Contents
Introduction
Kashin-Beck disease (KBD) is a type of endemic
osteoarthropathy. It is characterized by onset during childhood,
deformed phalangeal joints or even limbs and degenerative cartilage
damage (1). China has the broadest
endemic areas, the highest number of patients affected and the
highest prevalence of KBD worldwide. According the 2016 China
Health Statistical Yearbook, a total of 567,600 patients, including
12,730 juvenile patients (age, ≤13 years) in 378 counties, and
>104 million residents in China are currently at risk
(http://www.nhfpc.gov.cn). Furthermore, new cases
were recently diagnosed in Tibet. The X-ray-positive rate of
phalangeal damage in children, which indicates new KBD cases, was
6.67% in Re-yu Village of Bian-ba county in Tibet (2).
Identified environmental risk factors for KBD are
prevalent in China. Selenium supplementation has no longer been
applied nationwide since 2012, so that environmental selenium is
still low in KBD endemic areas. Mycotoxin contamination in food is
detected in Qinghai Province, a severely endemic region (3). Therefore, the threat of KBD in Chinese
populations persists. ‘Focus on prevention and control of endemic
diseases’ is one of the most important tasks stated in the 13th
Five-Year Plan for health and wellness of the public in China
(http://www.moh.gov.cn/). Hence, there is an
urgent demand to identify the underlying molecular mechanisms of
articular damage in patients induced by environmental risk factors,
and to develop effective prevention and treatment measures against
these risk factors to ‘eliminate the hazard of KBD’, as proposed in
the plan.
Dietary changes in endemic areas are closely
associated with the reduction of the prevalence of KBD
Despite the elusive etiology and pathology of KBD
after >160 years of investigation, various hypotheses have been
proposed by experts worldwide. The hypotheses commonly involve
environmental factors and may be summarized into two major models.
One view is that the occurrence of KBD is associated with a
specific geographical and ecological environment. The model
suggests that the overabundance, deficiency or disproportion of
certain elements in the endemic environment may cause an abnormal
nutritional status of certain elements (e.g., selenium deficiency)
in the body through the food chain, which then gradually induces
metabolic irregularities followed by disorders in physiological
function, and ultimately disease (4–7). The
other view suggests that food contamination is the primary cause of
KBD. It holds that mycotoxin contamination (e.g., T-2 toxin) of
locally produced cereals and organic poisoning (e.g., humus) in
drinking water may increase the levels of reactive oxygen species
and free radicals in the body, which may damage chondrocytes,
disturb the extracellular matrix and induce excessive apoptosis and
necrosis of chondrocytes in KBD patients (8,9).
The above hypotheses suggest that diet is
significantly associated with KBD. A number of epidemiological
investigations, pedigree studies and sib-pair studies have
indicated that KBD exhibits a familial aggregation but no familial
heredity (10,11). The incidence of KBD and the severity
of the disease were determined to be positively correlated with the
long-term intake of locally produced cereals (e.g., corn and
wheat), and drinking water (e.g., ditch and cellar water) polluted
by organic complexes (e.g., humic acid) (12). Low selenium in the environment tends
to cause low nutritional selenium in the body through the food
chain, which may increase the risk of KBD; by contrast, selenium
supplementation, e.g., through selenium-enriched salt, effectively
decreased the incidence of KBD in children, and may delay or
prevent articular cartilage lesions in patients (13). Long-term consumption of cereals
contaminated with mycotoxin (e.g., T-2 toxin) was reported to
significantly elevate the risk of KBD (14); furthermore, low selenium combined
with T-2 toxin induced human-KBD-like cartilage damage in rats as
determined via pathological, biochemical and molecular biology
analyses (15). Finally, a
cross-sectional study on nutrient intake of children aged 4–14
years from Lin-you and Yong-shou counties, which are severely
endemic areas, reported that the intake proportion of cereal
products was high, while the intake of meat, eggs and bean products
was low; furthermore, inadequate intake of proteins, minerals and
vitamins compared with the Estimated Average Requirement in China
was identified (16,17). This evidence suggested that an
undiversified diet structure and insufficient nutrient intake may
promote the occurrence of KBD.
Based on the above results, multivariate regression
analyses indicated that the major risk factors for KBD are low
nutritional selenium, insufficient consumption of bean products
(shortage of proteins), poor storage conditions for cereals, and
food contamination by mycotoxin (18). In recent decades, comprehensive
measures have been established to prevent KBD, including change of
cereal source, improvement of food storage conditions, diversified
diet structure, grain for green and relocation (19,20). The
incidence and severity of KBD have exhibited yearly decreases.
However, the mechanisms by which the diet affects the pathogenesis
of KBD remains elusive, and it is worth further investigating them
in order to develop novel, effective and accessible methods for
preventing KBD.
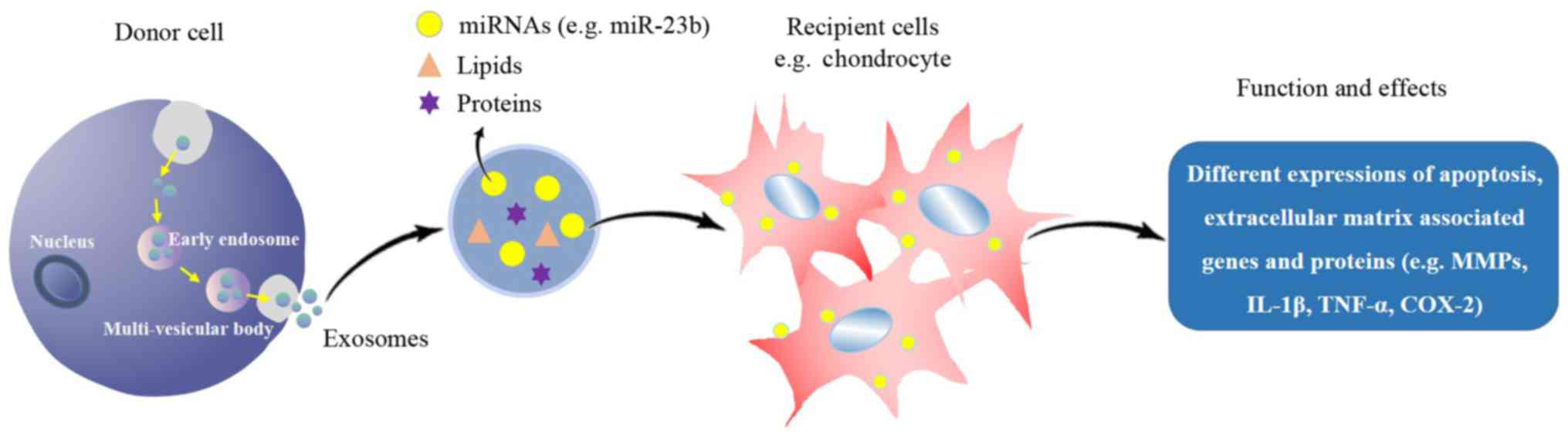
The exosome, an important carrier of
intercellular information, has a vital part in the etiology and
pathology of osteoarthropathy and may be studied as a therapeutic
strategy
Exosomes are a type of extracellular vesicle. The
prospect of clinical applications of exosomes in various fields has
been postulated in 2016 (21).
Exosomes widely exist in animals, plants and microbes, e.g., in
bodily fluids (synovial fluid, blood, urine, saliva and milk) and
the supernatant of cell cultures. Exosomes have a 40–100 nm bilayer
membrane structure formed by endocytosis, and they contain various
bioactive substances, including miRNAs, mRNAs, proteins and lipids
(22). These molecules are termed
‘cargo’ and are released into the intercellular space by exocytosis
in response to the body's requirements or pathogen stimulation.
Adjacent or long-distance cells may selectively absorb the released
substances by endocytosis for use in cellular processes, including
proliferation, differentiation, migration, apoptosis and
extracellular matrix degradation (Fig.
1) (23). The composition of
cargo affects the health status of organisms and vice versa.
Changes in cargo composition may induce abnormal expression of
genes and proteins in the body. In addition, psychological and/or
physiological disorders may lead to abnormal cargo composition in
exosomes (24).
Kato et al (25) and Withrow et al (26) reported that exosome and cargo
concentrations were abnormal in osteoarthropathy, as reflected by
differential expression of genes associated with cell apoptosis,
inflammatory factors and extracellular matrix. In 2016, a
pathogenesis study of osteoarthritis (OA) indicated that exosomes,
extracted from chondrocytes cultured with interleukin (IL)-1β,
increased the level of matrix metalloproteinase (MMP)-13 in
fibroblast-like synoviocytes up to 3-fold, accompanied by elevated
levels of IL-1β, tumor necrosis factor (TNF)-α and cyclooxygenase-2
(26). It suggests that exosomes of
chondrocytes affect the expression of inflammatory factors and MMP,
which further causes metabolic disorders of the cartilage matrix
and induces pathological changes.
Exosomes also have an important role in the
development of rheumatoid arthritis (RA) by influencing the
synthesis, transportation and activation of disease-associated
components, including immune complexes, micro (mi)RNA, inflammatory
factors and proteases (26). In an
animal model of RA, extracellular matrix degradation was induced by
exosomal cargo, including MMPs, a disintegrin and metalloproteinase
with thrombospondin motifs-5, hexosaminidase D and B-glucuronidase,
released by inflammatory cytokines, and resulting in cartilage
damage and aggravated inflammatory reaction (23).
The amount and activity of IL-1β and TNF-α in the
chondrocytes, serum and synovial fluid of KBD patients were
reported to be significantly increased compared with those in
normal controls. Overexpression of MMP-13 was also detected in
articular cartilage of KBD patients, in addition to substantial
loss of type II collagen and aggrecan (27). To date, it has not been explored
whether the pathological changes in KBD described above are
associated with abnormal exosomes. Furthermore, whether the level
of exosomal cargo may be affected by low selenium and T-2 toxin is
also a novel topic in this field.
As traditional therapies for diseases comprising
cartilage injury, including KBD and OA, intra-articular injection
of sodium hyaluronate may be performed to relieve pain and
arthroplasty may be applied to regain working capacity for advanced
patients. However, these therapies are limited by utility duration
and side-effects and are not able to decrease the incidence of the
disease. Exosomal cargo (e.g., miRNAs) derived from autologous
cells, when used for therapeutic purposes, may contribute to the
normal biological function without side effects, including
immunogenicity and tumourigenicity. Exosomal miRNA-140-5p derived
from human synovial mesenchymal stem cells (hSMSCs) promoted the
proliferation and migration of chondrocytes, and also depressed the
inhibition of extracellular matrix molecules, including type II
collagen and aggrecan (28,29). Exosomes derived from hSMSCs are also
able to promote osteochondral regeneration (30,31). Due
to these effects, the maintenance of cartilage homeostasis was
facilitated and tissue regeneration was enhanced, and exosomes may
therefore be an effective means to prevent OA.
Therefore, based on the therapeutic role of exosomes
in preventing cartilage injury-associated diseases, identification
of the link between exosomes and the pathogenesis of KBD is
necessary for the etiological study and development of novel
prevention measures for KBD.
Regulation of the PKA signaling pathway in
chondrocytes by miR-23b may be involved in the mechanism of
cartilage damage in KBD
miRs are small, non-coding RNAs consisting of 20–24
nucleotides, which regulate gene expression in humans and other
species (32). miR-23b has recently
been identified to be associated with osteoarticular diseases. It
is involved in several cellular functions, including proliferation,
differentiation, reconstruction of the cytoskeleton and migration.
miR-23b was identified to promote the differentiation of human
mesenchymal stem cells (hMSCs) into chondrocytes (33). It also depressed the expression of
Smad3 at the gene and protein level, which ameliorated
lipopolysaccharide-induced inhibition of osteogenic differentiation
in MC3T3-E1 pre-osteoblast cells (34).
Protein kinase A (PKA) is composed of two regulatory
subunits and two catalytic subunits and is normally inactive. PKA
is activated by the combination of cyclic AMP (cAMP) and its
regulatory subunits, and it is also known as cAMP-dependent protein
kinase (35). The activated form is
functional in numerous biological processes by phosphorylating
target proteins, including those involved in the regulation of
glycogen, sugar and lipid metabolism (36). Prostaglandin D2 dose-dependently
decreased the IL-1β-induced production of MMP-1 and MMP-13 protein
and mRNA in chondrocytes through the PKA signaling pathway. This
suggests that the PKA may be a promising therapeutic target for the
prevention of cartilage degradation (37,38). In
addition, Yokoyama et al (39) reported that the PKA pathway is
involved in the proliferation of chondrocytes in patients with
neonatal-onset multisystem inflammatory disease.
Interactions between miR-23b and the PKA pathway
have a role in maintaining normal physiological functions of
chondrocytes. miR-23b may promote the production of aggrecan,
SRY-box 9, as well as type II and type X collagens, and it also
suppresses the productions of MMPs. More importantly, it affects
the expression of phospho-cAMP response element binding protein, a
key phosphorylated response element, to inhibit the PKA pathway,
which then accordingly facilitates the differentiation of hMSCs
into chondrocytes (40,41).
KBD is characterized by upregulated apoptosis and
necrosis of chondrocytes in the deep zone of articular cartilage
(42). These pathological changes
are usually combined with the increased expression of type I
collagen and MMPs, as well as decreased productions of type II and
type X collagens and degradation of aggrecan (43). A meta-analysis of 139 studies
describing differentially expressed miRNAs in osteoarticular
diseases indicated that miR-23b was overexpressed in lesion
chondrocytes and cartilage tissue (44). Therefore, the pathological changes in
KBD may be regulated by miR-23b and the PKA pathway.
Dietary exosomal miRNAs are resistant to
digestion and exert functions across species
It has been confirmed that miRNAs are also contained
in vegetables, meat and animal products, and insufficiency of these
dietary sources of miRNAs cannot be compensated for by endogenous
synthesis (22). The cargo of
exogenous and endogenous exosomes is released and enters the
circulation, which is then taken up by different organs. However,
it is currently elusive how dietary exosomes are specifically
targeted to the recipient cells (45). It has been proven that dietary miRNAs
may be absorbed and utilized by mammalians as a form of exosomal
cargo to regulate vital pathways and participate in responses to
pathological triggers in the body to promote health, such as the
tumor-suppressive effect of milk-derived exosomal miRNAs (46) and the role of grape-derived exosomal
cargo (including miRNAs) in protecting mice from dextran sulfate
sodium-induced colitis (47).
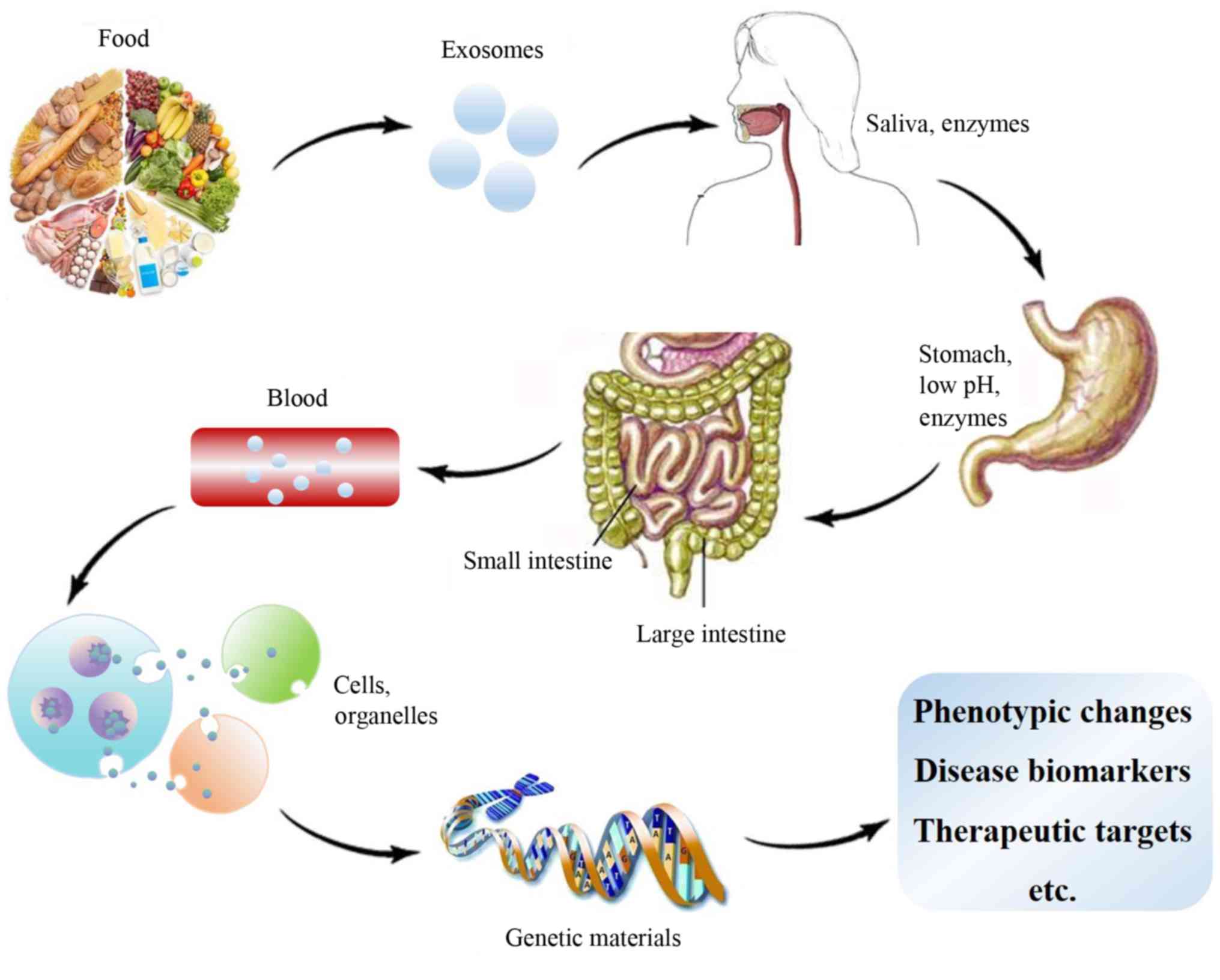
A simulation of the digestion process has indicated
that the protection of the exosome, their cargos, including RNA,
miRNAs and other degradable bioactive molecules, is able to resist
damage caused by stomach conditions and still retains bioactivity
in target cells, where they may be utilized (Fig. 2) (48). For instance, a computer-controlled
gastrointestinal model of TNO intestinal model-1 proved that a
large quantity of dairy milk Bos taurus (bta) miR-223 and
bta-miR-125b withstood digestion under simulated gastrointestinal
tract conditions. A large quantity of these 2 microRNAs was
detected in the upper small intestine compartments, which supports
their potential bioaccessibility (48). As another example, oral
administration of exosomal curcumin in Sprague Dawley rats
demonstrated 3–5 times higher levels in various organs compared
with those achieved by administration of the free substance
(49). miRNAs in a vegetarian diet,
including soy milk and rice, are not significantly reduced by
storage, processing and cooking. In addition, these dietary miRNAs
maintain relatively high bioactivity after entering the digestive
tract (50).
The results of previous studies suggest that
exosomal cargo may stimulate cell growth and differentiation in
vitro and may have therapeutic effects in in vivo models
of disease. Exosomes derived from fruit and vegetables may be
absorbed by macrophages and monocytes so that exosomal cargo may
have a beneficial effect by inhibiting the inflammatory reaction
(51,52). Grape exosome-like nanoparticles added
in culture media of stem cells facilitated the processes of
proliferation and organ differentiation, and they also effectively
elongated the life span of mice (47). Animal models of spontaneous
polyarthritis and collagen-induced arthritis indicated that
inflammatory regulation factor-associated miRNAs (miR-30a, −223 and
−92a) in milk-derived exosomes may delay the occurrence of
arthritis. This treatment was also effective in dose-dependently
alleviating physical and pathological manifestations, including
ankle swelling, cartilage damage and bone marrow inflammation.
Furthermore, the treatment may decrease the serum levels of
pro-inflammatory cytokines, including IL-6, growth-regulated alpha
protein, monocyte chemoattractant protein-1 and anti-collagen
immunoglobulin G2a, to facilitate the prevention and treatment of
articular cartilage injury (53).
Potential protective effect of dietary
exosome-miR-23b in residents of endemic regions and KBD patients
from articular cartilage injury stimulated by environmental risk
factors through regulation of the PKA pathway
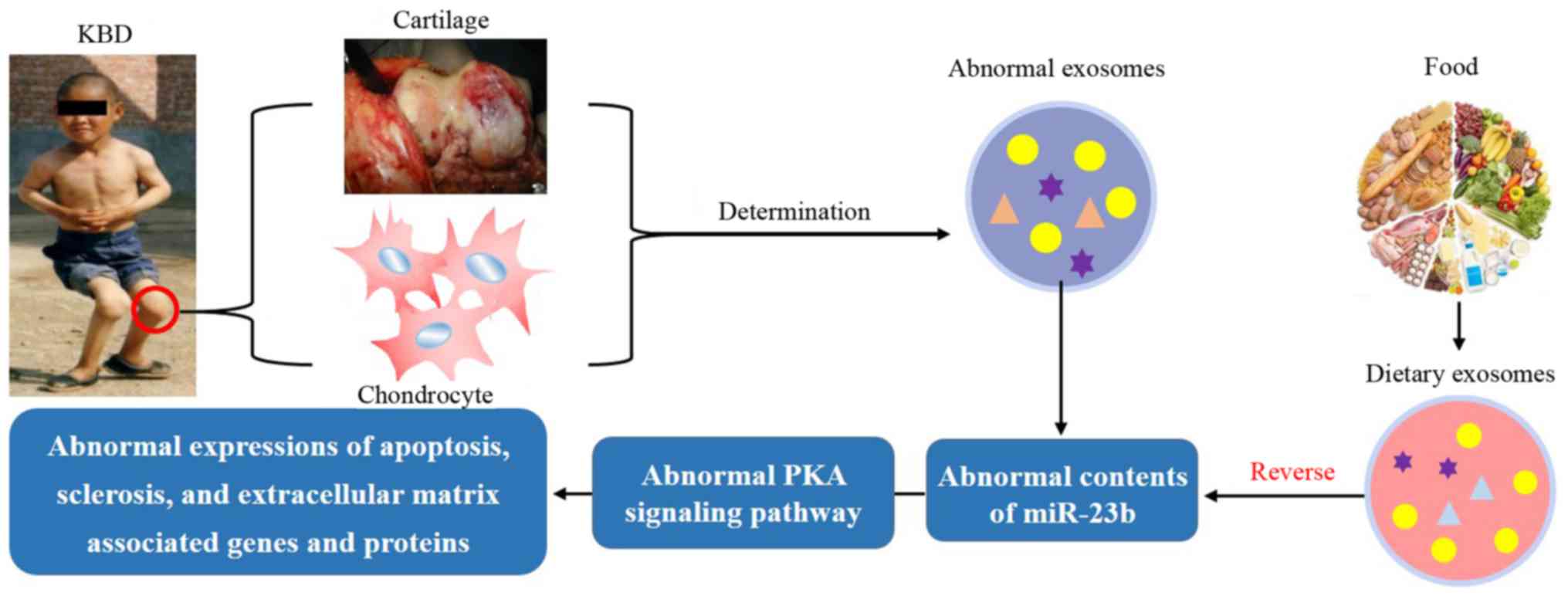
The preliminary studies mentioned above encourage
further investigation. There may be an abnormal quantity of
exosome-miR-23b in chondrocytes affected by KBD, which may lead to
the dysregulation of the PKA pathway and stimulate further
pathological changes.
If this is the case, to reverse the pathological
changes associated with KBD, intake of dietary (e.g., milk-derived)
exosome-miR-23b should be able to positively regulate the PKA
pathway to protect chondrocytes against damage induced by
environmental risk factors, namely low selenium and T-2 toxin
(Fig. 3).
Therefore, further study is warranted to i) clarify
the association between exosome and the disease; ii) verify the
interaction between miR-23b and the PKA pathway in KBD; and iii)
identify whether dietary exosome-miR-23b inhibits articular
cartilage injury stimulated by environmental risk factors through
regulating the PKA pathway.
Conclusions
The pathogenesis of KBD, which is stimulated by
environmental risk factors, is still a conundrum, and the
protective mechanisms of dietary components in preventing KBD
remains elusive. Supplementation of exosomal miRNA has been
suggested as a promising therapeutic method to prevent
osteoarticular diseases. miR-23b and the PKA pathway have been
identified to be abnormal in cartilage tissue and chondrocytes of
KBD patients. Hence, it is worth exploring whether dietary
exosome-miR-23b inhibits articular cartilage injury stimulated by
environmental risk factors through regulating the PKA pathway to
prevent KBD.
Acknowledgements
Not applicable.
Funding
This study was financially supported by the China
Postdoctoral Science Foundation (grant no. 2017M613153), the China
Postdoctoral Science Foundation (grant no. 2017M623197), the
National Key R&D Program of China (grant no. 2016YFE0119100)
and the Innovative Training Program Fund for College Students
(201610698090).
Availability of data and materials
Not applicable.
Authors' contributions
YN, XW and XG conceived the study and contributed in
writing the manuscript. PZ, AL, XQ and ML aquired and analyzed the
data.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interest to
declare.
References
|
1
|
Huang Q, Zhou ZK, Ma J, Li Y, Yang X, Shen
B, Yang J, Kang PD and Pei FX: The arthropathic and functional
impairment features of adult Kashin-Beck disease patients in Aba
Tibetan area in China. Osteoarthritis Cartilage. 23:601–606. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yaqun K, Ren Pbc, Jun W, Pei Jcq, Jia Zwg
and Ta Dzs: To investigate and analyze the condition of
Kaschin-Beck disease around 7–12 years old children in Bianba
county of Tibet in 2013. Chin J Infect Control. 5:350–351.
2014.
|
|
3
|
Lei R, Jiang N, Zhang Q, Hu S, Dennis BS,
He S and Guo X: Prevalence of selenium, T-2 toxin and
deoxynivalenol in kashin-beck disease areas in qinghai province,
Northwest China. Biol Trace Elem Res. 171:34–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J, Li H, Yang L, Li Y, Wei B, Yu J
and Feng F: Distribution and translocation of selenium from soil to
highland barley in the Tibetan plateau kashin-beck disease area.
Environ Geochem Health. 39:221–229. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dai X, Li Y, Zhang R, Kou Y, Mo X, Cao J
and Xiong Y: Effects of sodium selenite on c-Jun N-terminal kinase
signalling pathway induced by oxidative stress in human
chondrocytes and c-Jun N-terminal kinase expression in patients
with Kashin-Beck disease, an endemic osteoarthritis. Br J Nutr.
115:1547–1555. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dermience M, Lognay G, Mathieu F and
Goyens P: Effects of thirty elements on bone metabolism. J Trace
Elem Med Biol. 32:86–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dermience M, Mathieu F, Li XW,
Vandevijvere S, Claus W, De Maertelaer V, Dufourny G, Bin L,
Yangzom D and Lognay G: Minerals and trace elements intakes and
food consumption patterns of young children living in rural areas
of Tibet autonomous region, P.R. China: A cross-sectional survey.
Healthcare (Basel). 5(pii): E122017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang X, Zhang Y, Chang Y, Duan D, Sun Z
and Guo X: Elevation of IGFBP2 contributes to mycotoxin T-2-induced
chondrocyte injury and metabolism. Biochem Biophys Res Commun.
478:385–391. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen JH, Xue S, Li S, Wang ZL, Yang H,
Wang W, Song D, Zhou X and Chen C: Oxidant damage in Kashin-Beck
disease and a rat Kashin-Beck disease model by employing T-2 toxin
treatment under selenium deficient conditions. J Orthop Res.
30:1229–1237. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi XW, Guo X, Ren FL, Lü AL and Zhang YZ:
Familial aggregation and sibling heritability in Kashin-Beck
disease. Nan Fang Yi Ke Da Xue Xue Bao. 28:1187–1189. 2008.(In
Chinese). PubMed/NCBI
|
|
11
|
Shi XW, Lv AL, Ren FL, Li WR, Kang LL and
Guo X: Transmission disequilibrium test for 15 short tandem repeat
loci in Kashin-Beck disease and their interaction with low
selenium. Nan Fang Yi Ke Da Xue Xue Bao. 31:567–571. 2011.(In
Chinese). PubMed/NCBI
|
|
12
|
Guo X, Ma WJ, Zhang F, Ren FL, Qu CJ and
Lammi MJ: Recent advances in the research of an endemic
osteochondropathy in China: Kashin-Beck disease. Osteoarthritis
Cartilage. 22:1774–1783. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu FF, Han J, Wang X, Fang H, Liu H and
Guo X: Salt-rich selenium for prevention and control children with
Kashin-Beck disease: A meta-analysis of community-based trial. Biol
Trace Elem Res. 170:25–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun LY, Li Q, Meng FG, Fu Y, Zhao ZJ and
Wang LH: T-2 toxin contamination in grains and selenium
concentration in drinking water and grains in Kaschin-Beck disease
endemic areas of qinghai province. Biol Trace Elem Res.
150:371–375. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou X, Wang Z, Chen J, Wang W, Song D, Li
S, Yang H, Xue S and Chen C: Increased levels of IL-6, IL-1β, and
TNF-α in Kashin-Beck disease and rats induced by T-2 toxin and
selenium deficiency. Rheumatol Int. 34:995–1004. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ning YJ, Wang X, Wang S, Zhang F, Zhang L,
Lei Y and Guo X: Is it the appropriate time to stop applying
selenium enriched salt in Kashin-Beck disease areas in China?
Nutrients. 7:6195–6212. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ning YJ, Wang X, Ren L and Guo X: Effects
of dietary factors on selenium levels of children to prevent
Kashin-Beck disease during a high-prevalence period in an endemic
area: A cohort study. Biol Trace Elem Res. 153:58–68. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu FF, Liu H and Guo X: Integrative
multivariate logistic regression analysis of risk factors for
Kashin-Beck disease. Biol Trace Elem Res. 174:274–279. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang H, Li F, Yang X, et al: The
Evaluation result analysis of control and elimination of
kaschin-beck disease in 32 counties of sichuan province. J Prev Med
Inf. 33:355–360. 2017.
|
|
20
|
Gong H-q, Zhao S-c, Ni M-c-j, Guo M and Li
Q-w: Monitoring report of Kashin-Beck disease in Changdu Region of
Tibet in 2014. Foreign Med Sci Section Med. 36:270–273. 2015.
|
|
21
|
Votteler J, Ogohara C, Yi S, Hsia Y,
Nattermann U, Belnap DM, King NP and Sundquist WI: Designed
proteins induce the formation of nanocage-containing extracellular
vesicles. Nature. 540:292–295. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zempleni J, Aguilar-Lozano A, Sadri M,
Sukreet S, Manca S, Wu D, Zhou F and Mutai E: Biological activities
of extracellular vesicles and their cargos from bovine and human
milk in humans and implications for infants. J Nutr. 147:3–10.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yáñez-Mó M, Siljander PR, Andreu Z, Zavec
AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J,
et al: Biological properties of extracellular vesicles and their
physiological functions. J Extracell Vesicles. 4:270662015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun J, Aswath K, Schroeder SG, Lippolis
JD, Reinhardt TA and Sonstegard TS: MicroRNA expression profiles of
bovine milk exosomes in response to Staphylococcus aureus
infection. BMC Genomics. 16:8062015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kato T, Miyaki S, Ishitobi H, Nakamura Y,
Nakasa T, Lotz MK and Ochi M: Exosomes from IL-1β stimulated
synovial fibroblasts induce osteoarthritic changes in articular
chondrocytes. Arthritis Res Ther. 16:R1632014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Withrow J, Murphy C, Liu Y, Hunter M,
Fulzele S and Hamrick MW: Extracellular vesicles in the
pathogenesis of rheumatoid arthritis and osteoarthritis. Arthritis
Res Ther. 18:2862016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen J, Luo M, Wang W, Zhang Z, He Y,
Duance VC, Hughes CE, Caterson B and Cao J: Altered proteolytic
activity and expression of MMPs and aggrecanases and their
inhibitors in Kashin-Beck disease. J Orthop Res. 33:47–55. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tao SC, Yuan T, Zhang YL, Yin WJ, Guo SC
and Zhang CQ: Exosomes derived from miR-140-5p-overexpressing human
synovial mesenchymal stem cells enhance cartilage tissue
regeneration and prevent osteoarthritis of the knee in a rat model.
Theranostics. 7:180–195. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Yu D, Liu Z, Zhou F, Dai J, Wu B,
Zhou J, Heng BC, Zou XH, Ouyang H and Liu H: Exosomes from
embryonic mesenchymal stem cells alleviate osteoarthritis through
balancing synthesis and degradation of cartilage extracellular
matrix. Stem Cell Res Ther. 8:1892017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang S, Chu WC, Lai RC, Lim SK, Hui JH
and Toh WS: Exosomes derived from human embryonic mesenchymal stem
cells promote osteochondral regeneration. Osteoarthritis Cartilage.
24:2135–2140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Toh WS, Lai RC, Hui JHP and Lim SK: MSC
exosome as a cell-free MSC therapy for cartilage regeneration:
Implications for osteoarthritis treatment. Semin Cell Dev Biol.
67:56–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mao G, Zhang Z, Huang Z, Chen W, Huang G,
Meng F, Zhang Z and Kang Y: MicroRNA-92a-3p regulates the
expression of cartilage-specific genes by directly targeting
histone deacetylase 2 in chondrogenesis and degradation.
Osteoarthritis Cartilage. 25:521–532. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gabler J, Ruetze M, Kynast KL, Grossner T,
Diederichs S and Richter W: Stage-specific miRs in chondrocyte
maturation: Differentiation-dependent and hypertrophy-related miR
clusters and the miR-181 family. Tissue Eng Part A. 21:2840–2851.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu H, Hao W, Wang X and Su H: miR-23b
targets Smad 3 and ameliorates the LPS-inhibited osteogenic
differentiation in preosteoblast MC3T3-E1 cells. J Toxicol Sci.
41:185–193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van der Harg JM, Eggels L, Bangel FN,
Ruigrok SR, Zwart R, Hoozemans JJM, la Fleur SE and Scheper W:
Insulin deficiency results in reversible protein kinase A
activation and tau phosphorylation. Neurobiol Dis. 103:163–173.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu F, Xiao Y, Ji XL, Zhang KQ and Zou CG:
The cAMP-PKA pathway-mediated fat mobilization is required for cold
tolerance in C. elegans. Sci Rep. 7:6382017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zayed N, Afif H, Chabane N,
Martel-Pelletier J, Pelletier JP and Fahmi H: Prostaglandin D2
inhibits interleukin-1-b-induced matrix metalloproteinase-1 and −13
production by human chondrocytes via its DP1 receptor and cAMP/PKA
pathway. Osteoarthritis Cartilage. 16 Suppl 4:S90–S91. 2008.
View Article : Google Scholar
|
|
38
|
Zayed N, Afif H, Chabane N, Mfuna-Endam L,
Benderdour M, Martel-Pelletier J, Pelletier JP, Motiani RK, Trebak
M, Duval N and Fahmi H: Inhibition of interleukin-1beta-induced
matrix metalloproteinases 1 and 13 production in human
osteoarthritic chondrocytes by prostaglandin D2. Arthritis Rheum.
58:3530–3540. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yokoyama K, Ikeya M, Umeda K, Oda H,
Nodomi S, Nasu A, Matsumoto Y, Izawa K, Horigome K, Kusaka T, et
al: Enhanced chondrogenesis of induced pluripotent stem cells from
patients with neonatal-onset multisystem inflammatory disease
occurs via the caspase 1-independent cAMP/protein kinase A/CREB
pathway. Arthritis Rheumatol. 67:302–314. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ham O, Lee CY, Song BW, Lee SY, Kim R,
Park JH, Lee J, Seo HH, Lee CY, Chung YA, et al: Upregulation of
miR-23b enhances the autologous therapeutic potential for
degenerative arthritis by targeting PRKACB in synovial
fluid-derived mesenchymal stem cells from patients. Mol Cells.
37:449–456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ham O, Song BW, Lee SY, Choi E, Cha MJ,
Lee CY, Park JH, Kim IK, Chang W, Lim S, et al: The role of
microRNA-23b in the differentiation of MSC into chondrocyte by
targeting protein kinase A signaling. Biomaterials. 33:4500–4507.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Luo M, Chen J, Li S, Sun H, Zhang Z, Fu Q,
Li J, Wang J, Hughes CE, Caterson B and Cao J: Changes in the
metabolism of chondroitin sulfate glycosaminoglycans in articular
cartilage from patients with Kashin-Beck disease. Osteoarthritis
Cartilage. 22:986–995. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang W, Guo X, Chen J, Xu P and Lammi MJ:
Morphology and phenotype expression of types I, II, III, and X
collagen and MMP-13 of chondrocytes cultured from articular
cartilage of Kashin-Beck disease. J Rheumatol. 35:696–702.
2008.PubMed/NCBI
|
|
44
|
Wang X, Ning Y, Zhou B, Yang L, Wang Y and
Guo X: Osteoarthritis associated microRNA expression signature:
Integrated bioinformatics analysis. Mol Med Rep. 2017.
|
|
45
|
Guay C and Regazzi R: Exosomes as new
players in metabolic organ cross-talk. Diabetes Obes Metab. 19
Suppl 1:S137–S146. 2017. View Article : Google Scholar
|
|
46
|
Otsuka K, Yamamoto Y, Matsuoka R and
Ochiya T: Maintaining good miRNAs in the body keeps the doctor
away?: Perspectives on the relationship between food-derived
natural products and microRNAs in relation to
exosomes/extracellular vesicles. Mol Nutr Food Res. 62:2018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ju S, Mu J, Dokland T, Zhuang X, Wang Q,
Jiang H, Xiang X, Deng ZB, Wang B, Zhang L, et al: Grape
exosome-like nanoparticles induce intestinal stem cells and protect
mice from DSS-induced colitis. Mol Ther. 21:1345–1357. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Benmoussa A, Lee CH, Laffont B, Savard P,
Laugier J, Boilard E, Gilbert C, Fliss I and Provost P: Commercial
dairy cow milk microRNAs resist digestion under simulated
gastrointestinal tract conditions. J Nutr. 146:2206–2215. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Aqil F, Munagala R, Jeyabalan J, Agrawal
AK and Gupta R: Exosomes for the enhanced tissue bioavailability
and efficacy of curcumin. AAPS J. 19:1691–1702. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Philip A, Ferro VA and Tate RJ:
Determination of the potential bioavailability of plant microRNAs
using a simulated human digestion process. Mol Nutr Food Res.
59:1962–1972. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yarmarkovich M and Hirschi KD: Digesting
dietary miRNA therapeutics. Oncotarget. 6:13848–13849. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yang J, Farmer LM, Agyekum AA and Hirschi
KD: Detection of dietary plant-based small RNAs in animals. Cell
Res. 25:517–520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Arntz OJ, Pieters BC, Oliveira MC, Broeren
MG, Bennink MB, de Vries M, van Lent PL, Koenders MI, van den Berg
WB, van der Kraan PM and van de Loo FA: Oral administration of
bovine milk derived extracellular vesicles attenuates arthritis in
two mouse models. Mol Nutr Food Res. 59:1701–1712. 2015. View Article : Google Scholar : PubMed/NCBI
|