Introduction
Hepatocellular carcinoma (HCC) is the most common
liver cancer (1). Currently, HCC is
the third most deadly and fifth most common cancer worldwide
(1,2). Chronic infection with hepatitis B
virus, which affects ~5% of the global population, or hepatitis C
virus (HCV), affecting which affects 2% of the global population,
is a risk factor for the development of HCC (3). In Japan, a large number of patients are
infected with hepatitis and, in 2008, HCC was the fourth most
deadly and the sixth most common cancer in Japan (4).
It has been reported that inflammation serves
important roles in tumorigenesis. A number of environmental causes
and risk factors for cancer are associated with certain forms of
chronic inflammation; it has been suggested that ≤20% of cancers,
including HCC, are linked to chronic infections (5). Chronic inflammation has long been
associated with an increased incidence of malignancy and
similarities in regulatory mechanisms have been suggested. The
infiltration of innate immune cells, including macrophages and
neutrophils, into tumors promotes tumor development via various
mechanisms (5). Tumor-associated
macrophages (TAMs) are associated with myeloid-derived suppressor
cells and are key prototypic components of inflammation that drive
neoplastic progression (6). It is
known that solid tumors are generally infiltrated by macrophages
(7). Studies have revealed that a
high degree of macrophage infiltration is associated with poor
prognosis for a number of human malignancies, including
hepatocellular, colon, breast and lung carcinoma, and brain gliomas
(7–12).
Polarized M1 and M2 macrophages represent the
extremes of a continuum of functional states for TAMs. The
classically activated M1 macrophages are potent effector cells that
kill microorganisms and tumor cells and produce copious amounts of
proinflammatory cytokines (13). M2
macrophages tune inflammatory responses and adaptive Th1 immunity
to promote angiogenesis as well as tissue remodeling and repair
(13). Previous studies have
indicated that cluster of differentiation (CD)68 and CD163 are the
most common TAM markers (14,15).
CD68, first identified as a KP1 monoclonal antibody,
recognizes epitopes in a wide variety of tissue macrophages,
including Kupffer cells, germinal center, splenic and lamina
propria macrophages, and granulocyte precursors (16). CD163, first identified as an RM3/1
monoclonal antibody, was discovered during the search for specific
differentiation markers for mononuclear phagocytes (17). CD163 has been confirmed to be a
phenotypic marker of M2 macrophages that can be used to distinguish
M2 and M1 macrophages (17).
The folate receptor (FR) family includes four
members that bind folic acid with high affinity (18,19). The
FRβ gene encodes glycosyl phosphatidylinositol-anchored endocytic
receptors expressed in certain epithelial tissues, normal myeloid
tissues and acute myelogenous leukemia (20–22). FRβ
expression has been reported in TAMs, which exhibit an M2-like
functional profile and exert potent immunosuppressive functions
within the tumor environment (18).
In the present study the expression of CD68, CD163
and FRβ in TAMs from 105 HCC specimens was investigated using
immunohistochemistry. The association between these markers and
clinicopathological features in patients with HCC was also
assessed.
Patients and methods
Patient characteristics
A total of 105 patients with primary HCC were
treated using hepatic partial resection at the Department of
Digestive Surgery, Kagoshima University Graduate School of Medicine
(Kagoshima, Japan) between January 1996 and December 2002. The
patients comprised 83 men and 22 women, with a median age of 64.7
years. Of these patients, 19 patients tested positive for the
hepatitis B surface antigen, 73 were positive for the antibody to
HCV and 13 were negative for the two viruses. The mean tumor
diameter was 46.4 mm (range 10–150 mm). Macroscopically, 67 cases
(63.8%) had simple nodular tumors, whereas 38 cases (36.2%)
comprised other types. Microscopically, 20 tumors (19.0%) were
well-differentiated HCC, 74 tumors (70.5%) were moderately
differentiated and 8 tumors (7.6%) were poorly differentiated. A
total of 36 tumors had infiltrated blood vessels and 27 cases
presented with intrahepatic metastasis. For comorbidities, 41 cases
presented with hypertension, 34 cases with diabetes mellitus and 12
cases with hyperlipidemia (Table I).
Patients with a history of treatment for HCC and synchronous or
metachronous multiple cancers in other organs were excluded from
the present study. Follow-up data were obtained from all patients
post-surgery, with a median follow-up time of 53 months.
 | Table I.Patients characteristics. |
Table I.
Patients characteristics.
| Parameter | Value |
|---|
| No. | 105 |
| Age (years) | 64.7±16.3 |
| Gender;
male/female | 83/22 |
| Hepatitis
B/Hepatitis C/negative for both virus | 19/73/13 |
| Mean tumor diameter
(mm) | 46.4 |
| Gross structure;
simple nodular/other | 67/38 |
| Histological
differentiation; well/moderately/poorly | 20/74/8 |
| Infiltration to
blood vessel; yes/no | 36/69 |
| Intrahepatic
metastasis; yes/no | 27/78 |
| Hypertension;
yes/no | 41/64 |
| Diabetes Mellitus;
yes/no | 34/71 |
| Hyperlipidemia;
yes/no | 12/93 |
Informed consent
The present study was approved by the Ethics
Committees of the Graduate School of Medical and Dental Sciences,
Kagoshima University (Kagoshima, Japan; registration number 25–39)
and was conducted according to the Declaration of Helsinki. Written
informed consent was obtained from each patient, including consent
to publish and to report individual data from the participant.
Immunohistochemistry
Tissues were immersed into 10% neutral buffered
formalin for 24 h at room temperature immediately after resection
and then embedded with paraffin. Consecutive 4-µm sections were cut
from each paraffin-embedded block. Sections were immunostained with
anti-CD68 (cat. no. M0876; Dako; Agilent Technologies, Inc., Santa
Clara, CA, USA), anti-CD163 (cat. no. Mob460; Diagnostic
BioSystems, Inc., Pleasanton, CA, USA) and FRβ antibody, which was
kindly provided by Professor Matsuyama, Department of Immunology,
Graduate School of Medical and Dental Sciences, Kagoshima
University (23). Briefly, following
blocking with 0.3% H2O2/methanol for 30 min
at room temperature, specimens were blocked with PBS containing 5%
normal horse serum (Vector Laboratories Inc., Burlingame, CA, USA)
for 30 min at room temperature. Anti-CD68, anti-CD163 and FRβ
antibodies were used at a dilution of 1:100. Following overnight
incubation at 4°C with the primary antibodies, specimens were
briefly washed in PBS and incubated at room temperature for 30 min
with polymer detection system used at 1:1 dilution (cat. no.
424132; Histofine simple stain MAX PO; Nichirei Biosciences, Tokyo,
Japan). The specimens were washed with PBS and developed for 2 min
at room temperature using diaminobenzidine solution (Dako; Agilent
Technologies, Inc.). Specimens were subsequently washed with water
and counterstained with Meyer's hematoxylin for 30 sec at room
temperature (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
Evaluation of CD68, CD163 and FRβ
immunostaining
To evaluate the results of immunohistochemical
staining, the immunostained sections were scanned using a BX50-32
light microscope and DP71-SET-A digital camera (both Olympus
Corporation, Tokyo, Japan) at a magnification of ×40. The sections
were analyzed with cellSens Standard software (v1.11; Olympus
Corporation). The six fields of intra-tumor and peri-tumor lesions
with the greatest staining intensity in each specimen were
selected, the number positive cells in each field was counted
manually using high power (×200 magnification) light microscopy and
the mean number of positive cells for each specimen was calculated.
The peri-tumoral lesion was defined as the area within 2 mm from
the external edge of the tumor. The intra-tumoral lesion was
defined as the remaining area of the tumor. Two investigators
assessed the slides without knowing the clinicopathological
features and were blinded to each other's evaluation. They agreed
on all slides examined. The mean values for the positive cells in
the two locations were evaluated.
Clinicopathological factors
Clinicopathological factors selected for evaluation
included preoperative laboratory values [including indocyanine
green retention rate at 15 min (ICGR15) and tumor markers
α-fetoprotein (AFP) and des-gamma-carboxy prothrombin (DCP)].
Histopathological diagnosis was based on evaluation of tumor size,
the number of tumor nodules, lymph node metastasis and infiltration
of blood vessels (portal vein and hepatic artery and/or vein). The
tumor stage and pathological parameters were determined according
to the General Rules for the Clinical and Pathological Study of
Primary Liver Cancer (24). The
overall survival (OS) was calculated from the date of resection to
the date of mortality regardless of the cause. Recurrence-free
survival (RFS) was calculated from the date of resection to the
date that the tumor recurrence was diagnosed or from the date of
resection to the last visit if recurrence was not diagnosed.
Statistical analysis
An unpaired t-test was used to evaluate continuous
variables. Analysis of variance (ANOVA) was used to determine
whether there was a significant difference between groups of tumor
stage and histological differentiation. When ANOVA showed a
significant result, the Tukey honest significance difference test
was used to define between which groups there was a significant
difference. The cumulative OS and RFS rates were calculated using
the Kaplan-Meier method and tested using the Generalized Wilcoxon
test. Data are presented as the mean ± standard deviation.
Correlations were analyzed using Pearson's correlation coefficient.
Statistical analyses were performed using the SPSS statistical
software package (version 24; IBM Corp., Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
CD68, CD163 and FRβ expression in
lesions
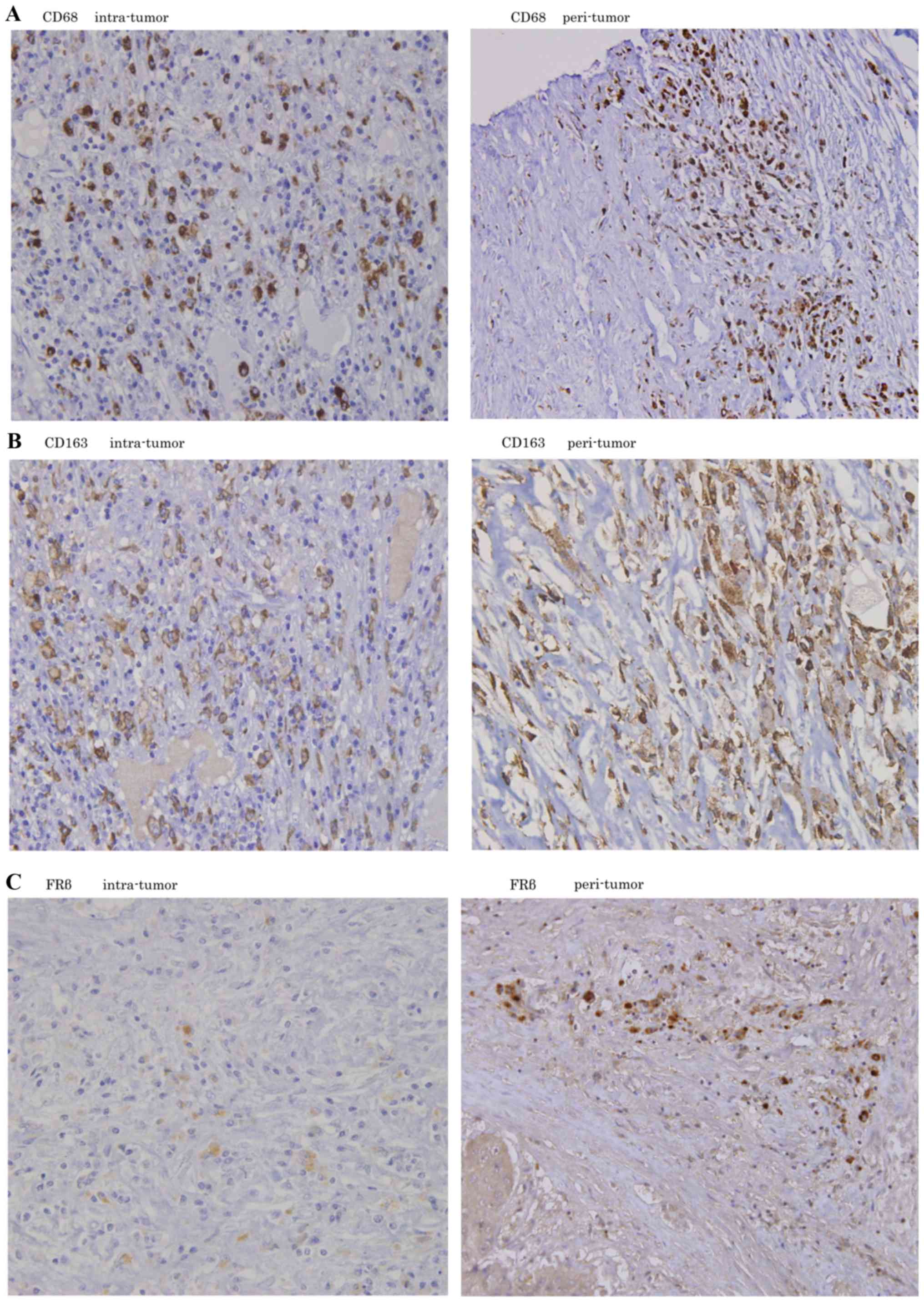
Positive staining for CD68, CD163 and FRβ was
assessed in intra-tumor and peri-tumor lesions, and was mainly
observed in the cytoplasm of stroma cells. The majority of tumor
cells and hepatic cells were negatively stained (Fig. 1).
Comparison of CD68 and CD163
expression with clinicopathological features
To elucidate the biological significance of CD68 and
CD163 expression in HCC, the number of CD68 and CD163 positive
cells was compared with the clinicopathological features of 105
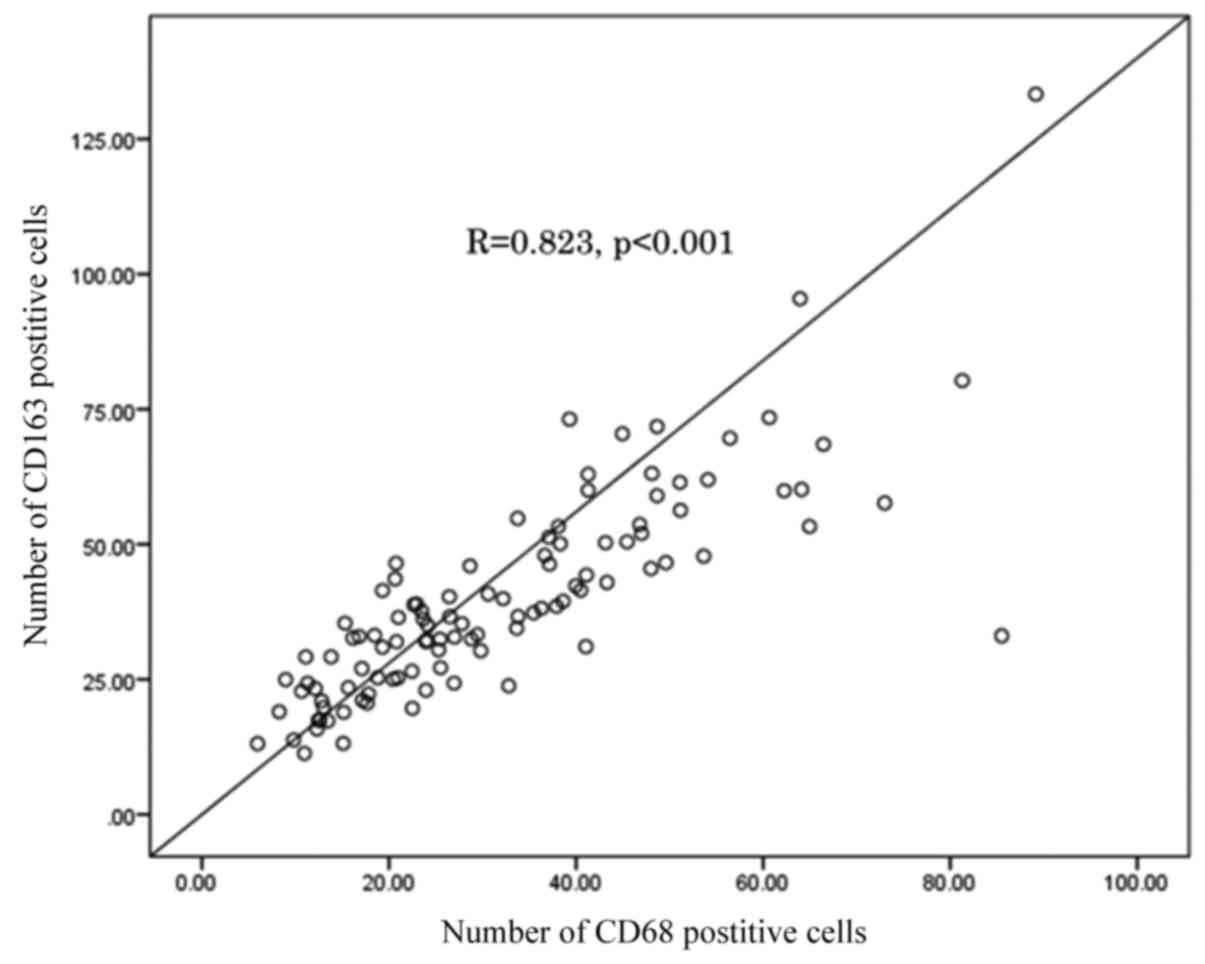
patients. The number of CD68 positive cells was significantly
correlated with that of CD163 positive cells (R=0.823; P<0.001;
Fig. 2). The number of CD68 positive
cells was significantly higher in patients with stage IV cancer
compared with stage III cancer (P=0.035; Table II) and lower in patients with simple
nodular type tumors, which had good prognosis, compared with
patients who were not diagnosed with simple nodular type tumors
(P=0.021; Table II). CD163 positive
cells were significantly more abundant in patients with median
tumor size ≥3.5 cm (P=0.049; Table
III) and in patients with poorly differentiated HCC (P=0.038;
Table III). The CD163/68 ratio was
also compared with clinicopathological features. The ratio was
significantly lower in patients with stage IV cancer (P=0.048 vs.
stage I; P=0.017 vs. stage III; Table
IV), DCP abnormalities (P=0.047; Table IV), infiltration to blood vessels
(P=0.016; Table IV) and
intrahepatic metastasis (P=0.050; Table
IV). A lower ratio appeared to be associated with the clinical
malignancy.
 | Table II.The comparison for the number of CD68
positive cells with the clinic-pathological features. |
Table II.
The comparison for the number of CD68
positive cells with the clinic-pathological features.
| Variables | CD68 | P-value |
|---|
| Male (83)/female
(22) | 32.207±18.466 | 32.184±16.543 | 0.996 |
| Tumor size ≥3.5
cm |
|
|
|
| Yes
(54)/No (51) | 35.288±18.003 | 28.935±17.590 | 0.070 |
| Tumor numbers
≥2 |
|
|
|
| Yes
(40)/No (65) | 34.959±18.654 | 30.506±17.521 | 0.220 |
| Tumor stage |
|
|
|
| I
(17) | 27.588±16.897 | 0.853a |
| II
(34) | 31.762±17.335 | 0.035b |
| III
(35) | 29.113±16.397 | 0.991c |
| IV
(19) | 42.811±20.056 | 0.051d |
|
|
|
| 0.922e |
|
|
|
| 0.128f |
| ICGR15 ≥10% |
|
|
|
| Yes
(63)/No (35) | 30.910±18.356 | 32.150±16.162 | 0.739 |
| AFP ≥10 ng/ml |
|
|
|
| Yes
(60)/No (31) | 31.552±17.693 | 33.308±17.210 | 0.652 |
| DCP ≥40 mAU/ml |
|
|
|
| Yes
(58)/No (19) | 33.324±18.234 | 25.824±15.745 | 0.112 |
| Pathological
parameter |
|
|
|
| Gross
structure; simple nodular type |
|
|
|
| Yes
(67)/No (38) | 29.168±15.992 | 37.553±20.218 | 0.021 |
| Histological
differentiation |
|
|
|
| Well
(13) | 21.623±12.117 | 0.096g |
|
Moderately (79) | 32.802±17.998 | 0.073h |
| Poorly
(10) | 32.802±17.998 | 0.635i |
| Infiltraion to
blood vessel |
|
|
|
| Yes
(36)/No (69) | 34.082±18.126 | 31.222±17.993 | 0.442 |
| Intrahepatic
metastasis |
|
|
|
| Yes
(27)/No (78) | 35.617±16.519 | 31.021±18.442 | 0.255 |
 | Table III.The comparison for the number of
CD163 positive cells with the clinic-pathological features. |
Table III.
The comparison for the number of
CD163 positive cells with the clinic-pathological features.
| Variables |
| CD163 |
| P-value |
|---|
| Male (83)/female
(22) | 39.848±19.999 |
| 40.311±15.644 | 0.996 |
| Tumor size ≥3.5
cm |
|
|
|
|
| Yes
(54)/No (51) | 43.507±20.927 |
| 36.173±16.321 | 0.049 |
| Tumor numbers
≥2 |
|
|
|
|
| Yes
(40)/No (65) | 40.706±16.880 |
| 39.476±20.460 | 0.750 |
| Tumor stage |
|
|
|
|
| I
(17)/II (34) | 35.977±14.359 |
| 40.407±23.241 | 0.865a |
|
|
|
|
| 0.709b |
|
|
|
|
| 0.958c |
| III
(35)/IV (19) | 38.844±18.076 |
| 44.695±16.604 | 0.527d |
|
|
|
|
| 0.987e |
|
|
|
|
| 0.863f |
| ICGR15 ≥10% |
|
|
|
|
| Yes
(63)/No (35) | 38.010±17.947 |
| 40.239±15.752 | 0.540 |
| AFP ≥10 ng/ml |
|
|
|
|
| Yes
(60)/No (31) | 37.773±16.114 |
| 41.515±16.794 | 0.304 |
| DCP ≥40 mAU/ml |
|
|
|
|
| Yes
(58)/No (19) | 39.627±16.721 |
| 33.766±13.859 | 0.172 |
| Pathological
parameter |
|
|
|
|
| Gross
structure; simple nodular type |
|
|
|
|
| Yes
(67)/No (38) | 38.353±20.044 |
| 42.751±17.208 | 0.259 |
| Histological
differentiation |
|
|
|
|
| Well
(13) |
| 29.715±9.846 |
| 0.164g |
|
Moderately (79) |
| 40.083±19.151 |
| 0.038h |
| Poorly
(10) |
| 49.455±24.717 |
| 0.305i |
| Infiltration to
blood vessel |
|
|
|
|
| Yes
(36)/No (69) | 38.131±15.935 |
| 40.891±20.606 | 0.485 |
| Intrahepatic
metastasis |
|
|
|
|
| Yes
(27)/No (78) | 40.917±15.603 |
| 39.608±20.251 | 0.761 |
 | Table IV.The comparison for the CD163/CD68
ratio with the clinic-pathological features. |
Table IV.
The comparison for the CD163/CD68
ratio with the clinic-pathological features.
| Variables |
| CD163/68 ratio |
| P-value |
|---|
| Male (83)/female
(22) | 1.363±0.426 |
| 1.390±0.439 | 0.792 |
| Tumor size
≥3.5cm |
|
|
|
|
| Yes
(54)/No (51) | 1.318±0.356 |
| 1.422±0.490 | 0.213 |
| Tumor numbers
≥2 |
|
|
|
|
| Yes
(40)/No (65) | 1.321±0.487 |
| 1.400±0.386 | 0.375 |
| Tumor stage |
|
|
|
|
| I
(17)/II (34) | 1.494±0.476 |
| 1.317±0.323 | 0.474a |
|
|
|
|
| 0.017b |
|
|
|
|
| 1.000c |
|
|
|
|
| 0.048d |
| III
(35)/IV (19) | 1.485±0.502 |
| 1.133±0.280 | 0.332e |
|
|
|
|
| 0.404f |
| ICGR15 ≥10% |
|
|
|
|
| Yes
(63)/No (35) | 1.392±0.474 |
| 1.353±0.368 | 0.680 |
| AFP ≥10 ng/ml |
|
|
|
|
| Yes
(60)/No (31) | 1.349±0.466 |
| 1.364±0.370 | 0.876 |
| DCP ≥40 mAU/ml |
|
|
|
|
| Yes
(58)/No (19) | 1.310±0.380 |
| 1.546±0.598 | 0.047 |
| Pathological
parameter |
|
|
|
|
| Gross
structure; simple nodular type |
|
|
|
|
| Yes
(67)/No (38) | 1.402±0.390 |
| 1.310±0.484 | 0.289 |
| Histological
differentiation |
|
|
|
|
| Well
(13) |
| 1.635±0.648 |
| 0.039g |
|
Moderately (79) |
| 1.322±0.373 |
| 0.548h |
| Poorly
(10) |
| 1.450±0.433 |
| 0.642i |
| Infiltration to
blood vessel |
|
|
|
|
| Yes
(36)/No (69) | 1.230±0.346 |
| 1.441±0.448 | 0.016 |
| Intrahepatic
metastasis |
|
|
|
|
| Yes
(27)/No (78) | 1.230±0.359 |
| 1.416±0.440 | 0.050 |
Correlation between FRβ expression,
clinicopathological features, CD68 and CD163 expression
To clarify the role of the M2 macrophage in HCC,
further examination was performed to evaluate the expression of FRβ
as another M2 macrophage marker, as well as the number of FRβ
positive cells compared with the clinicopathological features and
CD68 and CD163 expression. No significant correlation was observed
between FRβ positive cells and clinicopathological features
(Table V); however the number of FRβ
positive cells was significantly correlated with CD68 expression
and with CD163 expression (R=0.694, P<0.001; R=0.471,
P<0.001, respectively; data not shown).
 | Table V.The comparison for the number of FRβ
positive cells with the clinic-pathological features. |
Table V.
The comparison for the number of FRβ
positive cells with the clinic-pathological features.
| Variables |
| CD163/68 |
| P-value |
|---|
| Male (83)/female
(22) | 6.013±8.986 |
| 6.514±7.655 | 0.812 |
| Tumor size ≥3.5
cm |
|
|
|
|
| Yes
(54)/No (51) | 6.761±8.915 |
| 5.437±8.483 | 0.438 |
| Tumor numbers
≥2 |
|
|
|
|
| Yes
(40)/No (65) | 7.343±9.967 |
| 5.365±7.792 | 0.259 |
| Tumor stage |
|
|
|
|
| I
(17)/II (34) | 4.853±6.780 |
| 5.150±6.676 | 0.999a |
|
|
|
|
| 0.184b |
|
|
|
|
| 0.997c |
|
|
|
|
| 0.228d |
| III
(35)/IV (19) | 5.380±9.094 |
| 10.342±11.598 | 1.000e |
|
|
|
|
| 0.156f |
| ICGR15 ≥10% |
|
|
|
|
| Yes
(63)/No (35) | 6.589±9.568 |
| 4.309±6.252 | 0.208 |
| AFP ≥10 ng/ml |
|
|
|
|
|
Yes(60)/No(31) | 6.889±9.784 |
| 4.932±7.041 | 0.326 |
| DCP ≥40 mAU/ml |
|
|
|
|
| Yes
(58)/No (19) | 6.383±9.373 |
| 6.411±9.518 | 0.991 |
| Pathological
parameter |
|
|
|
|
| Gross
structure; simple nodular type |
|
|
|
|
| Yes
(67)/No (38) | 4.912±7.440 |
| 8.245±10.312 | 0.059 |
| Histological
differentiation |
|
|
|
|
| Well
(13) |
| 2.877±5.109 |
| 0.335g |
|
Moderately (79) |
| 6.461±9.103 |
| 0.973h |
| Poorly
(10) |
| 3.670±5.461 |
| 0.588i |
| Infiltration to
blood vessel |
|
|
|
|
| Yes
(36)/No (69) | 7.358±10.723 |
| 5.471±7.424 | 0.293 |
| Intrahepatic
metastasis |
|
|
|
|
| Yes
(27)/No (78) | 7.467±7.647 |
| 5.651±9.024 | 0.352 |
Association between prognosis and
CD68, CD163 and FRβ expression
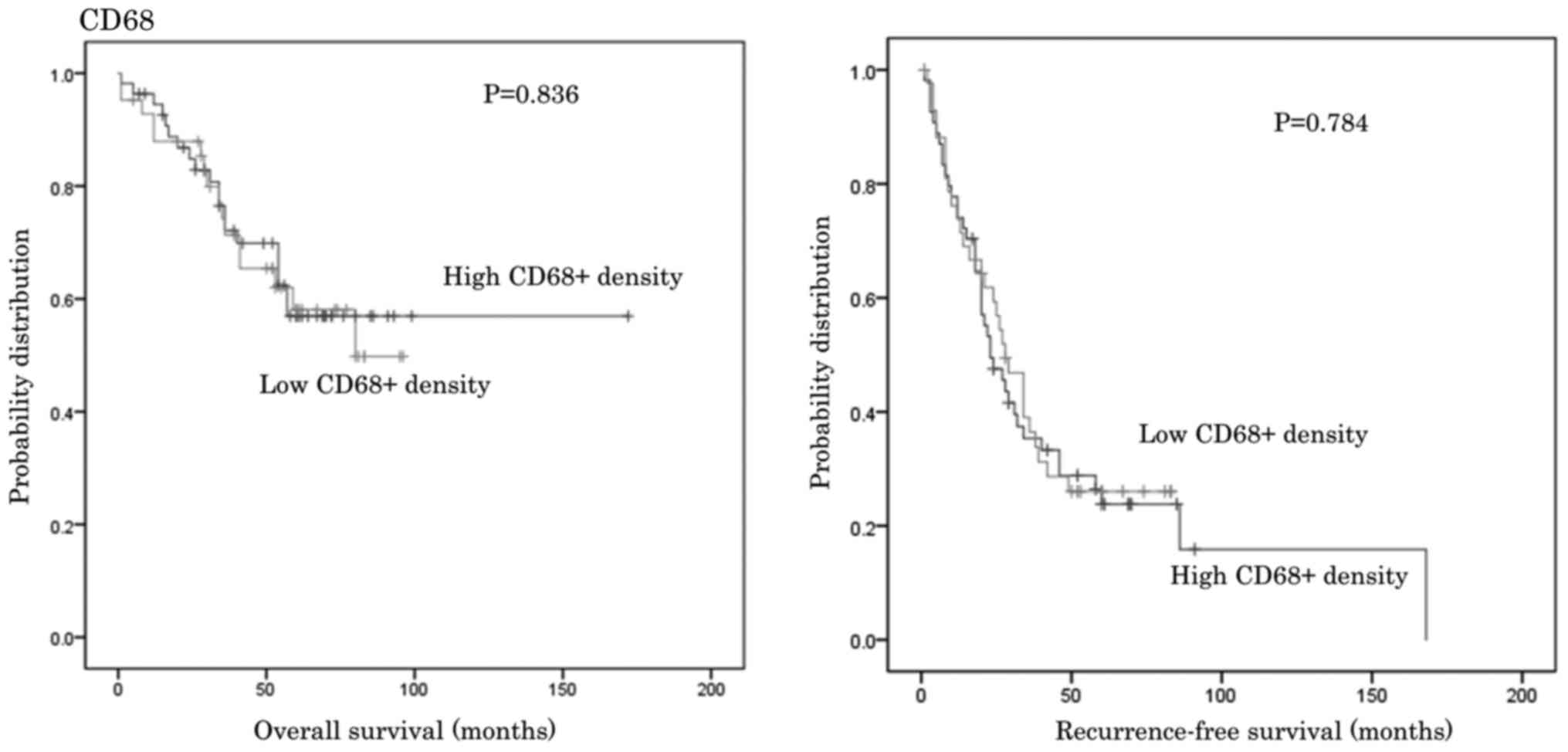
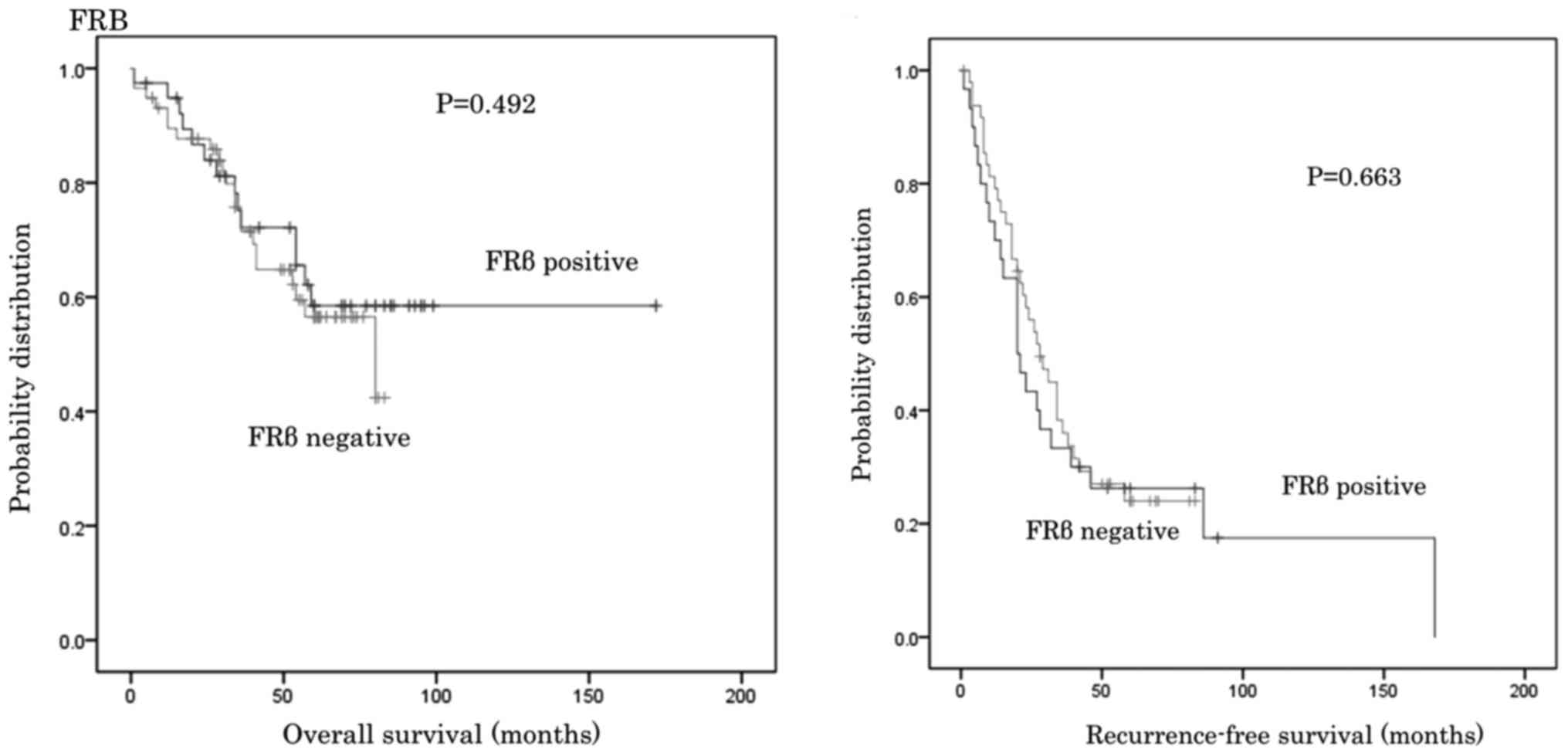
Patients were divided into subgroups depending on
the number of CD68 and CD163 cells observed and the CD163/68 ratio,
as well as into FRβ positive or negative groups. The OS and RFS for
the groups were then compared. No significant differences in OS and
RFS were identified between groups (Figs. 3–6).
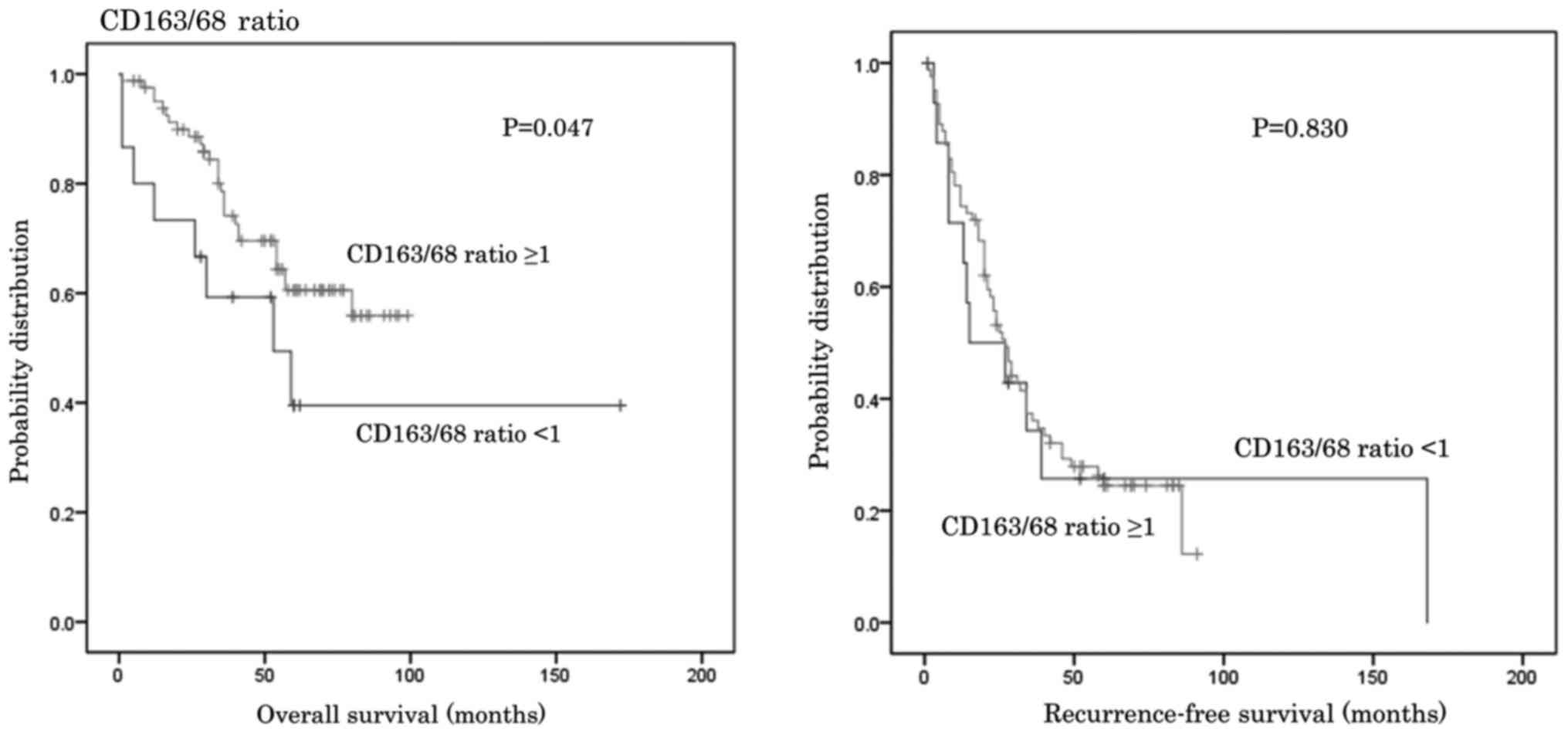
When a cut-off value of 1.0 was set for the CD163/68
ratio, OS (for the lower ratio group was significantly shorter
compared with the higher ratio group median survival 84.2 vs. 72.3
months; P=0.046; Fig. 7). However,
no significant differences in RFS were identified between the two
groups (Fig. 7).
Discussion
Monocytes are recruited from the circulation at
sites of injury, inflammation, infection and malignancy, where they
differentiate into tissue macrophages (25). The expression of numerous myeloid
lineage markers can change upon exposure to inflammatory mediators
and secreted factors from invading cancer cells (26). There have been a number of studies
involving macrophage surface markers and various classes of
macrophages have been proposed (16–18).
TAMs, which express CD68 and CD163 in HCC, were
evaluated using immunohistochemistry. The significance of CD68 and
CD163 positive cells in a cancer microenvironment is controversial.
Previous studies have indicated that CD68 and CD163 are the most
common TAM markers (14,15). CD68, as a pan-macrophage or M1
marker, and CD163 as an M2 marker, have frequently been used to
evaluate and classify TAMs (14,27). The
infiltration of CD68 and CD163 positive cells in tumors is
correlated with poor patient prognosis in cancers, including
hepatocellular, breast, bladder and ovarian cancer as well as hilar
cholangiocarcinoma (8,28–31). The
results of the present study demonstrated that high expression of
CD68 and CD163 was associated with worse outcome. However, Koelzer
et al (26), reported that
strong infiltration was correlated with favorable
clinicopathological features in colon cancer. They observed that
40% of all CD68 positive macrophages were CD163 positive, and 60%
were inducible nitric oxide synthase (M1 macrophage marker)
positive with double immunohistochemistry. In fact, a marked
correlation between CD163 and CD68 was observed in the present
study. These results may demonstrate that TAMs are not simply cells
with single markers or restricted M1 or M2 phenotypes and that they
are more diverse and heterogeneous, with cells exhibiting
considerable plasticity driven by environmental factors.
Additionally, organ type may influence the prognostic impact of
macrophages (26).
The correlation between the CD163/68 ratio and
clinicopathological features was assessed. The ratio was
significantly lower in patients with stage IV cancer, DCP
abnormalities, infiltration to blood vessels and intrahepatic
metastasis (Table IV). The OS of
the group with a CD163/68 ratio <1 was significantly shorter
than that for the higher ratio group (Fig. 7). A lower CD163/68 ratio appeared to
be associated with worse prognosis in the present study. Komohara
et al (32) examined the
association between the CD163/68 ratio and the patient prognosis in
glioma. They identified a significantly improved survival rate for
patients with a lower CD163/68 ratio, and their results are in
agreement with those of the present study. These results may also
demonstrate that TAMs are diverse and heterogeneous and that the
organ type influences the prognostic impact of macrophages.
The association between the number of FRβ positive
cells, M2 macrophage markers, and the clinicopathological features
was also evaluated. Nagai et al (33) and Nagayoshi et al (23), demonstrated that all FRβ-expressing
cells were CD68 positive macrophages in glioblastoma, that the
expression of FRβ was limited to activated macrophages, and that
TAM depletion by FRβ monoclonal antibody reduced tumor growth into
C6 glioma xenografts in nude mice. Puig-Kröger et al
(18), also reported that FRβ was a
marker for M2 macrophages. However, de Boer et al (34), reported that FRβ status did not
correlate with OS, RFS or other clinicopathological factors in
colon, ovarian, and breast cancer, and concluded that FRβ
positivity in tissue macrophages near an infiltrative tumor
reflected not only a tumor-specific phenomenon but also an
inflammatory process. The results of the current study also
demonstrated that the number of FRβ positive cells was not
correlated to the clinicopathological features. This is because FRβ
positivity may reflect an inflammatory process, and our study group
may possess not only tumor-specific but also inflammatory
macrophages.
In conclusion, the present study on HCC demonstrated
that high expression of CD68 and CD163 appeared to be associated
with worse outcome. Particularly, a low CD163/68 ratio strongly
correlated with worse results, and a CD163/68 ratio <1 was
associated with worse prognosis. However, the number of FRβ
positive cells was not correlated with the clinicopathological
features. As immunohistochemistry can only measure one or two
markers per sample, it may not fully reflect the complex factors
involved. This is a limitation of our and a number of
immunohistochemistry studies. More advanced studies using different
technologies are expected, and further studies are required to
determine the cross-interaction between diverse TAMs and the tumor
microenvironment.
Acknowledgements
The authors want to thank Professor Takami Matsuyama
from the Department of Immunology, Kagoshima University School of
Medicine (Kagoshima, Japan), for offering us the FRβ antibody.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KM, KH, SU, MS, HO and SN contributed to the
conception and design of this study. KM, KH, SU, MS, SI, YK, MH,
HK, YM, KM and HS collected the patient's data and provided the
figures. KM, KH, SU, MS, HO and SN were involved in drafting and
revising the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committees of the Graduate School of Medical and Dental Sciences,
Kagoshima University, Japan (registration number 25–39) and was
conducted according to the ethical guidelines of the Declaration of
Helsinki. Written informed consent was obtained from each
patient.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
HCV
|
hepatitis C virus
|
|
TAMs
|
tumor-associated macrophages
|
|
FR
|
Folate receptor
|
|
PBS
|
phosphate-buffered saline
|
|
ICGR15
|
indocyanine green retention rate at
15
|
|
AFP
|
α-fetoprotein
|
|
DCP
|
des-gamma-carboxy prothrombin
|
|
OS
|
overall survival
|
|
RFS
|
recurrence-free survival
|
References
|
1
|
El-Serag HB and Mason AC: Rising incidence
of hepatocellular carcinoma in the United States. N Engl J Med.
340:745–750. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bosch FX, Ribes J and Borràs J:
Epidemiology of primary liver cancer. Semin Liver Dis. 19:271–285.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Japan Ministry of Health, . Labor and
Welfare carried out in 2008.
|
|
5
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoshimura A: Signal transduction of
inflammatory cytokines and tumor development. Cancer Sci.
97:439–447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bacman D, Merkel S, Croner R, Papadopoulos
T, Brueckl W and Dimmler A: TGF-beta receptor 2 downregulation in
tumour-associated stroma worsens prognosis and high-grade tumours
show more tumour-associated macrophages and lower TGF-beta1
expression in colon carcinoma: A retrospective study. BMC Cancer.
7:1562007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kong LQ, Zhu XD, Xu HX, Zhang JB, Lu L,
Wang WQ, Zhang QB, Wu WZ, Wang L, Fan J, et al: The clinical
significance of the CD163+ and CD68+ macrophages in patients with
hepatocellular carcinoma. PLoS One. 8:e597712013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bolat F, Kayaselcuk F, Nursal TZ,
Yagmurdur MC, Bal N and Demirhan B: Microvessel density, VEGF
expression, and tumor-associated macrophages in breast tumors:
Correlations with prognostic parameters. J Exp Clin Cancer Res.
25:365–372. 2006.PubMed/NCBI
|
|
10
|
Chen JJ, Lin YC, Yao PL, Yuan A, Chen HY,
Shun CT, Tsai MF, Chen CH and Yang PC: Tumor-associated
macrophages: The double-edged sword in cancer progression. J Clin
Oncol. 23:953–964. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deininger MH, Meyermann R and Schluesener
HJ: Expression and release of CD14 in astrocytic brain tumors. Acta
Neuropathol. 106:271–277. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bingle L, Brown NJ and Lewis CE: The role
of tumour-associated macrophages in tumour progression:
Implications for new anticancer therapies. J Pathol. 196:254–265.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: Tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Elliott LA, Doherty GA, Sheahan K and Ryan
EJ: Human tumor-infiltrating myeloid cells: Phenotypic and
functional diversity. Front Immunol. 8:862017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pancione M, Giordano G, Remo A, Febbraro
A, Sabatino L, Manfrin E, Ceccarelli M and Colantuoni V: Immune
escape mechanisms in colorectal cancer pathogenesis and liver
metastasis. J Immunol Res. 2014:6868792014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pulford KA, Rigney EM, Micklem KJ, Jones
M, Stross WP, Gatter KC and Mason DY: KP1: A new monoclonal
antibody that detects a monocyte/macrophage associated antigen in
routinely processed tissue sections. J Clin Pathol. 42:414–421.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zwadlo G, Voegeli R, Osthoff Schulze K and
Sorg C: A monoclonal antibody to a novel differentiation antigen on
human macrophages associated with the down-regulatory phase of the
inflammatory process. Exp Cell Biol. 55:295–304. 1987.PubMed/NCBI
|
|
18
|
Puig-Kröger A, Sierra-Filardi E,
Domínguez-Soto A, Samaniego R, Corcuera MT, Gómez-Aguado F, Ratnam
M, Sánchez-Mateos P and Corbí AL: Folate receptor beta is expressed
by tumor-associated macrophages and constitutes a marker for M2
anti-inflammatory/regulatory macrophages. Cancer Res. 69:9395–9403.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leamon CP and Jackman AL: Exploitation of
the folate receptor in the management of cancer and inflammatory
disease. Vitam Horm. 79:203–233. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ross JF, Wang H, Behm FG, Mathew P, Wu M,
Booth R and Ratnam M: Folate receptor type beta is a neutrophilic
lineage marker and is differentially expressed in myeloid leukemia.
Cancer. 85:348–357. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pan XQ, Zheng X, Shi G, Wang H, Ratnam M
and Lee RJ: Strategy for the treatment of acute myelogenous
leukemia based on folate receptor beta-targeted liposomal
doxorubicin combined with receptor induction using all-trans
retinoic acid. Blood. 100:594–602. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hao H, Qi H and Ratnam M: Modulation of
the folate receptor type beta gene by coordinate actions of
retinoic acid receptors at activator Sp1/ets and repressor AP-1
sites. Blood. 101:4551–4560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nagayoshi R, Nagai T, Matsushita K, Sato
K, Sunahara N, Matsuda T, Nakamura T, Komiya S, Onda M and
Matsuyama T: Effectiveness of anti-folate receptor beta antibody
conjugated with truncated pseudomonas exotoxin in the targeting of
rheumatoid arthritis synovial macrophages. Arthritis Rheum.
52:2666–2675. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liver Cancer Study Group of Japan: The
General Rules for the Clinical and Pathological Study of Primary
Liver Cancer. The 6th edition. Kanehara syuppan; 2015
|
|
25
|
Atanasov G, Hau HM, Dietel C, Benzing C,
Krenzien F, Brandl A, Wiltberger G, Matia I, Prager I, Schierle K,
et al: Prognostic significance of macrophage invasion in hilar
cholangiocarcinoma. BMC Cancer. 15:7902015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Koelzer VH, Canonica K, Dawson H, Sokol L,
Karamitopoulou-Diamantis E, Lugli A and Zlobec I: Phenotyping of
tumor-associated macrophages in colorectal cancer: Impact on single
cell invasion (tumor budding) and clinicopathological outcome.
Oncoimmunology. 5:e11066772015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamaguchi T, Fushida S, Yamamoto Y,
Tsukada T, Kinoshita J, Oyama K, Miyashita T, Tajima H, Ninomiya I,
Munesue S, et al: Tumor-associated macrophages of the M2 phenotype
contribute to progression in gastric cancer with peritoneal
dissemination. Gastric Cancer. 19:1052–1065. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Balermpas P, Rödel F, Liberz R, Oppermann
J, Wagenblast J, Ghanaati S, Harter PN, Mittelbronn M, Weiss C,
Rödel C and Fokas E: Head and neck cancer relapse after
chemoradiotherapy correlates with CD163+ macrophages in primary
tumour and CD11b+ myeloid cells in recurrences. Br J Cancer.
111:1509–1518. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki
M, Kosuge T, Kanai Y and Hiraoka N: Immune cell infiltration as an
indicator of the immune microenvironment of pancreatic cancer. Br J
Cancer. 108:914–923. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang B, Wang Z, Wu L, Zhang M, Li W, Ding
J, Zhu J, Wei H and Zhao K: Circulating and tumor-infiltrating
myeloid-derived suppressor cells in patients with colorectal
carcinoma. PLoS One. 8:e571142013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mahmoud SM, Lee AH, Paish EC, Macmillan
RD, Ellis IO and Green AR: Tumour-infiltrating macrophages and
clinical outcome in breast cancer. J Clin Pathol. 65:159–163. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Komohara Y, Ohnishi K, Kuratsu J and
Takeya M: Possible involvement of the M2 anti-inflammatory
macrophage phenotype in growth of human gliomas. J Pathol.
216:15–24. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nagai T, Tanaka M, Tsuneyoshi Y, Xu B,
Michie SA, Hasui K, Hirano H, Arita K and Matsuyama T: Targeting
tumor-associated macrophages in an experimental glioma model with a
recombinant immunotoxin to folate receptor beta. Cancer Immunol
Immunother. 58:1577–1586. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
de Boer E, Crane LM, van Oosten M, van der
Vegt B, van der Sluis T, Kooijman P, Low PS, van der Zee AG, Arts
HJ, van Dam GM and Bart J: Folate receptor-beta has limited value
for fluorescent imaging in ovarian, breast and colorectal cancer.
PLoS One. 10:e01350122015. View Article : Google Scholar : PubMed/NCBI
|