Introduction
Choroidal neovascularization (CNV) is a serious
complication of exudative age-related macular degeneration, which
may result in significant loss of central vision (1). Retinal pigment epithelial (RPE) cells,
located between the neurosensory retina and the vascular choroids,
form the outer blood retinal barrier and have an important role in
the pathogenesis of CNV (2). Hypoxia
is also essential for the pathogenesis of CNV (3). Increasing research suggests that
vascular endothelial growth factor (VEGF) upregulation produced by
RPE cells under hypoxic conditions is a major angiogenic factor for
CNV (4,5). Therefore, inhibiting hypoxia-induced
VEGF expression may be a therapeutic approach for the treatment of
CNV.
The Dickkopf (DKK) family of proteins modulates Wnt
signaling. Members of the DKK protein family (DKK1, 2, 3 and 4) are
secreted proteins with an N-terminal signal peptide and two
conserved cysteine-rich domains separated by a linker region
(6). DKK2 is a putative Wnt
signaling inhibitor that is involved in tumor cell proliferation,
migration and invasion (7–9). In addition, it has been postulated that
DKK2 is involved in angiogenic processes. For example, one study
demonstrated that DKK2 promoted angiogenesis in cultured human
endothelial cells, and local injection of DKK2 protein
significantly improved tissue repair, with enhanced
neovascularization in animal models of both hind limb ischemia and
myocardial infarction (10).
Recently, a study by Park et al (11) demonstrated that DKK2 increased
retinal vessel density and reduced the avascular area in an in
vivo murine model of oxygen-induced retinopathy; however, the
mechanism by which DKK2 contributes to this process is not clear.
Therefore, the present study investigated the role of DKK2 in RPE
cells under hypoxic conditions, and the molecular mechanisms were
also explored.
Materials and methods
Cell culture and treatment
Human RPE cells were obtained from American Type
Culture Collection (Manassas, VA, USA) and cultured in Dulbecco's
modified Eagle Medium supplemented with F-12 nutrient mixture, 10%
fetal bovine serum (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany),
100 U/ml penicillin and 100 µg/ml streptomycin in a 5%
CO2 incubator at 37°C. Cells were plated in 6-well
culture dishes and used for experiments at 80–90% confluence. Cells
were placed in fresh serum-free medium for 24 h prior to use. For
culture under hypoxic conditions, RPE cells were incubated in a
hypoxic chamber (Forma Scientific, San Bruno, CA, USA) to maintain
the cells under low oxygen tension (5% CO2 with 1%
O2, balanced with N2).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
For DNase treatment 2 units of DNase I (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham MA, USA) per µg of total
RNA was added and incubated at 37°C for 30 min. Total RNA from RPE
cells was isolated using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Subsequently, 2 µg of total RNA was transcribed to first-strand
cDNA using TaqMan reverse transcription reagents (Applied
Biosystems; Thermo Fisher Scientific, Inc.). PCR was performed
using a CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) and a SYBR PrimeScript RT-PCR kit (Takara
Biotechnology Co., Ltd., Dalian, China), with a final reaction
volume of 20 µl, containing 50 ng of total RNA, 10 µl 2xSYBR Green
I reagent, 6.25 U Multi-Scribe reverse transcriptase, 10 U RNase
inhibitor and 0.1 mM primers. The specific primers for DKK2 were
sense 5′-AGTACCCGCTGCAATAATGG-3′ and antisense
5′-GAAATGACGAGCACAGCAAA-3′; and for β-actin, sense
5′-GATCATTGCTCCTCCTGAGC-3′ and antisense
5′-ACTCCTGCTTGCTGATCCAC-3′. PCR cycling conditions included a
holding step at 94°C for 10 min, and 35 cycles of 94°C for 15 sec,
59°C for 30 sec and 70°C for 30 sec. β-actin was used as an
internal control for normalizing gene expression. The data obtained
were quantified using the 2−ΔΔCq method (12).
Western blot analysis
Proteins were extracted from RPE cells using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China), and the protein concentration was
determined using a Bradford protein assay kit (Bio-Rad
Laboratories, Inc.), according to the manufacturer's protocol.
Equal amounts of protein sample (30 µg) were separated by 12%
SDS-PAGE and transferred to a nitrocellulose membrane (Amersham; GE
Healthcare Life Sciences, Little Chalfont, UK). Membranes were
subsequently blocked at room temperature in Tris-buffered saline
(TBS) containing 5% non-fat dry milk and incubated with the primary
antibodies blocking solution overnight at 4°C. Primary antibodies
included anti-DKK2 (1:3,000; PA5-18015; Invitrogen; Thermo Fisher
Scientific, Inc.), anti-hypoxia inducible factor-1α (HIF-1α)
(1:2,000; H6536; Sigma-Aldrich; Merck KGaA), anti-VEGF (1:2,500;
sc-7269; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-β-catenin (1:3,000; sc-7963; Santa Cruz Biotechnology) and
anti-glyceraldehyde 3-phosphate dehydrogenase (1:3,000; sc-47724;
Santa Cruz Biotechnology, Inc.). Subsequent to washing with
TBS-Tween 20 buffer, the membranes were incubated with bovine
anti-mouse horseradish peroxidase-conjugated secondary antibody
(1:3,000; sc-2380; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. The protein bands were visualized using enhanced
chemiluminescence reagents (Gibco; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The absorbance values of
the target proteins were obtained using Gel-Pro Analyzer v.4.0
software (Media Cybernetics, Inc., Rockville, MD, USA).
Transfection of small interfering (si)
RNA
The small interfering RNA expression vector that
expresses DKK2 was purchased from Shanghai GenePharma Co., Ltd.
(Shanghai, China). For in vitro transfection, RPE cells were
seeded in each well of 96-well microplates, grown for 24 h to reach
60% confluence and subsequently transfected with siRNA-DKK2 or
scramble control using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's
instructions.
Statistical analysis
Statistical analysis was performed using SPSS v.16.0
software (SPSS, Inc., Chicago, IL, USA). All experiments were
repeated three times. Results were presented as the mean ± standard
deviation. Statistical analysis was performed using one-way
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
Hypoxia induces DKK2 expression in RPE
cells
Hypoxia is the principal physiological stimulus that
induces angiogenesis to ensure sufficient levels of oxygen are
available to developing cells (13).
Therefore, the expression of DKK2 at both the mRNA and protein
expression level in RPE cells under normoxic and hypoxic conditions
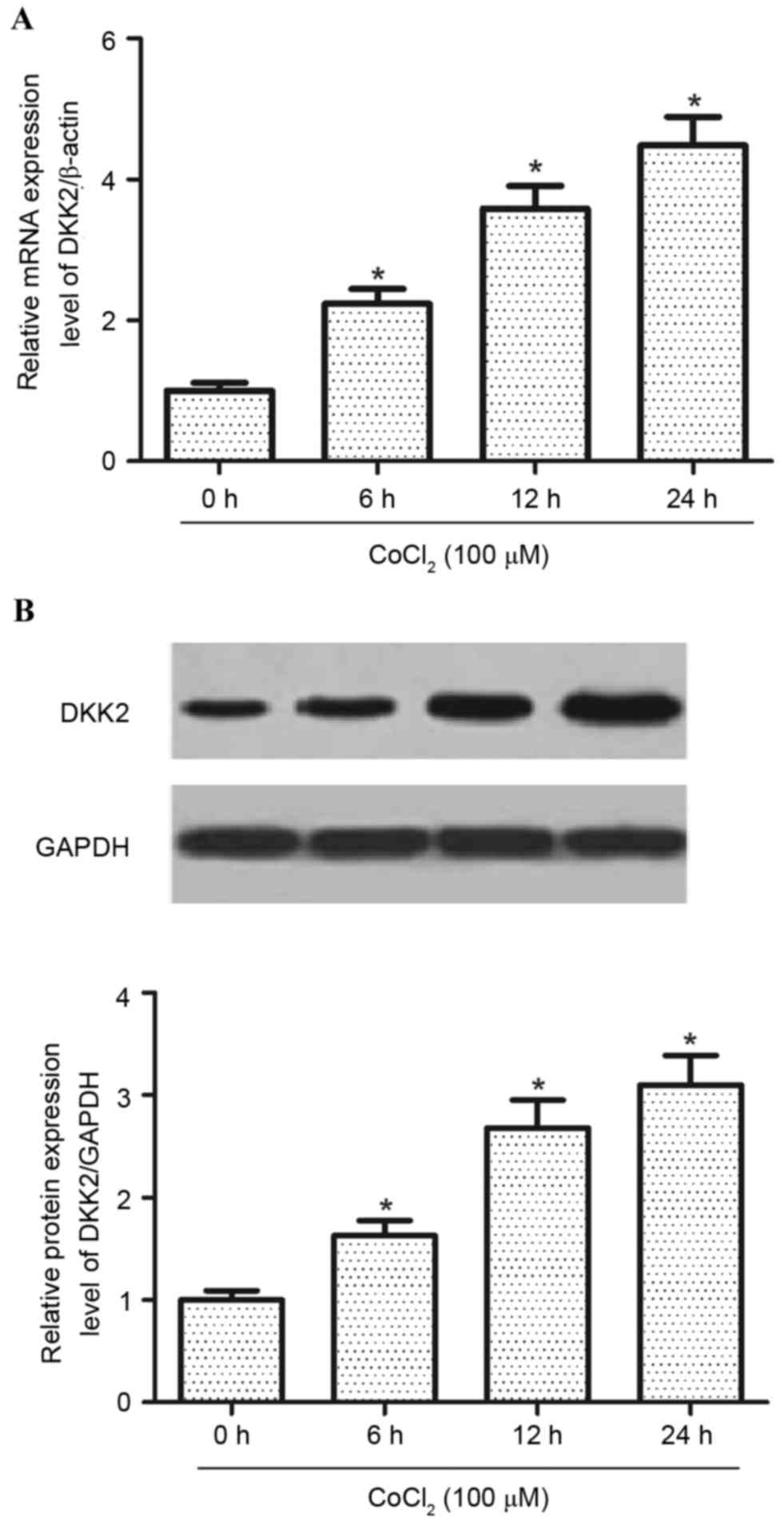
was investigated. As exhibited in Fig.
1A, the expression level of DKK2 mRNA was significantly
increased (P<0.05) by hypoxia treatment, as compared with the
normoxia group. In addition, hypoxia treatment significantly
increased (P<0.05) the protein expression levels of DDK2 in RPE
cells (Fig. 1B) compared with the
normoxia group.
RNA interference suppresses DKK2 mRNA
and protein expression
The knockdown of DKK2 was induced by a
lentivirus-mediated RNA interference vector in RPE cells and the
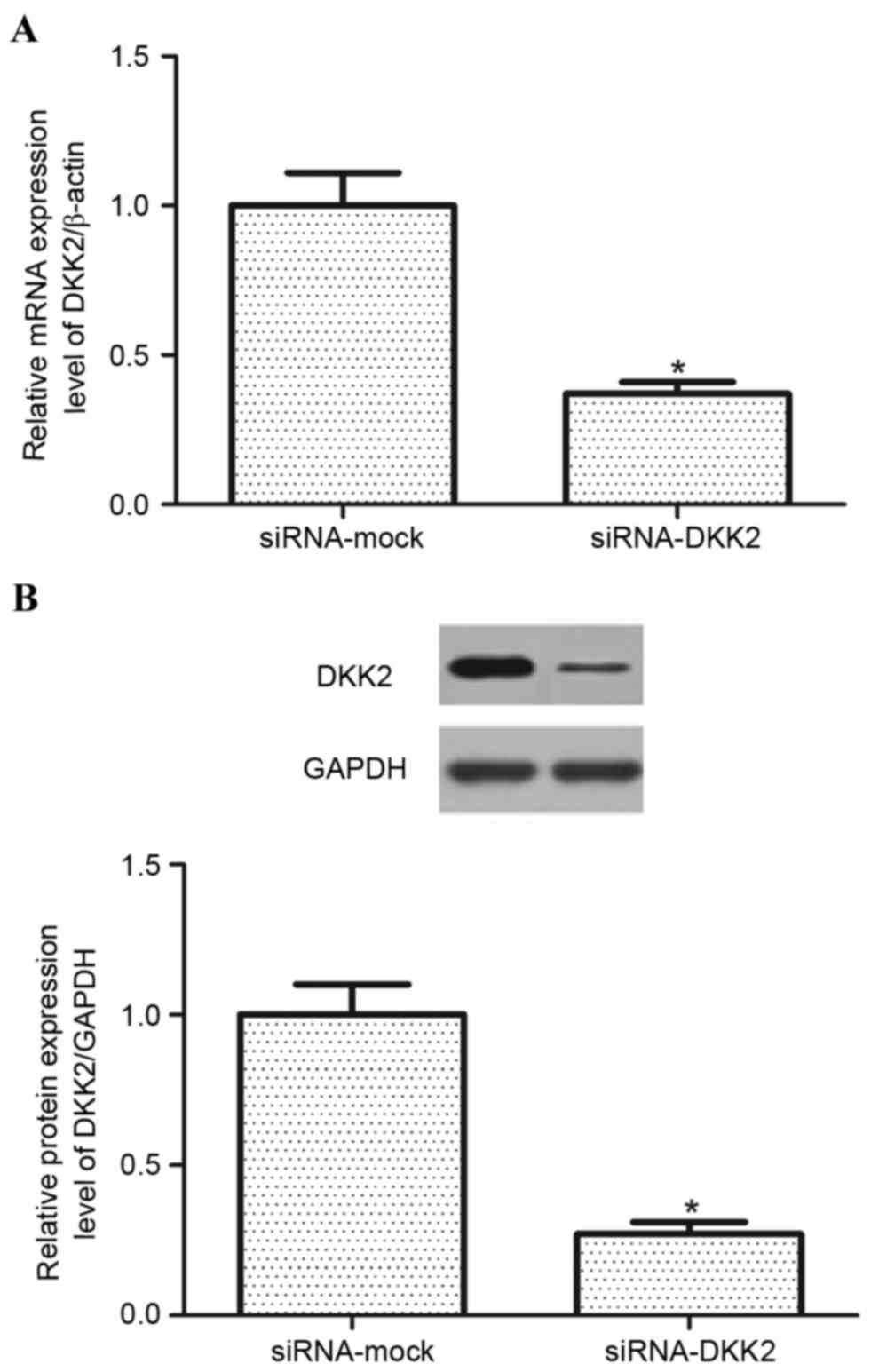
transfection efficiency was evaluated. As exhibited in Fig. 2, following transfection, the
expression of DKK2 was significantly decreased (P<0.05) at both
the RNA and protein expression levels in RPE cells under hypoxic
conditions compared with controls. The siRNA-DKK2 reduced DKK2 mRNA
levels to 36.7±2.4% of the siRNA-mock and decreased DKK2 protein
expression levels to 27.2±2.1% of the siRNA-mock.
Knockdown of DKK2 inhibits
hypoxia-induced HIF-1α and VEGF expression in RPE cells
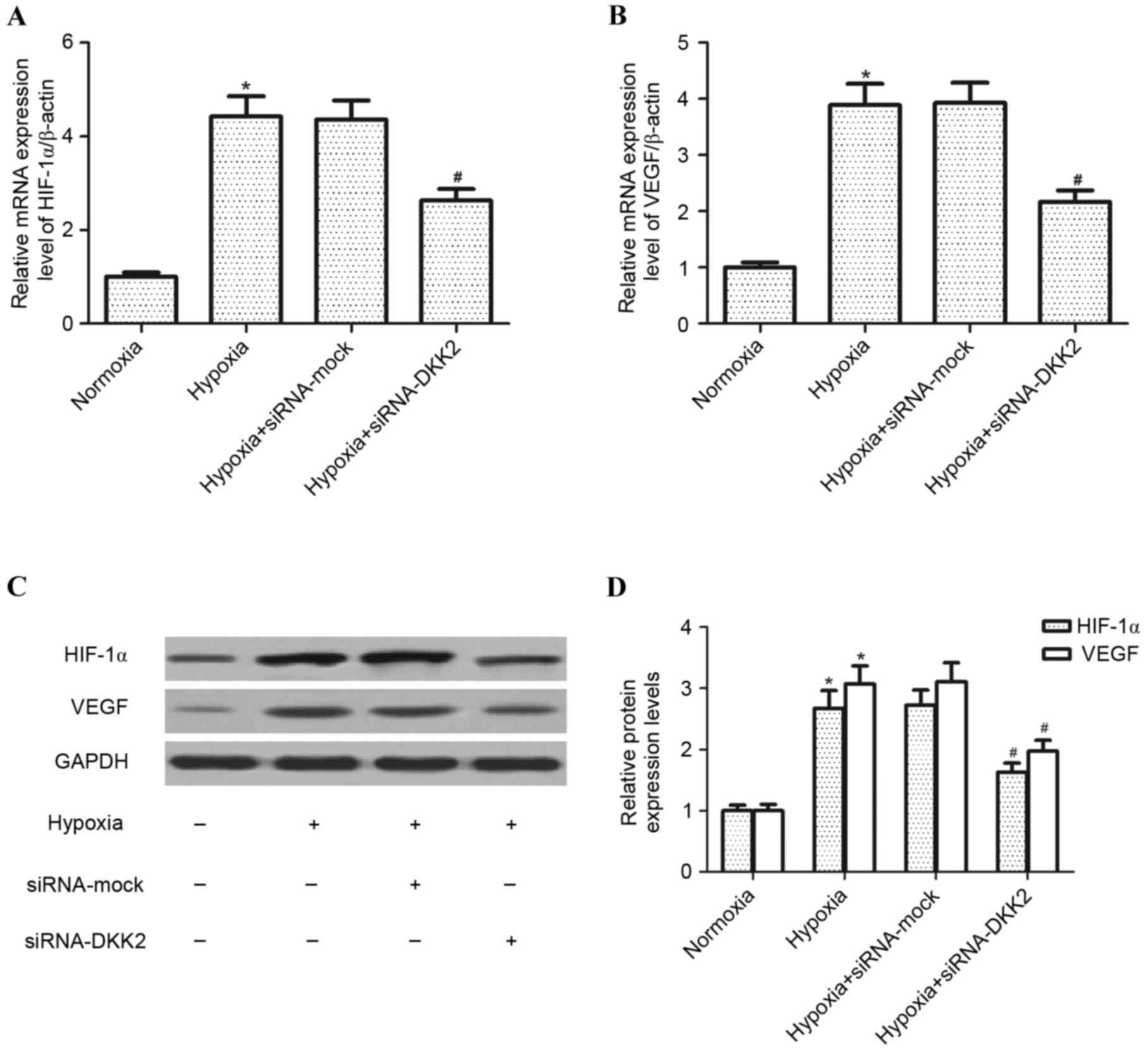
The effect of DKK2 on the expression of HIF-1α and
VEGF in hypoxia-stimulated RPE cells was investigated. Exposure to
hypoxia for 24 h resulted in a significant increase in HIF-1α
(P<0.05) and VEGF (P<0.05) mRNA expression levels in RPE
cells compared with the control group; however, knockdown of DKK2
significantly inhibited (P<0.05) this hypoxia-induced increase
in HIF-1α and VEGF mRNA (Fig. 3A and
B, respectively). Furthermore, DKK2 silencing significantly
inhibited the hypoxia-induced expression levels of HIF-1α
(P<0.05) and VEGF (P<0.05) protein in RPE cells compared with
the siRNA-mock control (Fig. 3B and
C).
Knockdown of DKK2 inhibits
hypoxia-induced Wnt/β-catenin activation in RPE cells
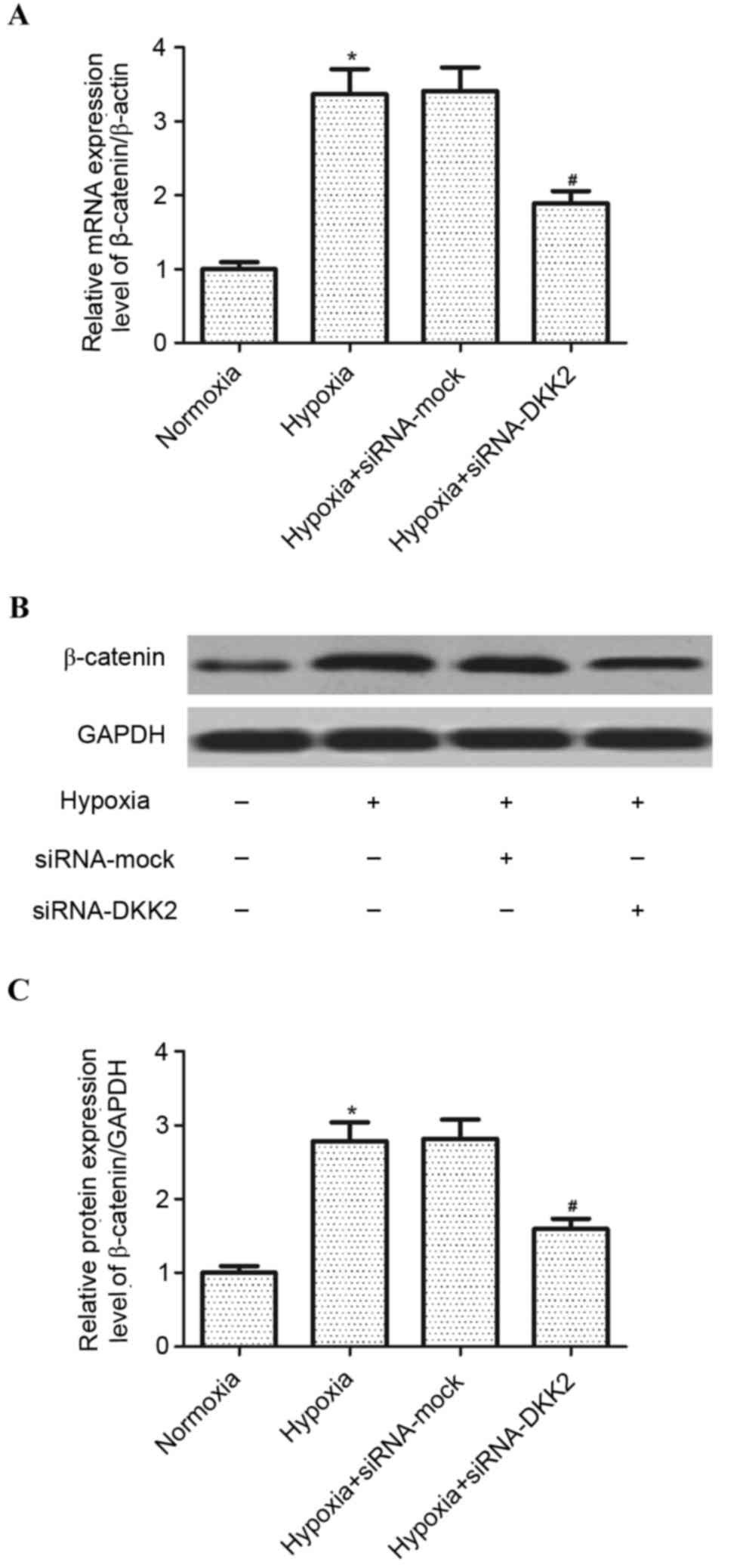
In order to explore the signaling pathway involved
in siRNA-DKK2-induced inhibition of VEGF expression in
hypoxia-stimulated RPE cells, the effect of DKK2 on Wnt/β-catenin
activation was investigated. As exhibited in Fig. 4, hypoxia treatment significantly
increased (P<0.05) the expression of β-catenin, at both the mRNA
and protein expression level, compared with the mock-transfected
control group; however, knockdown of DKK2 significantly inhibited
(P<0.05) the mRNA and protein expression of β-catenin induced by
hypoxia in RPE cells.
Discussion
CNV is the common pathological cause of irreversible
visual impairment encountered in a series of chorioretinal diseases
(1); however, the pathogenesis of
its development is complicated and poorly understood. In the
present study, it was demonstrated that hypoxic conditions induced
the expression of DKK2 in RPE cells and that knockdown of DKK2
inhibits the hypoxia-induced expression of HIF-1α and VEGF in RPE
cells. Furthermore, knockdown of DKK2 significantly inhibited the
hypoxia-induced expression of β-catenin in RPE cells.
Hypoxia is a common environmental stress that
influences signaling pathways and cell function and, through the
initiation of intracellular signaling pathways, induces the
activation of the transcription factor, HIF-1α (14,15). It
has been reported that hypoxia stimulates the expression of HIF-1,
HIF-2, and DKK2 in human osteoarthritis osteoblasts (16). Similarly, in the present study, it
was demonstrated that hypoxia induces the expression of DKK2 in RPE
cells. These results suggest that DKK2 may have a critical role in
the pathogenesis of CNV.
VEGF is one of the most well-characterized
angiogenic factors in CNV and is regulated by HIF-1 (17). Previous studies have demonstrated
that hypoxia induces VEGF production in several cell types
(18–20). For example, a study by Park et
al (21) reported that hypoxia
induced the transcriptional activity of HIF-1α, leading to an
increase in the expression of its downstream target, VEGF, in human
vascular endothelial cells. Hypoxia has been demonstrated to
increase the expression levels of VEGF via upregulation of HIF-1α
irrespective of p53 gene status in ovarian cancer cells (22). Consistent with these reports, in the
present study, it was observed that exposure to hypoxia for 24 h
resulted in a significant increase in HIF-1α and VEGF expression
levels in RPE cells; however, knockdown of DKK2 was able to inhibit
the hypoxia-induced HIF-1α and VEGF expression levels in RPE cells.
These results suggest that DKK2 silencing is able to partly block
the hypoxia-induced upregulation of VEGF in RPE cells by
downregulating the expression levels of HIF-1α.
Extensive data indicates that the Wnt/β-catenin
signaling pathway has a critical role in the regulation of
angiogenesis (23–25). A key regulator of this pathway is
intracellular β-catenin, which is a transcription coactivator
(26). It has been reported that the
aberrant upregulation of the Wnt/β-catenin signaling pathway at
multiple levels, including Wnt ligands, low-density lipoprotein
receptor-related protein 6 and β-catenin, has been observed in
laser-induced CNV models (27). In
addition, nuclear β-catenin regulates gene transcription by
interacting with Wnt target and activator genes (T-cell
factor-1/lymphoid enhancer binding factor-1) and knockdown of
HIF-1α inhibits the hypoxia-increased accumulation of β-catenin in
the nucleus (28). Therefore,
attenuation of the Wnt/β-catenin pathway may be a potential
strategy for treating CNV. Monoclonal antibody (Mab)2F1, a novel
inhibitor of the canonical Wnt pathway, has been demonstrated to
suppress the hypoxia-induced activation of Wnt signaling in
cultured RPE cells, thereby ameliorating CNV (27). Similar to the role of Mab2F1 in RPE
cells under hypoxic conditions, in the present study, it was
demonstrated that knockdown of DKK2 inhibited the hypoxia-induced
expression of β-catenin in RPE cells. These results suggest that
DKK2 silencing may mediate its anti-angiogenesis action by
inhibiting the Wnt/β-catenin signaling pathway, in turn suppressing
VEGF expression in human RPE cells.
In conclusion, the results of the present study
demonstrated that DKK2 is a vascular regulator involved in CNV
angiogenesis. Knockdown of DKK2 suppressed hypoxia-induced VEGF
expression via the inhibition of the Wnt/β-catenin signaling
pathway in RPE cells. These findings may facilitate further
understanding of the mechanisms that underlie CNV angiogenesis and
provide an innovative treatment strategy for ocular
angiogenesis.
References
|
1
|
Jager RD, Mieler WF and Miller JW:
Age-related macular degeneration. N Engl J Med. 358:2606–2617.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schwesinger C, Yee C, Rohan RM, Joussen
AM, Fernandez A, Meyer TN, Poulaki V, Ma JJ, Redmond TM, Liu S, et
al: Intrachoroidal neovascularization in transgenic mice
overexpressing vascular endothelial growth factor in the retinal
pigment epithelium. Am J Pathol. 158:1161–1172. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sheridan CM, Pate S, Hiscott P, Wong D,
Pattwell DM and Kent D: Expression of hypoxia-inducible
factor-1alpha and-2alpha in human choroidal neovascular membranes.
Graefes Arch Clin Exp Ophthalmol. 247:1361–1367. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dong X, Wang YS, Dou GR, Hou HY, Shi YY,
Zhang R, Ma K, Wu L, Yao LB, Cai Y and Zhang J: Influence of Dll4
via HIF-1a-VEGF signaling on the angiogenesis of choroidal
neovascularization under hypoxic conditions. PLoS One.
6:e184812011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mousa SA, Lorelli W and Campochiaro PA:
Role of hypoxia and extracellular matrix-integrin binding in the
modulation of angiogenic growth factors secretion by retinal
pigmented epithelial cells. J Cell Biochem. 74:135–143. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krupnik VE, Sharp JD, Jiang C, Robison K,
Chickering TW, Amaravadi L, Brown DE, Guyot D, Mays G, Leiby K, et
al: Functional and structural diversity of the human Dickkopf gene
family. Gene. 238:301–313. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kawakita A, Yanamoto S, Yamada S-i, Naruse
T, Takahashi H, Kawasaki G and Umeda M: MicroRNA-21 promotes oral
cancer invasion via the Wnt/β-catenin pathway by targeting DKK2.
Pathol Oncol Res. 20:253–261. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hirata H, Hinoda Y, Nakajima K, Kawamoto
K, Kikuno N, Kawakami K, Yamamura S, Ueno K, Majid S, Saini S, et
al: Wnt antagonist gene DKK2 is epigenetically silenced and
inhibits renal cancer progression through apoptotic and cell cycle
pathways. Clin Cancer Res. 15:5678–5687. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu J, Zhang S, Gu L and Di W: Epigenetic
silencing of DKK2 and Wnt signal pathway components in human
ovarian carcinoma. Carcinogenesis. 33:2334–2343. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Min JK, Park H, Choi HJ, Kim Y, Pyun BJ,
Agrawal V, Song BW, Jeon J, Maeng YS, Rho SS, et al: The WNT
antagonist Dickkopf2 promotes angiogenesis in rodent and human
endothelial cells. J Clin Invest. 121:1882–1893. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park H, Jung HY, Choi HJ, Kim DY, Yoo JY,
Yun CO, Min JK, Kim YM and Kwon YG: Distinct roles of DKK1 and DKK2
in tumor angiogenesis. Angiogenesis. 17:221–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-tie quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pugh CW and Ratcliffe PJ: Regulation of
angiogenesis by hypoxia: Role of the HIF system. Nat Med.
9:677–684. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Semenza GL: Hydroxylation of HIF-1: Oxygen
sensing at the molecular level. Physiology (Bethesda). 19:176–182.
2004.PubMed/NCBI
|
|
15
|
Semenza GL: HIF-1: Mediator of
physiological and pathophysiological responses to hypoxia. J Appl
Physiol (1985). 88:1474–1480. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bouvard B, Abed E, Yéléhé-Okouma M,
Bianchi A, Mainard D, Netter P, Jouzeau JY, Lajeunesse D and Reboul
P: Hypoxia and vitamin D differently contribute to leptin and
dickkopf-related protein 2 production in human osteoarthritic
subchondral bone osteoblasts. Arthritis Res Ther. 16:4592014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang XM, Wang YS, Zhang J, Li Y, Xu JF,
Zhu J, Zhao W, Chu DK and Wiedemann P: Role of PI3K/Akt and MEK/ERK
in mediating hypoxia-induced expression of HIF-1alpha and VEGF in
laser-induced rat choroidal neovascularization. Invest Ophthalmol
Vis Sci. 50:1873–1879. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hata Y, Nakagawa K and Sueishi K,
Ishibashi T, Inomata H, Ueno H and Sueishi K: Hypoxia-induced
expression of vascular endothelial growth factor by retinal glial
cells promotes in vitro angiogenesis. Virchows Arch. 426:479–486.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Y, Cox SR, Morita T and Kourembanas S:
Hypoxia regulates vascular endothelial growth factor gene
expression in endothelial cells identification of a 5′ enhancer.
Circ Res. 77:638–643. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Akeno N, Czyzyk-Krzeska MF, Gross TS and
Clemens TL: Hypoxia induces vascular endothelial growth factor gene
transcription in human osteoblast-like cells through the
hypoxia-inducible factor-2α. Endocrinology. 142:959–962. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park JJ, Hwang SJ, Park JH and Lee HJ:
Chlorogenic acid inhibits hypoxia-induced angiogenesis via
down-regulation of the HIF-1α/AKT pathway. Cell Oncol (Dordr).
38:111–118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Horiuchi A, Imai T, Shimizu M, Oka K, Wang
C, Nikaido T and Konishi I: Hypoxia-induced changes in the
expression of VEGF, HIF-1 alpha and cell cycle-related molecules in
ovarian cancer cells. Anticancer Res. 22:2697–2702. 2001.
|
|
23
|
Zhou T, Hu Y, Chen Y, Zhou KK, Zhang B,
Gao G and Ma JX: The pathogenic role of the canonical Wnt pathway
in age-related macular degeneration. Invest Ophthalmol Vis Sci.
51:4371–4379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Easwaran V, Lee SH, Inge L, Guo L,
Goldbeck C, Garrett E, Wiesmann M, Garcia PD, Fuller JH, Chan V, et
al: beta-Catenin regulates vascular endothelial growth factor
expression in colon cancer. Cancer Res. 63:3145–3153.
2003.PubMed/NCBI
|
|
25
|
Daneman R, Agalliu D, Zhou L, Kuhnert F,
Kuo CJ and Barres BA: Wnt/beta-catenin signaling is required for
CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci USA.
106:641–646. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu Y, Chen Y, Lin M, Lee K, Mott RA and Ma
JX: Pathogenic role of the Wnt signaling pathway activation in
laser-induced choroidal neovascularization. Invest Ophthalmol Vis
Sci. 54:141–154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mitani T, Harada N, Nakano Y, Inui H and
Yamaji R: Coordinated action of hypoxia-inducible factor-1α and
β-catenin in androgen receptor signaling. J Biol Chem.
287:33594–33606. 2012. View Article : Google Scholar : PubMed/NCBI
|