Introduction
The majority of early invasive ductal carcinomas are
diagnosed by pathological biopsy (1,2).
However, patients presenting with the same clinical pathological
stage that receive similar clinical treatments may have distinct
prognoses (3,4). Due to the heterogeneity of breast
cancer, the molecular subtype theory has been proposed, which
suggests that breast cancer should be divided into four subtypes
based on differential molecular expression (5,6). For
instance, the luminal A subtype of invasive ductal carcinoma
accounts for 65% of all breast cancer cases, and typically occurs
in postmenopausal women (7). The
maximum tumor diameter of the luminal A subtype is <2 cm.
Luminal A, namely the estrogen receptor (ER)-α (+)/progesterone
receptor (PR) (+)/Human epidermal growth factor receptor-2 (Her-2)
(−)/low expression of Ki-67, is the most common subtype that
accounts for 50% of all subtypes (8). In addition, luminal A subtype breast
cancer has the lowest rate of recurrence and metastasis (9). Her-2 overexpression is a subtype of
breast cancer that accounts for 20–30% of breast cancer cases
(6), while the triple-negative
subtype [Her-2 (−), ER (−) and PR (−)] accounts for 11–17% of cases
(10). Furthermore, Her-2
overexpression and triple-negative breast cancer typically occur in
pre-menopausal women (9,11). The tumor diameters associated with
the Her-2 and triple-negative subtypes are >2 cm (12,10), and
the recurrence rate for tumors with diameter >2 cm is relatively
high (12). As the fourth subtype,
basal-like type breast cancer has been associated with a familial
genetic tendency, and has the highest rates of recurrence and
metastasis compared with other subtypes (10).
MicroRNA (miRNA/miR) and its relevant signaling
pathways may be associated with the recurrence, metastasis and
prognosis of early invasive ductal carcinoma of the breast, along
with the efficacy of treatment (13,14). For
instance, Lowery et al (15)
reported that levels of miRNA expression were positively correlated
with the expression of ER-α, PR and Her-2. Furthermore, previous
studies have indicated that miR-10b was involved in early-stage
breast invasive ductal carcinoma (16,17).
Overexpression of miR-10b has also been observed in breast cancer
cells with high metastatic capability, and has been implicated in
the regulation of breast cancer metastasis (18). In addition, miR-10b may enable
non-metastatic breast cancer tumor cells to acquire potent invasive
and metastatic properties (19).
In the present study, the expression of miR-10b was
detected using in situ hybridization (ISH) in tumor samples
of patients with early invasive ductal carcinoma of the breast.
Immunohistochemistry was also performed to evaluate the expression
of ER-α, PR and Her-2 in the tumor samples. Based on the levels of
ER-α, PR and Her-2 expression, patient specimens were further
classified into different molecular subtypes, and the associations
between miR-10b expression with the expression of ER-α, PR and
Her-2 and the molecular subtypes were analyzed.
Materials and methods
Clinical data of patients
A database was established for patients with breast
cancer, which contained the data of 2,600 patients who received
treatment at the First Affiliated Hospital of Xinjiang Medical
University (Urumqi, China) between January 2000 and December 2013.
In the database, all patients with breast cancer were
pathologically confirmed (20), and
their clinical data and follow-up information were complete. The
follow-up data was collected for 2–15 years post-operation via
outpatient clinics, return visits and telephone interviews. The
interval of follow-up study was 1 year and the deadline was
December 2015. A lack of tumor recurrence during the follow-up
period was regarded as disease-free survival. Imaging (abdominal
ultrasound, bone scan, lung computed tomography) and
histopathological examining of tissues were used to confirm tumor
metastases and recurrence. The loss to follow-up rate was 6%. In
the present study, 193 patients with early invasive ductal
carcinoma of the breast were selected from the database. Patients
were excluded if they: i) had other cancers; ii) received
preoperative radiotherapy, chemotherapy; and iii) had
non-breast-derived early breast invasive ductal carcinoma. The
tumor diameter of enrolled patients was ≤2 cm. All of the patients
were women aged 34–78 years old (mean age, 46.5 years old). Tumor
samples were collected from the patients with early invasive ductal
carcinoma during radical surgery. All tumor samples were sectioned
into size of 1×1×0.5 cm, and subjected to immersion fixation with
10% neutral buffered formalin for 24 h at 4°C. Samples were then
embedded in paraffin. Prior written informed consent was obtained
from all patients and the study was approved by the Ethics Review
Board of the Ethics Committee of Xinjiang Medical University.
ISH
The enhanced sensitivity of an ISH detection kit I
(Boster Biological Technology, Pleasanton, CA, USA) was used to
perform an in situ hybridization assay. A hsa-miR-10b
miRCURY LNA Detection Probe was purchased from Exiqon A/S (Vedbaek,
Denmark). All procedures were performed following the
manufacturer's instructions. Paraffin slices of 4 µm of tumor
samples from 193 patients with early invasive ductal carcinoma were
deparaffinized with xylene and dehydrated with ethanol, and
subsequently treated with 3% H2O2, washed by distilled water twice,
combined with 3% citric acid and two drops of concentrated pepsin
according to the instructions of the kit (cat. no. MK1030; Boster
Biological Technology, Pleasanton, CA, USA). Following washing with
phosphate buffered saline (PBS) three times and distilled water
once, sample slices were prehybridized with digoxigenin-labeled
oligonucleotide probes (40 nM) at 37°C in a wet box for 3 h. Slices
were then washed with PBS for 2 times and incubated with 20 µl
digoxigenin-labeled oligonucleotide probes overnight at 37–40°C.
Following washing with PBS three times, 1X biotinylated mouse
anti-digoxin was added to slices for a further 2 h incubation at
4°C. Slices were respectively incubated with streptavidin biotin
complex and biotinylated peroxidase (3%) for 30 min at 37°C.
Finally, 3,3′-diaminobenzidine (DAB) was used for color
development, and samples were counterstained with hematoxylin.
Slices were mounted and observed under a light microscope (Olympus
Corporation, Tokyo, Japan). Cells with brown staining in the
cytoplasm were defined as miR-10b-positive cells. According to the
color intensity and number of positive cells, staining scores were
assigned, as described previously (21). Briefly, cells expressing miR-10b were
scored based on the degree of staining, as follows: 0 points, no
color; 1 point, yellowish, 2 points, brownish-yellow; and 3 points,
brown. According to the number of positive cells, the expression of
miR-10b was classified into four conditions: Score 1, the rate of
positive cells was 1–25%; score 2, the rate of positive cells was
26–50%; score 3, the rate of positive cells was 51–75%; and score
4, the rate of positive cells was >75%. The final evaluation
score for miR-10b expression in each sample was calculated as
follows: Final score = score of color intensity × score of positive
cell rate. In addition, the final score was divided into four
expression categories: 0, (−); 1–2 points, (+); 3–4 points (++);
and > 4, points (+++).
Immunohistochemistry
Immunohistochemistry was performed using an
immunohistochemistry kit (cat. no. SV00002; Boster Biological
Technology) according to the manufacturer's instructions. Paraffin
slices of 4 µm of tumor samples were deparaffinized and dehydrated.
Following the position of the antigen was detected by probes,
slices were processed with peroxidase blocking solution (3%) and
incubated at 37°C for 10 min with 5% bovine serum to block
non-specific antigens. A total of 50 µl primary antibody was added
to slices and incubated for 30 min at room temperature. The primary
antibodies used were rabbit anti-ER-α monoclonal antibody (ab16660;
1:100), rabbit anti-PR monoclonal antibody (ab32085; 1:100) and
rabbit anti-Her-2 monoclonal antibody (ab134182; 1:100), which were
purchased from Abcam (Cambridge, MA, USA). Following washing with
phosphate-buffered saline, 50 µl horseradish peroxidase-conjugated
goat anti-rabbit antibody (cat. no. SV00002; 1:200; Boster
Biological Technology) was added to the slices and incubated for
10–15 min at room temperature. Subsequently, 100 µl DAB was added
for color development. Following washing, slices were
counterstained with hematoxylin, mounted with neutral gum and
observed under an upright light microscope (BX43; Olympus
Corporation).
The expression levels of ER-α and PR were defined
according to the American Society of Clinical Oncology criteria
(22). When the rate of positive
tumor cells was ≤10%, samples were considered as ER-α or
PR-negative, while those with a rate >10% were considered as
ER-α or PR positive. To define the expression levels of Her-2, the
Hercep test standard was used (22),
as recommended by the US Food and Drug Inspection Bureau. The
expression of Her-2 was divided into four levels: (−), ≤10% cell
membrane was positively stained; (+), >10% cells were positively
stained, but cells were not contiguous or surrounding the cell
membrane; (++), >10% cells were positively stained with a weak
or moderate color surrounding the cell membrane and continuous; and
(+++), >10% cells were intensively and continuously stained
around the cell membrane. When the immunohistochemical staining
indicated Her-2 (+++) and the FISH test had confirmed Her-2 gene
amplification, the sample was considered to exhibit high expression
of Her-2.
Fluorescence ISH (FISH)
Tumor specimens from all patients were embedded in
paraffin and cut into 4-µm thick sections. Following
deparaffinization and dehydration, sections were immersed in 30%
acidic sodium sulfite at 50°C for 30 min. Following washing with
saline-sodium citrate buffer, samples were digested using 5 µg/ml
proteinase K solution (cat. no. 90003; Exiqon, Vedbaek, Denmark)
for 4–10 min at 37°C and immersed in 0.1 M HCl for 5–10 min.
Following fixation with 100% acetone for 10 min at 4°C and
air-drying, the tumor samples were hybridized with 10 µl 30 nM
probe-mixed solution (GLP HER-2/CSP17 dual-color fluorescent
probes, cat. no. D3571; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) overnight in a wet box at 40°C. Slices were air-dried in
the dark following washing with saline-sodium citrate five times
for 5 min, and 4′,6-diamidino-2-phenylindole was subsequently used
to stain the nucleus. Slices were mounted and observed using
fluorescence microscopy. The fluorescent probes GLP HER-2/CSP17
included two types of probes: CSP17 labeled the centromere of the
chromosome 17 with green fluorescence, and HER-2 labeled the Her-2
gene with orange fluorescence. In each visual field (selected
according to the results of immunohistochemical staining), 30 cells
were counted. The fluorescence ratio was calculated using the
following formula: Ratio = number of cells (orange)/number of cells
(green). When the ratio was <1.8, the sample was considered to
exhibit Her-2-negative expression, which indicated no
Her-2 amplification in the tumor sample. If the ratio was
>2.2, the sample was regarded to exhibit Her-2-positive
expression, which indicated that the Her-2 gene was
amplified in the tumor sample. When the ratio was 1.8–2.2, more
tumor cells were required to be counted or the FISH assay was
repeated. If the ratio was >20 or there was contiguous
fluorescence within clusters, this was considered to indicate gene
amplification (23).
Statistical analysis
SPSS v17.0 statistical software (SPSS, Inc.,
Chicago, IL, USA) was used for data analysis. A Pearson's
χ2 test was used to analyze the differential expression
of miR-10b in patients with distinct expression of ER-α, PR and
Her-2. The Kaplan-Meier method was used to determine the cumulative
disease-free survival time and a Log-rank test was performed to
further analyze differences. A multivariate Cox regression model
was applied to study different prognostic factors for early
invasive ductal carcinoma. The menstruation status was measure
according to if menopause was confirmed or not; the pathological
grades (I or II) were determined according the (American Joint
Committee on Cancer criteria; and treatment of chemotherapy and
radiotherapy was determined as received (yes) or never received
(no). P<0.05 was considered to indicate a statistically
significant difference.
Results
Determination of miR-10b
expression
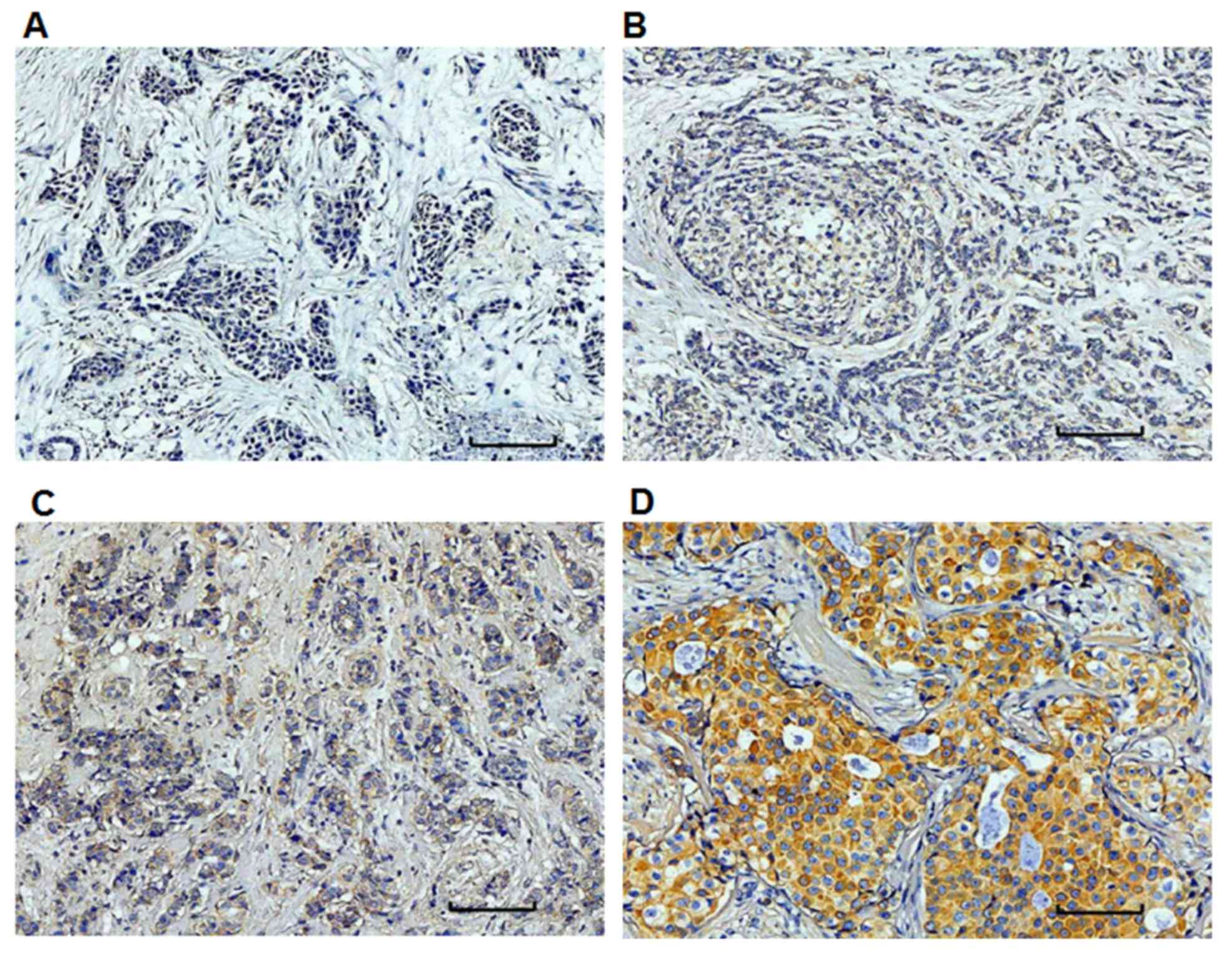
To determine the expression levels of miR-10b, in
situ hybridization was performed. The positivity or negativity
was determined by the cytoplasmic staining and miR-10b (+) cells
were stained brown. The negative and positive expression of miR-10b
was indicated in Fig. 1. Based on
staining intensity and the percentage of positive cells, the
expression levels of miR-10b were divided into negative expression
(−) (Fig. 1A), weak positive
expression (+) (Fig. 1B), positive
expression (++) (Fig. 1C), and
strong positive expression (+++) (Fig.
1D), respectively. Of all 193 included patients, 152 were
miR-10b (+) and 41 were miR-10b (−) (Table I).
 | Table I.Relationship between miR-10b
expression and ER-α, PR and Her-2 expression in early breast
invasive ductal cancer. |
Table I.
Relationship between miR-10b
expression and ER-α, PR and Her-2 expression in early breast
invasive ductal cancer.
| Clinical
indicator | N | miR-10b (−)
(%) | miR-10b (+)
(%) | χ2 | P-value |
|---|
| ER-α |
|
|
|
|
|
|
(+) | 110 | 29 (26.4) | 81 (73.6) | 4.008 | 0.045 |
|
(−) | 83 | 12 (14.5) | 71 (85.5) |
|
|
| PR |
|
|
|
|
|
|
(+) | 133 | 24 (18.0) | 109 (82.0) | 2.616 | 0.106 |
|
(−) | 60 | 17 (28.3) | 43 (71.7) |
|
|
| Her-2 |
|
|
|
|
|
|
(+) | 71 | 21 (29.6) | 50 (70.4) | 4.663 | 0.031 |
|
(−) | 122 | 20 (16.4) | 102 (83.6) |
|
|
Determination of ER-α and PR
expression
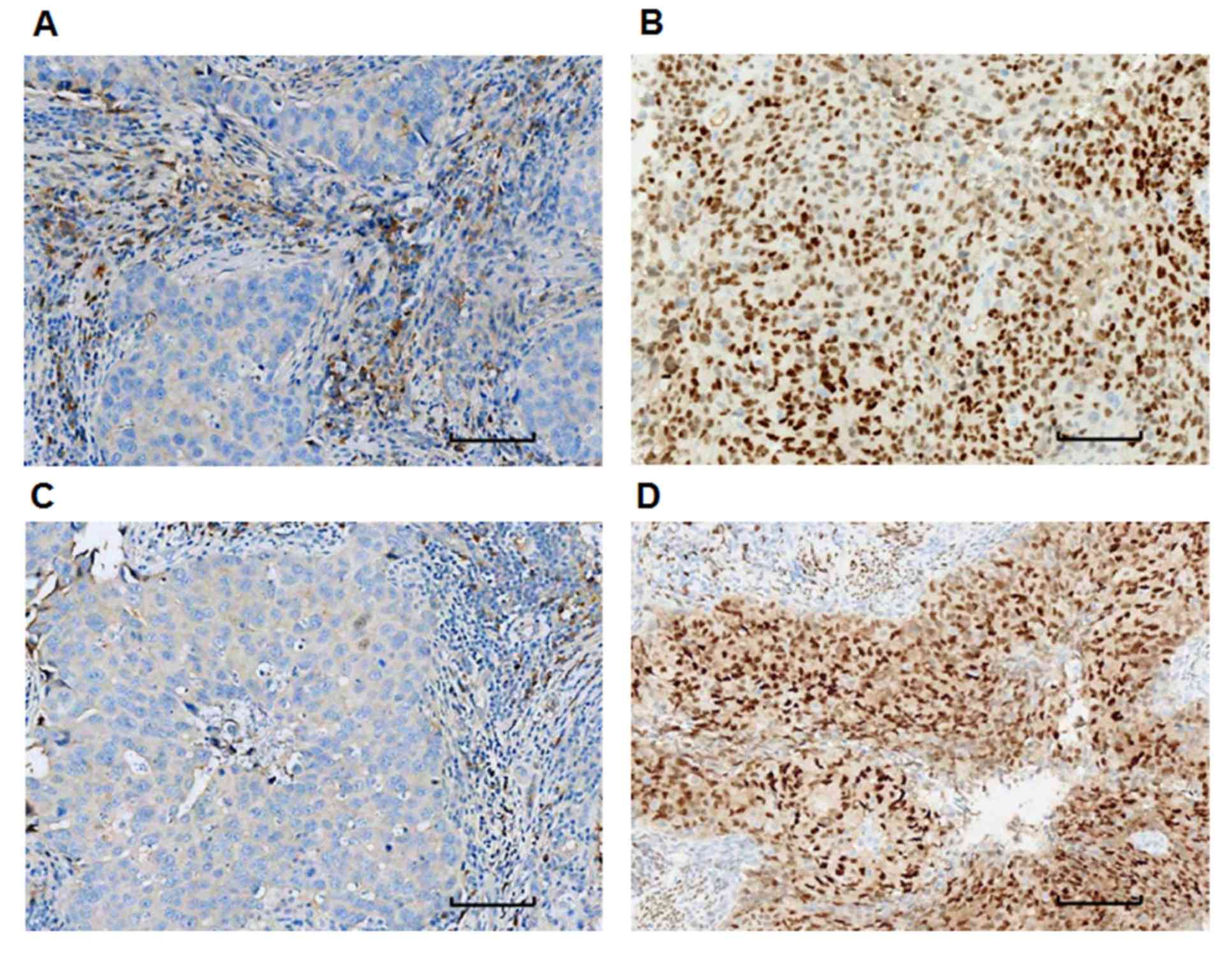
To detect the expression levels of ER-α and PR,
immunohistochemistry was performed. From the 193 patients, 110 were
ER-α (+), 83 were ER-α (−), 133 were PR (+) and 60 were PR (−)
(Table I). Fig. 2 depicts representative staining
images of tumor samples obtained from patients. Tumor samples with
a positive staining rate of ≤10% (ER-α- or PR-negative; Fig. 2A and C) and >10% (ER-α- or
PR-positive; Fig. 2B and D) were
identified.
Determination of Her-2 expression
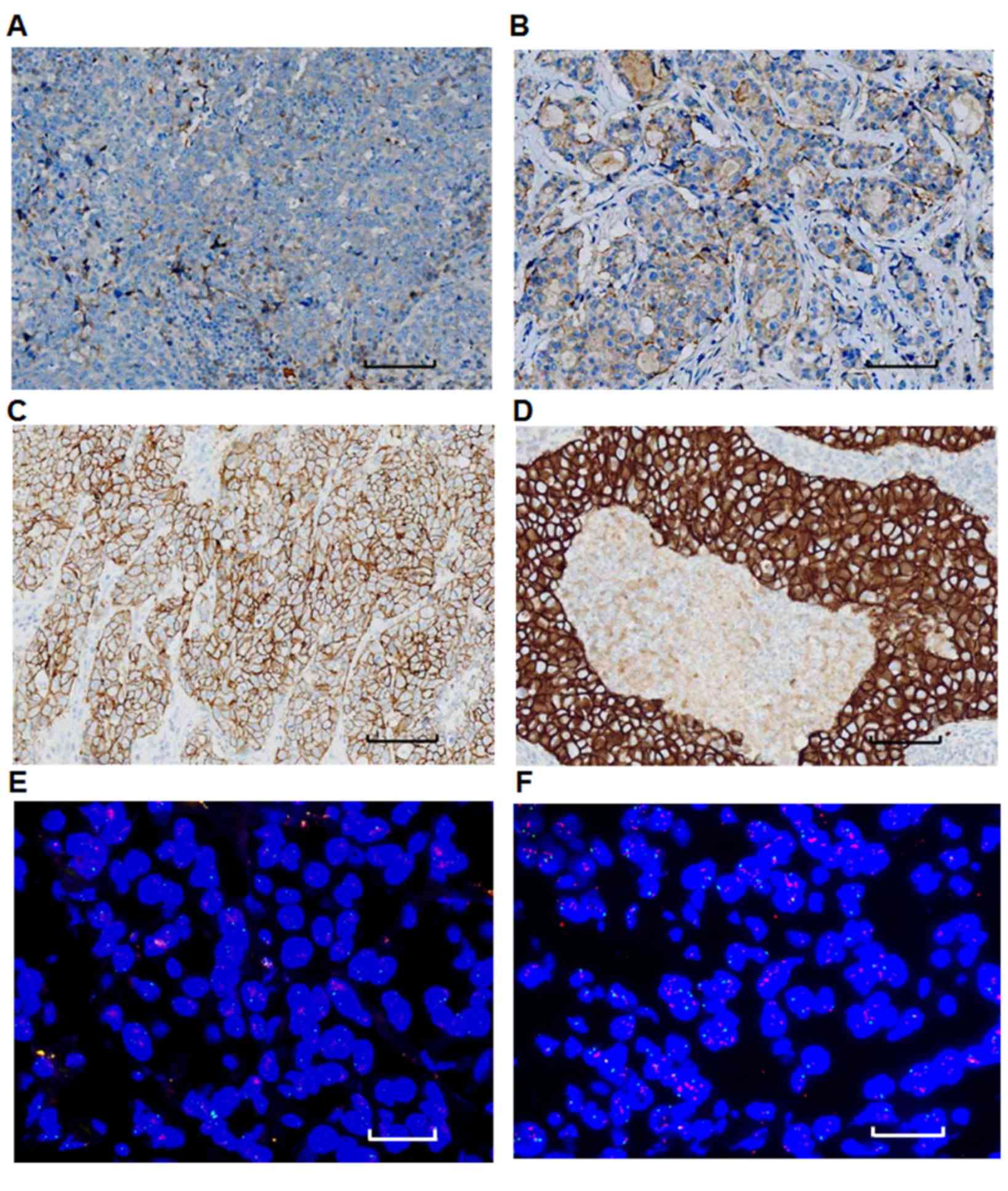
using immunohistochemistry and FISH
Her-2 expression was evaluated using
immunohistochemistry and FISH. There were 71 patients that were
Her-2 positive patients and 122 that were Her-2 negative (Table I). Fig.
3A depicts a representative tumor sample with Her-2 (−)
expression, while Figs. 3B-D
represent Her-2 (+), (++), (++) and (+++) samples, respectively.
Amplification of the Her-2 gene was detected using FISH. Fig. 3 depicts representative FISH images of
tumor samples. Samples with a fluorescence ratio of <1.8 were
considered to lack Her-2 expression, thus indicating that the Her-2
gene was not amplified in the tumor samples (Fig. 3E). A ratio of >2.2 identified
samples as Her-2-positive, which indicated that the Her-2 gene was
amplified in the tumor sample (Fig.
3F).
miR-10b expression and its correlation
with the expression of ER-α, PR and Her-2 in early invasive ductal
carcinoma
The association between miR-10b expression and the
expression of ER-α, PR or Her-2 in 139 cases of early invasive
ductal carcinoma was analyzed using a Pearson's χ2 test.
From the total 193 patients, there were 54 patients who showed
normal-like results in the immunohistochemistry test and were
therefore not categorized into the four subtypes, hence they were
not analyzed. Results indicated that the positive expression rate
of miR-10b was significantly increased in ER-α (−) samples when
compared with ER-α (+) samples (χ2=4.008, P=0.045;
Table I). Similarly, in tumor
samples that were Her-2 (−), the positive expression rate of
miR-10b was significantly increased when compared with Her-2 (+)
samples (χ2=4.663, P=0.031; Table I). By contrast, the miR-10b-positive
expression rate was greater in tumor samples that were PR (+)
compared with those that were PR (−), though this was not
statistically significant (χ2=2.616, P=0.106; Table I). These results indicate that the
positive expression rate of miR-10b was negatively correlated with
the expression of ER-α and Her-2.
miR-10b expression and its correlation
with different molecular subtypes
Based on the expression of ER-α, PR and Her-2 in 139
patients with early invasive ductal carcinoma, the patients were
divided into molecular subtypes, namely luminal A, luminal B, Her-2
overexpression and basal-like, as indicated in Table II. Among these distinct molecular
subtypes, the positive expression rate of miR-10b in luminal B was
significantly decreased when compared with the other subtypes
(χ2=8.250, P=0.037; Table
III). This data indicates that positive expression of miR-10b
may promote an increased risk of recurrence and metastasis of
luminal B type breast cancer.
 | Table II.Molecular subtypes of breast cancer
and their receptor expression. |
Table II.
Molecular subtypes of breast cancer
and their receptor expression.
| Molecular
subtype | Receptor
expression |
|---|
| Luminal A | ER-α (+), and/or PR
(+), Her-2(−) |
| Luminal B | ER-α (+), and/or PR
(+), Her-2(+) |
| Her-2
overexpression | ER-α (−), PR (−),
Her-2 (+) |
| Basal-like | ER-α (−), PR (−),
Her-2 (−) |
 | Table III.Association between miR-10b
expression and early breast invasive ductal cancer molecular
subtypes. |
Table III.
Association between miR-10b
expression and early breast invasive ductal cancer molecular
subtypes.
| Molecular
subtype | N | miR-10b (−)
(%) | miR-10b (+)
(%) | P-value |
|---|
| Luminal A | 52 | 7 (13.5) | 45 (86.5) | 0.037
(χ2 =8.25) |
| Luminal B | 35 | 13 (37.1) | 22 (62.9) | – |
| Her-2 | 20 | 3 (15.0) | 17 (85.0) | – |
| Basal-like | 32 | 4 (12.5) | 28 (87.5) | – |
Survival analysis of patients with
early invasive ductal carcinoma with miR-10b (+) expression and
variable ER-α, PR and Her-2 expression
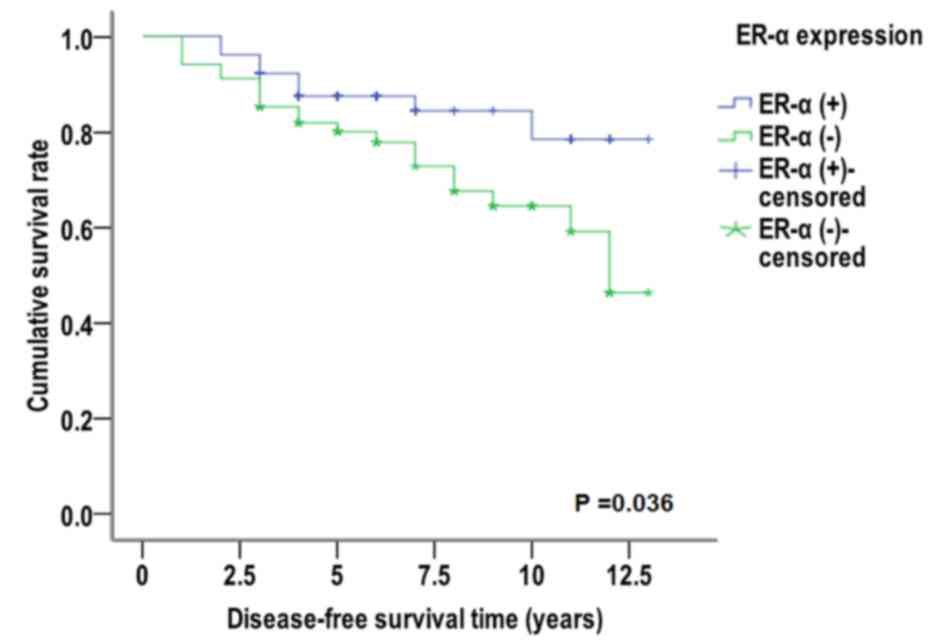
Cumulative disease-free survival analysis was
performed using the Kaplan-Meier method and Log-rank test. In tumor
samples that were identified as miR-10b (+), the median
disease-free survival (50% of the cumulative survival rate) time
was significantly increased in patients that were ER-α (+) (11.466
years) compared with patients that were ER-α (−) (9.994 years;
χ2=4.375, P=0.036; Fig.
4). Similarly, the median disease-free survival free time was
increased in patients that were miR-10b (+) and PR (+) (11.509
years) when compared with patients that were PR (−) (9.773 years;
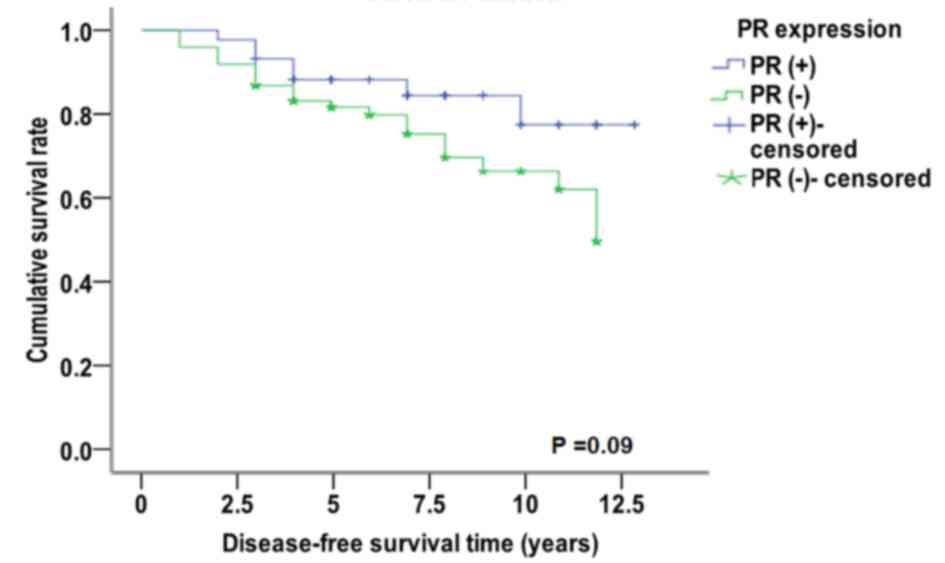
χ2=2.883, P=0.090; Fig.
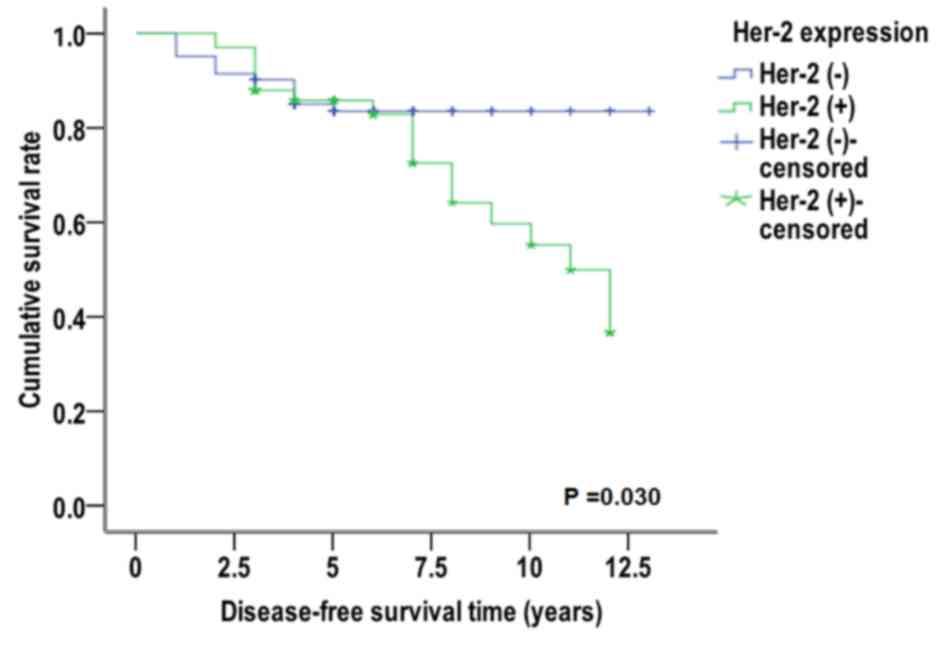
5). Conversely, in patients that were miR-10 (+) and Her-2 (−),
the median disease-free survival time was 11.346 years, which was
significantly increased compared with patients that were Her-2 (+)
(9.481 years) (χ2=4.704, P=0.030, Fig. 6). For patients with miR-10b (−),
miR-10b (−) expression had no effect on the median disease-free
survival time of different breast cancer molecular subtypes (data
not shown). These results suggest that miR-10b-positive expression
resulted in different expression levels of PR, which has no
significant effect on the disease-free survival curve of patients;
however, for miR-10b-positive expression, different expression
levels of ER-α and Her-2 may have a significant effect on the
disease-free survival curve of patients.
Survival analysis of patients with
early invasive ductal carcinoma with miR-10b (+) expression and
different molecular subtypes
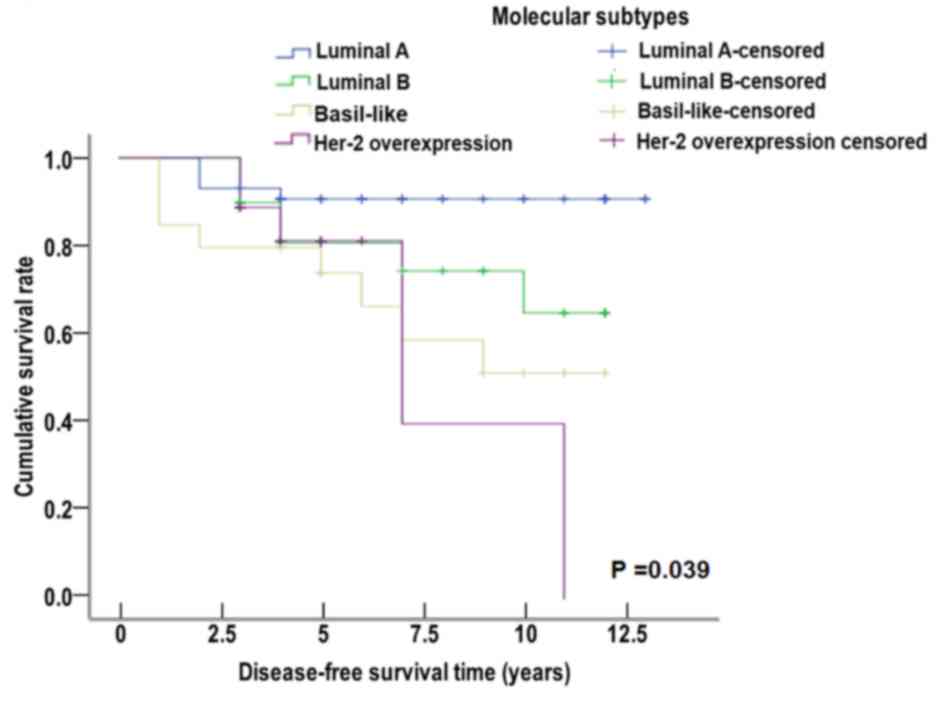
Patients that were miR-10b (+) with early invasive
ductal carcinoma were divided into several molecular subtypes.
These subtypes consisted of luminal A, luminal B, Her-2
overexpression and basal-like subtype. The Kaplan-Meier method and
Log-rank test were used to perform survival analysis. The median
disease-free survival time was 12.035 years for luminal A subtype,
9.882 years for luminal B subtype, 8.024 years for Her-2
overexpression subtype and 8.316 years for basal-like subtype. The
median disease-free survival time of patients that were miR-10b (+)
was significantly decreased in the Her-2 overexpression and
basal-like subtypes compared with luminal A subtype
(χ2=8.340, P=0.039; Fig.
7). These data suggested that the positive expression of
miR-10b was correlated with the median disease-free survival time
of different molecular subtypes.
Multivariate Cox regression analysis
of the prognostic factors for patients with early invasive ductal
carcinoma
To define the prognostic factors of early invasive
ductal carcinoma, multivariate Cox regression analysis was
performed. The prognostic factors included menstruation, clinical
stage, miR-10b expression, the molecular subtypes (luminal A,
luminal B, Her-2 overexpression and basal-like subtype),
chemotherapy and radiotherapy. Results indicated that the luminal A
molecular subtype may be an independent prognostic factor for early
breast invasive ductal carcinoma (P=0.049; Table IV). The basal-like and Her-2
overexpression subtypes were also identified as independent factors
for poor prognosis [odds ratio (OR)=5.232, P=0.007 and OR=4.214,
P=0.036, respectively]. In addition, the expression of miR-10b may
be a potential prognostic factor for early invasive ductal
carcinoma (OR=3.339); though this was not statistically significant
(P=0.108). These findings suggest that the molecular subtype may be
an independent prognostic factor for early invasive ductal
carcinoma of the breast.
 | Table IV.Multivariate Cox regression analysis
of early invasive ductal cancer molecular subtypes and prognostic
factors. |
Table IV.
Multivariate Cox regression analysis
of early invasive ductal cancer molecular subtypes and prognostic
factors.
| Clinicopathological
variable | β | Standard error | Wald value | P-value | OR | 95% confidence
interval |
|---|
| Menstruation status
(Entered menopause or not) | 0.171 | 0.409 | 0.174 | 0.676 | 1.186 | 0.532–2.645 |
| Pathological grade
(AJCC I or II) | 0.181 | 0.460 | 0.155 | 0.694 | 1.199 | 0.486–2.954 |
| miR-10b | 1.206 | 0.749 | 2.589 | 0.108 | 3.339 | 0.769–14.507 |
| Luminal A | – | – | 7.879 | 0.049 | – | – |
| Luminal B | 1.065 | 0.619 | 2.956 | 0.086 | 2.900 | 0.862–9.760 |
| Basal-like | 1.655 | 0.610 | 7.366 | 0.007 | 5.232 | 1.584–17.283 |
| Her-2
overexpression | 1.438 | 0.684 | 4.421 | 0.036 | 4.214 | 1.103–16.109 |
| Chemotherapy (yes
or no) | −0.394 | 1.065 | 0.137 | 0.711 | 0.674 | 0.084–5.433 |
| Radiotherapy (yes
or no) | 0.138 | 0.460 | 0.090 | 0.764 | 1.148 | 0.466–2.829 |
Discussion
Invasive ductal carcinoma has been indicated as the
most common pathological type of breast cancer, and accounts for
75% of all carcinomas of the breast (24,25).
Perou et al (5) and Sorlie
et al (6) reported that
breast cancer may be divided into four different subtypes (luminal
A, luminal B, Her-2 overexpression and basal-like subtype) in
accordance with the differential gene expression between tumor
cells and normal cells. Lowery et al (15) further classified breast cancer
subtypes based on their miRNA expression profiles and levels of
ER-α, PR and Her-2 expression. Lowery et al (15) also demonstrated that miRNA expression
may be positively correlated with the expression levels of ER, PR
and Her-2. Therefore, they proposed that breast cancer should be
further divided according to the miRNA expression level, thus
indicating that miRNA expression may be a diagnostic and prognostic
indicator for breast cancer. To the best of our knowledge, no
previous studies have investigated the association between miR-10b
expression and the different molecular subtypes of breast cancer.
The present results indicated that miR-10b expression was
associated with the expression of ER-α, PR, Her-2 and the different
molecular subtypes. The positive expression rate of miR-10b was
significantly increased in patients that were ER-α (−) compared
with those that were ER-α (+), and also significantly increased in
patients that were Her-2 (−) compared with those that were Her-2
(+). These data indicated that miR-10b expression was negatively
correlated with the expression of ER-α and Her-2. Furthermore,
among the four distinct molecular subtypes of breast cancer, the
positive expression rate of miR-10b in luminal B was the lowest, at
62.9%. These data suggest that the expression of miR-10b
potentially contributes to decreased expression of ER-α and Her-2,
which may further lead to a reduced risk of recurrence and
metastasis of the luminal B subtype. Notably, the present results
indicated that elevated expression of ER-α may be a protective
factor for breast cancer, whereas the upregulated expression of
Her-2 may be a risk factor, and the non-luminal B subtypes,
particularly Her-2 overexpression and basal-like, may increase the
possibility of tumor recurrence and metastasis.
At present, studies have indicated different
prognoses for the molecular subtypes of breast cancer. For
instance, the luminal A subtype is the most common breast cancer
subtype, and has been associated with a lower recurrence rate and
an improved prognosis compared with all other subtypes (3,26). The
luminal B subtype is characterized by an upregulation in
proliferation-associated genes, including CCNB1 and MYBL2, and
genes associated with the signaling pathways of growth factor
receptors (27). A previous study
indicated that the percentage of histological grade III in luminal
B was increased, despite lower expression levels of ER-related
genes in the luminal B subtype (28). Furthermore, luminal B is less
sensitive to endocrine therapy when compared with luminal A
(29). The Her-2 overexpression
subtype has the poorest prognosis among all the subtypes,
potentially due to its higher secretion level of proteolytic
enzymes (30), which stimulates cell
division, enhances the invasive ability of tumor cells and promotes
cancer metastasis (6,31). The basal-like subtype typically
occurs in pre-menopausal women, and ER-α (−), PR (−) and Her-2 (−)
expression has been implicated in this subtype (32,33). In
addition, the basal-like subtype has been associated with a higher
histological grade, poorer differentiation ability and stronger
invasive capability compared with the other subtypes, which may
result in early recurrence and distant metastasis (11,12). In
the present study, patients with miR-10b (+) breast cancer with
ER-α (+)/PR (+)/Her-2 (−) expression had a longer median
disease-free survival time and improved prognosis, which was
consistent with a previous study (8). Furthermore, results of the survival
analysis (K-M curve) indicated that the luminal A subtype indicated
the greatest prognosis in comparison with luminal B, basal-like
subtype and Her-2 overexpression subtype. Among the latter, Her-2
overexpression had the shortest median survival time, indicating
the poorest prognosis, and the result was in accordance with a
previous study (6). In addition,
miR-10b (−) expression had no effect on the median disease-free
survival time of different breast cancer molecular subtypes (data
not shown). There were 54 patients who showed normal-like results
in the immunohistochemistry test and were therefore not categorized
into the four subtypes, hence they were not analyzed in this
study.
With advances in molecular biology, a molecular
subtype theory has been proposed to explain the heterogeneity of
breast cancer (34,35), whereby different molecular subtypes
of breast cancer may indicate different prognosis. For instance,
the luminal A and B subtypes have been associated with an improved
prognosis and lower rate of recurrence in comparison with subtypes
Basal-like and Her-2 overexpression (36,37). In
addition, the Her-2 overexpression subtype has been associated with
a poor prognosis, and patients that are Her-2-positive are suitable
for targeted therapy (38). The
basal-like subtype, which is characterized by ER-α (−), PR (−) and
Her-2 (−), is typically associated with the poorest prognosis
(12,10). The present study indicated that the
luminal A molecular subtype was also an independent prognostic
factor in addition to basal-like and Her-2 overexpression.
Furthermore, miR-10b (+) may be a risk factor for the prognosis of
breast cancer.
In conclusion, the present study demonstrated that
miR-10b expression was correlated with the expression of ER-α, PR
and Her-2, and the different breast cancer molecular subtypes.
Furthermore, the different molecular subtypes of breast cancer
indicated different prognoses. The present findings indicate that
the expression of miR-10b may indirectly affect the prognosis of
breast cancer.
Acknowledgements
The present work was supported by the Special
Program for the Key Laboratory of Major Diseases and Medical
Science of Xinjiang Autonomous Region, Co-constructing National Key
Labs by Province and Ministry of Science and Technology of China
(grant no. SKLI-XJMDR-ZX-2014-1).
Glossary
Abbreviations
Abbreviations:
|
ER-α
|
estrogen receptor-α
|
|
PR
|
progesterone receptor
|
|
Her-2
|
human epidermal growth factor
receptor-2
|
|
miRNA/miR
|
microRNA
|
|
ISH
|
in situ hybridization
|
|
FISH
|
fluorescence in situ
hybridization
|
|
DAB
|
3,3′-diaminobenzidine
|
|
OR
|
odds ratio
|
References
|
1
|
Simpson PT, Reis-Filho JS and Lakhani SR:
Breast pathology: Beyond morphology. Semin Diagn Pathol. 27:91–96.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bevers TB, Anderson BO, Bonaccio E, Buys
S, Daly MB, Dempsey PJ, Farrar WB, Fleming I, Garber JE, Harris RE,
et al: NCCN clinical practice guidelinesin oncology: Breast cancer
screening and diagnosis. J Natl Compr Cancer Netw. 7:1060–1096.
2009. View Article : Google Scholar
|
|
3
|
Sorlie T, Tibshirani R, Parker J, Hastie
T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et
al: Repeated observation of breast tumor subtypes in independent
gene expression data sets. Proc Natl Acad Sci USA. 100:pp.
8418–8423. 2003; View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goldhirsch A, Ingle JN, Gelber RD, Coates
AS, Thürlimann B and Senn HJ: Panel members: Thresholds for
therapies: Highlights of the St Gallen international expert
consensus on the primary therapy of early breast cancer 2009. Ann
Oncol. 20:1319–1329. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sørlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implicatios. Proc Natl
Acad Sci USA. 98:pp. 10869–10874. 2001; View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blenkiron C, Goldstein LD, Thorne NP,
Spiteri I, Chin SF, Dunning MJ, Barbosa-Morais NL, Teschendorff AE,
Green AR, Ellis IO, et al: MicroRNA expression profiling of human
breast cancer identifies new markers of tumor subtype. Genome Biol.
8:R2142007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang M, Mo J, Huang P, et al:
Clinicopathologic profiles of breast cancer in young women: A
report of 85 cases. Chinese Journal of General Surgery. 23:665–669.
2014.
|
|
9
|
Dent R, Hanna WM, Trudeau M, Rawlinson E,
Sun P and Narod SA: Pattern of metastatic spread in triple-negative
breast cancer. Breast Cancer Res Treat. 115:423–428. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Onitilo AA, Engel JM, Greenlee RT and
Mukesh BN: Breast cancer subtypes based on ER/PR and Her2
expression: Comparison of clinicopathologic features and survival.
Clin Med Res. 7:4–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Buzdar AU, Ibrahim NK, Francis D, Booser
DJ, Thomas ES, Theriault RL, Pusztai L, Green MC, Arun BK, Giordano
SH, et al: Significantly higher pathologic complete remission rate
after neoadjuvant therapy with trastuzumab, paclitaxel, and
epirubicin chemotherapy: Results of a randomized trial in human
epidermal growth factor receptor 2-positive operable breast cancer.
J Clin Oncol. 23:3676–3685. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lund MJ, Butler EN, Bumpers HL, Okoli J,
Rizzo M, Hatchett N, Green VL, Brawley OW, Oprea-Ilies GM and
Gabram SG: High prevalence of triple-negative tumors in an urban
cancer center. Cancer. 113:608–615. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carrio M, Arderiu G, Myers C and Boudreau
NJ: Homeobox D10 induces phenotypic reversion of breast tumor cells
in a three-dimensional culture model. Cancer Res. 65:7177–7185.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grøndahl-Hansen J, Christensen IJ, Briand
P, Pappot H, Mouridsen HT, Blichert-Toft M, Danø K and Brünner N:
Plasminogen activator inhibitor type 1 in cytosolic tumor extracts
predicts prognosis in low-risk breast cancer patients. Clin Cancer
Res. 3:233–239. 1997.PubMed/NCBI
|
|
15
|
Lowery AJ, Miller N, Devaney A, McNeill
RE, Davoren PA, Lemetre C, Benes V, Schmidt S, Blake J, Ball G and
Kerin MJ: MicroRNA signatures predict oestrogen receptor,
progesterone receptor and HER-2/neu receptor status in breast
cancer. Breast Cancer Res. 11:R272009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Z, Zhu J, Cao H, Ren H and Fang X:
miR-10b promotes cell invasion through RhoC-AKT signaling pathway
by targeting HOXD10 in gastric cancer. Int J Oncol. 40:1553–1560.
2012.PubMed/NCBI
|
|
17
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ujifuku K, Mitsutake N, Takakura S,
Matsuse M, Saenko V, Suzuki K, Hayashi K, Matsuo T, Kamada K,
Nagata I and Yamashita S: miR-195, miR-455-3p and miR-l0a(*) are
implicated in acquired temozolomide resistance in glioblastoma
multiforme cells. Cancer lett. 296:241–248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Biagioni F, Bossel Ben-Moshe N, Fontemaggi
G, Canu V, Mori F, Antoniani B, Di Benedetto A, Santoro R, Germoni
S, De Angelis F, et al: miR-10b*, a master inhibitor of the cell
cycle, is down-regulated in human breast tumours. EMBO Mol Med.
4:1214–1229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Wen J, Tang J, Lai M and Bu H:
Breast Cancer Pathology. Eighth. People's Medical Publishing House
(China); pp. 373–377. 2005
|
|
21
|
Wolff AC, Hammond ME, Schwartz JN, Hagerty
KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer
A, et al: American society of clinical oncology/college of American
pathologists guideline recommendations for human epidermal growth
factor receptor 2 testing in breast cancer. Arch Pathol Lab Med.
131:18–43. 2007.PubMed/NCBI
|
|
22
|
Prisack HB, Karreman C, Modlich O,
Audretsch W, Danae M, Rezai M and Bojar H: Predictive biological
markers for response of invasive breast cancer to
anthracycline/cyclophos-phamide-based Primary (radio-)chemotherapy.
Anticancer Res. 25:4615–4621. 2005.PubMed/NCBI
|
|
23
|
Yarden Y: Biology of HER2 and its
importance in breast cancer. Oncology. 61 Suppl:S1–S13. 2001.
View Article : Google Scholar
|
|
24
|
Stewart BW and Wild CP: World cancer
report 2014. Int Age Res Can. 28:17–27. 2014.
|
|
25
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yuan ZY, Wang SS, Zhu MQ, Zheng L, Luo WB,
Zhou ZM and Guan ZZ: Clinical characteristics and prognosis of
different subtypes of breast cancer. Zhonghua Zhong Liu Za Zhi.
30:456–461. 2008.(In Chinese). PubMed/NCBI
|
|
27
|
Prat A and Perou C: Deconstructing the
molecular portraits of breast cancer. Mol Oncol. 5:5–23. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheang MC, Chia SK, Voduc D, Gao D, Leung
S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, et al: Ki67
iddex, HER2 status, and prognosis of patients with luminal B breast
cancer. J Natl Cancer Inst. 101:736–750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang J and Wu JH: The clinical value of
endocrine therapy for breast cancer in elderly women. J Can Cont
Treat. 23:3852010.
|
|
30
|
Durbecq V, Ameye L, Veys I, Paesmans M,
Desmedt C, Sirtaine N, Sotiriou C, Bernard-Marty C, Nogaret JM,
Piccart M and Larsimont D: A significant proportion of elderly
patients develop hormone-dependant ‘luminal-B’ tumours associated
with aggressive characteristics. Crit Rev Oncol Hematol. 67:80–92.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Onitilo AA, Engel JM, Greenlee RT and
Mukesh BN: Breast cancer subtypes based on ER/PR and Her2
expression: Comparison of clinicopathologic features and survival.
Clin Med Res. 7:4–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jones PA and Baylin SB: The epigenomics of
cancer. Cell. 128:683–692. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gee HE, Camps C, Buffa FM, Colella S,
Sheldon H, Gleadle JM, Ragoussis J and Harris AL: MicroRNA-10b and
breast cancer metastasis. Nature. 455:E8–E9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Green S, Walter P, Kumar V, Krust A,
Bornert JM, Argos P and Chambon P: Human oestrogen receptor cDNA:
Sequence, expression and homology to v-erb-A. Nature. 320:134–139.
1986. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Durbecq V, Ameye L, Veys I, Paesmans M,
Desmedt C, Sirtaine N, Sotiriou C, Bernard-Marty C, Nogaret JM,
Piccart M and Larsimont D: A significant proportion of elderly
patients develop hormone-dependant ‘luminal-B’ tumours associated
with aggressive characterristics. Crit Rev Oncol Hematol. 67:80–92.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Winer EP, Hudis C, Burstein HJ, Wolff AC,
Pritchard KI, Ingle JN, Chlebowski RT, Gelber R, Edge SB, Gralow J,
et al: American Society of Clinical Oncology technology assessment
on the use of aromatase inhibitors as adjuvant therapy for
postmenopausal women with hormone receptor-positive breast cancer:
Status report 2004. J clin Oncol. 23:619–629. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bartlett JM, Ellis IO, Dowsett M, Mallon
EA, Cameron DA, Johnston S, Hall E, A'Hern R, Peckitt C, Bliss JM,
et al: Human epidermal growth factor receptor 2 status correlates
with lymph node involvement in patients with estrogen receptor (ER)
negative, but with grade in those with ER-positive early-stage
breast cancer suitable for cytotoxic chemotherapy. J Clin Oncol.
25:4423–4430. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang Q, Chen J, Li HJ, Yu M, Tian CX and
Lü Q: Clinical features and prognosis analysis of different breast
cancer molecular subtypes. Zhonghua Zhong Liu Za Zhi. 33:42–46.
2011.(In Chinese). PubMed/NCBI
|