Introduction
Low back pain (LBP) is one of the most common
musculoskeletal diseases in the world, with an incidence of ~70% in
adults (1). Unfortunately, many of
those with LBP suffer from disability (1). Multiple causes could lead to LBP;
however, intervertebral disc degeneration (IDD) and disc herniation
are reported to be the two most common diagnoses and targets for
intervention (2), with IDD being
deemed as the main cause of LBP (3,4). IDD is
a multifactorial process that is characterized by cellular and
biochemical changes in the disc tissue, consequently generating
structural failure (5).
A normal disc is composed of two types of tissues,
the nucleus pulposus (NP) and the annulus fibrosus (AF), and they
serve different roles in load bearing. Gelatinous NP is
predominantly composed of type II collagen and proteoglycans, and
its relatively higher water content (compared to AF tissues) is
responsible for its resistance to compressive forces and
hydrostatic pressurization (6,7). In
degeneration, loss of proteoglycans and water signal intensity is
detected by changes in disc height and T2-weighted magnetic
resonance imaging (MRI) signal in the NP (8), which may lead to redistribution of load
onto fibrochondrocyte-like cells in the AF (9).
As IDD is associated with normal aging, many people
with IDD indications on MRI do not suffer with pain or disability
(10,11). However, current treatments for IDD,
including surgery, steroid injection and physical therapy, treat
symptoms and not disc structure/function regeneration.
Inflammation is correlated with IDD and it involves
various cells and molecules (12).
Multiple genes were reported to be associated with genetic
predisposition to IDD, including the inflammatory genes
cyclooxygenase (COX)-2, interleukin (IL)1-α, IL1-β and IL-6
(13).
The GDF-5 gene was demonstrated to be a
susceptibility gene for IDD (14,15) and
defects in this gene result in abnormalities of collagen and
proteoglycan discs in mice (16).
However, there was no report regarding overexpression of GDF-5 in
inhibiting inflammatory factors released by intervertebral disc
cells. Therefore, the present study aimed to demonstrate this
hypothesis in vitro by analyzing the levels of nuclear
factor (NF)-κB and inflammatory factors.
Materials and methods
Reagents and animal ethics
Lipopolysaccharide (LPS) was purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). pRL-TK plasmids
were purchased from Promega Corporation (Madison, WI, USA). A total
of 30 sprague Dawley (SD) rats (6–8 weeks old, 250–300 g),
regardless of their gender (24 male, 6 female), were purchased from
Animal Research Center of Beijing University of Chinese Medicine
(Beijing, China). All the rats were maintained at 27±3°C and a
humidity of 60±15% with a 12 h light/dark cycle with free access to
water and food. Then the rats were euthanized by abdominal
injection of a lethal dose (90 mg/kg) of pentobarbital sodium
(Sigma-Aldrich; Merck KGaA). All animal work was performed in
accordance with relevant national and international guidelines and
approved by the Animal Experimental Ethical Committee of Beijing
University of Chinese Medicine (Beijing, China).
NP cell isolation and culture
Following sacrifice, NP cells were isolated from the
lumbar spines of adult SD rats. The spines were separated between
each of the lumbar discs and, subsequently, a sterile scalpel blade
was applied to extract the NP completely. NP was rinsed with PBS to
remove other cells, followed by digestion with trypsin and
collagenase at 37°C for 25 min. NP cells were incubated in
high-glucose Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100
U/ml penicillin and 100 mg/ml streptomycin, cultured up to passage
2–3 at 37°C. In order to create an IDD model group, cells were
stimulated with 10 mg/ml LPS at 37°C for 24 h. Cells cultured with
normal medium were used as controls and cells treated with LPS +
GDF-5 were termed the LPS + GDP-5 group.
ELISA assessments
The levels of prostaglandin-E2 (PGE-2), tumor
necrosis factor (TNF)-α and IL-1β in the culture medium were
assessed using a Rat TNF-α Quantikine ELISA kit (cat. no. RTA00) or
Rat IL-1β/IL-1F2 Quantikine ELISA kit (cat. no. RLB00; R&D
Systems, Inc., Minneapolis, MN, USA), according to the protocols of
the manufacturer.
Measurement of nitric oxide (NO)
concentration
NO concentration was measured using Griess reagent
(Sigma-Aldrich; Merck KGaA). In brief, NP cells were transfected
with 100 ng/ml GDF-5 plasmid (Guangzhou Ribobio Co., Ltd.,
Guangzhou, China) using the Lipofectamine™ 2000 kit
(Thermo Fisher Scientific, Inc.) for 2 h followed by incubation
with 10 mg/ml LPS at 37°C for 24 h. Culture supernatant (50 µl)
from each group was incubated with the same volume (50 µl) of
Griess reagent at room temperature for 15 min in a 96-well plate.
Subsequently, the absorbance at 540 nm was read. The standard curve
was made by NaNO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from NP cells was isolated using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. RT was conducted from 1 mg RNA with a
1st Strand cDNA Synthesis kit (Takara Biotechnology Co., Ltd.,
Dalian, China) to obtain first-strand cDNA.
The relative gene expression levels were determined
using qPCR. qPCR was conducted with a SYBR Premix Ex Taq kit
(Takara Biotechnology Co., Ltd.) on an ABI Prism 7500 Fast
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. The primer
sequences used were as follows: Inducible NO synthase (iNOS)
forward, 5′-ACACAGTGTCGCTGGTTTGA-3′ and reverse,
5′-AGAAACTTCCAGGGGCAAGC-3′; COX-2 forward,
5′-ATCAGAACCGCATTGCCTCT-3′ and reverse, 5′-GCCAGCAATCTGTCTGGTGA-3′;
TNF-α forward, 5′-GACCCTCACACTCAGATCATCTT-3′ and reverse,
5′-CCACTTGGTGGTTTGCTACGA-3′; IL-1β forward,
5′-TGAAATGCCACCTTTTGACAG-3′ and reverse,
5′-CCACAGCCACAATGAGTGATAC-3′; and β-actin forward,
5′-AACCTTCTTGCAGCTCCTCCG-3′ and reverse,
5′-CCATACCCACCATCACACCCT-3′. β-actin was used as an internal
control. The thermocycling conditions of PCR were as follows: An
initial denaturation at 95°C for 10 min, followed by an
amplification cycle consisting of three steps; denaturation at 95°C
for 15 sec, annealing at 58°C for 30 sec and elongation at 72°C for
30 sec. Triplicate Cq values were averaged and the relative
expression levels were determined using the 2−ΔΔCq
method (17).
Western blotting
For the analysis of pathway-related protein levels,
NP cells were stimulated with 10 mg/ml LPS at 37°C for 30 min. For
collagen II and aggrecan protein levels, NP cells were cultured
with 10 mg/ml LPS at 37°C for 5 days. Subsequently, cells were
washed with PBS and total protein was extracted with
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China). The protein concentration was
assessed using a BCA Protein Assay kit (Thermo Fisher Scientific,
Inc.). Protein samples (20 mg) were separated by 10% SDS-PAGE and
transferred onto polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA). Membranes were blocked with 5% fat-free milk
at room temperature for 1 h. Subsequently, the membranes were
incubated with primary antibodies against phosphorylated (p)-IκB
(cat. no. 2859), p-p65 (cat. no. 3033), TNF-α (cat. no. 13377),
IL-1β (cat. no. 12703) (1:1,000; Cell Signaling Technology, Inc.,
Danvers, MA, USA), aggrecan (cat. no. ab3773) and collagen-II (cat.
no. ab188570) (all 1:1,000; Abcam, Cambridge, UK) at 4°C overnight.
Following three washes with Tris-buffered saline with Tween-20, the
membranes were incubated with horseradish peroxidase (HRP)-labeled
Goat Anti-Rabbit Immunoglobulin G (IgG; cat. no. A0208; Beyotime
Institute of Biotechnology) or HRP-labeled Goat Anti-Mouse IgG
(cat. no. A0216; Beyotime Institute of Biotechnology) that were
conjugated with IRDye 800CW at room temperature for 1 h.
Immunoreactive bands were detected using an Odyssey infrared
imaging system (LI-COR Biosciences, Lincoln, NE, USA). β-actin
(cat. no. 4970; 1:2,000; Cell Signaling Technology, Inc.) was used
as a control. Quantification was performed using ImageJ software
version 1.43 (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
All experiments were repeated at least three times.
Data were presented as the mean ± standard deviation. Statistical
analyses were conducted using one-way analysis of variance,
followed with Duncan's post hoc test. SPSS v. 19.0 (IBM Corp.,
Armonk, NY, USA) was used for statistical analyses. P<0.05 was
considered to indicate a statistically significant difference.
Results
GDF-5 overexpression inhibits
LPS-induced elevation of TNF-α and IL-1β in culture medium
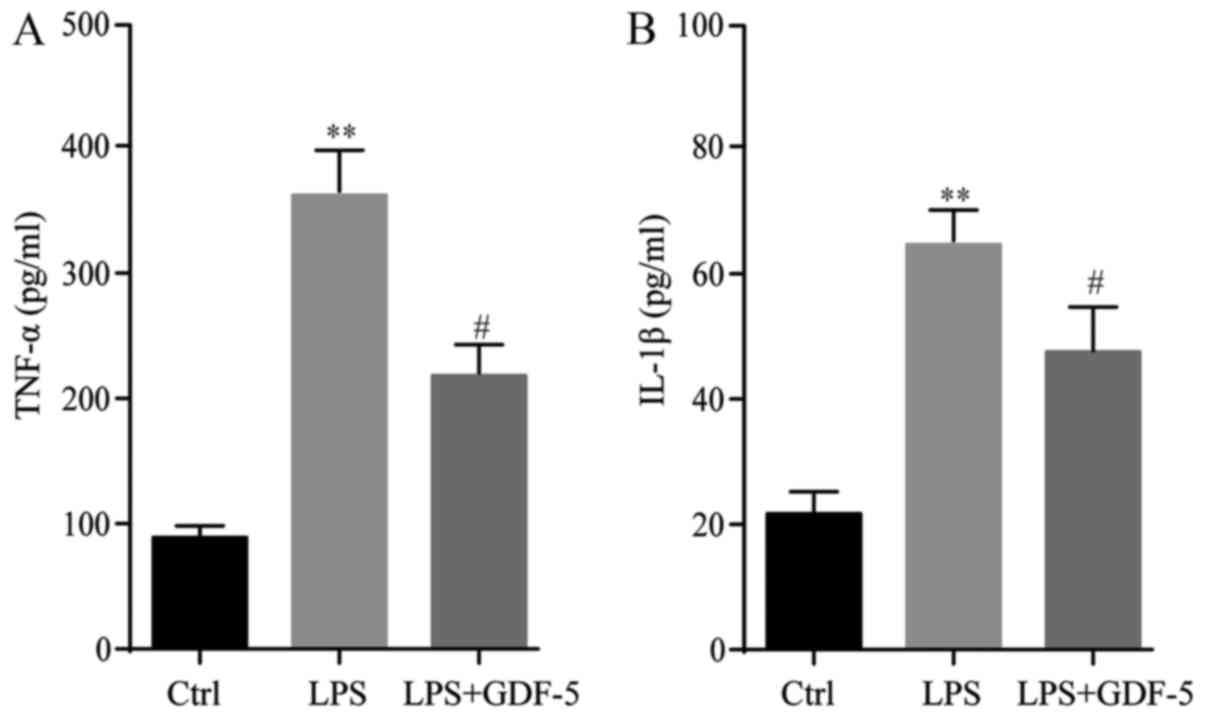
The protein levels of TNF-α and IL-1β in culture
medium were evaluated by ELISA. Results demonstrated that in the
control group, the expression level of TNF-α was only 95 pg/ml,
while in the LPS-induced IDD model group, the TNF-α concentration
was significantly elevated (P<0.01) compared with the levels in
the control group (361 pg/ml). This level was significantly
repressed to 203 pg/ml by GDF-5 overexpression (P<0.05; Fig. 1A). The changes to the IL-1β level
were similar to that of TNF-α. In the control group, IL-1β was only
at a level of 23 pg/ml and in the IDD group this level was
significantly higher than that in the control group (63 pg/ml;
P<0.01). This level was significantly reduced to 46 pg/ml
following GDF-5 overexpression (P<0.05; Fig. 1B).
GDF-5 overexpression represses
LPS-induced elevation of TNF-α and IL-1β in NP cells
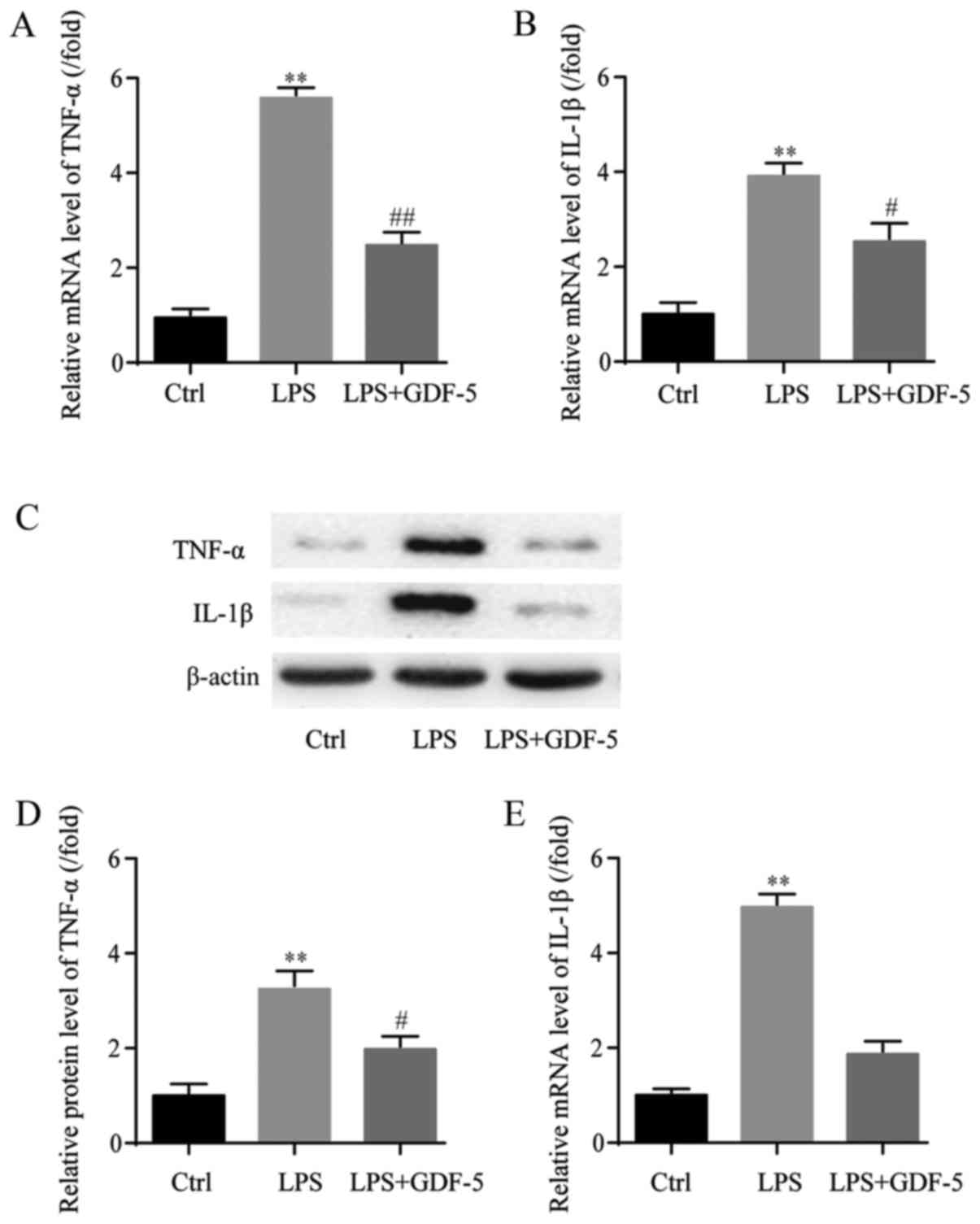
mRNA expression levels of TNF-α and IL-1β were
assessed in NP cells by RT-qPCR. In the LPS-induced IDD model
group, the mRNA expression level of TNF-α was ~6-fold higher that
in the control group (P<0.01) and GDF-5 overexpression
significantly inhibited TNF-α to near the normal level (P<0.01;
Fig. 2A). Similarly, in the IDD
group, the mRNA expression level of IL-1β was significantly
increased by almost 4-fold compared with the level in the control
group (P<0.01), which was significantly decreased by GDF-5
overexpression (P<0.05; Fig.
2B).
Following this, the protein expression levels of
TNF-α and IL-1β in NP cells were evaluated by western blotting
(Fig. 2C). In the IDD model group,
the TNF-α protein expression level was significantly higher
compared with the level in the control group (P<0.01).
Furthermore, GDF-5 overexpression significantly inhibited the level
of TNF-α (P<0.05; Fig. 2D). As
for the IL-1β protein expression level, in the IDD model group,
IL-1β was also significantly upregulated compared with the level in
the control group (P<0.01). This level was suppressed by GDF-5
overexpression; however, not to a significant level (Fig. 2E).
GDF-5 overexpression decreases
LPS-induced production of PGE2 and NO in NP cells
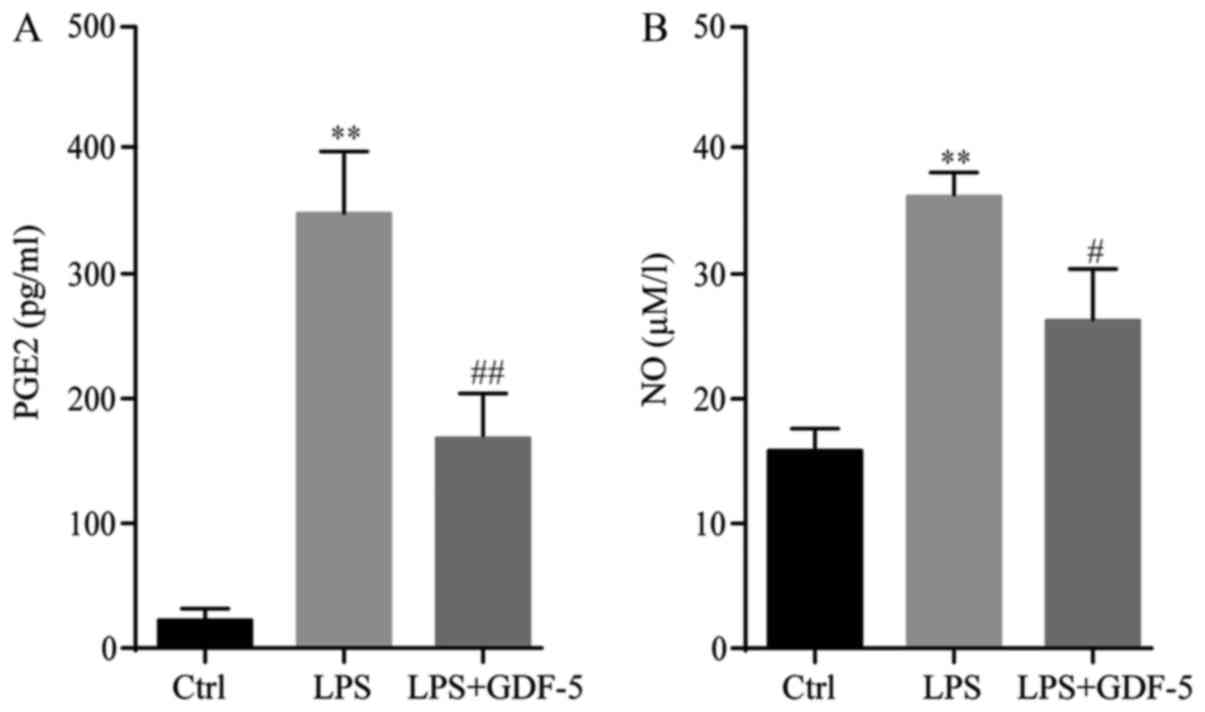
ELISA demonstrated that, in the control group, the
PGE2 level was only ~20 pg/ml. In the IDD model group, the PGE2
level was significantly increased by ~17-fold following LPS
stimulation compared with the level in the control group
(P<0.01). GDF-5 overexpression significantly inhibited
LPS-induced PGE2 production to ~156 pg/ml in the culture medium
(P<0.01; Fig. 3A).
Results of the Griess reaction indicated that, in
the culture supernatant of the control group, the NO concentration
was 16 µM/l, whereas in the LPS-stimulated cells, this level
increased significantly to 36 µM/l in the IDD group (P<0.01).
GDF-5 overexpression significantly inhibited LPS-induced NO
production to 25 µM/l in NP cells (P<0.05; Fig. 3B).
GDF-5 overexpression decreases
LPS-induced production of COX-2 and iNOS in NP cells
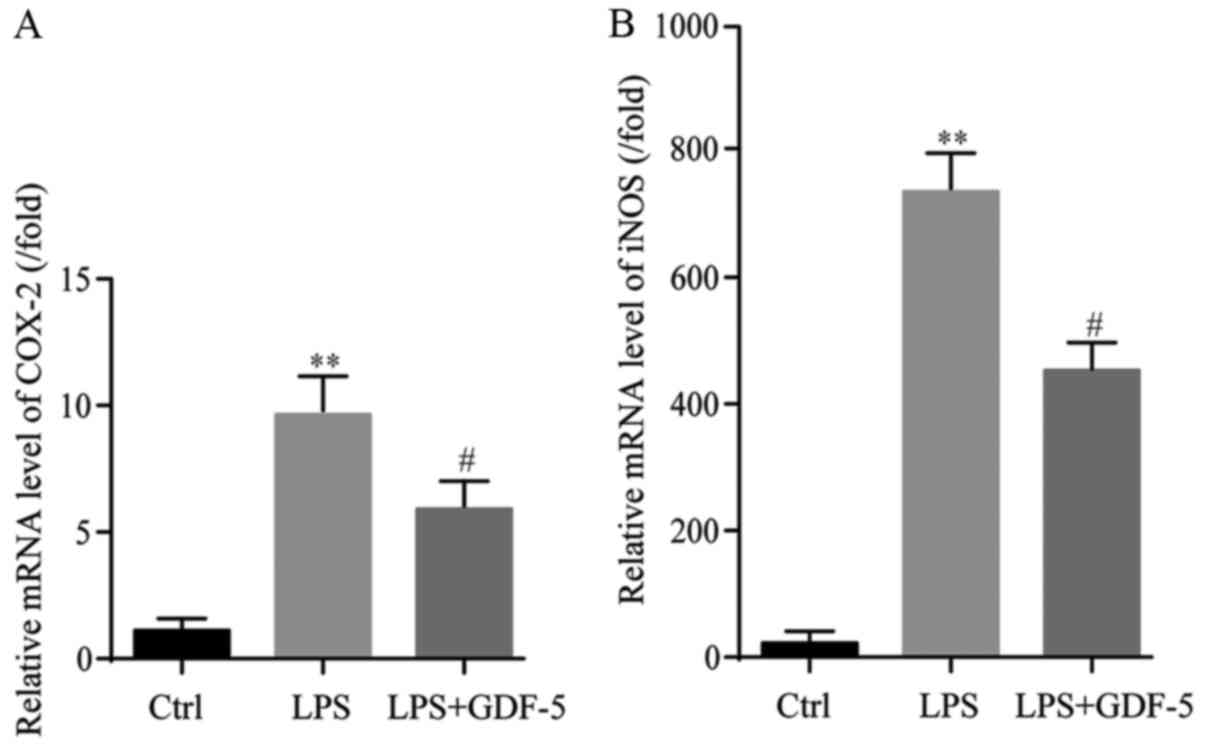
RT-qPCR indicated that LPS induced an ~10-fold
increase in gene expression of COX-2 in comparison with the level
in the control group (P<0.01). This increase induced by LPS
treatment was significantly reduced (by 6-fold) by GDF-5
overexpression (P<0.05; Fig.
4A).
Results from RT-qPCR demonstrated that LPS
significantly increased gene expression levels of iNOS, by 770-fold
of that in the control group (P<0.01). GDF-5 overexpression
significantly reduced the LPS-induced gene expression level of iNOS
by ~421-fold (P<0.05; Fig.
4B).
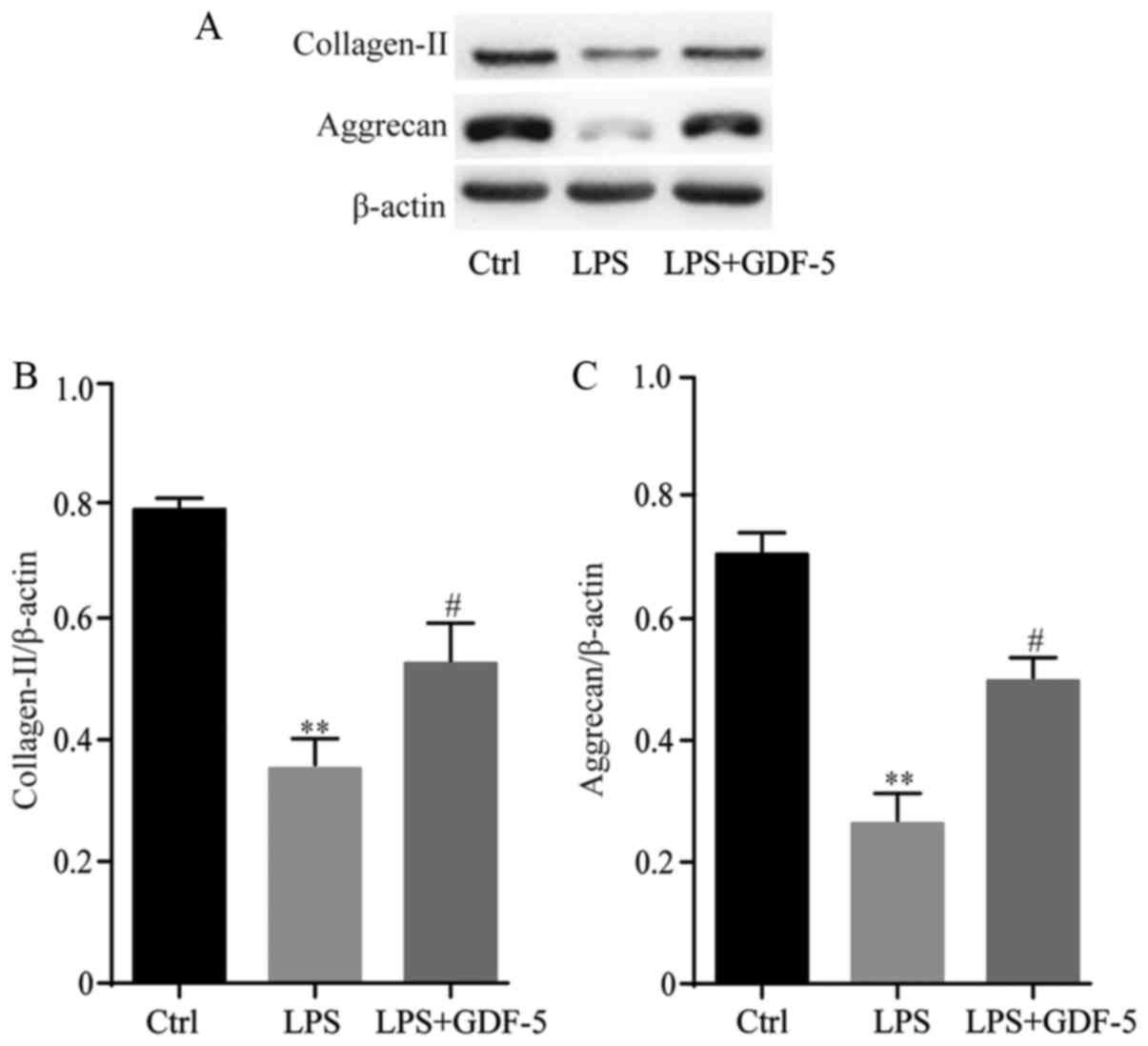
GDF-5 overexpression protects NP cells
from LPS-induced matrix degradation
GDF-5 overexpression effectively inhibited plentiful
matrix-degrading enzymes, which were induced by LPS; therefore,
analysis was performed to determine whether GDF-5 overexpression
antagonized LPS-induced matrix degradation of NP cells. NP cells
were stimulated with 10 mg/ml LPS in the presence or absence of
GDF-5 overexpression for 5 days, followed by western blot analysis.
Results indicated that stimulation of NP cells with LPS
significantly reduced collagen-II and aggrecan content compared
with the levels in the control cells (P<0.01). GDF-5
overexpression significantly inhibited the decrease of collagen-II
and aggrecan compared with that in LPS group (P<0.05; Fig. 5).
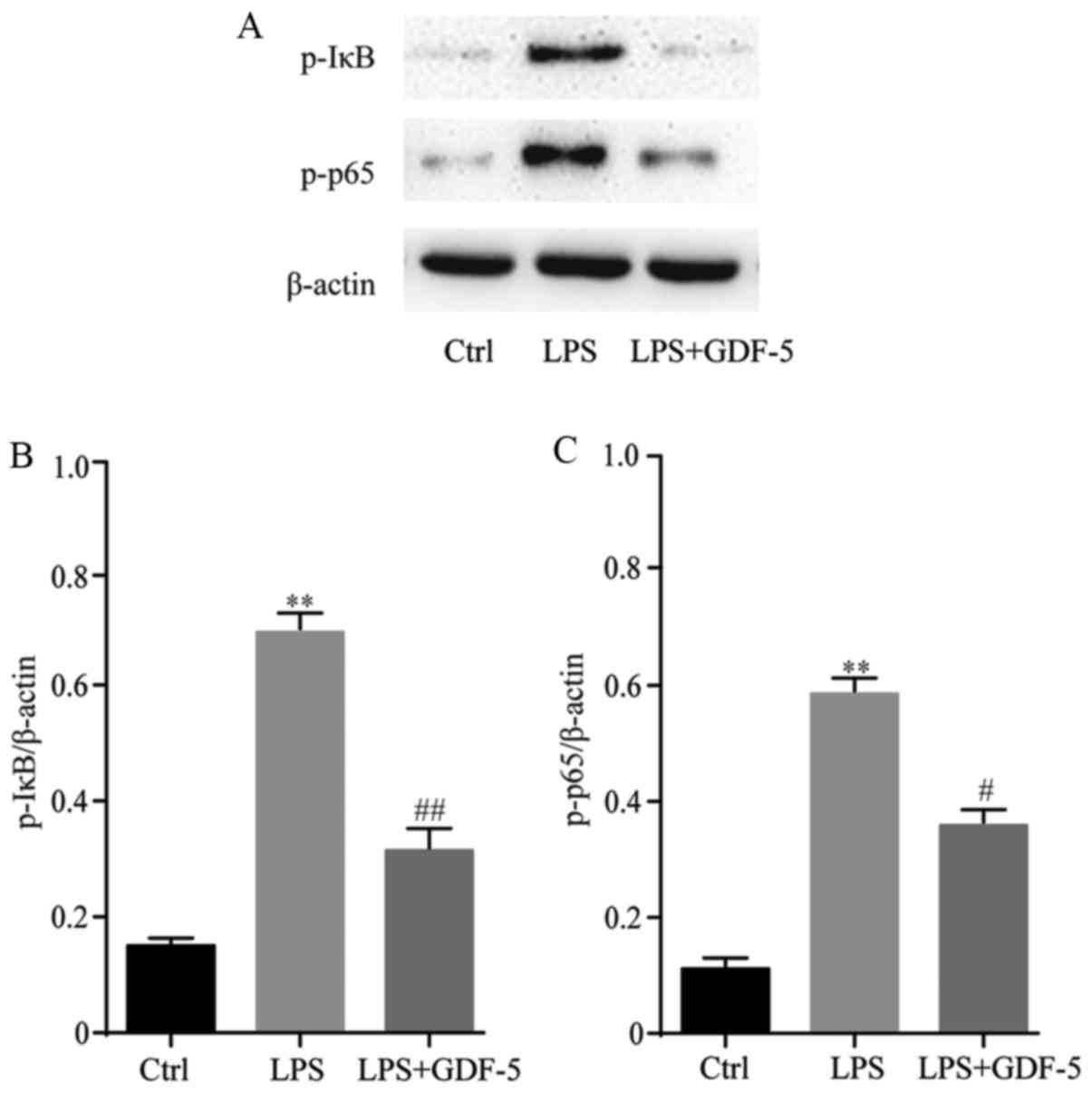
GDF-5 overexpression prevents NF-κB
over-activation from LPS-stimulated NP cells
NP cells were pretreated with GDF-5 plasmid for 2 h
and then stimulated with 10 mg/ml LPS for 24 h. Subsequently,
western blotting was performed to evaluate the mechanism of GDF-5
on LPS-treated NP cells (Fig. 6A).
GDF-5 significantly inhibited the phosphorylation of IκB
(P<0.01) and p65 (P<0.05) induced by LPS (Fig. 6B and C). These results demonstrated
that GDF-5 significantly inhibited the activation of the NF-κB
pathway induced by LPS.
Discussion
LBP is one of the most frequent musculoskeletal
diseases worldwide and results from IDD (1,3,4). In IDD, cellular and biochemical
deficits and structural failure occur in the discs (5). However, a large quantity of patients
with IDD exhibit neither pain nor disability (10,11).
GDF-5 defects have been reported to lead to abnormalities of
collagen and proteoglycan in discs in mice (16). Current treatments for IDD treat
symptoms but not disc structure/function regeneration. Therefore,
it is necessary to explore possible effective therapies for
IDD.
Inflammation is correlated with IDD (12); furthermore, various inflammatory
genes have been demonstrated to be correlated with IDD, including
COX-2, IL-1α, IL-1β and IL-6 (13).
The present study utilized ELISA to determine levels of TNF-α and
IL-1β in the culture medium, meanwhile, the mRNA and protein levels
of TNF-α and IL-1β in NP cells were evaluated by RT-qPCR and
western blotting, respectively. Results indicated that in culture
medium and NP cells, TNF-α and IL-1β levels were significantly
increased in the LPS-stimulated group, and GDF-5 significantly
decreased the aforementioned symptoms.
Mounting evidences have suggested that NO and PGE2
serve crucial roles in the modulation of cellular metabolism of
discs and the pathology of IDD (18–20).
PGE2 was found to be involved in the progression of sciatica
(21). Additionally, NO was
discovered to contribute to the development of radiculopathy
(22). In the present study, ELISA
and Griess reactions were performed to assess the levels of PGE2 in
culture medium and NO in NP cells, respectively. Results
demonstrated that GDF-5 overexpression significantly inhibited
LPS-induced upregulation of PGE2 and NO expression levels.
Furthermore, RT-qPCR was conducted to evaluate the
mRNA expression levels of COX-2 and iNOS in NP cells. Results
indicated that, GDF-5 overexpression significantly reversed
LPS-induced elevation of gene expression of COX-2 and iNOS. Taken
together, these results demonstrated that GDF-5 attenuated
LPS-induced IDD via inhibiting the production and release of
inflammatory factors; however, the possible molecular mechanisms
remain to be fully elucidated.
NP is predominantly composed of collagen-II and
aggrecan, and the decrease of collagen-II is associated with disc
degeneration (23,24). The present study demonstrated that
GDF-5 prevented the loss of collagen-II and aggrecan that was
induced by LPS. The NF-κB pathway modulates inflammation (25,26). The
results of the present study suggested that GDF-5 inhibited
phosphorylation of IκB and p65, which was stimulated by LPS in NP
cells. However, further research performed in vivo is
required to confirm this hypothesis.
In conclusion, the present study demonstrated that
GDF-5 possessed anti-inflammatory and anti-degenerative effects on
LPS-induced IDD via inhibition of NF-κB pathway activation in NP
cells; therefore, GDF-5 may be a potential novel agent for treating
IDD in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LS wrote the manuscript and interpreted the data. YW
analyzed the data and revised the manuscript. LH searched the
literature and collected the data. HZ designed the study.
Ethics approval and consent to
participate
All animal work was performed in accordance with
relevant national and international guidelines and approved by the
Animal Experimental Ethical Committee of Beijing University of
Chinese Medicine (Beijing, China).
Consent for publication
Not applicable.
Competing interests
All authors have no conflict of interest to
declare.
References
|
1
|
Andersson GB: Epidemiological features of
chronic low-back pain. Lancet. 354:581–585. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Deyo RA and Weinstein JN: Low back pain. N
Engl J Med. 344:363–370. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luoma K, Riihimäki H, Luukkonen R,
Raininko R, Viikari-Juntura E and Lamminen A: Low back pain in
relation to lumbar disc degeneration. Spine (Phila Pa 1976).
25:487–492. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takatalo J, Karppinen J, Niinimäki J,
Taimela S, Näyhä S, Mutanen P, Sequeiros RB, Kyllönen E and
Tervonen O: Does lumbar disc degeneration on magnetic resonance
imaging associate with low back symptom severity in young Finnish
adults? Spine (Phila Pa 1976). 36:2180–2189. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adams MA and Roughley PJ: What is
intervertebral disc degeneration, and what causes it? Spine (Phila
Pa 1976). 31:2151–2161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Antoniou J, Steffen T, Nelson F,
Winterbottom N, Hollander AP, Poole RA, Aebi M and Alini M: The
human lumbar intervertebral disc: Evidence for changes in the
biosynthesis and denaturation of the extracellular matrix with
growth, maturation, ageing, and degeneration. J Clin Invest.
98:996–1003. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohshima H, Tsuji H, Hirano N, Ishihara H,
Katoh Y and Yamada H: Water diffusion pathway, swelling pressure,
and biomechanical properties of the intervertebral disc during
compression load. Spine (Phila Pa 1976). 14:1234–1244. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mwale F, Iatridis JC and Antoniou J:
Quantitative MRI as a diagnostic tool of intervertebral disc matrix
composition and integrity. Eur Spine J. 17 Suppl 4:S432–S440. 2008.
View Article : Google Scholar
|
|
9
|
Iatridis JC, Nicoll SB, Michalek AJ,
Walter BA and Gupta MS: Role of biomechanics in intervertebral disc
degeneration and regenerative therapies: What needs repairing in
the disc and what are promising biomaterials for its repair? Spine
J. 13:243–262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boden SD, McCowin PR, Davis DO, Dina TS,
Mark AS and Wiesel S: Abnormal magnetic-resonance scans of the
cervical spine in asymptomatic subjects. A prospective
investigation. J Bone Joint Surg Am. 72:1178–1184. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jensen MC, Brant-Zawadzki MN, Obuchowski
N, Modic MT, Malkasian D and Ross JS: Magnetic resonance imaging of
the lumbar spine in people without back pain. N Engl J Med.
331:69–73. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Risbud MV and Shapiro IM: Role of
cytokines in intervertebral disc degeneration: Pain and disc
content. Nat Rev Rheumatol. 10:44–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mayer JE, Iatridis JC, Chan D, Qureshi SA,
Gottesman O and Hecht AC: Genetic polymorphisms associated with
intervertebral disc degeneration. Spine J. 13:299–317. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Williams FM, Popham M, Hart DJ, de
Schepper E, Bierma-Zeinstra S, Hofman A, Uitterlinden AG, Arden NK,
Cooper C, Spector TD, et al: GDF5 single-nucleotide polymorphism
rs143383 is associated with lumbar disc degeneration in northern
european women. Arthritis Rheum. 63:708–712. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mu J, Ge W, Zuo X, Chen Y and Huang C:
Analysis of association between il-1β, casp-9, and gdf5 variants
and low-back pain in chinese male soldier: Clinical article. J
Neurosurg Spine. 19:243–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X, Leo BM, Beck G, Balian G and
Anderson GD: Collagen and proteoglycan abnormalities in the
GDF-5-deficient mice and molecular changes when treating disk cells
with recombinant growth factor. Spine (Phila Pa 1976).
29:2229–2234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang IC, Ueng SW, Lin SS, Niu CC, Yuan LJ,
Su CI, Chen CH and Chen WJ: Effect of hyperbaric oxygenation on
intervertebral disc degeneration: An in vitro study with human
lumbar nucleus pulposus. Spine (Phila Pa 1976). 36:1925–1931. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takada T, Nishida K, Maeno K, Kakutani K,
Yurube T, Doita M and Kurosaka M: Intervertebral disc and
macrophage interaction induces mechanical hyperalgesia and cytokine
production in a herniated disc model in rats. Arthtitis Rheum.
64:2601–2610. 2012. View Article : Google Scholar
|
|
20
|
Hou G, Lu H, Chen M, Yao H and Zhao H:
Oxidative stress participates in age-related changes in rat lumbar
intervertebral discs. Arch Gerontol Geriatr. 59:665–669. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
England S, Bevan S and Docherty RJ: PGE2
modulates the tetrodotoxin-resistant sodium current in neonatal rat
dorsal root ganglion neurones via the cyclic AMP-protein kinase A
cascade. J Physiol. 495:429–440. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee SJ, Kim TU, Park JS and Ra JY:
Inhibition of nitric oxide mediated protein nitration: Therapeutic
implications in experimental radiculopathy. Spine (Phila Pa 1976).
38:1749–1753. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi Z, Gu T, Xin H, Wu J, Xu C, Zhang C,
He Q and Ruan D: Intervention of rAAV-hTERT-transducted nucleus
pulposus cells in early stage of intervertebral disc degeneration:
A study in canine model. Tissue Eng Part A. 21:2186–2194. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kozaci LD, Guner A, Oktay G and Guner G:
Alterations in biochemical components of extracellular matrix in
intervertebral disc herniation: Role of MMP-2 and TIMP-2 in type II
collagen loss. Cell Biochem Funct. 24:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tak PP and Firestein GS: NF-kappaB: A key
role in inflammatory diseases. J Clin Invest. 107:7–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Berenbaum F: Signaling transduction:
Target in osteoarthritis. Curr Opin Rheumatol. 16:616–622. 2004.
View Article : Google Scholar : PubMed/NCBI
|