Introduction
Amyotrophic lateral sclerosis (ALS) is a rapidly
progressive neurodegenerative disease. Patients gradually lose the
ability to control their muscles and 3–5 years following diagnosis,
they typically experience respiratory failure and succumb (1). Patient's ALS Assessment Questionnaire
(ALSAQ-40) scores decrease from 40 (normal motor capabilities) at
the onset of disease to 0 (total loss of motor capabilities) over
an average of 4.4 years (1,2). At present, no treatments are able to
prevent ALS progression. Riluzole is the only drug that is
currently approved for the treatment of ALS and typically increases
life expectancy by 3–6 months (3–7). Vitamin
E is also often prescribed to patients with ALS due to its
anti-oxidizing activity (8).
Potential novel treatments for ALS, including gabapentine,
copaxone, topiramate, creatine and the use of progenitor cells,
have been assessed; however, no beneficial effects have been
observed (9). Therefore, novel,
effective treatments for ALS are required.
Endotherapia is a novel therapeutic strategy that
has been used to treat chronic conditions, including autoimmune,
neurodegenerative and proliferative diseases (10–12).
This therapy uses small molecules, including vitamins, amino acids
and fatty acids, linked to poly-L-lysine (PLL) (10–12). PLL
linkage increases the availability of vitamins, amino acids and
fatty acids in vivo (10–12). Two
drug candidates, multiple sclerosis (MS)-Endotherapia (GEMSP) and
ALS-Endotherapia (GEMALS), have been assessed in patients with MS
and ALS, respectively (13–16). The beneficial effects of GEMALS on
ALS have previously been demonstrated in a superoxide dismutase 1
(SOD1) rat model of ALS (12) and in
a small clinical trial (n=12) (11).
In the rat model, GEMALS delayed the onset of disease and increased
the rat's lifespan (12). In humans
the results obtained were promising; 83% of patients exhibited
stabilization or improvement to their functional capacities
(11). Furthermore, treatment with
GEMALS reversed the functional capacity impairment (50.07%) and
increased patient life expectancy by 52.57 months (11).
The aim of the present study was to confirm the
beneficial action of GEMALS in a larger sample of patients with ALS
(n=31) and to further investigate the effect of GEMALS on specific
motor parameters that were not assessed in the previous study
(11).
Patients and methods
Patients
A total of 31 patients (10 women, 35.5%; 21 men,
64.5%) with ALS in the evolutive phase were enrolled in the present
study (age range, 38–82 years). The patients were recruited from
January 1994 to December 2011 (Table
I). There were no exclusion criteria. ALS diagnosis was
performed by a neurologist at the French University hospitals
located in Nantes, Marseille, Nîmes, Versailles, Dijon,
Henry-Mondor de Créteil, Nice, Grenoble, Paris, Lille, Poitiers,
Toulouse, St Etienne, Rennes, Metz, Montpellier and Angers using
standardised clinical criteria and electromyogram tests as
previously described (17). The
study was national, multicenter, non-randomized and non-blinded.
The duration of GEMALS treatment was between 3 and 140 months
(Table I) and patients were
evaluated 3 or 4 times per year. The following data were collected
for each patient: Age, sex and date of diagnosis, start/finish of
GEMALS treatment and ALS Assessment Questionnaire (ALSAQ-40) scores
over time (Table I). All patients
provided prior informed written consent for inclusion in the
current study.
 | Table I.Data for patients with amyotrophic
lateral sclerosis in the present study. |
Table I.
Data for patients with amyotrophic
lateral sclerosis in the present study.
|
| Diagnosis | GEMALS start | GEMALS end |
|
|---|
|
|
|
|
|
|
|---|
| Patient (sex) | Date | Age (years) | Score | Age (years) | Score | Age (years) | Duration of treatment
(months) |
|---|
| 1
(M) | 11/09 | 62 | 35.5 | 62 | 15 | 66 | 36 |
| 2
(M) | 01/08 | 60 | 31 | 63 | 13 | 65 | 28 |
| 3
(M) | 03/10 | 53 | 37 | 53 | 14.5 | 56 | 32 |
| 4
(F) | – | – | 17 | 64 | 15 | 64 | 7 |
| 5
(F) | 05/10 | 72 | 33 | 73 | 16 | 75 | 27 |
| 6
(F) | 01/09 | 59 | 12 | 60 | 12 | 62 | 19 |
| 7
(M) | 2007 | 65 | 27 | 69 | 23 | 71 | 23 |
| 8
(F) | 10/08 | 68 | 16 | 71 | 6 | 73 | 19 |
| 9
(M) | 09/10 | 36 | 28 | 38 | 20 | 39 | 9 |
| 10 (M) | 01/94 | 47 | 12 | 62 | 11 | 66 | 48 |
| 11 (M) | 01/04 | 53 | 33 | 60 | 35.5 | 63 | 36 |
| 12 (M) | 08/03 | 40 | 40 | 40 | 6.5 | 49 | 110 |
| 13 (M) | 01/94 | 47 | 12 | 62 | 11 | 66 | 48 |
| 14 (M) | 01/01 | 57 | 40 | 57 | 6 | 69 | 140 |
| 15 (M) | – | – | 35 | 74 | 27 | 76 | 15 |
| 16 (F) | – | – |
27.5 | 80 | 24 | 82 | 15 |
| 17 (M) | 03/08 | 44 | 31 | 46 | 17 | 49 | 24 |
| 18 (F) | 01/09 | 55 | 34 | 57 | 22 | 59 | 19 |
| 19 (F) | – | – | 37 | 63 | 32 | 64 | 11 |
| 20 (M) | 06/07 | 65 | 33 | 69 | 25 | 71 | 32 |
| 21 (M) | 07/07 | 61 | 34 | 65 | 18 | 67 | 26 |
| 22 (F) | – | – | 38 | 59 | 34.5 | 60 | 13 |
| 23 (M) | 02/08 | 73 | 37 | 74 | 25 | 76 | 21 |
| 24 (M) | 08/05 | 58 | 32 | 61 | 28 | 63 | 25 |
| 25 (F) | 11/08 | 44 | 35 | 47 | 36 | 49 | 28 |
| 26 (M) | 04/10 | 62 | 26 | 64 | 12 | 64 | 9 |
| 27 (M) | 12/11 | 68 | 27 | 70 | 23 | 70 | 3 |
| 28 (M) | 03/11 | 58 | 34 | 59 | 34 | 60 | 8 |
| 29 (M) | 07/07 | 57 | 29 | 59 | 17 | 63 | 50 |
| 30 (F) | 03/07 | 32 | 39 | 33 | 0 | 38 | 66 |
| 31 (M) | 01/01 | 46 | 20 | 53 | 28 | 58 | 57 |
GEMALS synthesis and dose
A dose of (60–180 µg/kg/day) GEMALS was administered
via the sublingual route. Initially all patients were administered
60 µg/kg/day, however the dosage was doubled (when there was a loss
of 1 point of the ALSQ-40 score in 1 month) or tripled (when
doubled dose was not enough to reduce the deterioration and there
was a decrease of ≥1 point of the ALSQ-40 score in 1 month) if the
patients condition deteriorated. As previously described, GEMALS
was synthesized according to patent numbers 792167 (EU) and 6114388
(USA) (12) and purchased from
Farmacia Legnani (Milan, Italy). GEMALS contains a mixture of
functional polypeptides: Fatty acids (including thioctic acid and
oleic acid), antioxidants (including ascorbic acid), free radical
scavengers and amino acids (including taurine and methionine), all
linked to PLL (Table II). If
patients presented with an acceleration of the disease during
follow up, the dosage of GEMALS was doubled to maintain its
protective effect.
 | Table II.Amyotrophic lateral
sclerosis-Endotherapia constituents. |
Table II.
Amyotrophic lateral
sclerosis-Endotherapia constituents.
| PLL
constituents | Final concentration
(M) |
|---|
|
Glucosamine-PLL |
1×10−04 |
| Agmatine-PLL |
1×10−04 |
| Uric acid-PLL |
1×10−04 |
|
α-Tocopherol-PLL |
3×10−05 |
| Ascorbic
acid-PLL |
3×10−05 |
| CoEnzyme
Q10-PLL-oleic acid |
3×10−05 |
| Retinoic
acid-PLL-oleic acid |
3×10−05 |
| Pantothenic
acid-PLL |
3×10−05 |
| Biotin-PLL |
3×10−05 |
| Oleic
acid-PLL-thioctic acid |
1×10−04 |
| Oleic
acid-PLL-myristic acid |
1×10−04 |
| Oleic
acid-PLL-lauric acid |
1×10−04 |
| Oleic
acid-PLL-linoleic acid |
1×10−04 |
| Oleic
acid-PLL-palmitic acid |
1×10−04 |
| Oleic
acid-PLL-palmitoleic acid |
1×10−04 |
| Lauric
acid-PLL-caprylic acid |
1×10−04 |
| T-T-Farnesyl-L.
cysteine-PLL-palmitic acid |
1×10−04 |
|
Cholesterol-PLL-oleic acid |
1×10−04 |
| L.
Cysteine-PLL |
2×10−04 |
| Taurine-PLL |
2×10−04 |
| L.
Methionine-PLL |
2×10−04 |
| L.
Glutathione-PLL |
1×10−04 |
Score
ALS evaluation was based on the evolution of
ALSAQ-40 scores throughout the follow-up period, with scores
ranging from 40 (normal) to 0 (total loss of motor capabilities).
Two ALSAQ-40 scores were defined and compared: M score, which was
the score of each patient in the present study determined during
follow-up visits; R score, which was the worldwide reference score
of theoretical disease progression without treatment (mean ALSAQ-40
score decrease is 0.769 points/month). Each score was measured as
previously described (18,19). ALSAQ-40 is a patient health status
report used to measure the subjective well being of ALS patients
and is primarily used in clinical trials for novel ALS drugs
(20,21). ALSAQ-40 is sensitive, reliable and an
acceptable tool for the assessment of quality of life in patients
with ALS (2,20). To estimate the effect of GEMALS
treatment, a reference curve of the mean individual evolution of
ALSAQ-40 score over time was constructed. Patient responses were
evaluated as follows: Progression, the individual mean speed of
evolution of ALSAQ-40 score with GEMALS was ≤R score (−0.769
points/month); decrease, the individual mean speed of evolution of
ALSAQ-40 score with GEMALS was between −0.769 and 0 points/month;
stabilization, the individual mean speed of evolution of ALSAQ-40
score with GEMALS was 0 points/month; and improvement, the
individual mean speed of evolution of ALSAQ-40 score with GEMALS
was >0 points/month.
Statistical analysis
Due to the high variability of the ALS cohort
included in the present study, a previously published method of
adapted valuation was used (11).
The Mann-Whitney U test was used to compare the study population
with the reference population. Analyses were performed using SAS
Version 9 (SAS Institute, Inc., Cary, NC, USA) and statistical
analysis was conducted externally by Stalphamis (Le Bourg, France).
P<0.05 was considered to indicate a statistically significant
difference.
A comprehensive study of the ALSAQ-40 score,
ALSAQ-40 evolution and the evolution of the ALSAQ-40 score based on
each item was performed. For descriptive analysis, the following
parameters were investigated: Strength, percentage, distribution,
minimum, 1st quartile, median, 3rd quartile, maximum,
mean, standard deviation and 95% confidence intervals. For
comparative analysis, Student's t test was used to compare final M
and R scores. The equality of variance was verified using the
folded F test. Evolution of the ALSAQ-40 score was analyzed using a
linear regression model and the following parameters were used to
analyze M and R scores: Coefficient of correlation (R2)
and associated P-value, intercept and slope (ax+b). A χ2
or Fisher's exact test were used to compare success and failure
rates according to the theoretical strength obtained by unilateral
assumption (% success >% of failure).
Results
General considerations
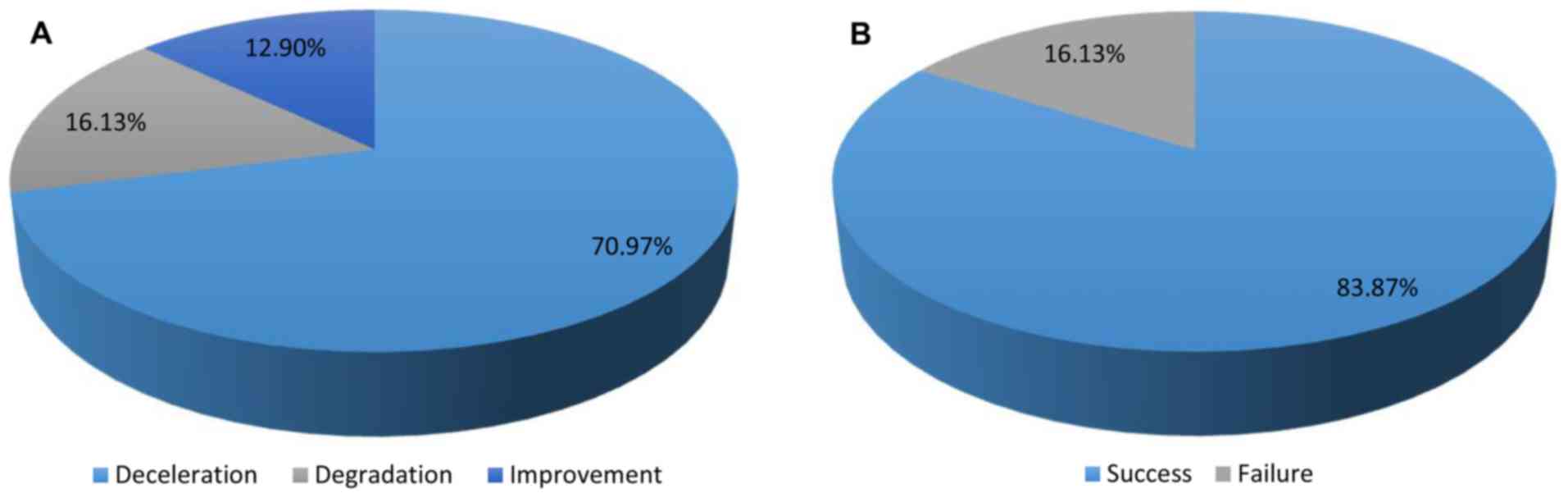
In the 31 patients treated with GEMALS, 16.13%
experienced a worsening of their condition (degradation), 70.97%
experienced a decrease in the progression of the disease
(deceleration) and 12.90% experienced a reversal of disease
evolution (improvement) (Fig. 1 and
Table III). When the final score
was higher than the initial score, the disease was considered to
have improved. When the initial and final scores were equal or the
final M score was higher than the R score, the disease was
considered to have decelerated. When the final M score was lower
than the R score, the evolution was considered have degraded
(Fig. 1 and Table III). The mean duration of GEMALS
treatment was of 988 days with a standard deviation of 902 days
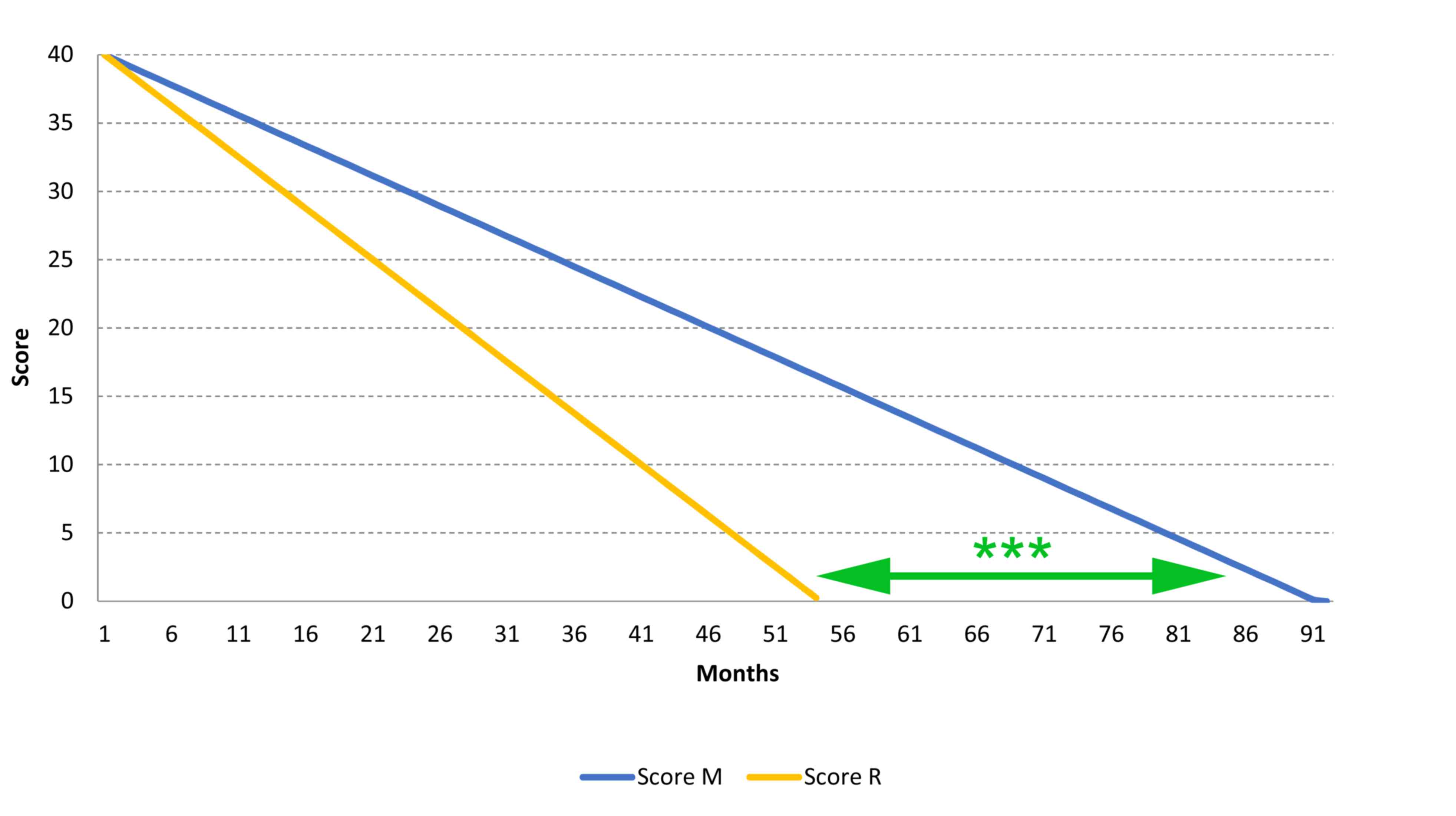
(minimum, 98 days; maximum, 4,287 days). The mean score evolution
of patients treated with GEMALS was −0.40 points/month and the
worldwide reference score evolution was −0.77 points/month
(Fig. 2); this represents a
significant increase in life expectancy of 38 months (3.16 years).
A total of 83.87% of patients treated with GEMALS had an
improvement in the evolution of ALS following GEMALS treatment
(P<0.0001; Table III).
Additionally, no side effects were reported and following rigorous
questioning of the patient no clinical symptoms including,
cutaneous alterations, flu-like symptoms, gastrointestinal
troubles, migraines, cephalea, insomnia, nauseas and vomiting were
described. Additionally the biological parameters (blood cell
count, ionograms, lipidic balance, hepatic enzymes, renal
filtration or glycaemia) were unaltered.
 | Table III.Effects of amyotrophic lateral
sclerosis-Endotherapia treatment (n=31). |
Table III.
Effects of amyotrophic lateral
sclerosis-Endotherapia treatment (n=31).
|
| No. patients
(%) |
|
|
|---|
|
|
|
|
|
|---|
| Evolution | Male (n=21) | Female (n=10) | Total | Success (%) | P-value |
|---|
| Improvement | 2 (9.52) | 2 (20.00) | 4 (12.90) | 26/31 (83.87) | <0.0001 |
| Deceleration | 14 (66.67) | 8 (80.00) | 22 (70.97) |
|
|
| Degradation | 5 (23.80) | 0 (0) | 5 (16.13) |
|
|
Item scores
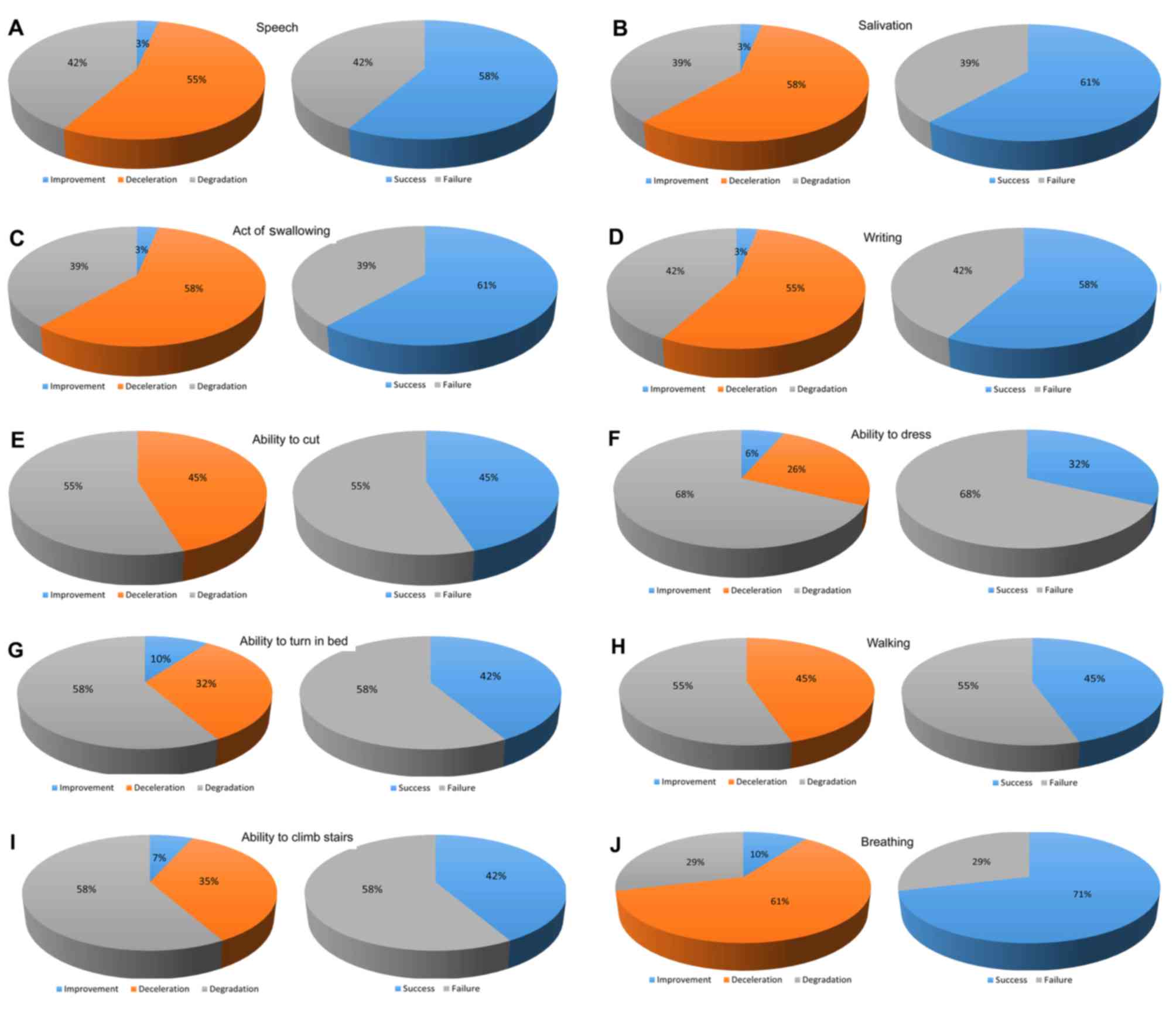
Following GEMALS treatment, the M score was
significantly higher than the R score for the following functions:
Speech (P<0.005), salivation (P<0.001), swallowing
(P<0.001), writing (P<0.05), ability to turn in bed
(P<0.05), walking (P<0.01) and breathing (P<0.001)
(Table IV). No significant
differences between M and R scores were observed for ability to cut
food, ability to dress and ability to climb stairs (Table IV).
 | Table IV.Scores according to each item. |
Table IV.
Scores according to each item.
| Item | Inclusion | M | R |
|---|
| Speech |
2.9±1.4 |
2.3±1.7c |
1.2±1.1 |
| Salivation |
3.3±0.9 |
2.6±1.4d |
1.3±1.1 |
| Swallowing |
3.4±0.9 |
2.8±1.3d |
1.4±1.1 |
| Writing |
2.9±1.0 |
1.8±1.6a |
1.1±1.1 |
| Ability to cut
food |
2.6±1.3 |
1.5±1.3 |
0.9±1.0 |
| Ability to
dress |
2.6±1.2 |
1.3±1.2 |
1.0±1.1 |
| Ability to turn in
bed |
3.0±1.3 |
2.0±1.5a |
1.2±1.2 |
| Walking |
2.6±0.9 |
1.7±1.1b |
1.0±1.0 |
| Ability to climb
stairs |
2.2±1.3 |
1.1±1.2 |
0.9±1.0 |
| Breathing |
3.1±1.2 |
2.7±1.4d |
1.3±1.2 |
Item score evolutions
Patients were considered to have improved if there
was an increase in the M score over time. If the M score decreased
over time and this decrease was <0.769 points/month, patients
were considered to have decelerated. Patients were considered to
have degraded if the M score decreased throughout the treatment
period at >0.769 points/month. Improvement and deceleration
indicate treatment success and degradation indicates treatment
failure. Items with a success rate >50% are presented in
Fig. 3. The results indicate that
the success rate for breathing was significantly greater than the
failure rate (P<0.05; Fig.
3).
Overall study
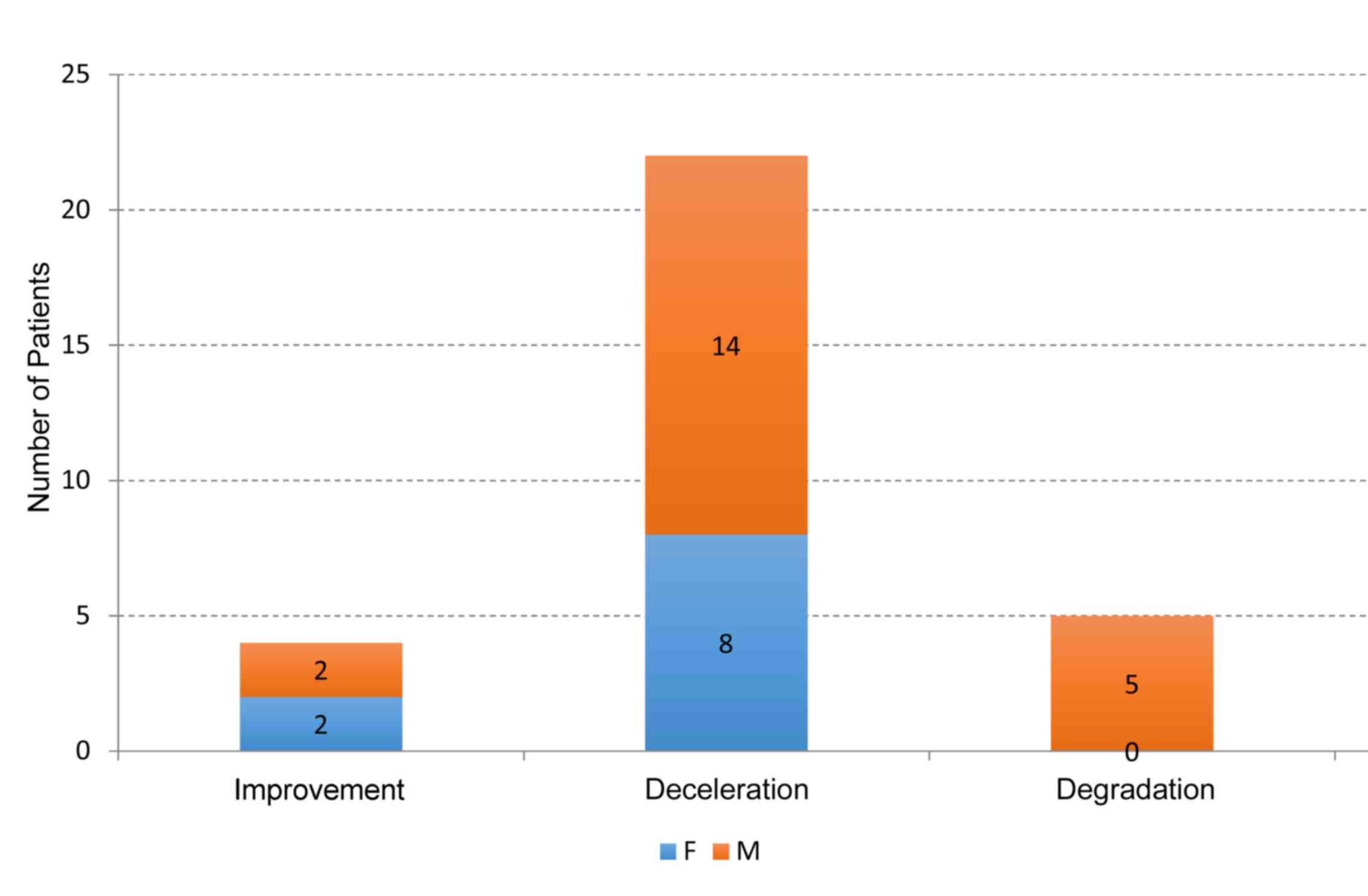
Overall evolutions for each patient were calculated
by linear regression, the evolution category (improvement,
deceleration and degradation) mean values are presented in Table V. The mean overall M score was 19.8,
which was significantly higher than the mean overall R score of
11.7 (P=0.0013). The mean values at the end of the study represent
an amelioration of the M group vs. the R group at 30.8% (P=0.0002).
The study population included 10 women and 21 men; the success rate
of treatment was 100% for women and 76.19% for men (Table III; P<0.0001). Overall the
treatment was significantly successful (P<0.0001; Table III and Fig. 4).
 | Table V.Global evolution mean values. |
Table V.
Global evolution mean values.
|
|
| Mean values |
|---|
|
|
|
|
|---|
| Evolution | No. cases | R2M
Group | P-value | M Slope | R2 R
Group | P-value | R Slope |
|---|
| Improvement | 4 | 0.58 | 0.125 | 0.00234 | −0.98 | <0.0004 | −0.02075 |
| Deceleration | 22 | −0.86 | <0.049 | −0.01357 | −0.98 | <0.0003 | −0.02325 |
| Degradation | 5 | −0.93 | <0.040 | −0.03118 | −0.94 | <0.0001 | −0.02120 |
Discussion
In a previous study using a SOD1 rat model, it was
demonstrated that GEMALS significantly extended lifespans and
improved electromyographic parameters (12). However, no significant increase in
life expectancy was observed when rats were treated with Riluzole
or other pharmacological agents, including minocycline and
nifedipine (12). Two GEMALS
concentrations were also assessed and the results suggested that
the effects of GEMALS may be dose-dependent (12). Based on these results, the higher
dose (3.75 mg/ml) administered to SOD1 rats was extrapolated for
use in the present study in humans (4–12.05 mg/day). In the SOD1
animal model and humans GEMALS demonstrated a beneficial
effect.
The effect of GEMALS in 12 patients with ALS has
been previously reported (11). The
results indicated that GEMALS treatment caused a 50.07% decrease in
the loss of functional capacities, corresponding to a mean life
expectancy increase of 52.57 months (4.38 years). In the present
study, there was an increase in life expectancy of 38 months (3.16
years). Each study indicates that GEMALS is an effective method of
treating ALS, with beneficial effects observed in 83% of patients
in the previous report (11) and
83.7% in the present study. In the previous study (11), the ALSAQ40 was reported; however,
data regarding specific functions, including speech, were not
available. In the present study, the effect of GEMALS treatment on
specific functions was evaluated and significant improvements in
breathing, walking, salivation, speech, swallowing and writing were
observed.
The glutamate release blocker Riluzole is, at
present, the only authorized treatment for ALS; however, Riluzole
treatment extends life expectancy by only a few months (3). It has been demonstrated that treatment
with Riluzole for 12 months reduces deterioration of muscular
function by 33.4% and extends life expectancy by 2.77 months
(4). It has also been reported that
treatment with Riluzole for 12 months extends survival by 4.2
months; however, this advantageous effect is transient and
disappears when the monitoring period is extended (5). This transient effect has been reported
in another study, with Riluzole only effective at treating patients
affected by the bulbar form of ALS, which represents ~30% of all
ALS cases (18).
It has previously been reported that GEMALS
treatment decreases the loss of functional capacities that occurs
in patients with ALS (11). No side
effects have been reported following treatment with GEMALS or GEMSP
(13). Similarly, in the current
study, no side effects following GEMALS treatment were reported.
This may be due to the low dose of GEMALS received by each patient.
The individual doses administered were calculated according to the
evolution of each patient at follow-up; thus, doses ranged from 60
to 180 µg/kg/day. PLL accounts for ~90% of the drug molecular
weight, therefore only 0.4–1.2 mg of small molecules were delivered
per administration.
To overcome the high variability of the ALS cohort
studied here, the Mann-Whitney U test was used as previously
reported (11). A total of 21 men
and 10 women were included in the present study and the treatment
success rates were 76.2 and 100%, respectively, however future
studies should use a larger population size to confirm the results
of the present study. Compared with Riluzole therapy, GEMALS
increased the life expectancy of patients with ALS from 4 to 38
months (3). Compared with the GEMALS
pilot study (11), the life
expectancy of patients decreased from 52.57 to 38 months. However,
if patients 26 and 27, who did not respond to treatment with
GEMALS, were excluded from further analyses, the mean increase in
life expectancy in the current study was 89 months (n=29).
The activity of Riluzole is based on the blockage of
excitatory amino acid pathways. It blocks sodium dependent voltage
channels, thus decreasing the release of glutamate (22). Taurine, one of the constituents of
GEMALS, also reduces glutamate excitotoxicity by enhancing
mitochondrial function and regulating intracellular calcium
homeostasis (23). ALS is a
multifactorial disease in which oxidative stress, mitochondrial
damage, neuroinflammation, zinc excitotoxicity and apoptosis serve
important roles. Treatment of ALS therefore requires a
multifactorial strategy; GEMALS, a tailor-made combination therapy
composed of functional polypeptides, was developed for this
purpose. In the present study, the constituents of GEMALS were
rigorously selected according to their known effects on the
regeneration or protection of neuronal components (10,11).
These constituents were selected with the aim of decreasing
oxidative and free radical stress, reducing inflammation,
inhibiting exogenous toxic factors and exhibiting neuroprotective
effects (12).
All PLL-linked molecules used in the present study
are endogenous and non-immunogenic, allowing for long-term
administration without the induction of toxicity and/or side
effects. The quantities of PLL-linked molecules represent a low
proportion (w/w) of the overall dosage, which may account for the
absence of side effects following treatment with GEMALS and GEMSP
(11). Free components not linked to
PLL have been reported to have no therapeutic effect in
experimental animal models (14–16,24). PLL
therefore appears to be more than a simple vector; PLL compounds
exhibit therapeutic properties. PLL-linked molecules are able to
cross the blood brain barrier, which is important as certain
molecules, including butyrilcholinesterase (molecular weight, 340
KDa), are unable to do so alone (25). Furthermore, PLL-linkage may increase
the active duration of molecules; for example, PLL-linked
butyrilcholinesterase remains active for ≥72 h (25). The results of a previous study
investigating GEMSP indicated that methionine-PLL, a constituent of
GEMSP, was present in the motor neurons of the spinal cord
(15), exerting potential
neuroprotective action. This observation is similar to that of a
different study, in which PLL was identified inside organelles and
the nuclear membrane (26).
The constituents of GEMALS exert the following
actions: i) Poly-unsaturated fatty acids exert a neuroprotective
effect and act as free radical scavengers to prevent oxidation of
unsaturated fatty acids in the cell membrane (27,28); ii)
α-tocopherol exerts a neuroprotective effect by potentially
attenuating the entry of Ca2+ via transient receptor
potential-like channels (29); iii)
ascorbic acid is necessary for the maintenance of neuronal
functions and exerts an antioxidant effect (30); iv) polyamine agmatine exerts a
neuroprotective effect against the excitotoxic action of glutamate
(31); v) cysteine, methionine,
glutathione, retinoic acid, pantothenic acid and biotin act as
antioxidants and scavengers to reduce the apoptosis and neuronal
death induced by reactive oxygen species (32); and vi) taurine modulates
mitochondrial calcium homeostasis (33).
In conclusion, a multifactorial strategy for the
treatment of ALS is required and GEMALS may be an effective
treatment. The present study is an in-depth investigation
demonstrating and confirming the effectiveness of GEMALS in a
larger cohort of patients with ALS. The results suggest that GEMALS
treatment significantly increases life expectancy and decreases the
loss of important motor functions in patients with ALS; however, a
phase IIb multicentric, randomized and double-blinded clinical
trial is required to confirm these results.
Acknowledgements
The present study was supported by the Institut pour
le Développement de la Recherche en Pathologie Humaine et
Thérapeutique. The authors would like to thank Mr. B. Combes
(Stalphamis; Le Bourg, France) for conducting statistical
analysis.
References
|
1
|
Shamshiri H, Fatehi F, Abolfazli R,
Harirchian MH, Sedighi B, Zamani B, Roudbari A, Razazian N, Khamseh
F and Nafissi S: Trends of quality of life changes in amyotrophic
lateral sclerosis patients. J Neurol Sci. 368:35–40. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jenkinson C, Norquist JM and Fitzpatrick
R: Deriving summary indices of health status from the amyotrophic
lateral sclerosis assessment questionnaires (ALSAQ-40 and ALSAQ-5).
J Neurol Neurosurg Psychiatry. 74:242–245. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boillée S and Lobsiger CS: Glial cells not
that supportive for motor neurons. Med Sci (Paris). 24:124–126.
2008.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bensimon G, Lacomblez L and Meininger V: A
controlled trial of Riluzole in amyotrophic lateral sclerosis.
ALS/Riluzole Study Group. New J Eng Med. 330:585–591. 1994.
View Article : Google Scholar
|
|
5
|
Traynor BJ, Alexander M, Corr B, Frost E
and Hardiman O: An outcome study of riluzole in amyotrophic lateral
sclerosis - a population-based study in Ireland, 1996–2000. J
Neurol. 250:473–479. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meininger V, Lacomblez L and Salachas F:
What has changed with riluzole? J Neurol. 247:19–22. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rossi S: Australian medicines handbook.
Australian Med Handbook Unit Trust Adelaide. 2013.
|
|
8
|
Ascherio A, Weisskopf MG, O'reilly EJ,
Jacobs EJ, McCullough ML, Calle EE, Cudkowicz M and Thun MJ:
Vitamin E intake and risk of amyotrophic lateral sclerosis. Ann
Neurol. 57:104–110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jones SP, Guillemin GJ and Brew BJ: The
kynurenine pathway in stem cell biology. Int J Tryptophan Res.
6:57–66. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Geffard M, de Bisschop L, Duleu S,
Hassaine N, Mangas A and Coveñas R: Endotherapia: A new frontier in
the treatment of multiple sclerosis and other chronic diseases.
Discov Med. 10:443–451. 2010.PubMed/NCBI
|
|
11
|
Geffard M, de Bisschop L, Duleu S, Pouns
O, Ferran G, Bessede A, Hassaine N, Autran JL, Bodet D, Mangas A
and Covenas R: Endotherapia. Antiinflamm Antiallergy Agents Med
Chem. 9:197–211. 2010. View Article : Google Scholar
|
|
12
|
Nicaise C, Coupier J, Dabadie MP, De
Decker R, Mangas A, Bodet D, Poncelet L, Geffard M and Pochet R:
Gemals, a new drug candidate, extends lifespan and improves
electromyographic parameters in a rat model of amyotrophic lateral
sclerosis. Amyotroph Lateral Scler. 9:85–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Geffard M, Duleu S, Bessede A, Vigier V,
Bodet D, Mangas A and Coveñas R: GEMSP: A new therapeutic approach
of multiple sclerosis. Cent Nerv Syst Agents Med Chem. 12:173–181.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mangas A, Covenas R, Bodet D, Dabadie MP,
Glaize G and Geffard M: Evaluation of the effects of a new drug on
brain leukocyte infiltration in an experimental model of autoimmune
encephalomyelitis. Lett Drug Des Discov. 3:138–148. 2006.
View Article : Google Scholar
|
|
15
|
Mangas A, Coveñas R, Bodet D, de León M,
Duleu S and Geffard M: Evaluation of the effects of a new drug
candidate (GEMSP) in a chronic EAE model. Int J Biol Sci.
4:150–160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mangas A, Coveñas R, Bodet D, Duleu S and
Geffard M: A new drug candidate (GEMSP) for multiple sclerosis.
Curr Med Chem. 16:3203–3214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Couratier P, Marin B, Laurette G, Nicol M
and Preux PM: Epidemiologie, clinical spectrum of ALS and
differential diagnoses. Presse Med. 43:538–548. 2014.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kimura F, Fujimura C, Ishida S, Nakajima
H, Furutama D, Uehara H, Shinoda K, Sugino M and Hanafusa T:
Progression rate of ALSFRS-R at time of diagnosis predicts survival
time in ALS. Neurology. 66:265–267. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Labra J, Menon P, Byth K, Morrison S and
Vucic S: Rate of disease progression: A prognostic biomarker in
ALS. J Neurol Neurosurg Psychiatry. 87:628–632. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jenkinson C, Fitzpatrick R, Brennan C,
Bromberg M and Swash M: Development and validation of a short
measure of health status for individuals with amyotrophic lateral
sclerosis/motor neurone disease: The ALSAQ-40. J Neurol. 3 246
Suppl:III16–III21. 1999. View Article : Google Scholar
|
|
21
|
Jenkinson C, Fitzpatrick R, Brennan C and
Swash M: Evidence for the validity and reliability of the ALS
assessment questionnaire: The ALSAQ-40. Amyotroph Lateral Scler
Other Motor Neuron Disord. 1:33–40. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dolfi SC, Medina DJ, Kareddula A, Paratala
B, Rose A, Dhami J, Chen S, Ganesan S, Mackay G, Vazquez A and
Hirshfield KM: Riluzole exerts distinct antitumor effects from a
metabotropic glutamate receptor 1-specific inhibitor on breast
cancer cells. Oncotarget. 8:44639–44653. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
El Idrissi A: Taurine increases
mitochondrial buffering of calcium: Role in neuroprotection. Amino
Acids. 34:321–328. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mangas A, Coveñas R and Geffard M: New
drug therapies for multiple sclerosis. Curr Opin Neurol.
23:287–292. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gaydess A, Duysen E, Li Y, Gilman V,
Kabanov A, Lockridge O and Bronich T: Visualization of exogenous
delivery of nanoformulated butyrylcholinesterase to the central
nervous system. Chem Biol Interact. 187:295–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Z, Zhu H, Li D and Yang X:
Preparation and application of single polyelectrolyte microcapsules
possessing tunable autofluorescent properties. Colloids Surfaces A:
Physicochem Eng Aspects. 329:58–66. 2008. View Article : Google Scholar
|
|
27
|
Blondeau N, Widmann C, Lazdunski M and
Heurteaux C: Polyunsaturated fatty acids induce ischemic and
epileptic tolerance. Neuroscience. 109:231–241. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yoshida H, Yanai H, Namiki Y,
Fukatsu-Sasaki K, Furutani N and Tada N: Neuroprotective effects of
edaravone: A novel free radical scavenger in cerebrovascular
injury. CNS Drug Rev. 12:9–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Crouzin N, de Jesus Ferreira MC,
Cohen-Solal C, Aimar RF, Vignes M and Guiramand J:
Alpha-tocopherol-mediated long-lasting protection against oxidative
damage involves an attenuation of calcium entry through TRP-like
channels in cultured hippocampal neurons. Free Radic Biol Med.
42:1326–1337. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qiu S, Li L, Weeber EJ and May JM:
Ascorbate transport by primary cultured neurons and its role in
neuronal function and protection against excitotoxicity. J Neurosci
Res. 85:1046–1056. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang WP, Iyo AH, Miguel-Hidalgo J,
Regunathan S and Zhu MY: Agmatine protects against cell damage
induced by NMDA and glutamate in cultured hippocampal neurons.
Brain Res. 1084:210–216. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Agar J and Durham H: Relevance of
oxidative injury in the pathogenesis of motor neuron diseases.
Amyotroph Lateral Scler Other Motor Neuron Disord. 4:232–242. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kumari N, Prentice H and Wu JY: Taurine
and its neuroprotective role. Adv Exp Med Biol. 775:19–27. 2013.
View Article : Google Scholar : PubMed/NCBI
|