution.
Anticancer Agents Med Chem. 13:1310–1324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pera A, Campos C, López N, Hassouneh F,
Alonso C, Tarazona R and Solana R: Immunosenescence: Implications
for response to infection and vaccination in older people.
Maturitas. 82:50–55. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma Y and Fang M: Immunosenescence and
age-related viral diseases. Sci China Life Sci. 56:399–405. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lotzová E, Savary CA, Freedman RS and
Bowen JM: Natural killer cell cytotoxic potential of patients with
ovarian carcinoma and its modulation with virus-modified tumor cell
extract. Cancer Immunol Immunother. 17:124–129. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moretta L, Bottino C, Pende D, Mingari MC,
Biassoni R and Moretta A: Human natural killer cells: Their origin,
receptors and function. Eur J Immunol. 32:1205–1211. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chretien AS, Le Roy A, Vey N, Prebet T,
Blaise D, Fauriat C and Olive D: Cancer-induced alterations of
NK-mediated target recognition: Current and investigational
pharmacological strategies aiming at restoring NK-mediated
anti-tumor activity. Front Immunol. 5:1222014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ivarsson MA, Michaëlsson J and Fauriat C:
Activating killer cell Ig-like receptors in health and disease.
Front Immunol. 5:1842014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ghoneum M: Anti-HIV activity in vitro of
MGN-3, an activated arabinoxylane from rice bran. Biochem Biophys
Res Commun. 243:25–29. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ghoneum M and Jewett A: Production of
tumor necrosis factor-alpha and interferon-gamma from human
peripheral blood lymphocytes by MGN-3, a modified arabinoxylan from
rice bran, and its synergy with interleukin-2 in vitro. Cancer
Detect Prev. 24:314–324. 2000.PubMed/NCBI
|
|
16
|
Ghoneum M and Gollapudi S: Modified
arabinoxylan rice bran (MGN-3/Biobran) sensitizes human T cell
leukemia cells to death receptor (CD95)-induced apoptosis. Cancer
Lett. 201:41–49. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Badr El-Din NK, Noaman E and Ghoneum M: In
vivo tumor inhibitory effects of nutritional rice bran supplement
MGN-3/Biobran on Ehrlich carcinoma-bearing mice. Nutr Cancer.
60:235–244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ghoneum M and Agrawal S: Activation of
human monocyte-derived dendritic cells in vitro by the biological
response modifier arabinoxylan rice bran (MGN-3/Biobran). Int J
Immunopathol Pharmacol. 24:941–948. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cholujova D, Jakubikova J, Czako B,
Martisova M, Hunakova L, Duraj J, Mistrik M and Sedlak J: MGN-3
arabinoxylan rice bran modulates innate immunity in multiple
myeloma patients. Cancer Immunol Immunother. 62:437–445. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ghoneum M, Badr El-Din NK, Ali DA and
El-Dein MA: Modified arabinoxylan from rice bran, MGN-3/biobran,
sensitizes metastatic breast cancer cells to paclitaxel in vitro.
Anticancer Res. 34:81–87. 2014.PubMed/NCBI
|
|
21
|
Ghoneum M: Enhancement of human natural
killer cell activity by modified arabinoxylane from rice bran
(MGN-3). Int J Immunothe. 14:89–99. 1998.
|
|
22
|
Alter G, Malenfant JM and Altfeld M:
CD107a as a functional marker for the identification of natural
killer cell activity. J Immunol Methods. 294:15–22. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ooi SL, McMullen D, Golombick T, Nut D and
Pak SC: Evidence-based review of BioBran/MGN-3 arabinoxylan
compound as a complementary therapy for conventional cancer
treatment. Integr Cancer Ther. Oct 1–2017.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ghoneum M and Brown J: NK
immunorestoration of cancer patients by MGN-3, a modified
arabinoxylan rice bran (study of 32 patients followed for up to 4
years)Wheat and rice in disease prevention and health. Anti-aging
medical therapeutics. Klatz R and Goldman R: III. Health Quest
Publications; Marina del Rey, CA: pp. 217–226. 1999
|
|
25
|
Ghoneum M and Abedi S: Enhancement of
natural killer cell activity of aged mice by modified arabinoxylan
rice bran (MGN-3/Biobran). J Pharm Pharmacol. 56:1581–1588. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pérez-Martínez A, Valentín J, Fernández L,
Hernández-Jiménez E, López-Collazo E, Zerbes P, Schwörer E, Nuñéz
F, Martín IG, Sallis H, et al: Arabinoxylan rice bran
(MGN-3/Biobran) enhances natural killer cell-mediated cytotoxicity
against neuroblastoma in vitro and in vivo. Cytotherapy.
17:601–612. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ghoneum M: From bench to bedside: The
growing use of arabinoxylan rice bran (MGN-3/Biobran) in cancer
immunotherapy. Austin Immunol. 1:10062016.
|
|
28
|
Ogata K, An E, Shioi Y, Nakamura K, Luo S,
Yokose N, Minami S and Dan K: Association between natural killer
cell activity and infection in immunologically normal elderly
people. Clin Exp Immunol. 124:392–397. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Smetana K Jr, Lacina L, Szabo P,
Dvořánková B, Brož P and Šedo A: Ageing as an important risk factor
for cancer. Anticancer Res. 36:5009–5017. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ghoneum M, Suzuki K and Gollapudi S:
Phorbol myristate acetate corrects impaired NK function of old
mice. Scand J Immunol. 34:391–397. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mariani E, Sgobbi S, Meneghetti A,
Tadolini M, Tarozzi A, Sinoppi M, Cattini L and Facchini A:
Perforins in human cytolytic cells: The effect of age. Mech Ageing
Dev. 92:195–209. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rukavina D, Laskarin G, Rubesa G, Strbo N,
Bedenicki I, Manestar D, Glavas M, Christmas SE and Podack ER:
Age-related decline of perforin expression in human cytotoxic T
lymphocytes and natural killer cells. Blood. 92:2410–2420.
1998.PubMed/NCBI
|
|
33
|
Chng WJ, Tan GB and Kuperan P:
Establishment of adult peripheral blood lymphocyte subset reference
range for an asian population by single-platform flow cytometry:
Influence of age, sex, and race and comparison with other published
studies. Clin Diagn Lab Immunol. 11:168–173. 2004.PubMed/NCBI
|
|
34
|
Wang WS, Lo AW, Siu LP, Leung JN, Tu SP,
Tai SW, Lam SC and Wong KF: Reference ranges for lymphocyte subsets
among healthy Hong Kong Chinese adults by single-platform flow
cytometry. Clin Vaccine Immunol. 20:602–606. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Al-Mawali A, Pinto AD, Busaidi RA and
Al-Zakwani I: Lymphocyte subsets: Reference ranges in an age- and
gender-balanced population of Omani healthy adults. Cytometry A.
83:739–744. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Krzewski K, Gil-Krzewska A, Nguyen V,
Peruzzi G and Coligan JE: LAMP1/CD107a is required for efficient
perforin delivery to lytic granules and NK-cell cytotoxicity.
Blood. 121:4672–4683. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aktas E, Kucuksezer UC, Bilgic S, Erten G
and Deniz G: Relationship between CD107a expression and cytotoxic
activity. Cell Immunol. 254:149–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun JC and Lanier LL: Natural killer cells
remember: An evolutionary bridge between innate and adaptive
immunity? Eur J Immunol. 39:2059–2064. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Godfrey DI, Hammond KJ, Poulton LD, Smyth
MJ and Baxter AG: NKT cells: Facts, functions and fallacies.
Immunol Today. 21:573–583. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Brigl M and Brenner MB: CD1: Antigen
presentation and T cell function. Annu Rev Immunol. 22:817–890.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shin JH and Park SH: The effect of
intracellular trafficking of CD1d on the formation of TCR
repertoire of NKT cells. BMB Rep. 47:241–248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nisticò R, Pignatelli M, Piccinin S,
Mercuri NB and Collingridge G: Targeting synaptic dysfunction in
Alzheimer's disease therapy. Mol Neurobiol. 46:572–587. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pignatelli M, Feligioni M, Piccinin S,
Molinaro G, Nicoletti F and Nisticò R: Synaptic plasticity as a
therapeutic target in the treatment of autism-related single-gene
disorders. Curr Pharm Des. 19:6480–6490. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nisticò R, Mori F, Feligioni M, Nicoletti
F and Centonze D: Synaptic plasticity in multiple sclerosis and in
experimental autoimmune encephalomyelitis. Philos Trans R Soc Lond
Ser B Biol Sci. 369:201301622013. View Article : Google Scholar
|
|
45
|
Musumeci G, Castrogiovanni P, Szychlinska
MA, Imbesi R, Loreto C, Castorina S and Giunta S: Protective
effects of high Tryptophan diet on aging-induced passive avoidance
impairment and hippocampal apoptosis. Brain Res Bull. 128:76–82.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Puzzo D, Staniszewski A, Deng SX,
Privitera L, Leznik E, Liu S, Zhang H, Feng Y, Palmeri A, Landry DW
and Arancio O: Phosphodiesterase 5 inhibition improves synaptic
function, memory, and amyloid-beta load in an Alzheimer's disease
mouse model. J Neurosci. 29:8075–8086. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Salama H, Medhat E, Shaheen M, Zekri AN,
Darwish T and Ghoneum M: Arabinoxylan rice bran (Biobran)
suppresses the viremia level in patients with chronic HCV
infection: A randomized trial. Int J Immunopathol Pharmacol.
29:647–653. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Daiwa Pharmaceutical and Co., Ltd., .
BioBran rice bran arabinoxylan compound. http://www.daiwa-pharm.com/english/product/biobran.htmlOctober
22–2017
|
|
49
|
Tazawa K: BioBran/MGN-3: Basic and
Clinical Application to Integrative Medicine. Iyakushuppan Co.
Publishers; Tokyo: pp. 18–22. 2003
|
|
50
|
Takahara K and Sano K: The life
prolongation and QOL improvement effect of rice bran arabinoxylan
derivative (MGN-3, Bio-Bran) for progressive cancer. Clin Pharmacol
Therapy. 14:267–271. 2004.
|
|
51
|
Hajto T, Horvath A and Papp S: Improvement
of quality of life in tumor patients after an immunomodulatory
treatment with standardized mistletoe lectin and arabinoxylan plant
extracts. Int J Neurorehabilitation. 3:1–3. 2016. View Article : Google Scholar
|
|
52
|
Shimotoyodome A, Suzuki J, Kameo Y and
Hase T: Dietary supplementation with hydroxypropyl-distarch
phosphate from waxy maize starch increases resting energy
expenditure by lowering the postprandial glucose-dependent
insulinotropic polypeptide response in human subjects. Br J Nutr.
106:96–104. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hood LF, Vancampen DR, House WA and
Szatkowski E: Effect of modified and unmodified tapioca starches on
59Fe retention in rats. J Nutr. 106:1768–1772. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kishida T, Nakai Y and Ebihara K:
Hydroxypropyl-distarch phosphate from Tapioca starch reduces zinc
and iron absorption, but not calcium and magnesium absorption, in
rats. J Nutr. 131:294–300. 2001. View Article : Google Scholar : PubMed/NCBI
|