Introduction
Traumatic brain injury (TBI) has a high incidence
rate worldwide, and results in immediate neuronal and glial cell
loss, in addition to associated neurological deficits (1). In recent years, neural stem cells
(NSCs) have been used as a novel approach in the treatment of
certain neurodegenerative disorders, as well as in the management
of central neural system injuries (2). NSCs are able to self-renew and to
differentiate into mature neuronal and glial cell types, and
thereby have become a potential target for cell transplantation
therapy following injury (3). A
previous study showed that transplanted NSCs could significantly
improve neurological function following TBI (4), suggesting NSC transplantation is a
potential treatment strategy for TBI. However, the success of this
approach has been limited, largely due to the complexity of the
injury microenvironment (5).
TBI results in both primary and secondary injury to
the brain (6). The primary injury
leads to direct neural cell loss and necrotic cell death, while the
secondary injury involves neuronal cell death following TBI, which
exacerbates the damage from the primary injury (7). Oxidative stress, characterized by
excessively produced reactive oxygen species (ROS), is one of the
events that occurs subsequent to TBI that contributes to secondary
neuronal cell death (8). After TBI,
the increased level of cellular damage to cellular macromolecules
caused by ROS, including that to proteins, DNA and membrane
phospholipids, leads to lipoperoxidation of the cell membrane,
resulting in the dysfunction of numerous structures and organelles,
such as mitochondria. This then results in apoptotic cell death of
neurons (9,10). In the nervous system, superoxide
dismutases (SODs) are important antioxidant enzymes involved in
superoxide detoxification in normal cellular metabolism and after
cell injury (11). Therefore,
overexpression of SODs may improve the efficacy of the
transplantation of NSCs in the treatment of TBI.
SOD1 is a specific antioxidant enzyme that is able
to counteract superoxide anions. Transplantation of NSCs that
overexpress SOD1 prolongs the survival of these grafted cells and
enhances functional recovery in animal models of ischemic stroke
and intracerebral hemorrhage (12,13).
PEP-1, a 21-residue peptide, has been used as a carrier for the
delivery of biologically active molecules, such as SOD1, into cells
(14,15). PEP-1-SOD1 has been demonstrated to
protect neurons from ROS once delivered to the site of the injury,
and also to improve functional recovery after stroke or spinal cord
injury (14,15). The aforementioned studies suggest
that PEP-1-SOD1 may also be a potential therapeutic agent for the
treatment of TBI. Therefore, in the present study, the effect of
the transplantation of NSCs in combination with PEP-1-SOD1 was
investigated for the treatment of experimental TBI in rats, and the
underlying mechanisms were assessed.
Materials and methods
Isolation and culture of NSCs
Brain tissue was isolated from embryonic 14-day-old
fetal Sprague-Dawley (SD) rats as described in a previous study
(16). The SD rats were purchased
from the Experimental Animal Center of The Third People's Hospital
of Changzhou (Changzhou, China). The rats were maintained in cages
with free access to water and food, at a temperature of 25±2°C and
humidity of 50±5%, with a 12 h light/dark cycle. The pia mater and
choroid plexus were peeled off. The brain tissue was then cut into
~1-mm3 sections. DMEM/F12 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was added to the shredded
tissue and incubated for 10 min at room temperature. Gentle
pipetting was used to generate a single cell suspension. The
suspension was centrifuged for 10 min at 1,200 × g, resuspended
with culture medium supplemented with 2% B27, 20 ng/ml basic
fibroblast growth factor and 20 ng/ml epidermal growth factor (all
PeproTech, Inc., Rocky Hill, NJ, USA) and then seeded in
25-cm2 T-flasks. Cells were maintained at 37°C in a
humidified atmosphere with 5% CO2. The experiments were
approved by the Ethics Committee of the Third People's Hospital of
Changzhou.
Expression and purification of
PEP-1-SOD1 fusion protein
Construction of the PEP-1-SOD1 expression vector,
expression of PEP-1-SOD1 in E. coli, and purification of the
expressed fusion protein were performed according to a method
described previously (15). Briefly,
to produce the PEP-1-SOD1 fusion protein, the plasmid
pET15b-PEP-1-SOD1 was transformed into E. coli and grown in
100 ml Luria-Bertani medium (cat. no. 28200; Newborn Co. Ltd.,
Shenzhen, China) at 37°C to an optimal density (which was examined
in the following experiments). Harvested cells were lysed by
sonication at 4°C and cell extracts were centrifuged at 15,000 × g
for 10 min at 4°C. The fusion protein was purified using an
ProteoPrep Top 20 protein affinity column (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) according to the manufacturer's
instructions. The protein concentration was determined by the BAC
method using bovine serum albumin (cat. no. NIST927E;
Sigma-Aldrich; Merck Millipore) as a standard.
MTT assay
The in vitro biological activity of PEP-1-SOD
fusion proteins in NSCs was assessed by measuring the viability of
NSCs. The cells were seeded into 96-well plates at a density of
1×105/ml. The cells were pretreated with 0, 0.5, 2.5 and
4.5 µM PEP-1-SOD for 24, 48 or 72 h. Subsequently, an MTT assay was
performed according to a previously described method (17,18).
Immunocytochemical analysis
To investigate the differentiation of the NSCs,
immunocytochemistry was performed according to a previous method
(19). In brief, cells were fixed
with 4% paraformaldehyde. After washing with phosphate-buffered
saline, NSCs were processed for immunohistochemical staining using
cell-specific markers of differentiation via the detection of
receptor interacting protein (RIP), neural nuclear antigen (NeuN)
and glial fibrillary acidic protein (GFAP), using rabbit anti-rat
RIP polyclonal antibody (cat. no. ab106393), rabbit anti-rat NeuN
polyclonal antibody (cat. no. ab104225) and rabbit anti-rat GFAP
polyclonal antibody (cat. no. ab7260), respectively (all 1:200;
Abcam, Cambridge, UK). Lastly, the reaction was developed using
3,3′-diaminobenzidine (cat. no. D8001; Sigma-Aldrich; Merck
Millipore), which resulted in brown-yellow staining. The cells were
observed under a light microscope (BX51; Olympus, Tokyo,
Japan).
Establishment of the TBI model
Male SD rats (n=80; age, 2–3 months; weight, 250±10
g) were purchased from Beijing HFK Bioscience Co. Ltd. (Beijing,
China). The rats were housed with a 12 h dark/light cycle and were
allowed free access to food and water. All the animals used in the
study received humane care.
The TBI model was established according to a
previously described method (20).
Briefly, the rats were anesthetized with 7% chloral hydrate
(Sigma-Aldrich; Merck Millipore) at a dose of 5 ml/kg by
intraperitoneal (i.p.) injection. The rectal temperature was
maintained within the range of 37±0.5°C with a heating pad. A right
parietal craniotomy (diameter, 5 mm) was conducted with a drill
under aseptic conditions. The center of the craniotomy was 2 mm
anterior to the lambdoid suture and 2 mm lateral to the skull
midline. A contusion was produced by allowing a counterweight
weighing 30 g to drop onto a piston resting on the dura from a
height of 15 cm through a guide tube. After this trauma, the rats
were returned to their cages, and the room temperature was
maintained at 25±0.5°C.
Treatment groups
At 24 h post trauma, the rats were randomly assigned
into four groups as follows: i) Saline group, ii) NSCs group, iii)
PEP-1-SOD1 group, and iv) NSCs + PEP-1-SOD1 group, with 20 animals
in each group. The rats were anesthetized and the craniotomy was
performed again. The rats were then treated with the NSC suspension
or recombinant PEP-1-SOD1 as follows: i) Saline group, received 0.1
ml saline injected at the injury site; ii) NSCs group, treated with
0.1 ml NSC suspension (2×107/ml) injected at the site of
injury; iii) PEP-1-SOD1 group, treated with 200 µg PEP-1-SOD1 by
i.p. injection; iv) NSCs + PEP-1-SOD1 group, treated with a 0.1 ml
NSC suspension (2×107/ml) injected at the site of injury
and 200 µg PEP-1-SOD1 by i.p. injection. The neurological function
of the rats was assessed at designated times (days 1, 3, 7, 14, 21
and 28 after TBI). Four days post-TBI, 6 rats in each group were
anesthetized with 7% chloral hydrate (Sigma-Aldrich; Merck
Millipore; 5 ml/kg, i.p.) and sacrificed by decapitation. The brain
tissues were then isolated and frozen immediately in liquid
nitrogen in preparation for the subsequent analyses.
Neurological function analysis
(Bederson score)
The first assessment was performed 30 min prior to
treatment administration to verify the establishment of TBI. Any
rat not displaying a score of ≥2 was eliminated from the study.
The neurological function of each rat was carefully
evaluated by members in a laboratory at the Department of
Pathology, who were blinded to the group information using a
modified Bederson score (21,22) as
follows: 0, no apparent neurological deficits; 1, offside forelimb
flexion; 2, weakness of the offside forelimb gripping strength when
holding the tail; 3, circling to the left when holding the tail; 4,
circling to the left when moving freely.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Approximately 50–100 mg brain tissue was homogenized
in 1 ml TRIzol reagent, and then centrifuged at 12,000 × g for 10
min at 4°C. The supernatant was collected and total RNA was
extracted. The RNA concentration was then measured and the purity
was confirmed. Treatment with DNase (Invitrogen Life Technologies;
Thermo Fisher Scientific, Inc.) was conducted prior to the RT
reaction. A SuperScript III reverse transcription kit (cat. no.
18080093; Invitrogen Life Technologies; Thermo Fisher Scientific,
Inc.) was used to convert RNA into cDNA at 4°C, and then qPCR was
performed using SYBR-Green I dye (cat. no. S7585; Invitrogen Life
Technologies; Thermo Fisher Scientific, Inc.) using the FTC-2000
Real-Time Quantitative PCR system. The primers used in the
experiments are as follows: aquaporin-4 (AQP4) forward,
5′-TCGCCAAGTCCGTCTTCTACA-3′ and reverse, 5′-CCGTGGTGACTCCCAATCC-3′;
β-actin forward, 5′-CCCATCTATGAGGGTTACGC-3′ and reverse,
5′-TTTAATGTCACGCACGATTTC-3′. The PCR reaction system included 2 µl
cDNA, 2 µl polymerase, 25 µl 2X PCR buffer, 0.5 µl 20X SYBR-Green I
(all purchased from Invitrogen Life Technologies; Thermo Fisher
Scientific, Inc.), 1 µl 25 mM forward and reverse primers and
distilled water, to ensure the total volume of the mixture was 50
µl. The PCR program was as follow: 94°C for 4 min, followed by 94°C
for 20 sec, 60°C 30 sec, 72°C 30 sec and a total of 35 reaction
cycles. The absence of non-specific products was verified after
each run by melting curve analysis. The relative gene quantities
were calculated by the 2−ΔΔCq method (23) in comparison with the expression
levels of β-actin.
Western blot analysis
The tissues were lysed utilizing RIPA buffer (1
ml/100 mg) at 0°C, and centrifuged at a speed of 12,000 × g for 10
min. The total protein concentration was determined using a BCA
assay kit (cat. no. 23227; Pierce Biotechnology, Rockford,
Illinois, USA) according to the instructions of the manufacturer.
The total protein (40 µg) was loaded for 15% SDS-PAGE
electrophoresis, and then the proteins were transferred to PVDF
membranes. The PVDF membranes were blocked with 5% non-fat dry milk
at 37°C for 1 h, and then incubated with anti-rat AQP4 (cat. no.
ab156924; 1:2,000; Abcam) or anti-rat β-actin (cat. no. ab8227;
1:2,000; Abcam) antibody at 4°C for 12 h. Finally, the membranes
were incubated with HRP-labeled goat anti-mouse IgG antibody (cat.
no. ab6789; 1:1,000; Abcam) at 37°C for 1 h. The immunoreactivity
of the bands was detected using an enhanced chemiluminescence
reagent (ECL; Amersham, Piscataway, New Jersey, USA) on X-ray film.
The densities of AQP4 and β-actin were quantified using a Gel Image
Analysis System (Labworks 4.6; UVP LLC, Upland, CA, USA). The
relative AQP4 expression was normalized to those of β-actin.
Immunocytochemistry assay
The cells were fixed using 4% paraformaldehyde for
24 h. The endogenous peroxidase in the cells was inactivated by
incubation with 3% hydrogen peroxide, and slices were blocked using
5% BSA for 20 min. The slices were incubated with rabbit anti-rat
nestin polyclonal antibody (cat. no. ab93157; 1:2,000; Abcam) at
4°C overnight. The slices were then incubated with goat anti-rabbit
peroxidase-conjugated IgG (cat. no. ab205718; 1:500; Abcam) at 25°C
for 1 h. Finally, the slices were immersed in alkaline
phosphatase-labeled diaminobenzidine (DAB; ZSGB Bio Co., Ltd.,
Beijing, China). Finally, the images were analyzed utilizing a
Medical Image Analysis system (HMIAS22000; Wuhan Champion Image
Technology Co., Ltd., Wuhan, China).
Statistical analysis
All results are expressed as the means ± standard
deviation. SPSS statistical software for windows (version 17.0;
SPSS, Inc., Chicago, IL, USA) was used to analyze the data. One-way
analysis of variance was performed for multiple comparisons
followed by Fisher's least significant difference post-hoc
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Isolation and identification of
NSCs
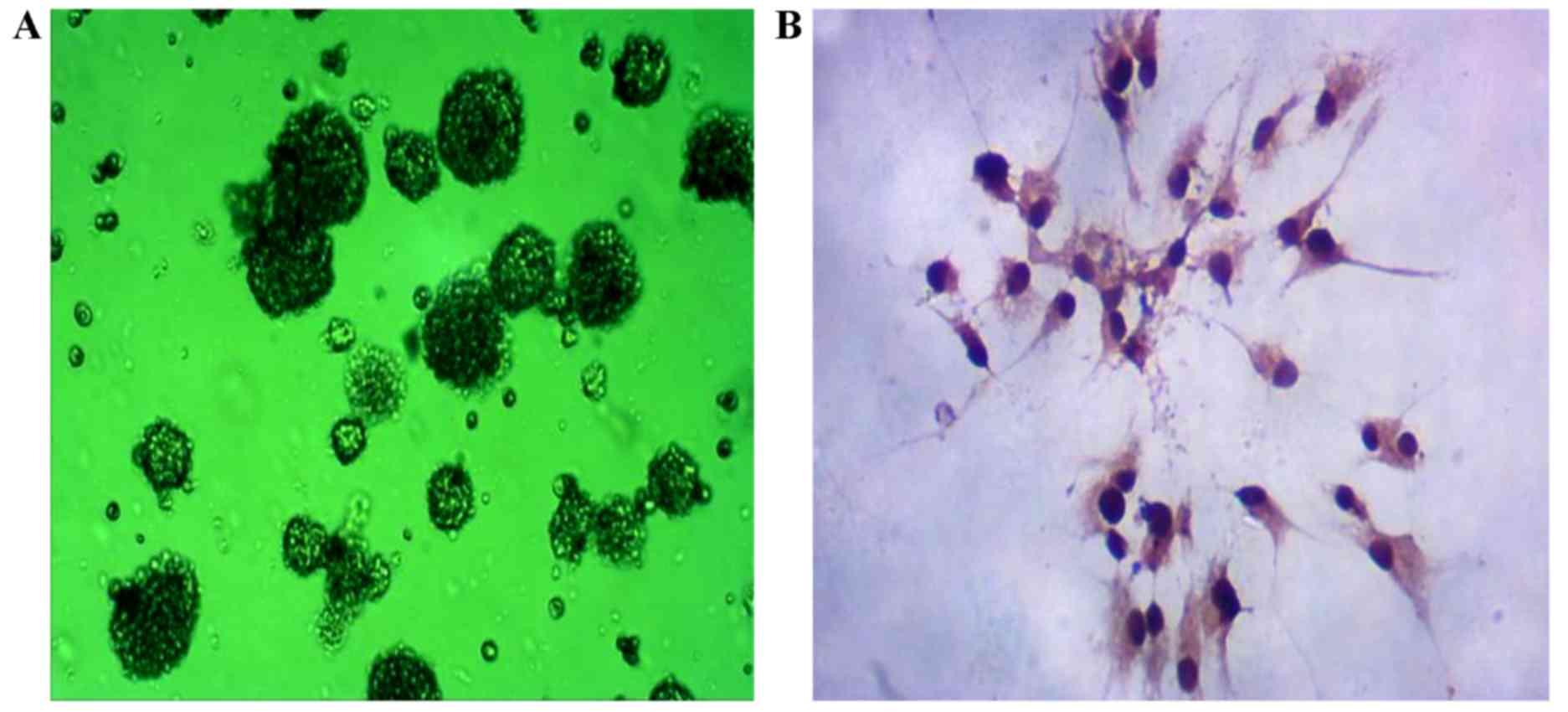
Fetal rat central NSCs were isolated from the
fetuses of rats on day 15 (Fig. 1A).
The expression of nestin was detected by immunocytochemistry. As
shown in Fig. 1B, positive nestin
expression was observed in the isolated cells.
PEP-1-SOD1 promotes the proliferation
and differentiation of NSCs in vitro
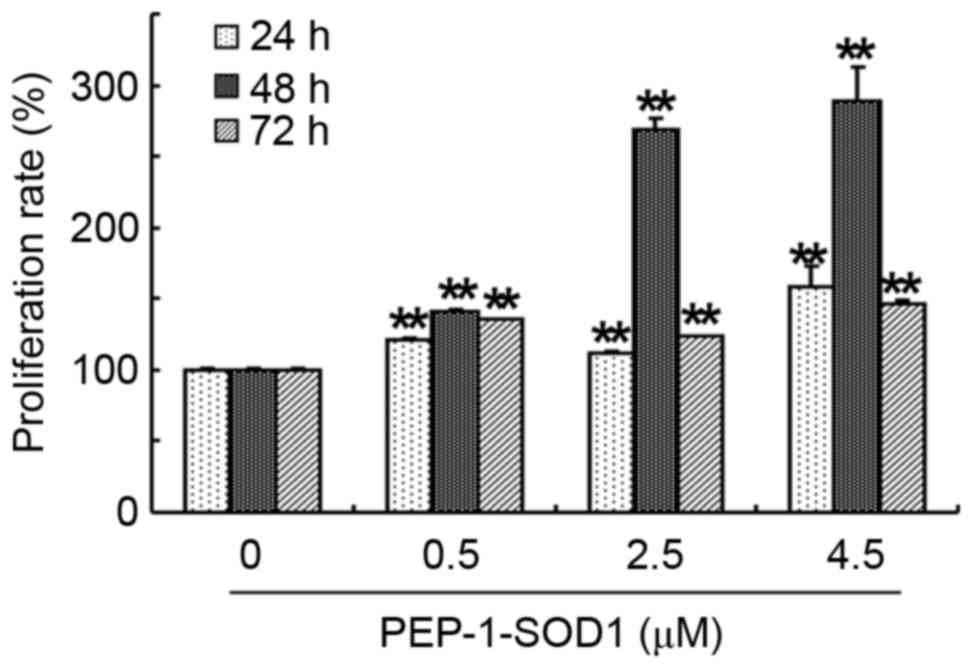
The effect of recombinant PEP-1-SOD1 protein on the
proliferation of primary cultured rat NSCs was evaluated by MTT
assay. PEP-1-SOD1 (0.5, 2.5 and 4.5 µM) significantly increased the
proliferation rates of NSCs compared with the control group at 24,
48 and 72 h in a dose-dependent manner (P<0.01; Fig. 2). Among the three time points,
treatment with PEP-1-SOD1 induced the highest proliferation rate at
48 h.
In order to determine whether PEP-1-SOD1 was able to
promote the differentiation of NSCs, the expression of RIP, NeuN
and GFAP was detected by immunocytochemistry. As shown in Fig. 3, untreated cells showed a low rate of
differentiation. Exposure to PEP-1-SOD1 resulted in an increase in
the expression levels of RIP, NeuN and GFAP.
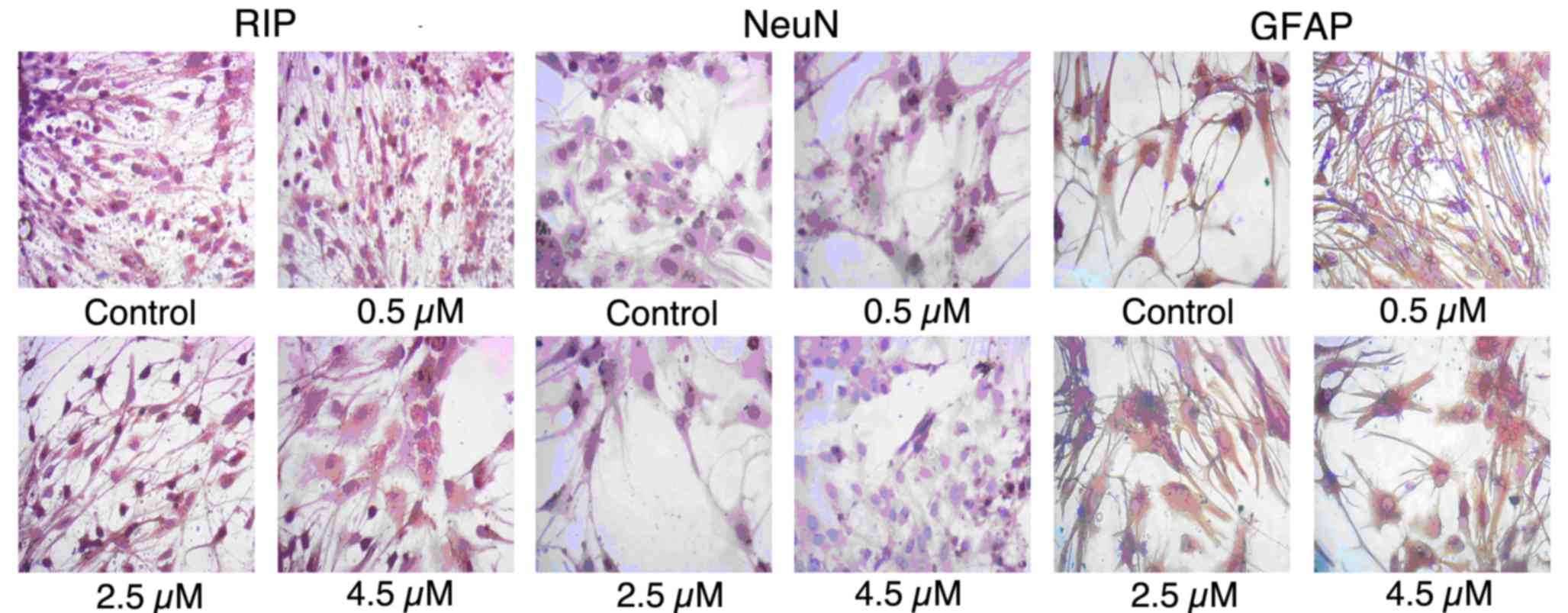 | Figure 3.PEP-1-SOD1 promoted the
differentiation of NSCs. NSCs were incubated with different
concentrations (0, 0.5, 2.5 and 4.5 µM) of PEP-1-SOD1 for 6 days
and then immunocytochemical staining was performed to detect the
expression of RIP, NeuN and GFAP (magnification, ×400). NSCs,
neural stem cells; SOD1, Cu, Zn-superoxide dismutase; RIP, receptor
interacting protein; NeuN, neural nuclear antigen; GFAP, glial
fibrillary acidic protein. |
PEP-1-SOD1 improves the effect of NSCs
transplantation on neurological recovery after TBI
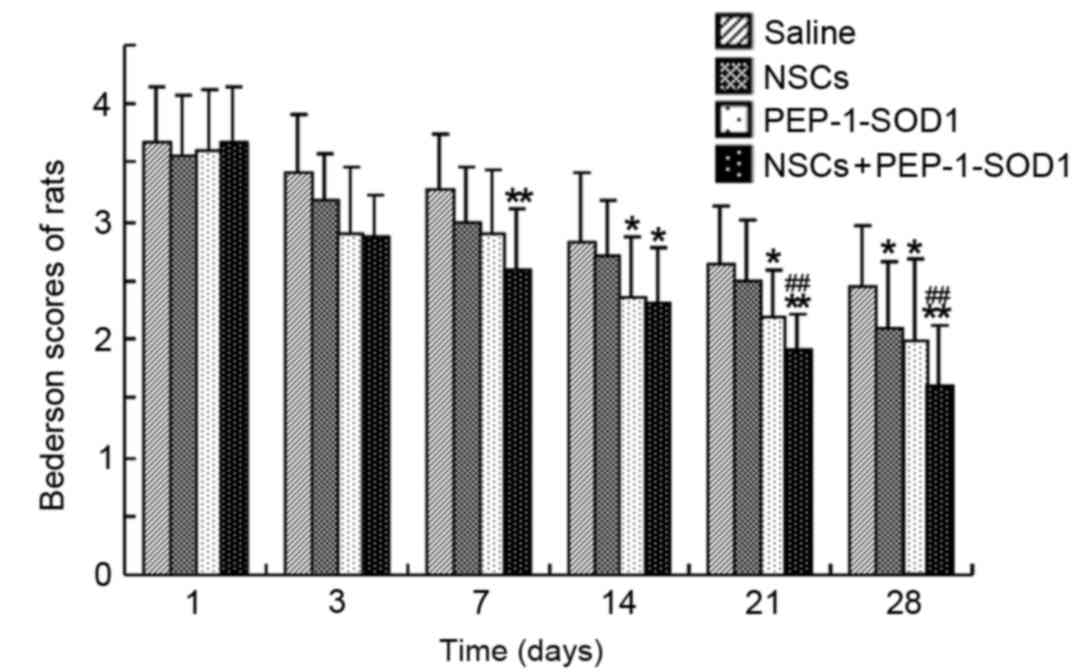
Neurological functions of rats with TBI were
evaluated on days 1, 3, 7, 14, 21 and 28 after treatment (Fig. 4). The Bederson score system reflects
the neurological functions of rats following TBI and was used to
assess post-traumatic neurological impairment. The functional
recovery of rats in all groups began 3 days post-TBI and was
observed to gradually improve at the subsequent time points. The
Bederson score was decreased in the NSCs, PEP-1-SOD1 and NSCs +
PEP-1-SOD1 groups on day 3 following TBI, although no significant
difference was observed determined between these groups and saline
group. At day 7 post-TBI, rats treated with NSCs + PEP-1-SOD1
showed a significantly lower Bederson score compared with rats
treated with saline (P<0.05; Fig.
4). The Bederson scores for the NSCs + PEP-1-SOD1 group were
significantly decreased on days 21 and 28 after TBI, compared with
those in the saline and NSCs groups (P<0.01; Fig. 4), suggesting that PEP-1-SOD1 improved
the effect of NSC transplantation in the treatment of TBI.
PEP-1-SOD1 enhanced the effect of NSC
transplantation on brain aquaporin-4 expression
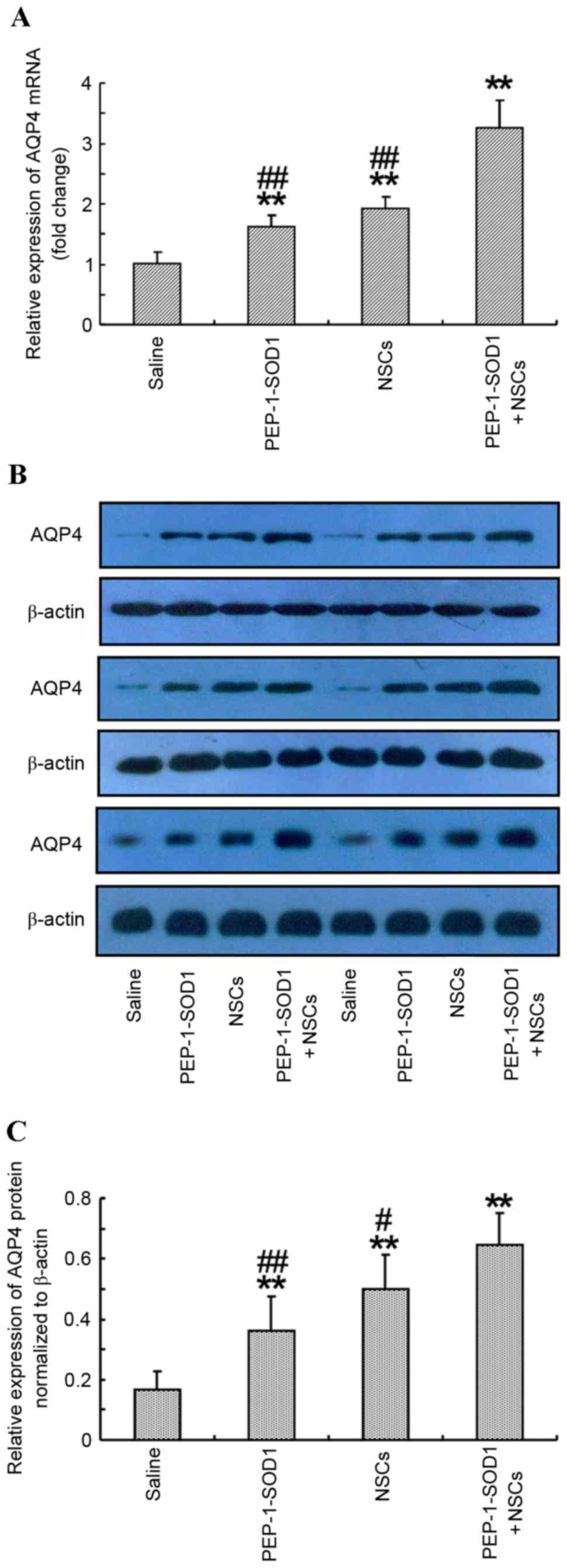
AQP4 serves an important role in the formation of
brain edema. Therefore, the effect of PEP-1-SOD1 in combination
with NSCs on the expression of AQP4 in the brains of rats was
determined (Fig. 5). Both PEP-1-SOD1
and NSCs transplantation significantly elevated the AQP4 mRNA and
protein expression levels compared with those in saline-treated
rats. In the NSCs + PEP-1-SOD1 group, the mRNA and protein
expression levels of AQP4 were further increased compared with
those in the PEP-1-SOD1 and NSCs groups (P<0.01; Fig. 5).
Discussion
The data of the present study indicated that
recombinant PEP-1-SOD1 was able to promote the proliferation and
differentiation of NSCs. Subsequently, the effect of PEP-1-SOD1 in
combination with NSCs in rat models of TBI was investigated.
PEP-1-SOD1 + NSC transplantation accelerated the functional
recovery of neurological functions following TBI. Further
experiments showed that PEP-1-SOD1 in combination with NSCs
transplantation led to further increase in the mRNA and protein
expression levels of AQP4.
The proliferation and differentiation of NSCs are
key factors for NSC transplantation (24). It has been shown that chronic and
acute oxidative stress may inhibit the proliferation of NSCs and
the attenuation of oxidative damage may preserve the proliferation
of NSCs (25). Therefore, SOD1 may
serve an important role in protecting the proliferation of NSCs
against oxidative damage. Thus, the current study determined the
effect of recombinant PEP-1-SOD1 protein on the proliferation and
differentiation of rat NSCs. The current data showed that treatment
with three concentrations of PEP-1-SOD1 for 24, 48 and 72 h
increased the proliferation of stem cells. PEP-1-SOD1 also promoted
the expression of RIP, NEUN and GFAP in stem cell-derived neurons,
suggesting that PEP-1-SOD1 is able to promote the differentiation
of NSCs.
The Bederson score has been widely used for the
assessment of functional recovery in studies concerning brain
injury (26,27). To obtain further information
regarding the rats' recovery, the Bederson score was evaluated by a
member of our laboratory, who was blinded to the group information,
at 1, 3, 7, 14, 21 and 28 days after treatment. In the present
study, the Bederson score was significantly increased in all the
groups 24 h after TBI. Thereafter, the Bederson score in all the
groups gradually decreased. The Bederson score in the NSCs +
PEP-1-SOD1 group was lower compared with that of the control group
from 7 days post-TBI. From 21 days post TBI, the Bederson score in
the NSCs + PEP-1-SOD1 group was lower compared with that of the
NSCs group. Cho et al (14)
demonstrated that PEP-1-SOD1 treatment in ischemic animals
displayed a neuroprotective effect in the ischemic hippocampus for
an extended duration. In an intracerebral hemorrhage (ICH) mouse
model, overexpression of SOD1 protected the grafted NSCs by
decreasing the production of ROS (13). In addition, SOD1 overexpression
resulted in progressive improvement in the behavioral recovery of
mouse models of ICH (13). The
current study suggests that PEP-1-SOD1 is able to promote the
proliferation and differentiation of NSCs, and thereby facilitate
the functional recovery of rats with TBI when administered in
combination with NSC transplantation.
Brain edema is one of the hallmarks of TBI (28). AQP4, which is the water channel
protein and the most abundant aquaporin in the brain, has been
shown to have a dual role in the formation and resolution of edema
after TBI (29). The formation of
brain edema is due to the disruption of the blood-brain barrier and
the entrance of water due to the AQP4 protein (30). The mRNA and protein expression levels
of AQP4 increase rapidly following TBI (31). In the early stages post-TBI,
inhibition of AQP4 has positive effects in the prevention of edema
formation (32,33). AQP4 knockout in rats has been shown
to exert a neuroprotective effect and improve functional recovery
after TBI (34). However, in the
late stages following TBI (a few days after the injury), AQP4 is
important for the clearance of water from the brain to the blood
vessels (35). Consistent with the
results of a previous study (36), a
significant increase in AQP4 was observed 4 days post-TBI in the
PEP-1-SOD1, NSCs and PEP-1-SOD1 + NSCs groups compared with the
control group. The PEP-1-SOD1 + NSCs group showed a further
increase of AQP4 mRNA and protein expression levels compared with
the NSCs + PEP-1-SOD1 group, suggesting that SOD1 may improve the
effect of NSC transplantation on the functional recovery of TBI
rats through upregulating AQP4 expression.
Although this study generated some notable results,
there were also a few limitations. Firstly, the animal model used
is limited, which may affect the precision of the results.
Secondary, the mechanism underlying the effect of PEP-1-SOD1 on the
functional recovery following neural stem cell transplantation in
rats has not been fully clarified. Thirdly, other factors
associated with the potential neuroprotective effect on functional
recovery have not been investigated. In future studies, a larger
number of animals may be used to investigate the effects of
PEP-1-SOD-1 or other factors on functional recovery. Furthermore,
the specific mechanism responsible for the effects of PEP-1-SOD-1
on the functional recovery also merit investigation in a further
study.
In conclusion, SOD1 may promote the proliferation
and differentiation of NSCs and thereby improve the functional
recovery of TBI rats following NSC transplantation. SOD1 also
further increased the expression levels of AQP4 after NSCs
transplantation to facilitate the efflux of water into the blood,
and subsequently accelerate the regression of brain edema. The
current data indicates that recombinant SOD1 in combination with
NSC transplantation is a promising potential therapy for TBI.
References
|
1
|
Moppett IK: Traumatic brain injury:
Assessment, resuscitation and early management. Br J Anaesth.
99:18–31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rossi F and Cattaneo E: Opinion: Neural
stem cell therapy for neurological diseases: Dreams and reality.
Nat Rev Neurosci. 3:401–409. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xiong Z, Zhao S, Mao X, Lu X, He G, Yang
G, Chen M, Ishaq M and Ostrikov K: Selective neuronal
differentiation of neural stem cells induced by nanosecond
microplasma agitation. Stem Cell Res. 12:387–399. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma H, Yu B, Kong L, Zhang Y and Shi Y:
Transplantation of neural stem cells enhances expression of
synaptic protein and promotes functional recovery in a rat model of
traumatic brain injury. Mol Med Rep. 4:849–856. 2011.PubMed/NCBI
|
|
5
|
Addington CP, Roussas A, Dutta D and
Stabenfeldt SE: Endogenous repair signaling after brain injury and
complementary bioengineering approaches to enhance neural
regeneration. Biomark Insights. 10 Suppl 1:S43–S60. 2015.
|
|
6
|
Lozano D, Gonzales-Portillo GS, Acosta S,
de la Pena I, Tajiri N, Kaneko Y and Borlongan CV:
Neuroinflammatory responses to traumatic brain injury: Etiology,
clinical consequences and therapeutic opportunities. Neuropsychiatr
Dis Treat. 11:97–106. 2015.PubMed/NCBI
|
|
7
|
Kabadi SV and Faden AI: Neuroprotective
strategies for traumatic brain injury: Improving clinical
translation. Int J Mol Sci. 15:1216–1236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dasuri K, Zhang L and Keller JN: Oxidative
stress, neurodegeneration, and the balance of protein degradation
and protein synthesis. Free Radic Biol Med. 62:170–185. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mutinati M, Pantaleo M, Roncetti M,
Piccinno M, Rizzo A and Sciorsci RL: Oxidative stress in
neonatology: A review. Reprod Domest Anim. 49:7–16. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang JW, Wang HD, Cong ZX, Zhou XM, Xu JG,
Jia Y and Ding Y: Puerarin ameliorates oxidative stress in a rodent
model of traumatic brain injury. J Surg Res. 186:328–337. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peluffo H, Acarin L, Faiz M, Castellano B
and Gonzalez B: Cu/Zn superoxide dismutase expression in the
postnatal rat brain following an excitotoxic injury. J
Neuroinflammation. 2:122005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakata H, Niizuma K, Wakai T, Narasimhan
P, Maier CM and Chan PH: Neural stem cells genetically modified to
overexpress cu/zn-superoxide dismutase enhance amelioration of
ischemic stroke in mice. Stroke. 43:2423–2429. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wakai T, Sakata H, Narasimhan P, Yoshioka
H, Kinouchi H and Chan PH: Transplantation of neural stem cells
that overexpress SOD1 enhances amelioration of intracerebral
hemorrhage in mice. J Cereb Blood Flow Metab. 34:441–449. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cho JH, Hwang IK, Yoo KY, Kim SY, Kim DW,
Kwon YG, Choi SY and Won MH: Effective delivery of Pep-1-cargo
protein into ischemic neurons and long-term neuroprotection of
Pep-1-SOD1 against ischemic injury in the gerbil hippocampus.
Neurochem Int. 52:659–668. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yune TY, Lee JY, Jiang MH, Kim DW, Choi SY
and Oh TH: Systemic administration of PEP-1-SOD1 fusion protein
improves functional recovery by inhibition of neuronal cell death
after spinal cord injury. Free Radic Biol Med. 45:1190–1200. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Piao J, Major T, Auyeung G, Policarpio E,
Menon J, Droms L, Gutin P, Uryu K, Tchieu J, Soulet D and Tabar V:
Human embryonic stem cell-derived oligodendrocyte progenitors
remyelinate the brain and rescue behavioral deficits following
radiation. Cell Stem Cell. 16:198–210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kabeer FA, Sreedevi GB, Nair MS,
Rajalekshmi DS, Gopalakrishnan LP, Kunjuraman S and Prathapan R:
Antineoplastic effects of deoxyelephantopin, a sesquiterpene
lactone from Elephantopus scaber, on lung adenocarcinoma (A549)
cells. J Integr Med. 11:269–277. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Y, Wang X, Cheng S, Du J, Deng Z, Zhang
Y, Liu Q, Gao J, Cheng B and Ling C: Diosgenin induces G2/M cell
cycle arrest and apoptosis in human hepatocellular carcinoma cells.
Oncol Rep. 33:693–698. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yao ZG, Sun XL, Li P, Liu HL, Wu HL, Xi ZQ
and Zheng ZH: Neural stem cells transplantation alleviate the
hyperalgesia of spinal cord injured (SCI) associated with
down-regulation of BDNF. Int J Clin Exp Med. 8:404–412.
2015.PubMed/NCBI
|
|
20
|
Zhao J, Chen N, Shen N, Zhao H, Wang D,
Shi J, Wang Y, Cui X, Yan Z and Xue H: Transplantation of human
umbilical cord blood mesenchymal stem cells to treat a rat model of
traumatic brain injury. Neural Regen Res. 7:741–748.
2012.PubMed/NCBI
|
|
21
|
Crumrine RC, Marder VJ, Taylor GM, Lamanna
JC, Tsipis CP, Scuderi P, Petteway SR Jr and Arora V:
Intra-arterial administration of recombinant tissue-type
plasminogen activator (rt-PA) causes more intracranial bleeding
than does intravenous rt-PA in a transient rat middle cerebral
artery occlusion model. Exp Transl Stroke Med. 3:102011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bederson JB, Pitts LH, Tsuji M, Nishimura
MC, Davis RL and Bartkowski H: Rat middle cerebral artery
occlusion: Evaluation of the model and development of a neurologic
examination. Stroke. 17:472–476. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Arocho A, Chen B, Ladanyi M and Pan Q:
Validation of the 2-DeltaDeltaCt calculation as an alternate method
of data analysis for quantitative PCR of BCR-ABL P210 transcripts.
Diagn Mol Pathol. 15:56–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ryu HH, Lim JH, Byeon YE, Park JR, Seo MS,
Lee YW, Kim WH, Kang KS and Kweon OK: Functional recovery and
neural differentiation after transplantation of allogenic
adipose-derived stem cells in a canine model of acute spinal cord
injury. J Vet Sci. 10:273–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takemura S, Kayama T, Kuge A, Ali H,
Kokubo Y, Sato S, Kamii H, Goto K and Yoshimoto T: Correlation
between copper/zinc superoxide dismutase and the proliferation of
neural stem cells in aging and following focal cerebral ischemia. J
Neurosurg. 104:129–136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun F, Jin K and Uteshev VV: A type-II
positive allosteric modulator of α7 nAChRs reduces brain injury and
improves neurological function after focal cerebral ischemia in
rats. PLoS One. 8:e735812013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Crumrine RC, Marder VJ, Taylor GM, Lamanna
JC, Tsipis CP, Novokhatny V, Scuderi P, Petteway SR Jr and Arora V:
Safety evaluation of a recombinant plasmin derivative lacking
kringles 2–5 and rt-PA in a rat model of transient ischemic stroke.
Exp Transl Stroke Med. 4:102012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marmarou A: Pathophysiology of traumatic
brain edema: Current concepts. Acta Neurochir Suppl. 86:7–10.
2003.PubMed/NCBI
|
|
29
|
Badaut J, Ashwal S and Obenaus A:
Aquaporins in cerebrovascular disease: A target for treatment of
brain edema? Cerebrovasc Dis. 31:521–531. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Panikashvili D, Shein NA, Mechoulam R,
Trembovler V, Kohen R, Alexandrovich A and Shohami E: The
endocannabinoid 2-AG protects the blood-brain barrier after closed
head injury and inhibits mRNA expression of proinflammatory
cytokines. Neurobiol Dis. 22:257–264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lopez-Rodriguez AB, Acaz-Fonseca E,
Viveros MP and Garcia-Segura LM: Changes in cannabinoid receptors,
aquaporin 4 and vimentin expression after traumatic brain injury in
adolescent male mice. Association with edema and neurological
deficit. PLoS One. 10:e01287822015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ding Z, Zhang J, Xu J, Sheng G and Huang
G: Propofol administration modulates AQP-4 expression and brain
edema after traumatic brain injury. Cell Biochem Biophys.
67:615–622. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cui T and Zhu G: Ulinastatin attenuates
brain edema after traumatic brain injury in rats. Cell Biochem
Biophys. 71:595–600. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fukuda AM, Adami A, Pop V, Bellone JA,
Coats JS, Hartman RE, Ashwal S, Obenaus A and Badaut J:
Posttraumatic reduction of edema with aquaporin-4 RNA interference
improves acute and chronic functional recovery. J Cereb Blood Flow
Metab. 33:1621–1632. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fukuda AM and Badaut J: Aquaporin 4: A
player in cerebral edema and neuroinflammation. J
Neuroinflammation. 9:2792012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tourdias T, Mori N, Dragonu I, Cassagno N,
Boiziau C, Aussudre J, Brochet B, Moonen C, Petry KG and Dousset V:
Differential aquaporin 4 expression during edema build-up and
resolution phases of brain inflammation. J Neuroinflammation.
8:1432011. View Article : Google Scholar : PubMed/NCBI
|