Introduction
Liver cirrhosis is a kind of metabolic disease that
is reversible and may be treated when it is identified at an early
stage (1). Liver cirrhosis is
divided into hepatitis B cirrhosis and hepatitis C cirrhosis
according to pathogenesis in clinical research (2,3).
Previous reports have indicated risks for alcoholic liver
cirrhosis, and regression of fibrosis/cirrhosis by glycine
propionyl-l-carnitine treatment has been also investigated in
D-Galactosamine-induced chronic liver damage (4,5). The
main pathogenesis of liver cirrhosis is progressive fibrosis
(6). A comprehensive review has
evaluated the management of patients with autoimmune hepatitis with
decompensated cirrhosis (7).
Furthermore, a study by Wang et al (7) suggested that chronic hepatitis B and
hepatitis B virus-related cirrhosis contributes to other metabolic
syndromes, which further influences renal function and increases
the risk of renal damage, hypophosphatemia, and adrenal
insufficiency (8–10).
Fibroblast growth factor (FGF)-21 is an atypical
member of the FGF family, as well as a multifunctional protein
predominantly secreted by adipose tissue, the pancreas and liver,
which has been regarded as an efficient polypeptide for the
treatment of metabolic disorders (11,12).
Previous research has reported that metabolic hormone effects of
FGF-21 on energy metabolism were essential for human vascular
endothelial cells (13,14). A study by Wang et al (15) indicated that FGF-21 is positively
associated with atrial fibrosis in patients with atrial
fibrillation with rheumatic heart disease. FGF-21 has been reported
as a novel liver safeguard (16), as
well as being identified as a momentous controller and regulator of
glucose and lipid metabolism, and long-term energy balance
(17,18). Notably, transplantation of basic
FGF-pretreated adipose tissue-derived stromal cells enhances
regression of liver fibrosis in mice (19). However, the molecular mechanisms of
liver fibrosis associated with FGF-21 are not well understood or
clearly elaborated.
Chronic inflammation associated with hepatitis C
virus infection contributes to hepatic transforming growth factor
(TGF)-β signaling that promotes cirrhosis and hepatocellular
carcinoma (20). Research has also
indicated protective effects of allopurinol against acute liver
damage and cirrhosis induced by carbon tetrachloride through
modulation of nuclear factor (NF)-κB, cytokine production and
oxidative stress (21). The present
study analyzed the potential diagnostic value of human (h)FGF-21
and investigated the hFGF-21-mediated signaling pathway of
hepatitis B cirrhosis combined with adrenal insufficiency in liver
cells. The present data indicated that plasma concentration levels
of hFGF-21 were downregulated in patients with hepatitis B
cirrhosis combined with adrenal insufficiency (PhbA), which may be
associated with the NF-κB-mediated TGF-β signaling pathway.
Patients and methods
Patients and healthy volunteers
A total of 186 PhbA (90 male and 96 female) and 68
healthy volunteers (35 male and 33 female) were recruited in the
present clinical investigation following presentation to Beijing
You'an Hospital, Capital Medical University (Beijing, China)
between May 2014 and October 2015. The mean age was 38.5 (16.4–62.5
years) and 34.2 (22.5–46.2 years) in PhbA and healthy volunteers,
respectively. A total of 10 patients [male/female, 5/5; 34.2 years
old (22.5–46.2)] who had recovered from hepatitis B cirrhosis
combined with adrenal insufficiency (PPhbA) were also recruited to
the present study. Patients with diabetes mellitus and digestive
tract diseases were excluded from the present study. Patients were
diagnosed with PhbA as described previously (22). All participants were required to
provide written informed consent prior to initiation of the study.
The present study was approved by the Ethics Committee of Beijing
You'an Hospital, Capital Medical University (Beijing, China).
Cell culture
Liver and renal epithelial cells were obtained from
PhbA using a biopsy needle as previously described (23). Cells were cultured in minimal
essential medium supplemented with 10% fetal bovine serum
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Liver and renal
epithelial cells were incubated with hFGF-21 (1.0 mg/ml,
Sigma-Aldrich; Merck KGaA) for 24 h to analyze purpose protein
expression with non-treated cells used as controls. The cells were
cultured in a humidified atmosphere containing 5% of CO2
at 37°C.
ELISA
Serum levels of hFGF-21 (cat. no. DF2100), tumor
necrosis factor (TNF)-α (cat. no. DTA00C), interleukin (IL)-1β
(cat. no. DLB50), IL-6 (cat. no. D6050) and IL-8 (cat. no. D8000C)
were detected in PhbA and healthy volunteers using ELISA kits (IBL
International GmbH, Hamburg, Germany), according to the
manufacturer's protocol. The serum levels of hFGF-21 were also
analyzed between PhbA and PPhbA on day 30 following treatments. The
serum concentration levels of hFGF-21, TNF-α, IL-1β, IL-6 and IL-8
were measured by an enzyme microplate reader at 450 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from liver and renal
epithelial cells using an RNeasy Mini kit (Qiagen Sciences, Inc.,
Gaithersburg, MD, USA), according to the protocol provided by the
manufacturer. RNA was reversed transcribed using a PrimeScript RT
Master Mix kit (Takara Bio, Inc., Otsu, Japan). All forward and
reverse primers were synthesized by Invitrogen (Thermo Fisher
Scientific, Inc., Waltham, MA, USA; Table I). For amplification diluted cDNA was
combined with a reaction mixture containing SYBR-Green PCR Core
Reagents (cat. no. 4304886; Applied Biosystems; Thermo Fisher
Scientific, Inc.). Relative mRNA expression levels were calculated
using the 2−ΔΔCq method (24). PCR cycling was performed under the
following conditions: 94°C for 30 sec and 45 cycles of 95°C for 5
sec, 57°C for 10 sec and 72°C for 10 sec. The results were
expressed as the n-fold of the control.
 | Table I.Primer sequences used in the study
for polymerase chain reaction. |
Table I.
Primer sequences used in the study
for polymerase chain reaction.
|
| Sequences
(5′-3′) |
|---|
|
|
|
|---|
| Gene name | Reverse | Forward |
|---|
| FGF-21 |
CTGCTGGGGGTCTACCAAG |
CTGCGCCTACCACTGTTCC |
| TNF-α |
TCCAGACTTCCTTGAGACA |
GGCGATTACAGACACAACT |
| IL-6 |
CCACACAGACAGCCACTCA |
CATCCATCTTTTTCAGCCATCT |
| IL-1β |
GGCTGCTTCCAAACCTTTGA |
GAAGACACGGATTCCATGGT |
| IL-8 |
TACTCCAAACCTTTCCACCC |
AACTTCTCCACAACCCTCTG |
| β-actin |
CGGAGTCAACGGATTTGGTC |
AGCCTTCTCCATGGTCGTGA |
Western blot analysis
Liver and renal epithelial cells from PhbA were
incubated with hFGF-21 (2 mg/ml) for 12 h at 37°C. Cells not
treated with hFGF-21 were used as the controls. Cells were
homogenized in a lysate buffer containing protease-inhibitor
(P3480; Sigma-Aldrich; Merck KGaA) and were centrifuged at 4,000 ×
g at 4°C for 10 min. Western blot analysis was subsequently
performed as previously described (25). Protein concentration was measured by
a BCA protein assay kit (Thermo Fisher Scientific, Inc.). Protein
samples (20 µg/lane) were resolved by 15% SDS-PAGE and then
transferred to polyvinylidene fluoride membranes (Merck KGaA).
Monoclonal rabbit anti-human epidermal growth factor (EGF),
platelet-derived growth factor (PDGF; ab32570), hFGF-21 (ab64857),
TNF-α (ab6671), IL-1β (ab2105), IL-6 (ab6672) and IL-8 (ab7747),
TGF-β (ab31013), NF-κB (ab32360) and Kruppel-like factor 6 (KLF6;
ab135783), extracellular matrix (ECM; ab28666), reactive oxygen
species (ROS; ab5512) and β-actin (ab8227) antibodies (all 1:200;
Abcam, Shanghai, China) were incubated with protein samples for 1 h
at room temperature. After blocking with 1% bovine serum albumin
(Sigma-Aldrich; Merck KGaA), followed by incubation with
horseradish peroxidase-conjugated polyclonal anti-rabbit
immunoglobulin G antibodies (1:10,000; PV-6001; OriGene
Technologies, Inc., Beijing, China) for 1 h at room temperature.
Signals were visualized by chemiluminescence detection (Z370398;
Sigma-Aldrich; Merck KGaA). Densitometric quantification of the
immunoblot data was performed using Quantity-One software (version
3.24; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Gene knockdown with small interfering
RNA (siRNA)
Liver cells (1×104/well) were incubated
with hFGF-21 (2 mg/ml) for 5 days at 37°C in a six-well plate. To
silence NF-κB gene expression, liver cells were transfected with
100 pmol siRNA-NF-κB sense, 5-′CUUGGUCAAUCUCAAGAUAtt-3′ and
antisense, 5′-UAUCUUGAGAUUGACCAAGca-3′; with siRNA-vector sense,
5′-CGGACAAACGGCUCACUUUtt-3′ and antisense,
5′-AAAGUGAGCCGUUUGUCCGgg-3′ as a control (Applied Biosystems;
Thermo Fisher Scientific, Inc.) using a Cell Line Nucleofector kit
L (Lonza Group, Ltd., Basel, Switzerland), according to the
manufacturer's protocol (26). The
cells were analyzed 48 h following transfection.
Flow cytometry
The following antibodies were used: FITC-conjugated
anti-CD11b (cat. no. 557686; clone M1/70; BD Biosciences, Franklin
Lakes, NJ, USA), allophycocyanin-conjugated anti-Ly-6B.2 (cat. no.
NBP2-13077APC; clone 7/4; Bio-Rad Laboratories, Inc.),
FITC-conjugated anti-CD4 (cat. no. MCA2649; clone RM 4–5;
eBioscience; Thermo Fisher Scientific, Inc.), PercP-conjugated
anti-CD8a (cat. no. 555369; clone, 53–6.7; BD Biosciences),
PE-conjugated anti-CD45R/B220 (cat. no. A15835; clone RA3-6B2;
eBioscience; Thermo Fisher Scientific, Inc.) for 12 h at 4°C after
blocking with 1% bovine serum albumin for 2 h at 37°C. All
antibodies were used at a dilution of 1:100. B-lymphocytes were
identified as CD11bhiLy6G-7/4hi/lo and macrophagocytes were
identified as CD11chi. Cells were washed three times with 0.1%
tris-buffered saline-Tween-20. Cells were analyzed using a flow
cytometer (LSR II; BD Biosciences). Data was analyzed using BD
FACSDiva™ software version 8.0.1 (BD Biosciences).
Statistical analysis
All data were presented as the mean ± standard
deviation of triplicate independent trials in each experiment. All
data were analyzed using SPSS Statistics 19.0 (IBM Corp., Armonk,
NY, USA). Statistical differences between groups were assessed
using analysis of variance with the post hoc Dunnett's test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Analysis of expression levels of
hFGF-21 in PhbA
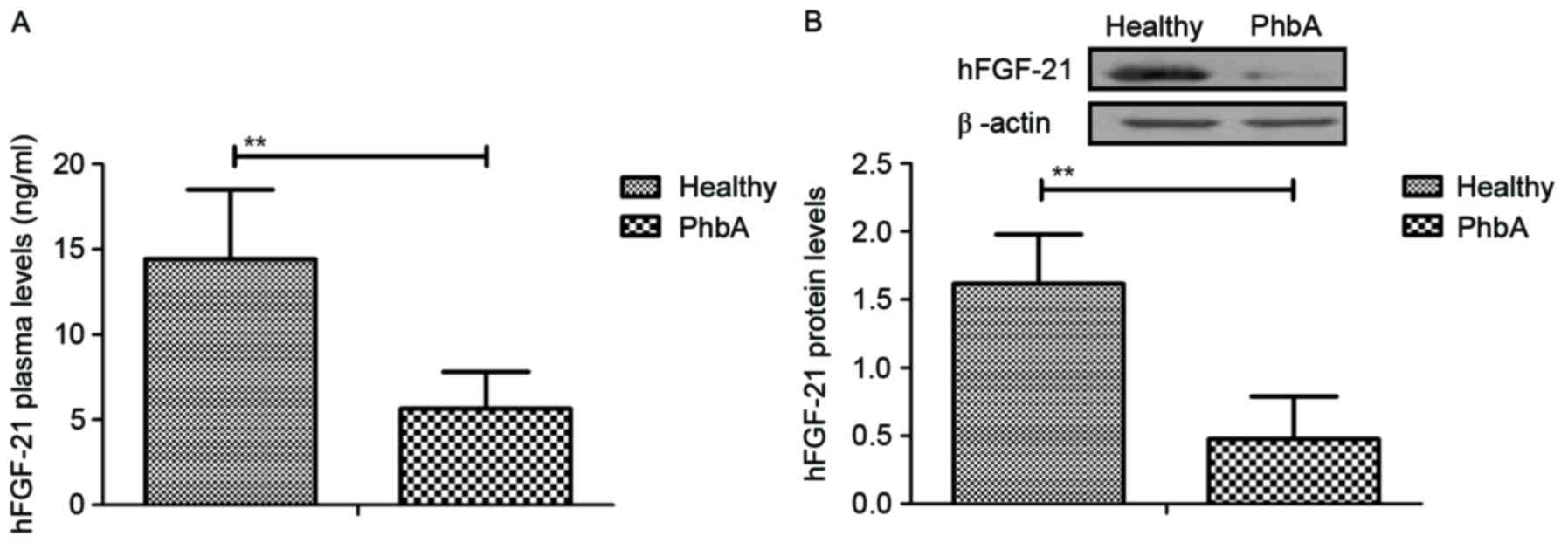
Expression levels of hFGF-21 were analyzed in serum
and cellular units in PhbA. Characteristics of patients were
summarized in Table II. Plasma
concentration levels of hFGF-21 were significantly downregulated in
PhbA compared with the those in healthy volunteers (P<0.01)
(Fig. 1A). Western blotting
demonstrated that hFGF-21 protein expression levels were
significantly downregulated in liver cells isolated from PhbA
compared to those from healthy volunteers (P<0.01) (Fig. 1B). These outcomes suggested that
hFGF-21 is downregulated in PhbA. Healthy.
 | Table II.Characteristics of patients and
healthy volunteers. |
Table II.
Characteristics of patients and
healthy volunteers.
|
Characteristics | Patients | Healthy
volunteers |
|---|
| Number | 186 | 68 |
| Age, years
(range) | 16.4–62.5 | 22.5–46.2 |
| Sex, n |
|
|
|
Male | 70 | 30 |
|
Female | 116 | 38 |
Analysis of expression levels of
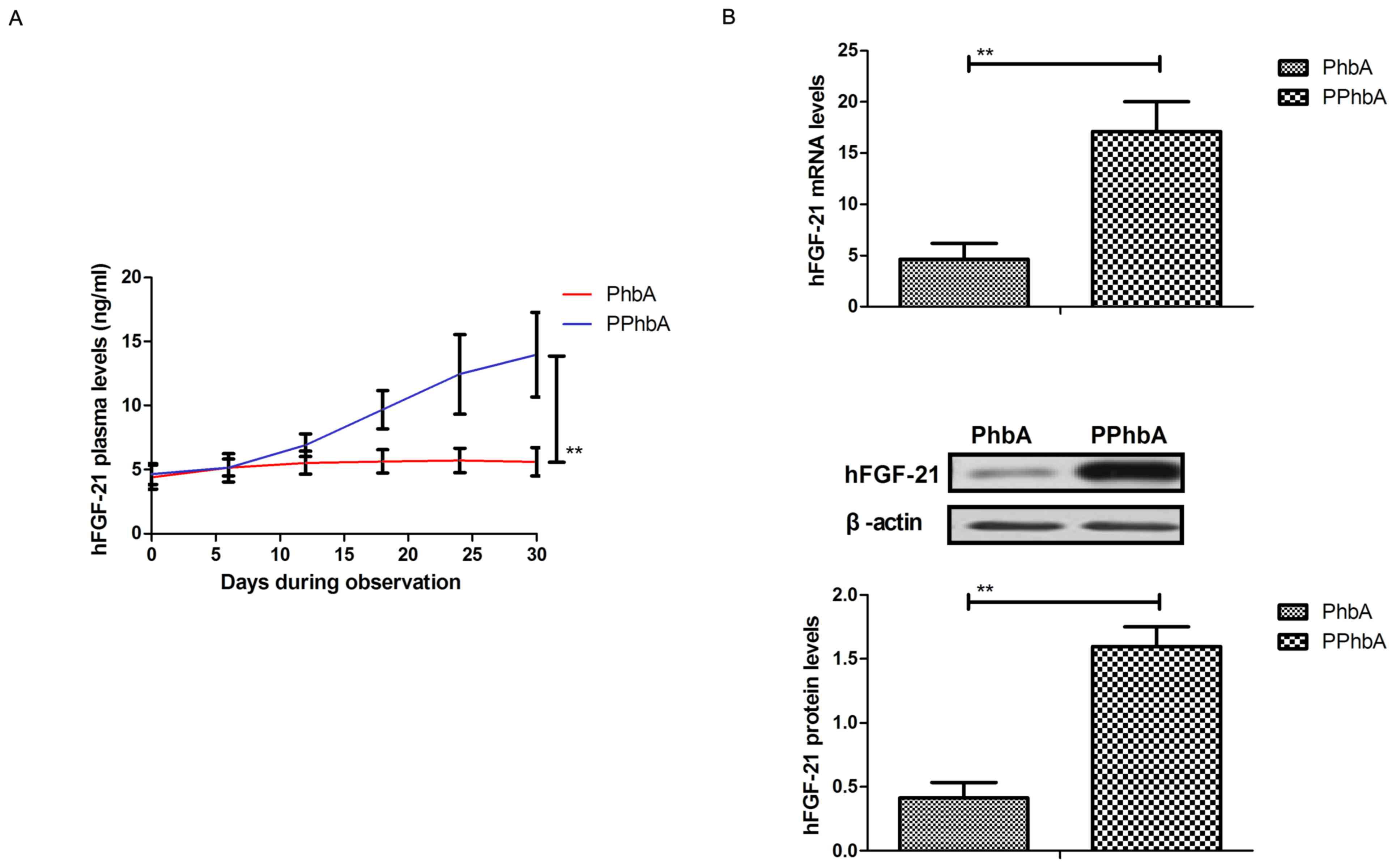
hFGF-21 in PhbA and PPhbA
Expression levels of hFGF-21 were detected in PPhbA.
As demonstrated in Fig. 2A, plasma
concentration levels of hFGF-21 were significantly increased in
PPhbA compared with those in PhbA on day 30 (P<0.01). Cellular
hFGF-21 mRNA and protein expression levels in liver cells isolated
from PPhbA were significantly upregulated compared with those
isolated from PhbA (P<0.01) (Fig.
2B). These results indicated that hFGF-21 may be a prognostic
indicator in PhbA.
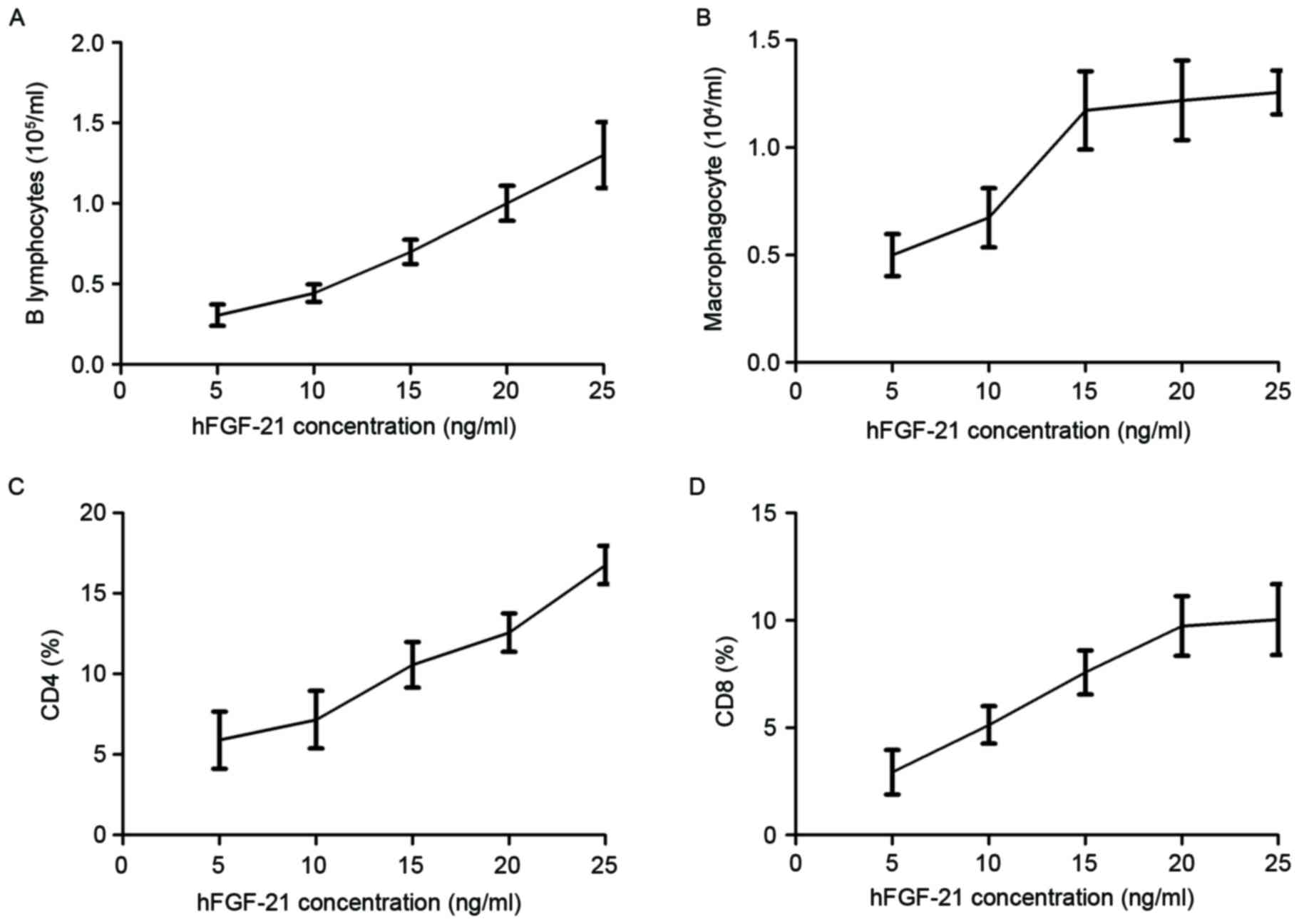
Association of hFGF-21 plasma
concentration with cellular immunity and humoral immunity in
PhbA
Characteristics of cellular immunity and humoral
immunity were investigated in clinical PhbA prior and post
treatments. Results demonstrated that the B lymphocyte level
increased as the hFGF-21 plasma concentration increased during
treatment (Fig. 3A). Macrophagocyte
concentration levels were demonstrated to be positively associated
with hFGF-21 plasma concentration during treatment (Fig. 3B). Results indicated that the
percentage of cluster of differentiation (CD)4+ and
CD8+ cells increased in serum as the hFGF-21 plasma
concentration increased during treatment (Fig. 3C and D). These results indicated that
hFGF-21 plasma concentration may be associated with cellular
immunity and humoral immunity in PhbA during treatment.
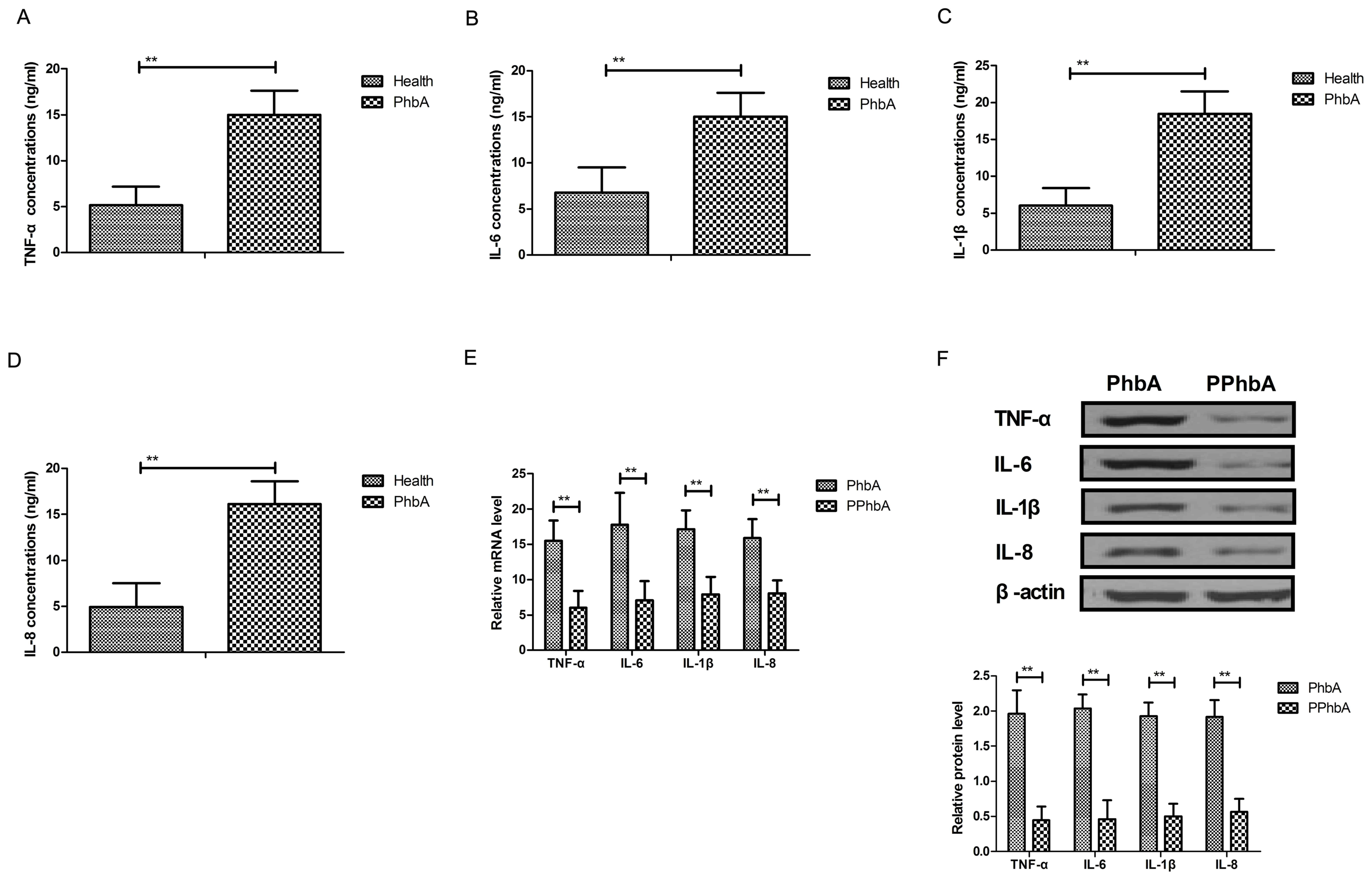
Effects of hFGF-21 on inflammatory
cytokine expression levels in liver cells isolated from clinical
patients
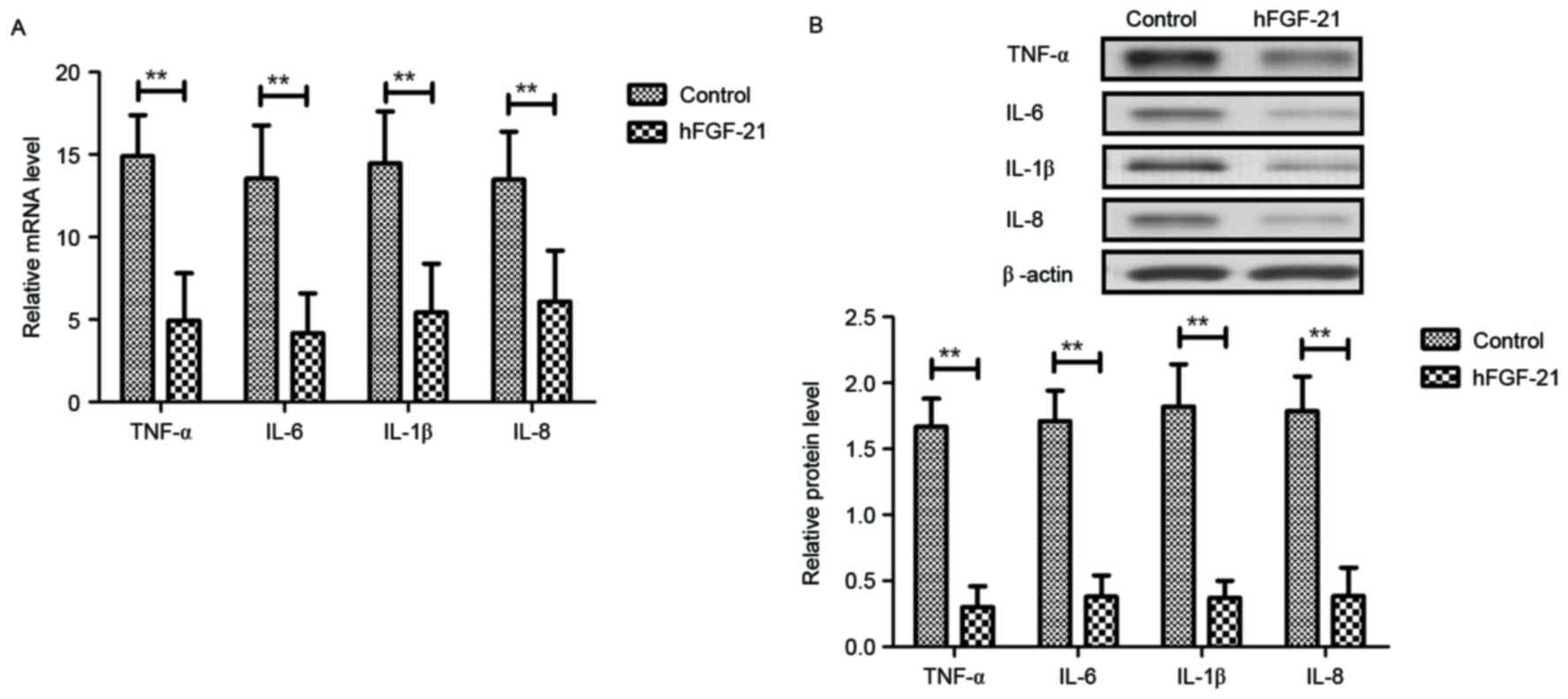
Inflammatory cytokine levels were investigated in
PhbA. As demonstrated in Fig. 4A-D,
plasma concentration levels of TNF-α, IL-6, IL-1β and IL-8 were
significantly upregulated in PhbA compared with those in healthy
volunteers (P<0.01). Western blot analysis and RT-qPCR indicated
that protein and mRNA expression levels of TNF-α, IL-6, IL-1β and
IL-8 were significantly downregulated in the PPhbA groups compared
with PhbA groups (P<0.01) (Fig. 4E
and F). These results indicated that hFGF-21 treatment
decreases inflammatory cytokine expression levels in liver cells
isolated from clinical patients.
Effects of hFGF-21 on inflammatory
cytokine expression levels in renal epithelial cells isolated from
clinical patients
Inflammatory cytokines in renal epithelial cells
isolated from clinical patients were analyzed following treatment
with hFGF-21. As demonstrated in Fig. 5A
and B, gene and protein expression levels of TNF-α, IL-6, IL-1β
and IL-8 were significantly downregulated by hFGF-21 treatment in
renal epithelial cells isolated from clinical patients compared to
the levels in the control cells (P<0.01). These outcomes
indicated that hFGF-21 suppresses inflammatory cytokine expression
in renal epithelial cells isolated from clinical patients.
hFGF-21 regulates inflammatory
cytokines through downregulation of the NF-κB-mediated TGF-β
signaling pathway
In order to analyze the potential mechanism mediated
by hFGF-21, the NF-κB-mediated TGF-β signal pathway was
investigated in liver cells isolated from clinical patients.
Results demonstrated that hFGF-21 treatment significantly inhibited
deposition of ECM and ROS expression levels in liver cells compared
with the levels in control cells (P<0.01) (Fig. 6A). Western blotting indicated that
expression levels of TGF-β, NF-κB and KLF6 were significantly
downregulated and PDGF and EGF expression levels were significantly
upregulated by hFGF-21 treatment in liver cells compared with the
levels in the control cells (P<0.01) (Fig. 6B and C). Knockdown of NF-κB with
siRNA-NF-κB significantly inhibited the hFGF-21-induced suppression
of TGF-β and KLF6 expression and hFGF-21-promoted PDGF and EGF
expression levels in liver cells compared with the levels in cells
transfected with siRNA-vector (P<0.01; Fig. 6D and E). Findings also indicated that
knockdown of NF-κB significantly inhibited the suppression of
protein expression levels of TNF-α, IL-6, IL-1β and IL-8 in liver
cells induced by hFGF-21 compared with the levels in cells
transfected with siRNA-vector (P<0.01; Fig. 6F). These results indicated that
hFGF-21 regulates inflammatory cytokines through downregulation of
the NF-κB-mediated TGF-β signaling pathway.
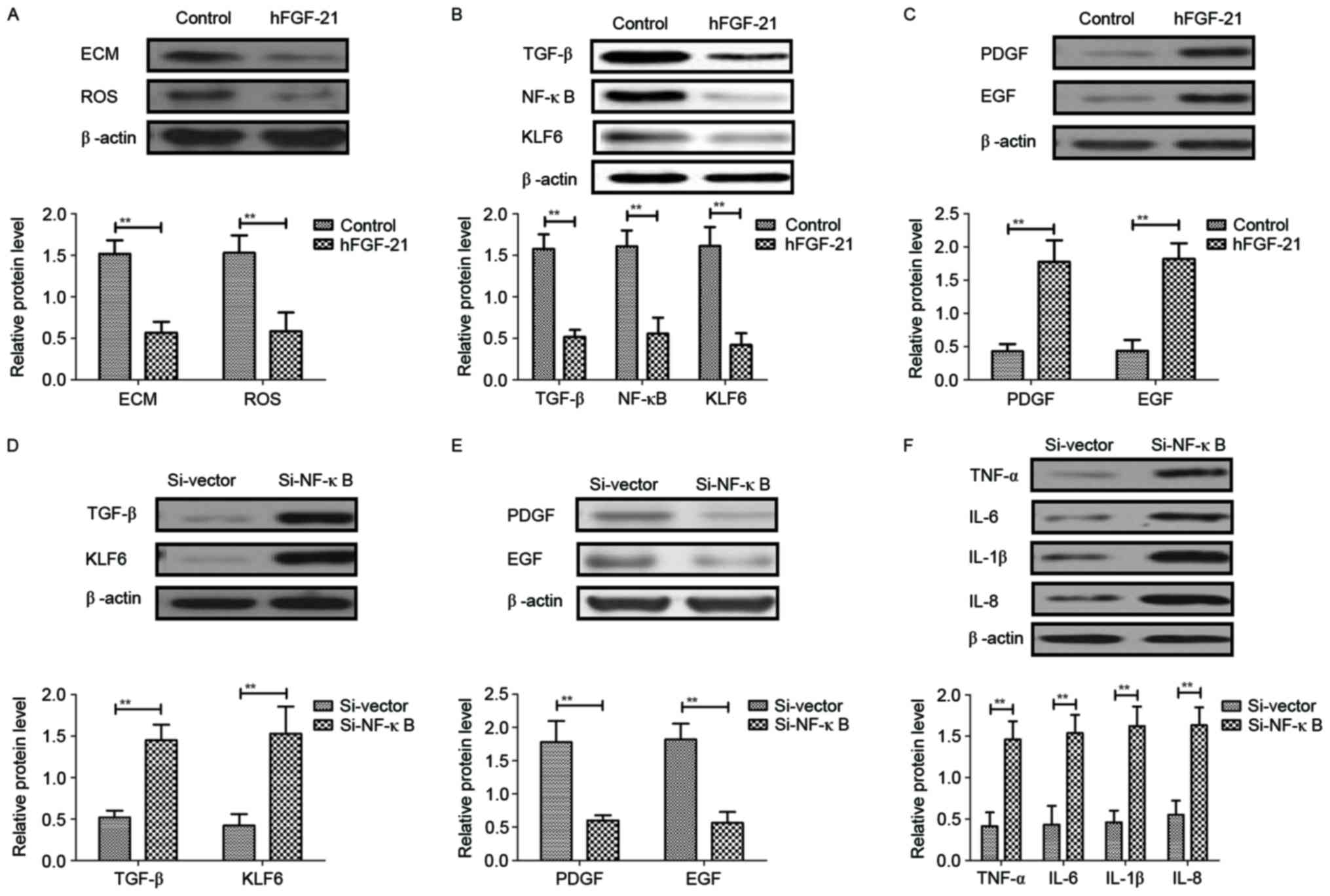 | Figure 6.hFGF-21 regulates inflammatory
cytokines through downregulation of the NF-κB-mediated TGF-β
signaling pathway. (A) Effects of hFGF-21 on deposition of ECM and
ROS expression levels in liver cells. (B) Effects of hFGF-21 on
expression levels of TGF-β, NF-κB and KLF6 in liver cells. (C)
Effects of hFGF-21 on expression levels of PDGF and EGF in liver
cells. (D) Knockdown of NF-κB with Si-NF-κB increases TGF-β and
KLF6 expression in liver cells. (E) Knockdown of NF-κB with
Si-NF-κB suppresses PDGF and EGF expression in liver cells. (F)
Effects of Si-NF-κB on protein expression levels of TNF-α, IL-6,
IL-1β and IL-8 in liver cells. **P<0.01. hFGF-21, human
fibroblast growth factor-21; NF, nuclear factor; TGF, transforming
growth factor; ECM, extracellular matrix; ROS, reactive oxygen
species; KLF6, Kruppel-like factor 6; PDGF, platelet-derived growth
factor; EGF, epidermal growth factor; TNF, tumor necrosis factor;
IL, interleukin; Si, small interfering RNA. |
Discussion
Hepatitis B-induced liver cirrhosis poses a great
threat to health and frequently leads to adrenal insufficiency that
further affects the endocrine system and disturbs liver metabolism
(27,28). Pathophysiologic and clinical
evidences have suggested that inflammation is associated with
hepatitis B-induced liver cirrhosis and inflammatory cytokines,
including TNF and IL-1, which may be potential target agents in
decompensated cirrhosis (29).
Research has also indicated that FGF is altered and molecular
signaling pathways are regulated by attenuating the expression of
TGF-β (30). The present study
detected hFGF-21 serum concentration and expression levels in PhbA.
Outcomes indicated that hFGF-21 suppressed inflammatory cytokine
levels in liver cells isolated from clinical specimens through
regulation of the NF-κB-mediated TGF-β signaling pathway. These
findings suggested that hFGF-21 may serve as a predictor and
prognostic factor in PhbA.
Beneficial effects of inhibition of oxidative stress
and inflammation have been reported in hepatitis C virus-positive
patients with liver cirrhosis and findings indicate that
inflammation inhibition influences microinflammation and the
metabolism of iron in hepatitis C virus-positive patients with
liver cirrhosis, which subsequently appeared to reduce the
production of oxidative stress, possibly leading to a decrease in
the occurrence of hepatocellular carcinoma (31). A study by Prystupa et al
(32) indicated that proinflammatory
cytokines (IL-1β and IL-6) and hepatocyte growth factor were
upregulated in patients with alcoholic liver cirrhosis.
Additionally, the levels of ghrelin, leptin, TNF-α and IL-8 in
liver cirrhosis were increased following hepatitis B and hepatitis
D virus infection (33,34). The present findings suggested that
hFGF-21 treatment inhibits mRNA and protein expression levels of
TNF-α, IL-6, IL-1β and IL-8 in liver cells. Inhibitory effects of
hFGF-21 were demonstrated in the present study, indicating that
hFGF-21 regulates inflammatory cytokines by downregulation of the
NF-κB-mediated TGF-β signaling pathway.
Target-specific systemic delivery of siRNA for TGF-β
has been proposed for the treatment of liver cirrhosis and has
demonstrated feasible therapeutic effects on liver cirrhosis by
reduction of nodule formation, collagen content and hepatic
stellate cell numbers (35). A study
by Chávez et al (36)
suggested that Sulfasalazine prevents the increase in TGF-β,
cyclooxygenase-2 and NF-κB translocation and fibrosis in carbon
tetrachloride-induced liver cirrhosis in rats. A study by
Aldaba-Muruato et al (21)
indicated that modulation of NF-κB, cytokine production and
oxidative stress may protect the liver against allopurinol-induced
acute liver damage and cirrhosis induced by carbon tetrachloride.
Therefore, we assumed that the regulation of inflammatory cytokines
by hFGF-21 may be associated with the NF-κB signaling pathway. The
present results supported this hypothesis and the findings
suggested that hFGF-21 treatment suppresses ECM and ROS expression
levels and downregulates TGF-β, NF-κB and KLF6 expression levels in
liver cells.
Previous research has demonstrated that FGF-21
resulted in insulin resistance by inhibiting the activation of
NF-κB (37). FGF-21 also served an
endocrine hormone role in blocking somatic growth, leading to
growth hormone resistance (38).
Furthermore, FGF-21 has been reported to be associated with lipid
metabolism and the incidence of cardiovascular disease (39), as well as various human diseases and
metabolic syndromes, including geriatric obesity, type 2 diabetes
mellitus and congenital hypothyroidism (40–42). In
the present study, changes of hFGF-21 plasma concentration levels
in PhbA were analyzed. Outcomes suggested that hFGF-21 is
downregulated in clinical patients suffering with hepatitis B
cirrhosis combined with adrenal insufficiency. Therefore, hFGF-21
may serve as a predictor and prognostic factor for hepatitis B
cirrhosis combined with adrenal insufficiency.
In conclusion, the present study indicated that
hFGF-21 improved inflammatory cytokine expression levels in renal
epithelial cells and liver cells isolated from clinical patients.
The results demonstrated the potential molecular mechanism mediated
by hFGF-21 in liver cells in the progression of hepatitis B
cirrhosis combined with adrenal insufficiency. The present study
suggested that hFGF-21 administration downregulates inflammatory
cytokine levels through the NF-κB-mediated TGF-β signaling pathway.
Changes in hFGF-21 plasma concentration prior and post treatment
were observed for PhbA, suggesting that hFGF-21 possesses the
potential to act as an alternative predictor and prognostic
indicator for the evaluation of prognosis of hepatitis B cirrhosis
combined with adrenal insufficiency.
References
|
1
|
Acharya UR, Raghavendra U, Fujita H,
Hagiwara Y, Koh JE, Jen Hong T, Sudarshan VK, Vijayananthan A,
Yeong CH, Gudigar A and Ng KH: Automated characterization of fatty
liver disease and cirrhosis using curvelet transform and entropy
features extracted from ultrasound images. Comput Biol Med.
79:250–258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dezső K, Rókusz A, Bugyik E, Szücs A,
Szuák A, Dorogi B, Kiss M, Nemeskéri Á, Nagy P and Paku S: Human
liver regeneration in advanced cirrhosis is organized by the portal
tree. J Hepatol. 66:778–786. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aguirre Valadez JM, Rivera-Espinosa L,
Méndez-Guerrero O, Chávez-Pacheco JL, García Juárez I and Torre A:
Intestinal permeability in a patient with liver cirrhosis. Ther
Clin Risk Manag. 12:1729–1748. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ganai AA, Ganaie IA, Verma N and Farooqi
H: Regression of fibrosis/cirrhosis by Glycine
propionyl-l-carnitine treatment in d-Galactosamine induced chronic
liver damage. Chem Biol Interact. 260:117–128. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Askgaard G, Leon DA, Kjaer MS, Deleuran T,
Gerds TA and Tolstrup JS: Risk for alcoholic liver cirrhosis after
an initial hospital contact with alcohol problems: A nationwide
prospective cohort study. Hepatology. 65:929–937. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chiriac S, Stanciu C and Trifan A:
Corticosteroid treatment in the setting of decompensated liver
cirrhosis with relative adrenal insufficiency: A case report and a
brief review of the literature. Rev Med Chir Soc Med Nat Iasi.
120:288–292. 2016.PubMed/NCBI
|
|
7
|
Wang Z, Sheng L, Yang Y, Yang F, Xiao X,
Hua J, Guo C, Wei Y, Tang R, Miao Q, et al: The management of
autoimmune hepatitis patients with decompensated cirrhosis:
Real-world experience and a comprehensive review. Clin Rev Allergy
Immunol. 52:424–435. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han Y, Zeng A, Liao H, Liu Y, Chen Y and
Ding H: The efficacy and safety comparison between tenofovir and
entecavir in treatment of chronic hepatitis B and HBV related
cirrhosis: A systematic review and meta-analysis. Int
Immunopharmacol. 42:168–175. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fialla AD, Israelsen M, Hamberg O, Krag A
and Gluud LL: Nutritional therapy in cirrhosis or alcoholic
hepatitis: A systematic review and meta-analysis. Liver Int.
35:2072–2078. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Manne V, Akhtar E and Saab S: Cirrhosis
regression in patients with viral hepatitis B and C: A systematic
review. J Clin Gastroenterol. 48:e76–e84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eto K: FGF-21, a newcomer in the field of
hypertension research. J Hum Hypertens. 27:343–344. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reinehr T, Woelfle J, Wunsch R and Roth
CL: Fibroblast growth factor 21 (FGF-21) and its relation to
obesity, metabolic syndrome, and nonalcoholic fatty liver in
children: A longitudinal analysis. J Clin Endocrinol Metab.
97:2143–2150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dushay J, Chui PC, Gopalakrishnan GS,
Varela-Rey M, Crawley M, Fisher FM, Badman MK, Martinez-Chantar ML
and Maratos-Flier E: Increased fibroblast growth factor 21 in
obesity and nonalcoholic fatty liver disease. Gastroenterology.
139:456–463. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hotta Y, Nakamura H, Konishi M, Murata Y,
Takagi H, Matsumura S, Inoue K, Fushiki T and Itoh N: Fibroblast
growth factor 21 regulates lipolysis in white adipose tissue but is
not required for ketogenesis and triglyceride clearance in liver.
Endocrinology. 150:4625–4633. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang R, Yi X, Li X and Jiang X: Fibroblast
growth factor-21 is positively associated with atrial fibrosis in
atrial fibrillation patients with rheumatic heart disease. Int J
Clin Exp Pathol. 8:14901–14908. 2015.PubMed/NCBI
|
|
16
|
Cariello M and Moschetta A: Fibroblast
growth factor 21: A new liver safeguard. Hepatology. 60:792–794.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suomalainen A, Elo JM, Pietiläinen KH,
Hakonen AH, Sevastianova K, Korpela M, Isohanni P, Marjavaara SK,
Tyni T, Kiuru-Enari S, et al: FGF-21 as a biomarker for
muscle-manifesting mitochondrial respiratory chain deficiencies: A
diagnostic study. Lancet Neurol. 10:806–818. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin Z, Wu Z, Yin X, Liu Y, Yan X, Lin S,
Xiao J, Wang X, Feng W and Li X: Serum levels of FGF-21 are
increased in coronary heart disease patients and are independently
associated with adverse lipid profile. PLoS One. 5:e155342010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kamada Y, Yoshida Y, Saji Y, Fukushima J,
Tamura S, Kiso S and Hayashi N: Transplantation of basic fibroblast
growth factor-pretreated adipose tissue-derived stromal cells
enhances regression of liver fibrosis in mice. Am J Physiol
Gastrointest Liver Physiol. 296:G157–G167. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsuzaki K, Murata M, Yoshida K, Sekimoto
G, Uemura Y, Sakaida N, Kaibori M, Kamiyama Y, Nishizawa M,
Fujisawa J, et al: Chronic inflammation associated with hepatitis C
virus infection perturbs hepatic transforming growth factor beta
signaling, promoting cirrhosis and hepatocellular carcinoma.
Hepatology. 46:48–57. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aldaba-Muruato LR, Moreno MG, Shibayama M,
Tsutsumi V and Muriel P: Protective effects of allopurinol against
acute liver damage and cirrhosis induced by carbon tetrachloride:
Modulation of NF-κB, cytokine production and oxidative stress.
Biochim Biophys Acta. 1820:65–75. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
de Lédinghen V, Douvin C, Kettaneh A, Ziol
M, Roulot D, Marcellin P, Dhumeaux D and Beaugrand M: Diagnosis of
hepatic fibrosis and cirrhosis by transient elastography in
HIV/hepatitis C virus-coinfected patients. J Acquir Immune Defic
Syndr. 41:175–179. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Iguchi T, Hiraki T, Matsui Y, Fujiwara H,
Sakurai J, Masaoka Y, Gobara H and Kanazawa S: CT
fluoroscopy-guided renal tumour cutting needle biopsy:
Retrospective evaluation of diagnostic yield, safety, and risk
factors for diagnostic failure. Eur Radiol. 28:283–290. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Almeida Mde A, Pizzini CV, Damasceno LS,
Muniz Mde M, Almeida-Paes R, Peralta RH, Peralta JM, Oliveira Rde
V, Vizzoni AG, de Andrade CL and Zancopé-Oliveira RM: Validation of
western blot for Histoplasma capsulatum antibody detection assay.
BMC Infect Dis. 16:872016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mattheolabakis G, Ling D, Ahmad G and
Amiji M: Enhanced anti-tumor efficacy of lipid-modified platinum
derivatives in combination with survivin silencing siRNA in
resistant non-small cell lung cancer. Pharm Res. 33:2943–2953.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiao S, Chen H, Wang Y, Zhu J, Tan J and
Gao J: Splenectomy versus partial splenic embolization for massive
splenomegaly secondary to hepatitis B-related liver cirrhosis: A
case-control study. Gastroenterol Res Pract. 2016:34716262016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maan R, van Tilborg M, Deterding K, Ramji
A, van der Meer AJ, Wong F, Fung S, Sherman M, Manns MP, Cornberg
M, et al: Safety and effectiveness of direct-acting antiviral
agents for treatment of patients with chronic hepatitis C virus
infection and cirrhosis. Clin Gastroenterol Hepatol.
14:1821–1830.e6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Artigas A, Wernerman J, Arroyo V, Vincent
JL and Levy M: Role of albumin in diseases associated with severe
systemic inflammation: Pathophysiologic and clinical evidence in
sepsis and in decompensated cirrhosis. J Crit Care. 33:62–70. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen X, Shi C, Meng X, Zhang K, Li X, Wang
C, Xiang Z, Hu K and Han X: Inhibition of Wnt/β-catenin signaling
suppresses bleomycin-induced pulmonary fibrosis by attenuating the
expression of TGF-β1 and FGF-2. Exp Mol Pathol. 101:22–30. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ohno T, Tanaka Y, Sugauchi F, Orito E,
Hasegawa I, Nukaya H, Kato A, Matunaga S, Endo M, Tanaka Y, et al:
Suppressive effect of oral administration of branched-chain amino
acid granules on oxidative stress and inflammation in HCV-positive
patients with liver cirrhosis. Hepatol Res. 38:683–688. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Prystupa A, Kiciński P, Sak J,
Boguszewska-Czubara A, Toruń-Jurkowska A and Załuska W:
Proinflammatory cytokines (IL-1α, IL-6) and hepatocyte growth
factor in patients with alcoholic liver cirrhosis. Gastroenterol
Res Pract. 2015:5326152015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ganji SH, Kashyap ML and Kamanna VS:
Niacin inhibits fat accumulation, oxidative stress, and
inflammatory cytokine IL-8 in cultured hepatocytes: Impact on
non-alcoholic fatty liver disease. Metabolism. 64:982–990. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cesaratto L, Codarin E, Vascotto C,
Leonardi A, Kelley MR, Tiribelli C and Tell G: Specific inhibition
of the redox activity of ape1/ref-1 by e3330 blocks tnf-α-induced
activation of IL-8 production in liver cancer cell lines. PLoS One.
8:e709092013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park K, Hong SW, Hur W, Lee MY, Yang JA,
Kim SW, Yoon SK and Hahn SK: Target specific systemic delivery of
TGF-β siRNA/(PEI-SS)-g-HA complex for the treatment of liver
cirrhosis. Biomaterials. 32:4951–4958. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chávez E, Castro-Sánchez L, Shibayama M,
Tsutsumi V, Moreno MG and Muriel P: Sulfasalazine prevents the
increase in TGF-β, COX-2, nuclear NFκB translocation and fibrosis
in CCl4-induced liver cirrhosis in the rat. Hum Exp Toxicol.
31:913–920. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Salehi MH, Kamalidehghan B, Houshmand M,
Aryani O, Sadeghizadeh M and Mossalaeie MM: Association of
fibroblast growth factor (FGF-21) as a biomarker with primary
mitochondrial disorders, but not with secondary mitochondrial
disorders (Friedreich Ataxia). Mol Biol Rep. 40:6495–6499. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gahete MD, Córdoba-Chacón J, Luque RM and
Kineman RD: The rise in growth hormone during starvation does not
serve to maintain glucose levels or lean mass but is required for
appropriate adipose tissue response in female mice. Endocrinology.
154:263–269. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu D, Sun CY, Sun GP, Ren GP, Ye XL, Zhu
SL, Wang WF, Xu PF, Li SJ, Wu Q, et al: The synergistic effect of
FGF-21 and insulin on regulating glucose metabolism and its
mechanism. Yao Xue Xue Bao. 49:977–984. 2014.(In Chinese).
PubMed/NCBI
|
|
40
|
Ren G, Yin J, Wang W, Li L and Li D:
Fibroblast growth factor (FGF)-21 signals through both FGF
receptor-1 and 2. Sci China Life Sci. 53:1000–1008. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kharitonenkov A, Dunbar JD, Bina HA,
Bright S, Moyers JS, Zhang C, Ding L, Micanovic R, Mehrbod SF,
Knierman MD, et al: FGF-21/FGF-21 receptor interaction and
activation is determined by betaKlotho. J Cell Physiol. 215:1–7.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kharitonenkov A, Shiyanova TL, Koester A,
Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers
JS, Owens RA, et al: FGF-21 as a novel metabolic regulator. J Clin
Invest. 115:1627–1635. 2005. View
Article : Google Scholar : PubMed/NCBI
|