Introduction
Sepsis is characterized by stimulation of a systemic
inflammatory response related to an infection (1), usually complicated with multiple organ
failure (MOF). Sepsis can lead to various complications. Acute
kidney injury (AKI), as a common type of MOF, is a frequent and
serious complication of sepsis in intensive care unit (ICU)
patients (2), whose morbidity and
mortality increase dramatically (3).
As sepsis-induced AKI is quite complicated, its
pathophysiology has not been completely explicit and not fully
understood. Emerging publications have shed light upon its
underlying mechanisms, including the following aspects: i) renal
macrocirculatory and microcirculatory disorder, ii) surge of
inflammatory cytokines and oxidative stress, iii) coagulation
cascade activation, and iv) bioenergetics adaptive response with
controlled cell-cycle arrest aiming to prevent cell death (4). So it is critical to follow the
pathogenesis of AKI in sepsis and then develop corresponding
therapeutic approaches to treat sepsis-induced AKI, which can
restore perfusion of the renal microcirculation, scavenge or
suppress inflammatory markers and oxidative stress.
Pterostilbene (Pte), a natural analog of
resveratrol, is a well-recognized antioxidant that primarily exists
in blueberries, grapevines and heartwood of red sandalwood
(5). And it has been suggested that
the in vivo biological activity of equimolar doses of Pte
may be greater than that of resveratrol (6), and the antioxidant activity of Pte are
superior to resveratrol due to its higher lipophilicity, which
increases its ability to be absorbed compared to resveratrol
(6). Studies have demonstrated that
resveratrol has salutary effect on sepsis-induced multiple organ
dysfunction, such as myocardial depression (7), AKI (8–10).
However, the effect of Pte on sepsis-induced AKI remains
unclear.
Therefore, the aim of this study is to explore the
potential protective role of Pte in the mouse model of
sepsis-induced AKI, and its potential underlying mechanisms.
Materials and methods
Reagents
Pte was purchased from Sigma-Aldrich (St. Louis, MO,
USA). Mouse serum creatinine (Scr) assay kit was purchased from
Crystal Chem Inc. (Downers Grove, IL, USA). Mouse blood urea
nitrogen (BUN) detection kit was purchased from Bio-Medical Assay
Co., Ltd (Beijing, China). Mouse tumor necrosis factor-alpha
(TNF-α) and Interleukin-1 beta (IL-1β) ELISA kits were purchased
from Thermo Fisher Scientific Inc. (Waltham, MA, USA). The rabbit
polyclonal antibody against B-cell lymphoma 2 (Bcl-2) and
Bcl-2-associated protein × (Bax) was purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). Ultrapure RNA kit was obtained
from Takara Biotechnology Co., Ltd (Dalian, China).
Animals
Male C57BL/6J mice aged 6–8 weeks were purchased
from the Laboratory Animal Center of Wenzhou Medical University.
The mice were kept at 25°C (humidity 50%) and 12 h light/12 h dark
cyclical alternates. This study was performed according to the
Guide for the Care and Use of Laboratory Animals, which is
published by the US National Institutes of Health (National
Institutes of Health Publication no. 85–23, revised 1996) and was
approved by the Ethics Committee of Wenzhou Military Medical
University.
Animal model of cecal ligation and
puncture (CLP)
Sepsis was induced by CLP as previously reported
(11). Briefly, mice were
anesthetized by 50 mg/kg pentobarbital sodium (i.p.) and a midline
incision (1–2 cm) was made to expose the internal organs. The cecum
was ligated and punctured with a 20-gauge needle in two places, and
a small amount of cecal contents was expressed through the puncture
wound to induce sepsis. The bowel was then sent back to the
abdomen, and the incision was sutured with a sterile 6-0 silk. The
mice in the sham-operated group underwent the same operation
without CLP.
Experimental groups and drug
treatment
Mice were randomly divided into the following three
groups: The Sham group, CLP + vehicle group, CLP + Pte 15 mg/kg
group. Pte was first dissolved in DMSO and then diluted in normal
saline and then Pte was administered intraperitoneally twice (0.5
and 4 h after CLP induction). The CLP + vehicle group was
administered the same volume of vehicle. 24 h after CLP induction,
all the animals were euthanized and peripheral blood and kidney
tissues were collected for further experiments.
Evaluation of survival rate
The 7-day survival rate was evaluated after CLP
induction. After the survival study, animals were euthanized upon
exhibition of certain symptoms/features i.e., discomfort/pain.
Histopathological examination
Kidney samples were collected 24 h after CLP
induction and fixed in 10% formalin solution. Then the samples were
embedded in paraffin and sectioned into 5-µm-thick sections.
Sections were stained with hematoxylin and eosin (H&E) and
assessed under light microscopy at magnification, ×200. Sections
were examined to evaluate the degree of pathological changes based
on glomerular epithelial hyperplasia, tubular dilatation and
protein cast formation, and inflammatory cell infiltrations. These
were scored from 0 to 3 (normal to severe, total score was 0 to 9).
The total score for each sample was evaluated.
Assessment of renal function
Renal functions were evaluated by detecting BUN and
Scr levels with commercially available kits according to the
manufactures' instructions.
Detection of inflammatory
cytokines
Kidney tissues from different groups were
homogenized with PBS on ice, and then centrifuged at 4°C for 40 min
at 12,000 rpm. Subsequently, the supernatants were collected to
perform ELISA. The levels of inflammatory cytokines, including
TNF-α and IL-1β in kidney tissue were detected by commercially
available ELISA kits according to the manufacturer's
recommendation.
RT-qPCR
Total RNA was extracted using Ultrapure RNA kit
according to the manufacturer's instructions. Total RNA (400 ng)
was then reverse transcribed. Amplification conditions were 95°C
(10 min) followed by 50 cycles of 95°C (30 sec) and 60°C (20 sec).
Primers were as follows: Bax forward, 5′-CAGGATGCGTCCACCAAGAA-3′
and reverse, 5′-AGTAGAAGAGGGCAACCACG-3′; Bcl-2 forward,
5′-GAGTACCTGAACCGGCATCT-3′ and reverse, 5′-GGTATGCACCCAGAGTGATG-3′;
and GAPDH forward, 5′-AAGAGGGATGCTGCCCTTAC-3′ and reverse
5′-TACGGCCAAATCCGTTCACA-3′. The amount of mRNA for each gene was
normalized by GAPDH, and the relative expression levels were
calculated using the 2−ΔΔCt method (12). Relative mRNA levels of each gene in
the Sham group samples were adjusted to 1. The mRNA levels in the
experimental groups were expressed as fold-changes as compared with
that of the Sham samples.
Western blot analysis
The kidney samples were collected and protein
extractions were obtained. Total proteins from renal lysates (50
µg) were separated by 10% sodium dodecyl sulfate-polyacrylamide
gels electrophoresis (SDS-PAGE), and transferred to polyvinylidene
fluoride (PVDF) membranes. The membranes were then blocked with 5%
non-fat milk for 2 h at room temperature, followed by incubating
with primary antibodies against Bcl-2 and Bax at 4°C overnight.
β-actin was used as the loading control. Membranes were developed
with ECL western blotting substrate (GE Healthcare,
Buckinghamshire, UK) and western blots were visualized and analyzed
on the Bio-Rad Image System (Bio-Rad, Berkeley, CA, USA).
Statistical Analysis
All analyses were performed using GraphPad Prism 6
(GraphPad Software, La Jolla, CA). Data are expressed as mean ±
standard error of the mean (SEM) and compared via one-way analysis
of variance (ANOVA) followed by Bonferroni multiple comparisons
test. Survival rate was determined by the Kaplan-Meier estimator
and compared by a log-rank test. P<0.05 was considered to
indicate statistically significant difference.
Results
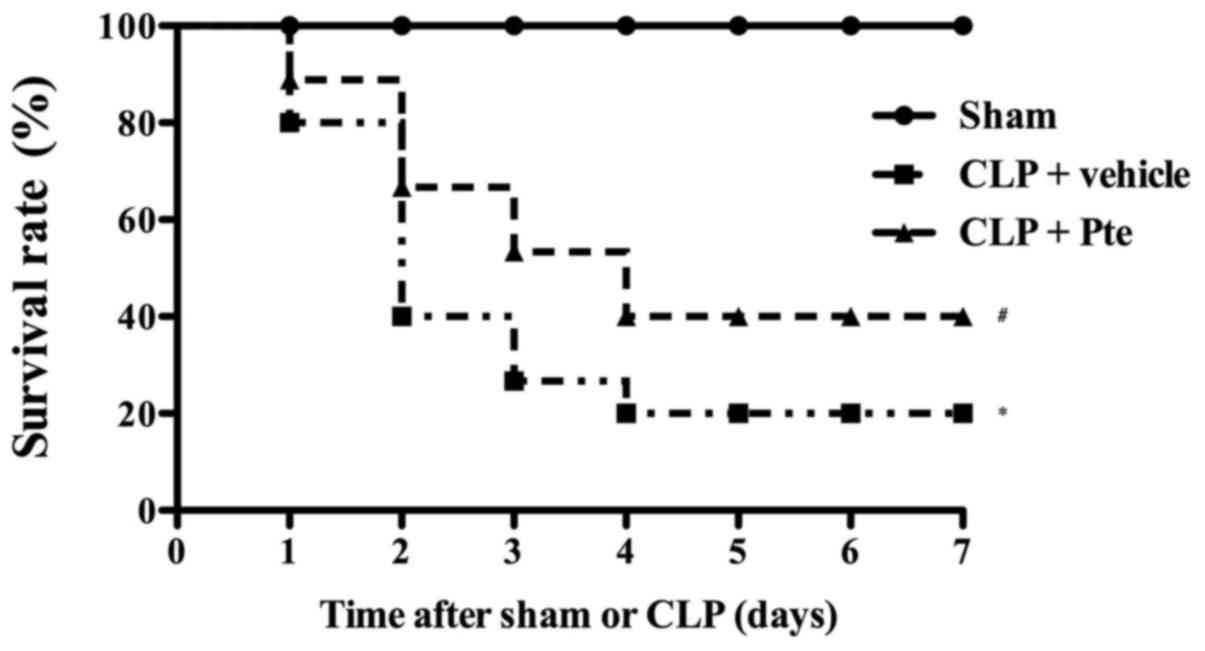
Pte treatment improves survival rate
after CLP induction
To investigate the protective effect of Pte against
lethality induced by CLP induction, we first evaluated the 7-day
survival rate in the three groups. As shown in Fig. 1, the 7-day survival rate in sham
group was almost 100%. However, 7 days after CLP surgery, the
survival rate decreased dramatically (vs. the sham group,
P<0.05). Pte administration the significantly increased survival
rate in the CLP + Pte group (vs. the CLP group, P<0.05).
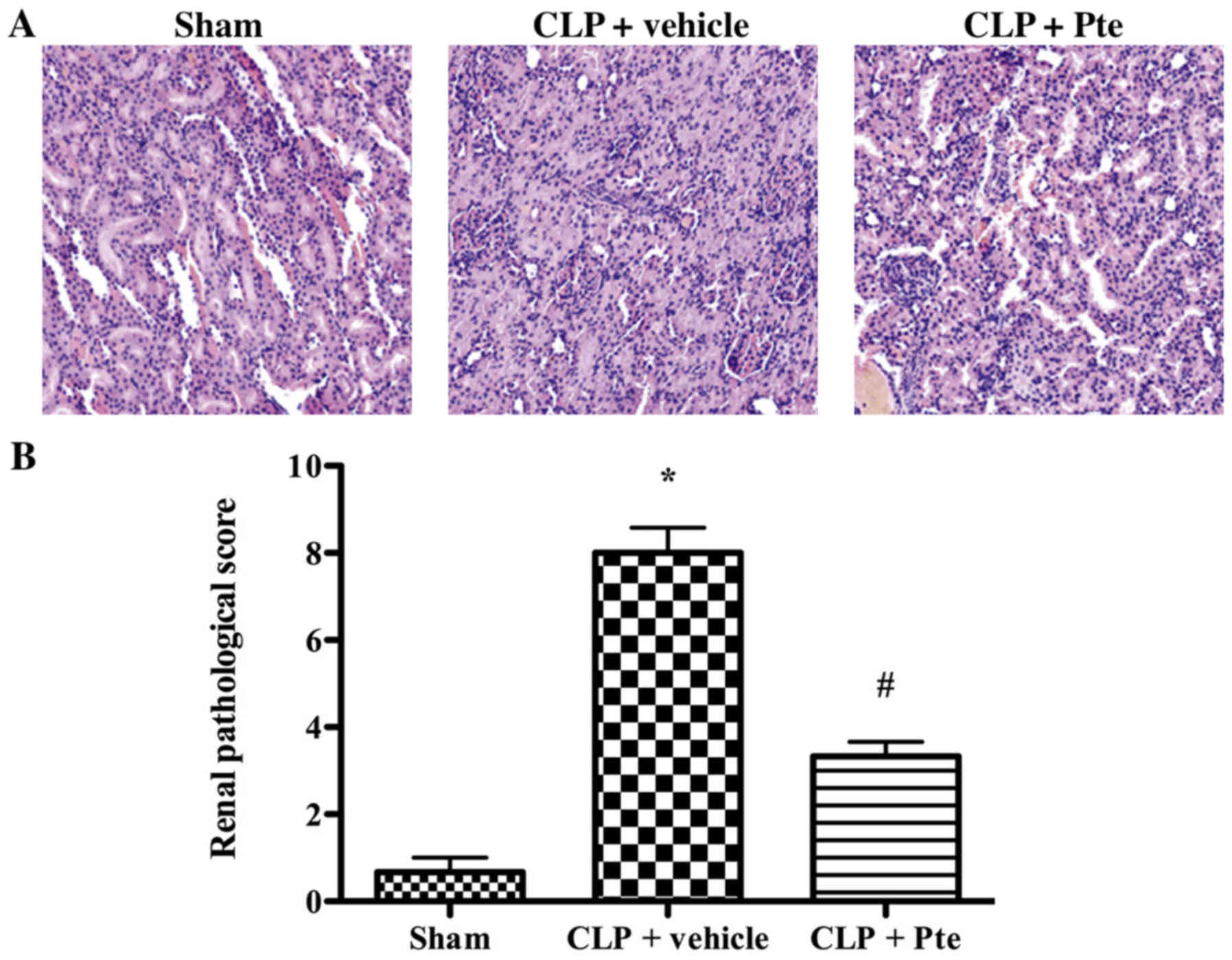
Pte attenuates the histopathological
injury of kidney in septic mice
Histopathological evaluation was performed using
H&E staining (Fig. 2). In the
Sham group, the renal tissue was intact. Histologic evaluation of
the kidney in the CLP + vehicle group indicated severe pathological
changes, including glomerular epithelial hyperplasia, tubular
dilatation, abundant protein exudation, and numerous inflammatory
cell infiltrations in the renal interstitium. Pte treatment
significantly attenuated the severity of pathological changes as
compared with the CLP + vehicle group.
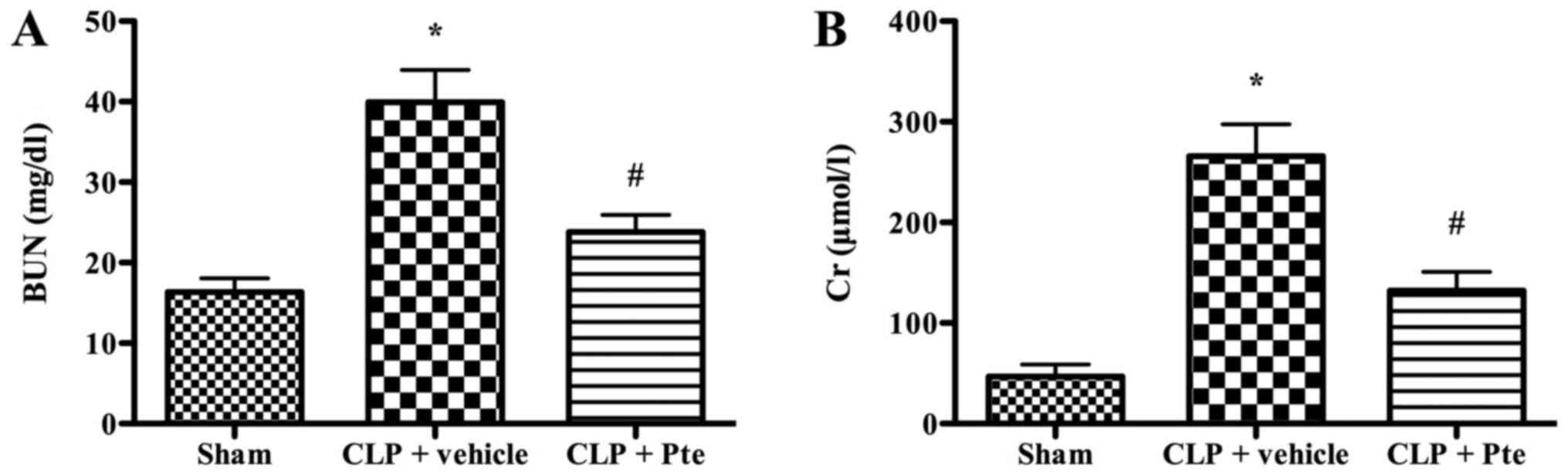
Pte reduces Scr and BUN levels after
CLP
Scr and BUN levels are regarded as critical
indicators of renal function. The levels of BUN and Scr were
dramatically elevated after CLP (Fig.
3). In Pte-treated mice, the levels of BUN and Scr reduced
dramatically compared to the CLP + vehicle group (P<0.05).
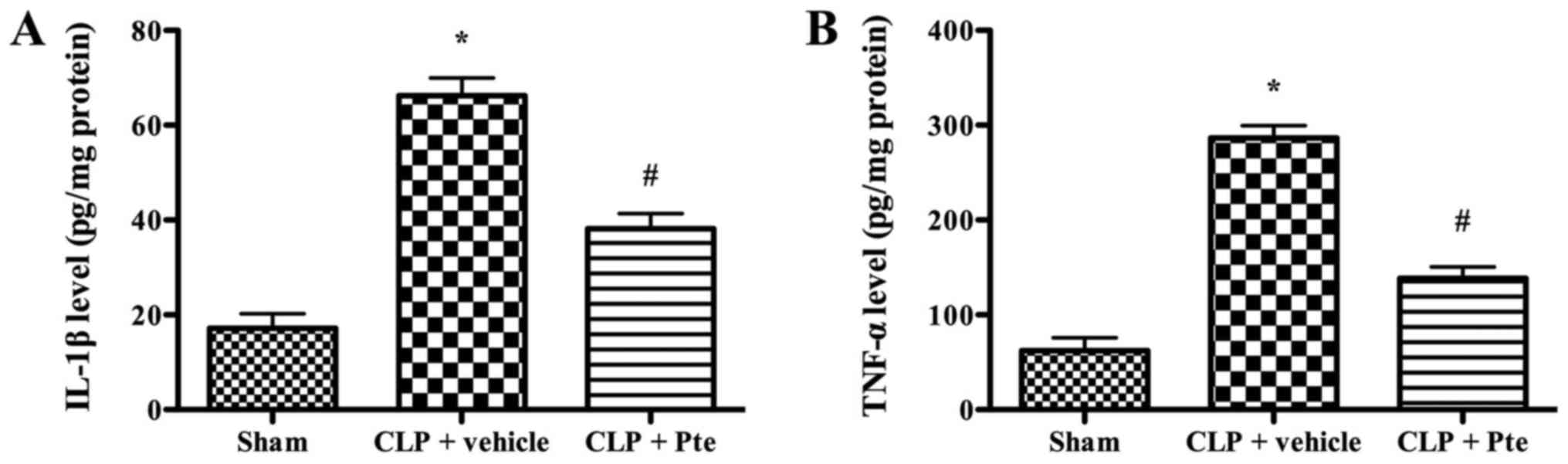
Pte suppresses inflammatory response
in vivo
To investigate the effect of Pte on inflammatory
response in vivo, representative inflammatory cytokines were
determined by ELISA. As shown in Fig.
4, the levels of TNF-α and IL-1β were higher in sepsis group
(P<0.05). Pte treatment significantly decreased the levels of
TNF-α and IL-1β (P<0.05).
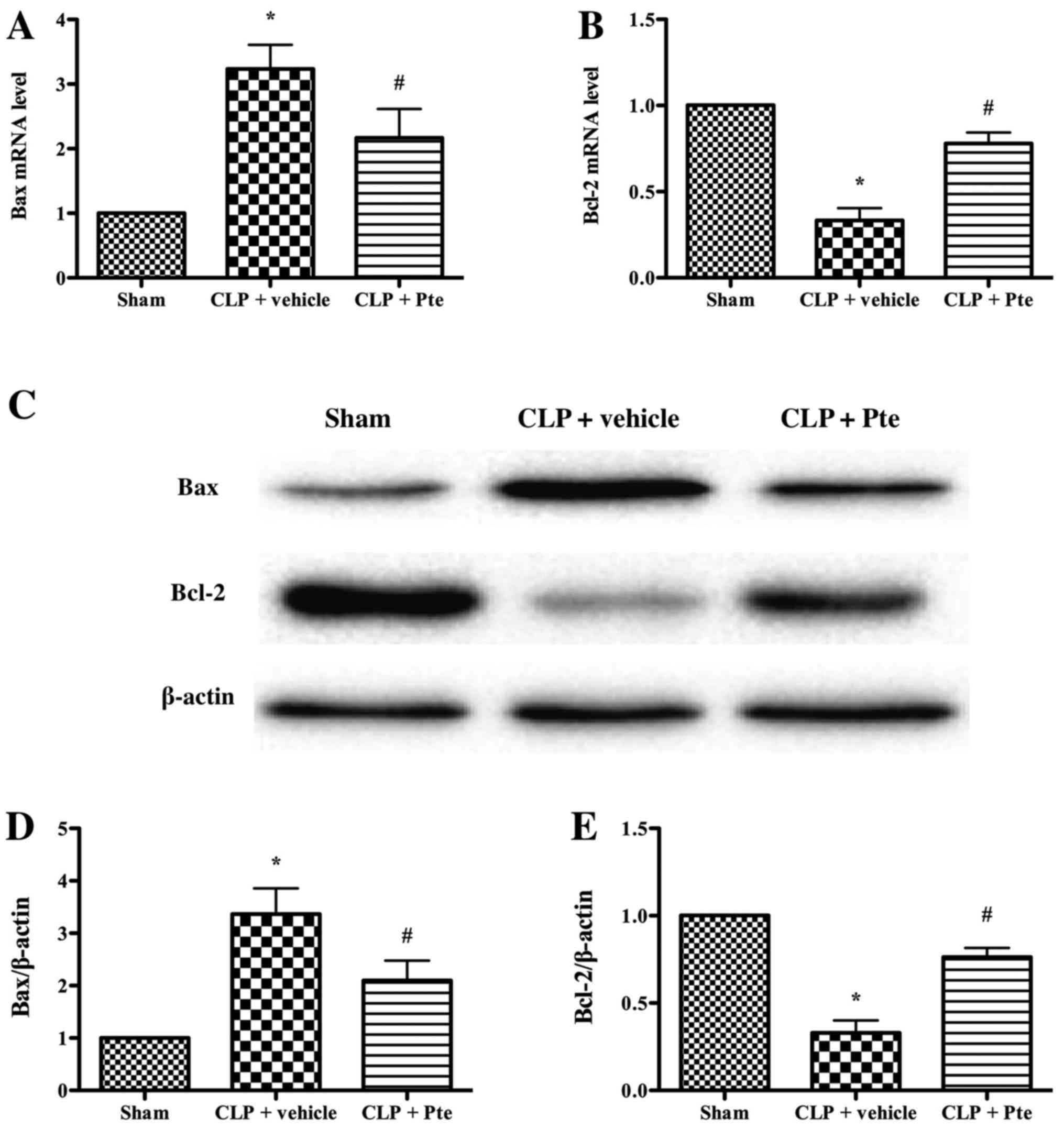
Effect of Pte on apoptosis-related
proteins expression after CLP
To further investigate the effects of Pte on
apoptosis-related proteins expression in the renal tissue of septic
mice, the expression of Bax and Bcl-2 in was analyzed using qRT-PCR
and western blot (Fig. 5). Our data
indicated that the expression of Bax mRNA and protein markedly
decreased in the Pte-treated group (P<0.05) compared with the
CLP + vehicle group. The expression of Bcl-2 mRNA and protein
significantly increased in the Pte-treated group (P<0.05). This
result suggested that Pte confers anti-apoptotic effect against AKI
induced by CLP.
Discussion
In the current study, we established a CLP-induced
sepsis model to evaluate the effects of Pte on sepsis-induced AKI.
CLP is the most widely used animal model of sepsis and it was
considered as a reliable and clinically relevant animal model of
the human sepsis (13). The major
findings in the present study are: (1) Pte treatment improves the survival rate
in mice subjected to CLP surgery. (2) Pte treatment attenuates kidney injury in
septic mice, as evidenced by the lowered pathological changes and
decreased BUN and Scr levels. (3)
Pte treatment suppresses inflammatory response and apoptosis in
septic mice.
We first evaluate whether Pte treatment can improve
the survival rate in septic mice. Our results suggest that Pte
treatment significantly improves 7-day survival rate after CLP
induction, indicating the protective effects of Pte against sepsis.
Then, we evaluate the effect of Pte on the pathological changes of
kidney. Sepsis results in obvious inflammatory cells infiltration
in renal interstitium, glomeruli and nephric tubules. This injury
can be attenuated dramatically by Pte treatment. The BUN and Scr
levels increase significantly in mice subjected to CLP, indicating
the severe injury of the kidney. Pte treatment alleviates kidney
injury as evidenced by the decreased levels of BUN and Scr
levels.
Sepsis-induced AKI accounts for nearly 50% of cases
of AKI in the ICU (14). Moreover,
AKI is the leading cause of death in the ICU (15). Although previous studies have
suggested that hypotension, renal vasoconstriction and
ischemia-reperfusion injury as the primary pathophysiological
mechanisms involved in sepsis-induced AKI, recent studies have
focused on other pathophysiological mechanisms, including
microcirculatory flow abnormalities, inflammation, cell
bioenergetic adaptive responses to injury, microparticles, which
are involved in septic AKI (16).
Inflammation triggered by sepsis leads to the activation of
leukocytes and release of pro-inflammatory cytokines.
Pro-inflammatory cytokines, especially IL-1β and TNF-α, damage the
main function of vascular endothelial cells, which plays a critical
role in maintaining the fluidity of blood. In addition, IL-1β and
TNF-α enhance clot formation by impairing the anticoagulant pathway
via the inhibition of the production of activated protein C
(17). In the current study, our
results suggest that Pte treatment dramatically decrease the levels
of IL-1β and TNF-α, indicating the anti-infalmmatory effect of Pte
in AKI induced by sepsis. It has been reported that Pte exerts
protection against sepsis-induced liver injury (18). Liu et al (18) suggested that Pte decrease the
infiltration of neutrophil into the liver. So, neutrophil may be,
at least, one of the target cells of Pte.
Finally, we evaluate the effect of Pte on renal
apotosis induced by sepsis. Apoptosis is a programmed cell death,
and it is involved in sepsis induced kidney injury (19). Apoptosis is regulated by the
anti-apoptotic family and the pro-apoptotic family. Bcl-2, known as
a key protein in the anti-apoptotic family, is localized in the
outer membrane of mitochondria, and it plays an important role in
promoting cellular survival and inhibiting the actions of
pro-apoptotic proteins (20). It has
been suggested that Bcl-2 can inhibit the release of cytochrome c,
which can trigger apoptosis, from the mitochondria (21). On the contrary, Bax is a key protein
in the pro-apoptotic family. Bax can promote the permeabilization
and release of cytochrome c and ROS, resulting the activation of
the apoptosis cascade (22). In the
current study, our data indicates that CLP induces a dramatic
increase in Bax expression and a decrease in Bcl-2 expression in
the kidney, which were alleviated by Pte treatment.
In summary, Pte exerts protective effects via
suppressing inflammation and apoptosis in sepsis-induced AKI in
mice. The current study suggests that administration of Pte may be
a potential management for the treatment of sepsis. However, the
precise mechanisms still need to be further elucidated before its
clinical application.
References
|
1
|
van der Poll T and Opal SM: Host-pathogen
interactions in sepsis. Lancet Infect Dis. 8:32–43. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bagshaw SM, George C and Bellomo R; ANZICS
Database Management Committee, : Early acute kidney injury and
sepsis: A multicentre evaluation. Crit Care. 12:R472008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kissoon N, Daniels R, van der Poll T,
Finfer S and Reinhart K: Sepsis-the final common pathway to death
from multiple organ failure in infection. Crit Care Med.
44:e4462016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shum HP, Yan WW and Chan TM: Recent
knowledge on the pathophysiology of septic acute kidney injury: A
narrative review. J Crit Care. 31:82–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McCormack D and McFadden D: A review of
pterostilbene antioxidant activity and disease modification. Oxid
Med Cell Longev. 2013:5754822013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kapetanovic IM, Muzzio M, Huang Z,
Thompson TN and McCormick DL: Pharmacokinetics, oral
bioavailability, and metabolic profile of resveratrol and its
dimethylether analog, pterostilbene, in rats. Cancer Chemother
Pharmacol. 68:593–601. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Smeding L, Leong-Poi H, Hu P, Shan Y,
Haitsma JJ, Horvath E, Furmli S, Masoom H, Kuiper JW, Slutsky AS,
et al: Salutary effect of resveratrol on sepsis-induced myocardial
depression. Crit Care Med. 40:1896–1907. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Holthoff JH, Wang Z, Seely KA, Gokden N
and Mayeux PR: Resveratrol improves renal microcirculation,
protects the tubular epithelium, and prolongs survival in a mouse
model of sepsis-induced acute kidney injury. Kidney Int.
81:370–378. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sebai H, Ben-Attia M, Sani M, Aouani E and
Ghanem-Boughanmi N: Protective effect of resveratrol in
endotoxemia-induced acute phase response in rats. Arch Toxicol.
83:335–340. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen L, Yang S, Zumbrun EE, Guan H,
Nagarkatti PS and Nagarkatti M: Resveratrol attenuates
lipopolysaccharide-induced acute kidney injury by suppressing
inflammation driven by macrophages. Mol Nutr Food Res. 59:853–864.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen LW, Chen W, Hu ZQ, Bian JL, Ying L,
Hong GL, Qiu QM, Zhao GJ and Lu ZQ: Protective effects of growth
arrest-specific protein 6 (Gas6) on sepsis-induced acute kidney
injury. Inflammation. 39:575–582. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Doi K, Leelahavanichkul A, Yuen PS and
Star RA: Animal models of sepsis and sepsis-induced kidney injury.
J Clin Invest. 119:2868–2878. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Umbro I, Gentile G, Tinti F, Muiesan P and
Mitterhofer AP: Recent advances in pathophysiology and biomarkers
of sepsis-induced acute kidney injury. J Infect. 72:131–142. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hutchins NA, Unsinger J, Hotchkiss RS and
Ayala A: The new normal: Immunomodulatory agents against sepsis
immune suppression. Trends Mol Med. 20:224–233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pettilä V and Bellomo R: Understanding
acute kidney injury in sepsis. Intensive Care Med. 40:1018–1020.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weiler H and Ruf W: Activated protein C in
sepsis: The promise of nonanticoagulant activated protein C. Curr
Opin Hematol. 15:487–493. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu X, Yang X, Han L, Ye F, Liu M, Fan W,
Zhang K, Kong Y, Zhang J, Shi L, et al: Pterostilbene alleviates
polymicrobial sepsis-induced liver injury: Possible role of SIRT1
signaling. Int Immunopharmacol. 49:50–59. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gómez H and Kellum JA: Sepsis-induced
acute kidney injury. Curr Opin Crit Care. 22:546–553. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu H, Zhao L, Yue L, Wang B, Li X, Guo H,
Ma Y, Yao C, Gao L, Deng J, et al: Pterostilbene attenuates early
brain injury following subarachnoid hemorrhage via inhibition of
the NLRP3 inflammasome and Nox2-related oxidative stress. Mol
Neurobiol. Sep 24–2016.(Epub ahead of print).
|
|
21
|
Jemmerson R, Dubinsky JM and Brustovetsky
N: Cytochrome C release from CNS mitochondria and potential for
clinical intervention in apoptosis-mediated CNS diseases. Antioxid
Redox Signal. 7:1158–1172. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luna-Vargas MP and Chipuk JE:
Physiological and pharmacological control of BAK, BAX, and beyond.
Trends Cell Biol. 26:906–917. 2016. View Article : Google Scholar : PubMed/NCBI
|