Introduction
Each year, 338,000 patients are newly diagnosed with
kidney cancer worldwide (1). Renal
cell carcinoma (RCC) accounts for 3% of all adult malignancies and
90% of neoplasms arising from the kidney (2). Of patients with initially localized
disease who undergo nephrectomy, 20–40% develop metastases
(3). Patients with metastatic
disease have a poor prognosis, with 5-year survival rates <10%
(3). Molecular targeted therapies
have markedly improved the prognosis of patients with metastatic
RCC. In metastatic renal cell carcinoma (mRCC) the tyrosine-kinase
inhibitor sunitinib leads to progression-free survival (PFS) of
11.5 months and an overall survival (OS) of 26 months, which may
reach 40 months with adequate sequential therapy (4–7).
Patients are now able to receive different treatments, including
surgery, molecular target therapies and immunotherapies depending
on their age and past history.
In Japan, four types of tyrosine kinase inhibitors
(TKIs), sorafenib, sunitinib, axitinib and pazopanib; and two types
of mammalian targets of rapamycin (mTOR) everolimus and
temsirolimus are available for patients with metastatic RCC as a
first-line molecular targeted therapy. However, physicians are
currently required to select the appropriate treatment for each
patient based on the stage of cancer and age of the patient
etc.
In the present study, the cases of two patients with
metastatic RCC from very different backgrounds and at very
different life stages were examined. Both patients provided written
informed consent for inclusion in the study.
Case report
Case 1
The first patient was an 80-year-old Japanese female
who had been diagnosed with a left 7 cm renal mass and was referred
to Tokyo Medical University Ibaraki Medical Center (Ibaraki, Japan)
in June, 2011. The patient had a history of cerebral infarction and
impaired eyesight. Laboratory findings were as follows: Hemoglobin
(Hb), 13.8 g/ml (normal range, 12~16 g/ml); hematocrit (Ht), 40.4%
(normal range, 34~42%); leukocytes, 4,600 cells/ml (normal range,
4,000~9,000 cells/ml); blood urea nitrogen (BUN), 8.4 mg/dl (normal
range, 8~23 mg/dl); and creatinine (Cr), 0.98 mg/dl (normal range,
0.6~1.2 mg/dl). Urinalysis indicated 0–1 (normal range, <5)
white and 0–1 (normal range, <5) red blood cells per high-power
field.
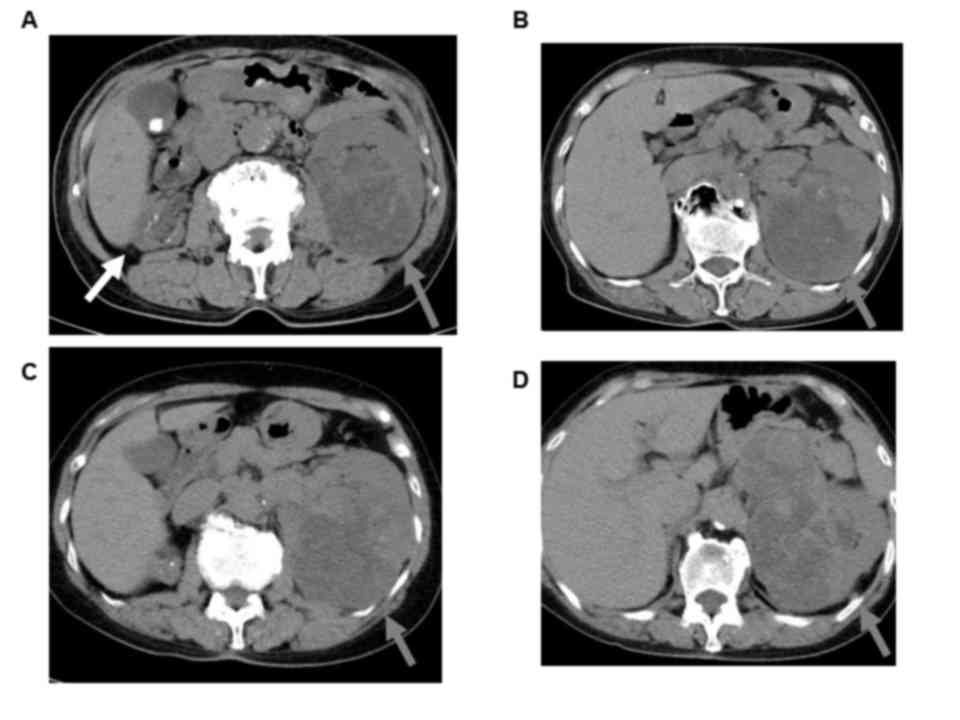
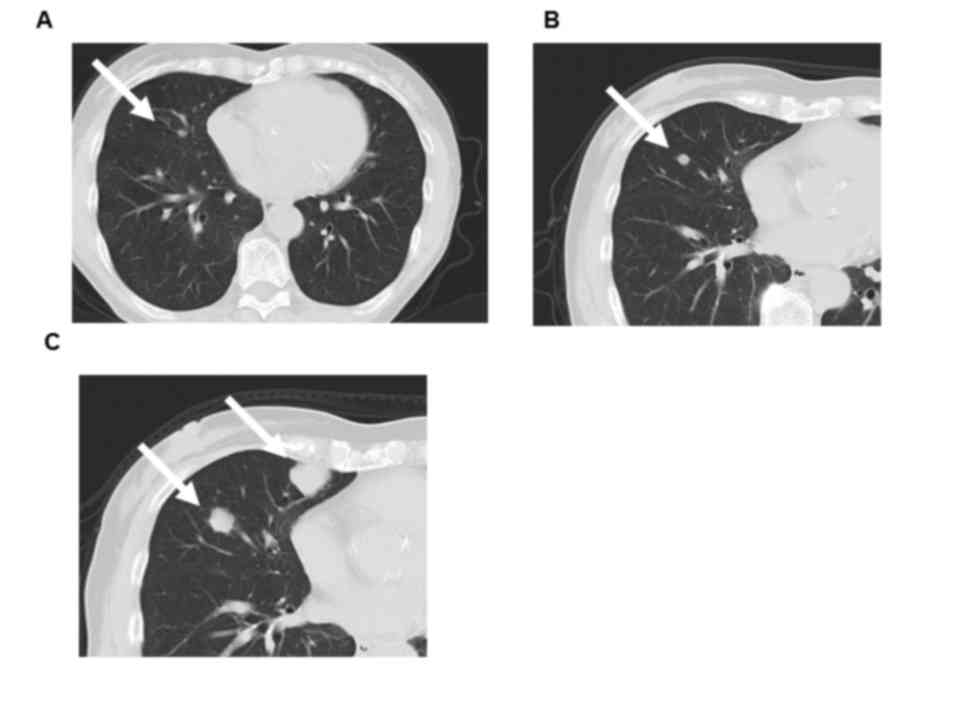
Computed tomography (CT) scan and magnetic resonance
imaging (MRI) revealed a solid mass of ~7 cm in the left kidney
with small pulmonary metastases and indicated that the patient's
right kidney was atrophic (Figs. 1
and 2). This renal tumor was
hypervascular and suggestive of RCC.
Following consultation with the patient and family
regarding the treatment, conservative treatment was selected, as
the patient would have required dialysis, due to having a right
atrophic kidney, if a left radical nephrectomy was performed.
Furthermore, the patient was already wheelchair bound, due to
impaired eyesight and brain infarction.
Therefore, the patient began treatment with oral
sorafenib (Bayer, Leverkusen, Germany) at 200 mg/day. With a
prominent rise in the blood levels of creatine phosphokinase (CPK)
up to 452 IU (normal range, 32~187 IU), the dose of oral sorafenib
was reduced to 200 mg every 2 days. This dose was well tolerated by
the patient without adverse events.
Disease progression was observed on a follow-up CT
scan every 6 months for 5 years, which demonstrated that the left
renal tumor and pulmonary metastases remained stable for first two
years but they were slowly progressing and new pulmonary metastases
had emerged after 2 years (Fig. 2).
However, the patients' quality of life (QOL), including renal
function, was well maintained up to 5 years following the
initiation of sorafenib treatment. In addition, the patient has
continued to use sorafenib on this schedule despite mid disease
progression as no severe adverse events have occurred.
Case 2
The second patient was a 39-year-old Japanese male
who had been diagnosed with a left 19 cm of renal mass and was
referred to Tokyo Medical University Ibaraki Medical Center
November, 2015. Laboratory findings were as follows: Hb, 13.6 g/ml
(normal range, 14~17 g/ml); Ht, 41.8% (normal range, 40~48%);
leukocytes, 6,500 cells/ml (normal range, 4,000~9,000 cells/ml);
BUN, 12.7 mg/dl (normal range, 8~23 mg/dl); and Cr, 1.01 mg/dl
(normal range, 0.6~1.2 mg/dl). Urinalysis indicated 10–19 (normal
range, <5) white and >100 (normal range, <5) red blood
cells per high-power field.
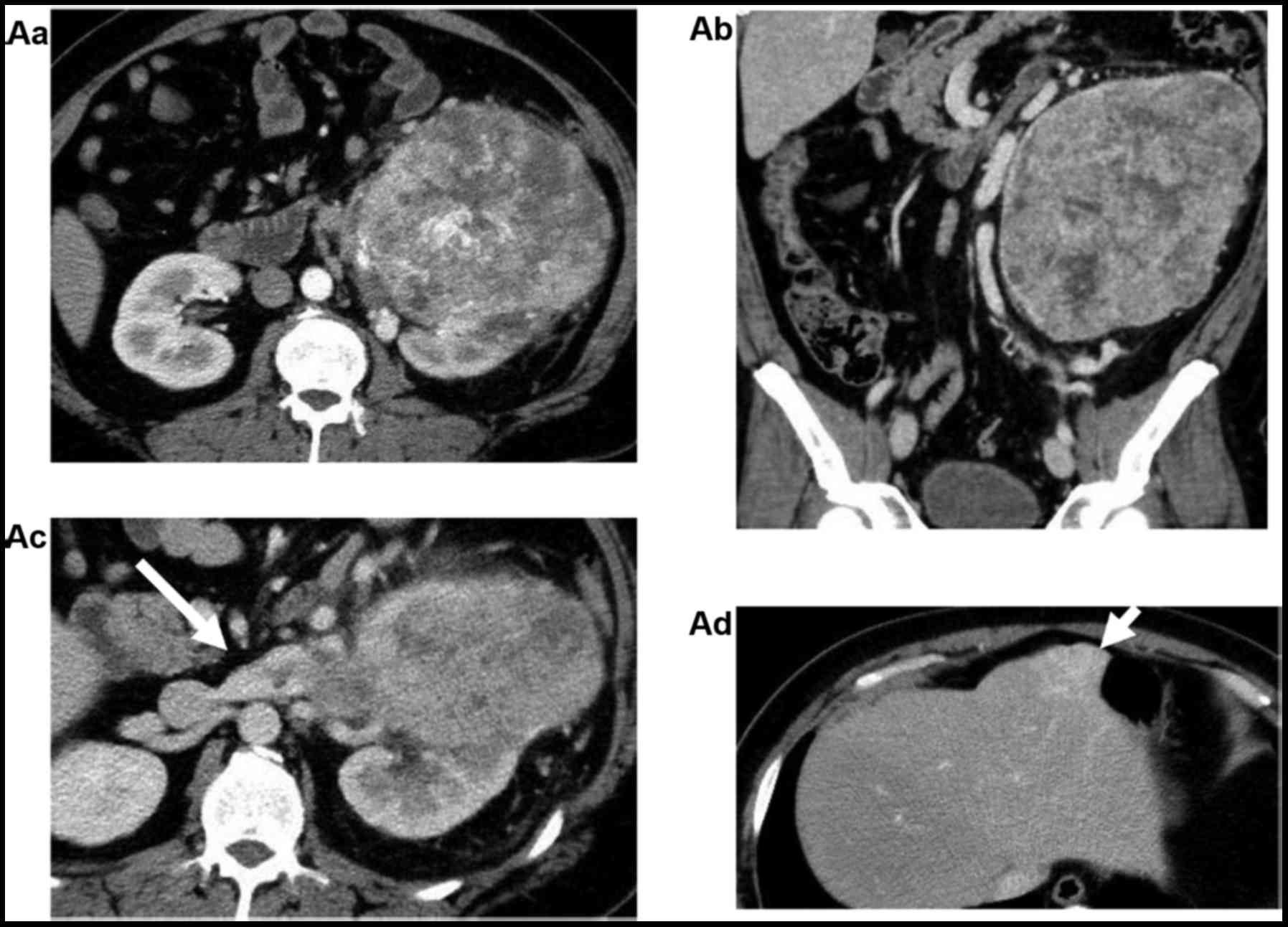
CT and MRI identified a solid mass, ~19 cm in the
left kidney with multiple pulmonary, hepatic and lymph node
metastases and a long embolus in the left renal vein (Fig. 1A). This renal tumor was hypervascular
and suggestive of RCC.
Due to the large size of the renal tumor, the
patient began treatment with oral pazopanib (GlaxoSmithKline plc,
London, UK) at 800 mg/day to reduce the tumor volume. The pazopanib
treatment was continued with only slight fatigue without major
adverse effects.
Following 6 months from the initiation of pazopanib
treatment, a CT scan indicated a ~10% regression based on the
Response Evaluation Criteria in Solid Tumors (8). In addition, the long embolus in the
left renal vein was reduced, reaching from across the aortic
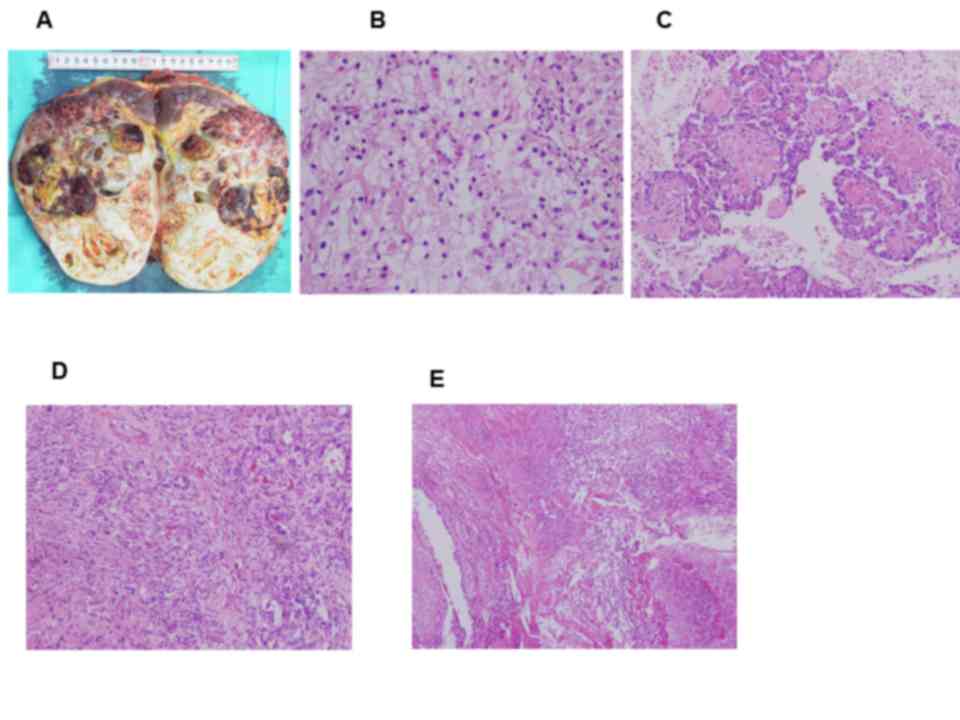
atresia to the left side of aortic atresia (Fig. 3). A left radical nephrectomy was
performed. The histology of the renal tumor indicated clear cell
RCC, Fuhrman nuclear grade 2 (9),
with features of papillary RCC and sarcomatoid carcinoma that was
classified as pathological stage T3aN1M1 according to TMN
classification (Fig. 4) (10). In addition, some areas exhibited
necrosis. The patient has continued to use pazopanib at 800 mg/day
following left radical nephrectomy.
Discussion
With the introduction of molecular targeted therapy,
treatment of metastatic RCC has improved markedly; previously, only
immunotherapies, including interferon-α and -γ or interleukin
(IL)-2, were used to treat renal cancer (11). However, immunotherapies do not have a
prominent effect on the response or survival of late stage RCC
(11).
Molecular targeted therapy exhibits prominent
effects in the treatment of RCC. However, the adverse effects,
including general fatigue or myelosuppression are severe and the
treatment often has to be discontinued (12–15). The
continuity of molecular targeted therapy is important and further
consideration of the prescription plan depending on the state of
the patient and tumor status is required. In addition, it has been
reported that there are differences in the clinical features of
patients with RCC, for example elderly patients with RCC display
significantly higher cancer-specific mortality and other-cause
mortality rates in comparison with their younger counterparts
(16). There is a possibility that
treatment according to standard guidelines may not be suitable in
every case.
In the present study, case 1 was an elderly patient
with comorbidities. The patients' renal tumor exhibited slow
progression; however, QOL was well maintained and the molecular
targeted therapy was well tolerated without severe adverse events.
Generally, in case of elderly patients, treatment is discontinued
due to adverse events such as fatigue and loss of appetite;
however, sorafenib induces fewer adverse effects than other TKIs
and the use of sorafenib is recommended for such patients (17). In particular, sorafenib has little
influence on the renal function, so it is better for older patients
such as case 1, enabling them to avoid further deterioration of
kidney function (18). However,
vascular endothelial growth factor inhibitors induce major adverse
effects, including myelosuppression, cardiotoxicity, hypertension,
thrombocytopenia, fatigue and loss of appetite (19). Therefore, patients with poor
conditions should consider treatment with mTOR inhibitors such as
everolimus and temsirolimus.
Sorafenib does not induce evident adverse events;
however, the rate of continuation of sorafenib treatment >1 year
is only 30% in Japan (20). To
continue treatment, optimal adjustment dose or a prescription
interval is required.
Nephrectomy was performed on case 2 following
cytoreduction using pazopanib. In the cytokine therapy period
(interferon and Il-2), nephrectomy to resect the primary tumor for
patients with metastatic RCC is useful in improving the survival
rate (cytoreductive nephrectomy) (21–23). A
number of studies report that cytoreductive nephrectomy improves
the survival rate in patients with metastatic RCC undergoing
molecular targeted therapy (24,25).
However, the usefulness of molecular targeted therapy administered
prior to surgery as neoadjuvant therapy for patients with
metastatic RCC remains controversial (26–28).
Setting an end point for neoadjuvant therapy is difficult and the
timing of the surgery might be missing by the therapy if molecular
targeted therapy dose not effect for RCC and tumor would be
growing.
However, molecular targeted therapy is able to
reducing the primary tumor size (19), therefore, there is a possibility that
molecular targeted therapy administered prior to surgery may reduce
the invasiveness of surgery for patients with local invasive
RCC.
However, care must be taken prior to administering
molecular targeted therapy prior to surgery to avoid adverse
effects which may, in turn, influence surgical treatment.
Case 2 was a young patient with a high PS; thus,
resection of the primary tumor by surgery was recommended. In this
case, primary tumor resection was safely performed following a
reduction of the primary tumor volume by administration of
molecular targeted therapy, which did not induce any severe adverse
effects.
In conclusion, the present study examined the cases
of two patients with metastatic RCC with very different backgrounds
who were at different life stages. Case 1 was elderly and with a
past medical history that included cerebral infarction, therefore
the dose of sorafenib used for treatment was reduced. Case 2 was
younger and exhibited enough PS, therefore surgical resection of
the primary tumor was deemed desirable. The current study indicated
that molecular targeted therapy is effective at reducing the tumor
burden of RCC prior to radical nephrectomy. A number of different
methods of molecular targeted therapy may be used, depending on the
state of the patient and the tumor status. The current case report
furthers understanding of the characteristics of molecular targeted
therapy in the treatment of renal cell carcinoma.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chow WH, Devesa SS, Warren JL and Fraumeni
JF Jr: Rising incidence of renal cell cancer in the United States.
JAMA. 281:1628–1631. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fry DE, Amin M and Harbrecht PJ: Rectal
obstruction secondary to carcinoma of the prostate. Ann Surgery.
189:488–492. 1979.
Motzer RJ, Bander NH and Nanus DM: Renal
cell carcinoma. N Engl J Med. 335:865–875. 1996.
|
|
4
|
Escudier B, Eisen T, Stadler WM, Szczylik
C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA,
et al: Sorafenib in advanced clear-cell renal-cell carcinoma. N
Engl J Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hudes G, Carducci M, Tomczak P, Dutcher J,
Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi
I, et al: Temsirolimus, interferon alfa, or both for advanced
renal-cell carcinoma. N Engl J Med. 356:2271–2281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik
C, Kim ST, et al: Sunitinib versus interferon alfa in metastatic
renal-cell carcinoma. N Engl J Med. 356:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rini BI, Escudier B, Tomczak P, Kaprin A,
Szczylik C, Hutson TE, Michaelson MD, Gorbunova VA, Gore ME,
Rusakov IG, et al: Comparative effectiveness of axitinib versus
sorafenib in advanced renal cell carcinoma (AXIS): A randomised
phase 3 trial. Lancet. 378:1931–1939. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fuhrman SA, Lasky LC and Limas C:
Prognostic signoficance of morphologic parameters in renal cell
carcinoma. Am J Surg Pathol. 6:655–663. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM classification of malignant tumours. 7th. Wiley-Blackwell;
New Jersey: pp. 255–257. 2009
|
|
11
|
Pantuck AJ, Zisman A and Belldegrun AS:
The changing natural history of renal cell carcinoma. J Urol.
166:1611–1623. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili
R, Bjarnason GA, et al: Overall survival and updated results for
sunitinib compared with interferon alfa in patients with metastatic
renal cell carcinoma. J Clin Oncol. 22:3584–3590. 2009. View Article : Google Scholar
|
|
13
|
Faivre S, Delbaldo C, Vera K, Robert C,
Lozahic S, Lassau N, Bello C, Deprimo S, Brega N, Massimini G, et
al: Safety, pharmacokinetic, and antitumor activity of SU11248, a
novel oral multitarget tyrosine kinase inhibitor, in patients with
cancer. J Clin Oncol. 1:25–35. 2006. View Article : Google Scholar
|
|
14
|
Gore ME, Szczylik C, Porta C, Bracarda S,
Bjarnason GA, Oudard S, Hariharan S, Lee SH, Haanen J, Castellano
D, et al: Safety and efficacy of sunitinib for metastatic
renal-cell carcinoma: An expanded-access trial. Lancet Oncol.
8:757–763. 2009. View Article : Google Scholar
|
|
15
|
Sternberg CN, Davis ID, Mardiak J,
Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA,
Kavina A, et al: Pazopanib in locally advanced or metastatic renal
cell carcinoma: Results of a randomized phase III trial. J Clin
Oncol. 6:1061–1068. 2010. View Article : Google Scholar
|
|
16
|
May M, Cindolo L, Zigeuner R, De Cobelli
O, Rocco B, De Nunzio C, Tubaro A, Coman I, Truss M, Dalpiaz O, et
al: Results of a comparative study analyzing octogenarians with
renal cell carcinoma in a competing risk analysis with patients in
the seventh decade of life. Urol Oncol. 32:1252–1258. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Escudier B, Szczylik C, Hutson TE, Demkow
T, Staehler M, Rolland F, Negrier S, Laferriere N, Scheuring UJ,
Cella D, et al: Randomized phase II trial of first-line treatment
with sorafenib versus interferon Alfa-2a in patients with
metastatic renal cell carcinoma. J Clin Oncol. 8:1280–1289. 2009.
View Article : Google Scholar
|
|
18
|
Zhang HL, Qin XJ, Wang HK, Gu WJ, Ma CG,
Shi GH, Zhou LP and Ye DW: Clinicopathological and prognostic
factors for long-term survival in Chinese patients with metastatic
renal cell carcinoma treated with sorafenib: A single-center
retrospective study. Oncotarget. 6:36870–36883. 2015.PubMed/NCBI
|
|
19
|
Motzer RJ, Escudier B, Tomczak P, Hutson
TE, Michaelson MD, Negrier S, Oudard S, Gore ME, Tarazi J,
Hariharan S, et al: Axitinib versus sorafenib as second-line
treatment for advanced renal cell carcinoma: Overall survival
analysis and updated results from a randomised phase 3 trial.
Lancet Oncol. 14:552–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Akaza H, Naito S, Ueno N, Aoki K, Houzawa
H, Pitman Lowenthal S and Lee SY: Real-world use of sunitinib in
Japanese patients with advanced renal cell carcinoma: Efficacy,
safety and biomarker analyses in 1689 consecutive patients. Jpn J
Clin Oncol. 45:576–583. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mickisch GH, Garin A, van Poppel H, de
Prijck L and Sylvester R; European organisation for research and
treatment of cancer (EORTC) genitourinary Group, : Radical
nephrectomy plus interferon-alfa-based immunotherapy compared with
interferon alfa alone in metastatic renal-cell carcinoma: A
randomised trial. Lancet. 358:966–970. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Flanigan RC, Salmon SE, Blumenstein BA,
Bearman SI, Roy V, McGrath PC, Caton JR Jr, Munshi N and Crawford
ED: Nephrectomy followed by interferon alfa-2b compared with
interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J
Med. 345:1655–1659. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Flanigan RC, Mickisch G, Sylvester R,
Tangen C, Van Poppel H and Crawford ED: Cytoreductive nephrectomy
in patients with metastatic renal cancer: A combined analysis. J
Urol. 171:1071–1076. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choueiri TK, Xie W, Kollmannsberger C,
North S, Knox JJ, Lampard JG, McDermott DF, Rini BI and Heng DY:
The impact of cytoreductive nephrectomy on survival of patients
with metastatic renal cell carcinoma receiving vascular endothelial
growth factor targeted therapy. J Urol. 185:60–66. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heng DY, Xie W, Regan MM, Warren MA,
Golshayan AR, Sahi C, Eigl BJ, Ruether JD, Cheng T, North S, et al:
Prognostic factors for overall survival in patients with metastatic
renal cell carcinoma treated with vascular endothelial growth
factor-targeted agents: results from a large, multicenter study. J
Clin Oncol. 27:5794–5799. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wood CG and Margulis V: Neoadjuvant
(presurgical) therapy for renal cell carcinoma: A new treatment
paradigm for locally advanced and metastatic disease. Cancer. 115
10 Suppl:S2355–S2360. 2009. View Article : Google Scholar
|
|
27
|
Powles T, Blank C, Chowdhury S, Horenblas
S, Peters J, Shamash J, Sarwar N, Boleti E, Sahdev A, O'Brien T, et
al: The outcome of patients treated with sunitinib prior to planned
nephrectomy in metastatic clear cell renal cancer. Eur Urol.
60:448–454. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bex A, Blank C, Meinhardt W, van Tinteren
H, Horenblas S and Haanen J: A phase II study of presurgical
sunitinib in patients with metastatic clear-cell renal carcinoma
and the primary tumor in situ. Urology. 78:832–837. 2011.
View Article : Google Scholar : PubMed/NCBI
|