Introduction
Oral cancer is considered one of the most common
cancer types around the world (1).
The estimated incidence of oral cancer and associated fatalities
per year worldwide are 300,000 and 145,000, respectively (1). Typically, oral squamous cell carcinoma
(OSCC) is preceded by epithelial precancerous lesions, which are
defined as morphologically altered tissue in which cancer is more
likely to occur compared with its normal mucosae (2). The presence of epithelial oral
dysplasia (OD) in precancerous lesions is an indicator of
malignancy transformation (3). Early
diagnosis of such lesions may therefore aid in preventing the
emergence of OSCC and ameliorating the burden of the disease
(2,3).
Lichen planus is one of the most common inflammatory
mucocutaneous diseases with an incidence of 0.5–2.0% in the adult
population and a female to male ratio of 3:1 (4). Lichen planus affects the skin, mucosa
(including the oral mucosa) or a combination of both (4). In 1978, the World Health Organization
(WHO) indicated that oral lichen planus (OLP) is a precancerous
condition (5). In this regard, the
association between OLP and oral cancer has been extensively
studied. However, whether OLP lesions have an increased risk of
malignant transformation and the extent of this remains a
controversial issue (6–8).
The identification of reliable biomarkers for
detecting malignant transformation poses a unique role for the
development of standardized screening and improved follow-up in
patients with oral precancerous lesions. There is a good
perspective for the feasible coupling of these techniques with
other strategies, such as multimodal cell analysis for brush
biopsies in the early detection of potentially premalignant lesions
(9). The antibody for the nuclear
antigen Ki-67 is a well-established marker of cell proliferation
and has been widely studied in OSCC and precancerous oral lesions
(10,11). Although Ki-67 has been evaluated as a
risk factor in the development of oral cancer from precancerous
lesions, only a limited number of studies have explored the
expression of Ki-67 in OLP in comparison with other epithelial
lesions (10,11). In addition, previous results have
suggested the diagnostic and predictive value of p16 in different
types of cancer, particularly in that of the cervix (12). Notably, P16 is a cell cycle
progression inhibitor that is involved in the inhibition of
cyclin-dependent kinase (CDK)4 and CDK6 (12). Despite this important role in the
cell cycle, its value as a predictor of malignancy progression
remains controversial and is dependent on the anatomical site; for
example, in cervical or breast cancer (12).
Various novel potential markers have been studied
for their association with malignancy in preclinical and clinical
trials. For example, budding uninhibited by benzimidazoles 3
(Bub-3) is known as a mitotic checkpoint gene that inhibits mitosis
(13). The expression of this gene
has been associated with low-grade, as opposed to high-grade,
luminal breast cancer (14).
Furthermore, mutations in BUB3 have been associated with colorectal
cancer (15). Additionally, the
overexpression of the transcription factor sex-determining region
Y-related high mobility group box 4 (SOX4), which is also
considered a novel proliferation marker, is hypothesized to be an
unfavorable prognostic factor in patients with breast cancer
(16). SOX4 is broadly expressed in
small-cell lung carcinoma and esophageal squamous cell carcinoma,
and has therefore been considered as a potential target for lung
cancer vaccines (16,17).
The aim of the present study was to evaluate the
expression levels of p16, Ki-67, Bub-3 and SOX4 in order to assess
their role as potential markers of malignant transformation in OLP.
To the best of our knowledge, no studies thus far have determined
the expression of Bub-3 and SOX4 in OLP. The analysis of these
expression levels may elucidate the mechanism and provide novel
evidence concerning malignant transformation in OLP lesions.
Materials and methods
Study design
In the present retrospective study design, biopsy
tissue samples of lesions of OLP, OD, cutaneous lichen planus (CLP)
and oral fibrous hyperplasia (OFH) were obtained from 120 patients
(44 males and 76 females; mean age, 49 years), prior to being fixed
in 10% formalin (24 h at room temperature) and paraffin-embedded
for a maximum of 5 years. The patient selection was made by
convenience, according to the given criteria for each study group,
as described below. The data from each subject, including age, sex
and lesion location were collected using medical record files of
the Brasília University Hospital, Brasília, Brazil. The following
exclusion criteria were applied: Lack of data regarding clinical
diagnosis, divergence between clinical and histological aspects and
poor amount of material for immunohistochemical analysis. The
present study was reviewed and approved by the Institutional Review
Board (IRB) from the School of Medicine, University of Brasília
(Brasília, Brazil; approval number, CEPFM 042/2010) and conducted
in accordance with the principles of the Declaration of Helsinki.
Informed consent was obtained in the majority of cases; however,
some patients were not available following several contact attempts
or had experienced mortality. Therefore, the IRB approved not
having the formal consent in these cases. In order to improve the
evaluation of the present study, the inclusion of specific groups
was incorporated. CLP was selected as the negative control as it is
the skin counterpart of the disease that is not associated with
malignant transformation (6,8). The most well-known oral lesions that
have a tendency towards cancer progression are OD lesions (5) and therefore this was used as the
positive control. OFH was also considered in the present study as
negative control, despite being reactive lesions with increased
proliferation, the risk of malignancy progression is negligible
(5).
Samples
The diagnosis of OLP and CLP was based on clinical
and histological features. All OLP cases exhibited bilateral
involvement and were divided into three subtypes: Reticular, when
white plaques and/or reticular lesions were reported; erythematous,
when erythematous areas (reddish in color) were described; and
erosive (eroOLP), when ulcerations were added to the previous
features. The histologic criteria for OLP diagnosis was determined
as previously described (18). As
exclusion criteria, no signs of epithelial dysplasia could be
present in cases classified as OLP. The same histological
parameters were used for the CLP group. Additionally, histological
criteria used for selection of OD cases were as previously defined
by WHO (5). Lesions were categorized
as OFH when there was a detection of proliferative fibrous
connective tissue with dense collagen arrangement that was lined by
squamous epithelium.
Immunohistochemistry
Immunohistochemistry was performed on all tissue
samples, using the streptavidin-biotin method. Information
associated with the primary antibodies used, including the
manufacturer, clone, dilution, incubation time, temperature and
antigen retrieval buffer is summarized in Table I. All primary antibodies were diluted
in 1% buffered bovine serum albumin (Sigma-Aldrich; Merck KGaA,
Darmstadt Germany). Sections (4-µm thick) from paraffin-embedded
blocks were deparaffinized and rehydrated, then fixed in a mixture
of methanol and acetone for 15 min at room temperature. Specimens
were then immersed in antigen retrieval buffer and incubated for 20
min at 98°C. Endogenous peroxidase activity was blocked using 0.3%
hydrogen peroxide, for 20 min at room temperature. The primary
antibodies were detected using the Polyvalent HRP Plus kit (Spring
Bioscience Corporation, Pleasanton, CA, USA) and
3,3′-diaminobenzidine tetrahydrochloride chromogen with 2 drops at
room temperature for 10 min (Dako; Agilent Technologies, Inc.,
Santa Clara, CA, USA). Incubation with secondary antibodies using a
Dako Cytomation LSAB System-HRP Kit (Dako Agilent Technologies,
Inc., Santa Clara, CA, USA) was conducted in a humid chamber at
37°C for 1 h. A light microscope was used to observe the slides at
a magnification of ×400.
 | Table I.Primary features of the
immunohistochemical antibodies p16, Ki-67, BUB3 and SOX4. |
Table I.
Primary features of the
immunohistochemical antibodies p16, Ki-67, BUB3 and SOX4.
| Antibody | Clone | Manufacturer | Dilution | Incubation
(h/°C) | Antigen
retrieval | Positive
controls |
|---|
| p16 | G175-405 | BD Pharmingen; BD
Biosciences, San Jose, CA, USA | 1:200 | 18/4 | Citrate pH 6.0 | Cervical
carcinoma |
| Ki-67 | SP6 | Biocare Medical,
Concord, CA, USA | 1:50 | 18/4 | Citrate pH 6.0 | Palatine
tonsil |
| BUB3 | EPR5319 (2) | Abcam, Cambridge,
MA, USA | 1:500 | 18/4 | Citrate pH 6.0 | Breast gland |
| SOX4 | Polyclonal | Abcam,
Cambridge | 1:800 | 18/4 | Citrate pH 6.0 | Breast gland |
Evaluation of Ki-67, p16, Bub-3 and
SOX4 expression levels
Histological images were acquired using a ScanScope
CS System histological scanner (Aperio ePathology Solutions; Leica
Microsystems GmbH, Wetzlar, Germany) at a magnification of ×400
(resolution: 0.25 µ/pixel), using ImageScope (Leica Microsystems
GmbH). Only histological areas that indicated a typical appearance
of the studied lesions were assessed. For instance, a field of OFH
in which epithelium hyperplasia was not detected indicated that
this field was not eligible for further analysis. Following this
initial histological screening, at least six fields were randomly
and systematically selected for each case. Positive and negative
epithelial cells were counted using ImageJ version 1.51 g; Software
(National Institutes of Health, Bethesda, MD, USA). Quantitative
assessment was performed by counting 500 cells at the basal and
suprabasal epithelial cell layers in each case and for each
biomarker studied. The percentage of positive cells (n/500) was
obtained and recorded as the positivity index (PI) for each marker.
Epithelial cells indicating nuclear brown staining were considered
positive for BUB3 and Ki-67. Nuclear or cytoplasmic staining was
considered positive for p16 and SOX4 antibodies.
Statistical analysis
Data analyses were performed using SPSS version 20.0
for Windows (IBM Corp., Armonk, NY, USA). All tests were two-tailed
and P<0.05 was considered to indicate a statistically
significant difference. The Kolmogorov-Smirnov test was used to
determine the frequency distribution of the variables. In cases of
normal distribution, a one-way analysis of variance with pairwise
comparisons was performed. The Tukey´s post-hoc test was performed
when statistical differences were identified, if necessary. For
non-normal distribution, Kruskal-Wallis test was applied to detect
any difference among groups associated with continuous variables
and the Dunn post-hoc test was applied when statistical
significance was indicated. The χ2 test was used to
assess differences associated with categorical variables, such as
sex, among groups. Spearman's correlation tests were used to
evaluate the expression of the four antibodies studied.
Results
General data
A total of 120 paraffin block samples were evaluated
that were equally distributed into the OLP (n=30), OD (n=30), OFH
(n=30) and CLP (n=30) groups. Results are indicated in Table II. No significant differences
between the demographic characteristics were revealed. The mean age
of subjects and the female-to-male ratio demonstrated no
significant differences among groups (P=0.71 and P=0.83,
respectively).
 | Table II.Demographic and clinical
characteristics of each study group. |
Table II.
Demographic and clinical
characteristics of each study group.
|
| Oral lesions | Skin lesions |
|---|
|
|
|
|
|---|
|
Characteristics | Oral lichen planus
(n=30) | Oral dysplasia
(n=30) | Oral fibrous
hyperplasia (n=30) | Cutaneous lichen
planus (n=30) |
|---|
| Age, years |
|
|
|
|
|
Mean | 51.8 | 49.4 | 49 | 47.2a |
| 95%
confidence interval | 46.2–57.1 | 43.2–55.6 | 43.6–54.4 | 41.9–52.4 |
| Sex, n (%) |
|
|
|
|
|
Male | 9
(30) | 12 (40) | 11 (36.7) | 12
(40)b |
|
Female | 21 (70) | 18 (60) | 19 (63.7) | 18 (60) |
| Biopsy site, n
(%) |
|
|
|
|
| Buccal
mucosa | 15 (50) | 3
(10) | 5
(16.7) | – |
|
Lips | 2
(6.7) | 5
(16.7) | 2 (6.7) | – |
|
Gums | 3
(10) | 2
(6.7) | 2 (6.7) | – |
|
Tongue | 3
(10) | 3
(10) | 1 (3.3) | – |
|
Palate | 1
(3.3) | 3
(10) | 2 (6.7) | – |
| Buccal
fornix | 2
(6.7) | – | 2 (6.7) | – |
|
Retromolar triangle | – | 1
(3.3) | 1 (3.3) | – |
|
Alveolar ridge | – | – | 1 (3.3) | – |
| Floor
of the mouth | – | 1
(3.3) | – | – |
| Upper
limbs | – | – | – | 7
(23.3) |
|
Trunk | – | – | – | 5
(16.3) |
| Lower
limbs | – | – | – | 4
(13.3) |
|
Neck | – | – | – | 3
(10) |
|
Penis | – | – | – | 1
(3.3) |
|
Face | – | – | – | 1
(3.3) |
|
Head | – | – | – | 1
(3.3) |
| Not
available | 4
(13.3) | 12 (40) | 14 (46.7) | 8
(26.7) |
| Total | 30
(100) | 30
(100) | 30 (100) | 30
(100) |
Immunohistochemical data are summarized in Figs. 1 and 2. In the following text, PI is represented
in percentage (%) and the 25 and 75th percentiles follow in
parenthesis. Fig. 3 indicates case
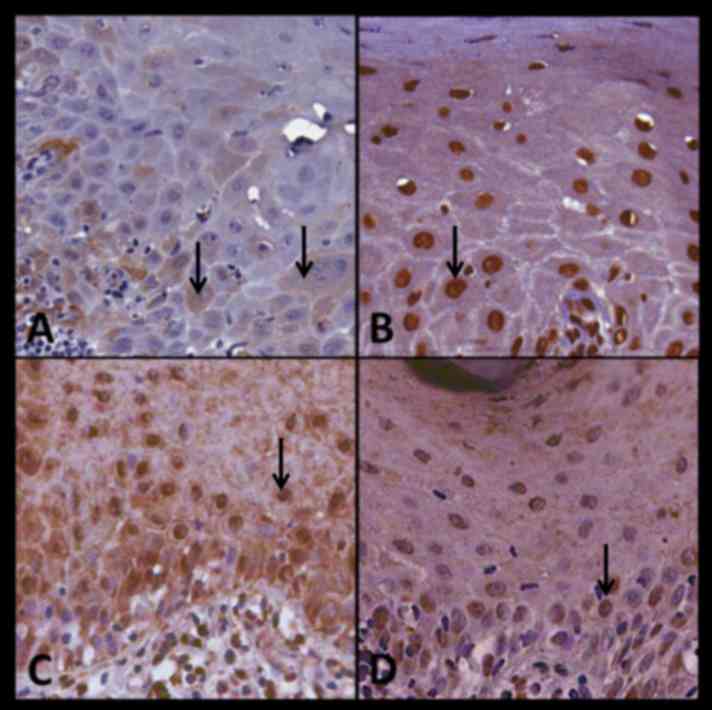
no. 22 and presents the expression of P16, BUB3, SOX4 and Ki67 in
OLP. In this particular case, Bub-3 demonstrated the highest level
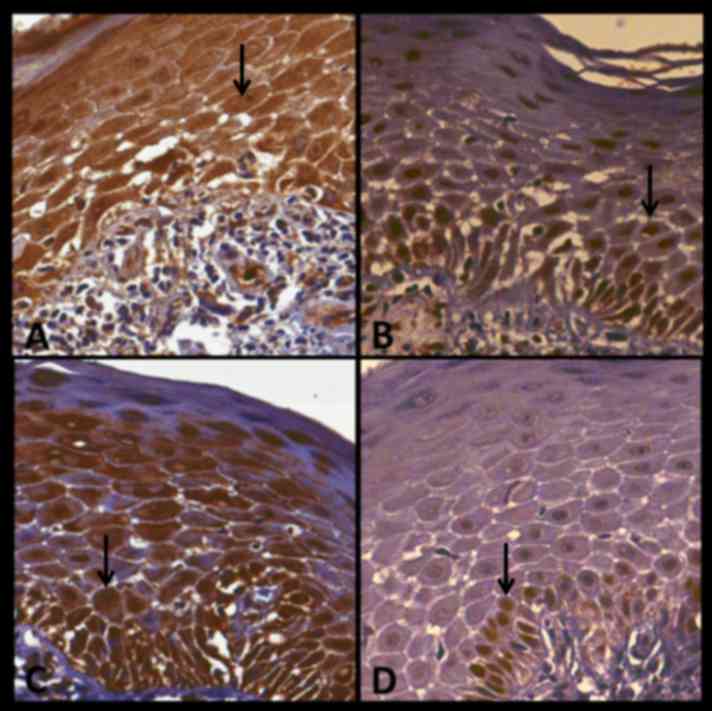
of immunostaining. Fig. 4 indicates
case no. 12 and presents the expression of p16, Bub-3, SOX4 and
Ki-67 in CLP. In this case, all antibodies demonstrated strong
immunostaining with the exception of Ki67, which stained weakly.
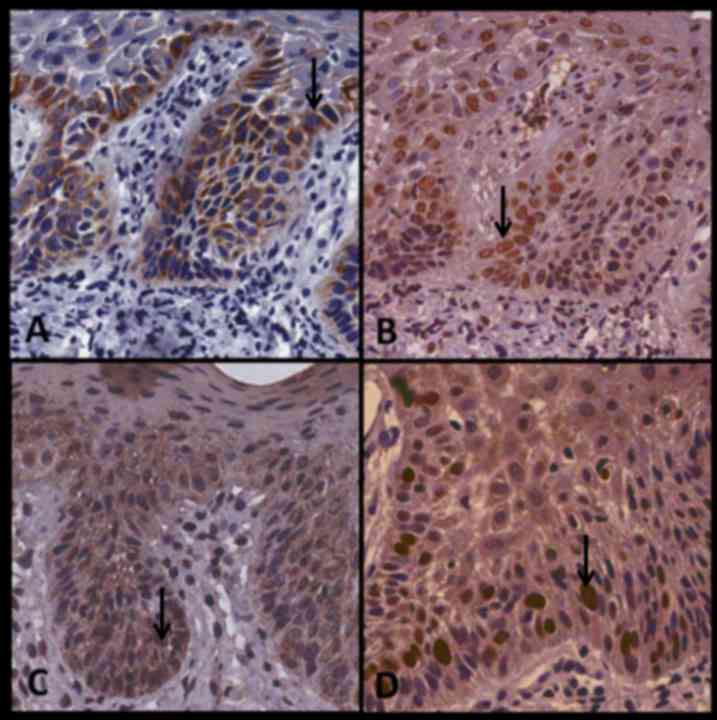
Fig. 5 presents case no. 29,
demonstrating the expression of p16, BUB3, SOX4 and Ki-67 in OD.
All antibodies exhibited strong immunostaining with the exception
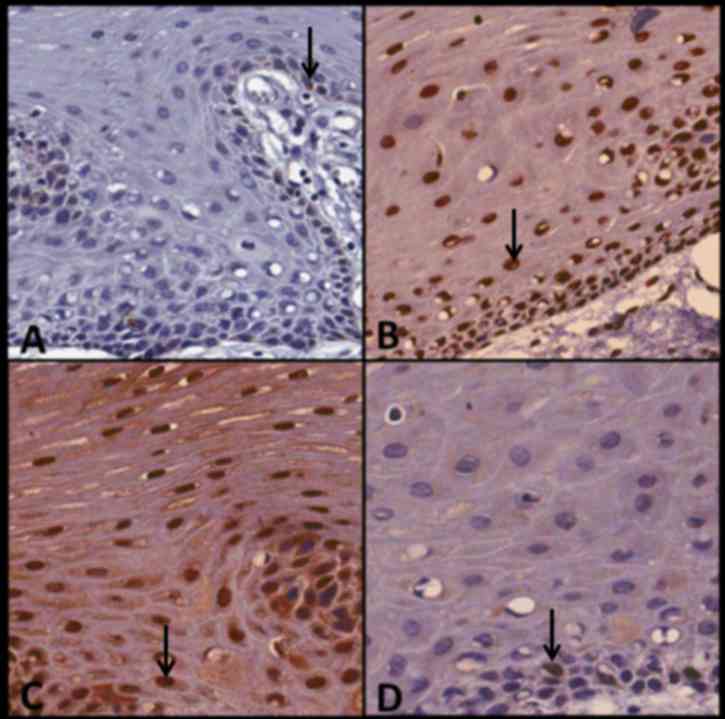
of SOX4, which was only moderately stained. Fig. 6 presents case no. 23 and demonstrates
the expression of P16, BUB3, SOX4 and Ki67 in OFH. Bub-3 and SOX4
exhibited strong immunostaining.
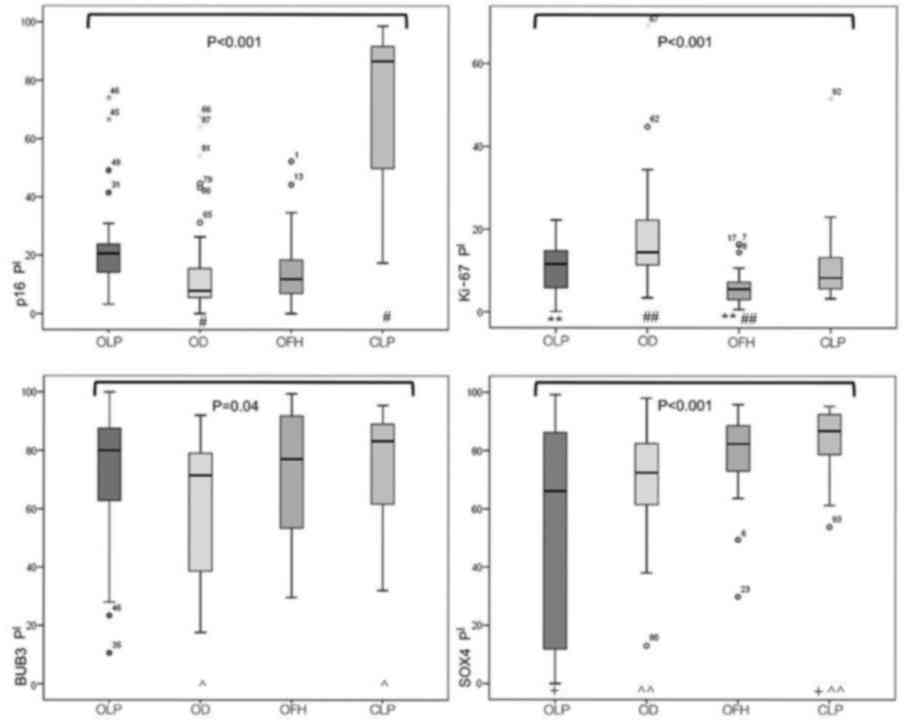 | Figure 1.Expression of p16, Ki-67, BUB3 and
SOX4 antibodies in OLP, OD, OFH and CLP. Differences among all
antibodies were determined using the Kruskal-Wallis test (P-values
written in full). The groups with indicators represent P-values
that were determined following the Dunn's post hoc test:
#P<0.002; **P<0.005; ##P=0.012;
^P=0.04; +P<0.001; ^^P=0.001.
SOX4, sex determining region Y-box 4; BUB3, budding uninhibited by
benzimidazoles 3; OLP, oral lichen planus; OD, oral dysplasia; OFH,
oral fibrous hyperplasia; CLP, cutaneous lichen planus; PI,
positivity index (percentage of positive cells n/500). |
P16 data
The PI for p16 was 20.65% (14.02–23.95) for OLP,
7.85% (5.22–18.12) for OD, 86.59% (49.80–91.80) for CLP and 11.8%
(6.82–18.50) for OFH, and the difference between these groups was
statistically significant (P<0.001). Notably, the Dunn post hoc
test indicated a significant difference between OLP and OD
(P<0.002). Although the distribution was not statistically
significant, the expression of p16 by OLP macroscopic type was
unevenly distributed (Fig. 2). The
widest distribution was observed in the eroOLP type (P=0.403).
Ki-67 data
PIs of Ki-67 were indicated as 11.6% (5.60–15.15)
for OLP, 14.4% (11.25–22.8) for OD, 8.24% (5.40–13.45) for CLP and
5.5% (3.00–7.45) for OFH, and a statistically significant
difference was observed between groups (P<0.001). Dunn post hoc
tests indicated that a significant difference existed between OLP
and OFH (P<0.005), and between OD and OFH (P=0.012).
Bub-3 data
Assessment of BUB3 expression resulted in PIs of
80.0% (60.15–88.30) for OLP, 71.4% (38.10–79.75) for OD, 83.2%
(60.95–89.40) for CLP and 77% (53.10–91.90) for OFH, and a
statistically significant difference between these groups was
indicated (P=0.04). Pairwise comparisons demonstrated a significant
difference between only CLP and OD (P=0.04).
SOX4 data
PIs for SOX4 were 66.1% (11.20–86.20) for OLP, 72.4%
(61.05–82.55) for OD, 86.8% (78.65–92.4) for CLP and 82.3%
(71.95–88.65) for OFH, and a statistically significant difference
between all groups was demonstrated (P<0.001). Pairwise
comparisons revealed a significant difference between OLP and CLP
(P<0.001) and between CLP and OD (P=0.001).
Discussion
To the best of our knowledge, the present study is
the first to evaluate the expression of p16, Ki-67, Bub-3 and SOX4
as markers of malignant transformation in OLP. To explore the roles
of these factors in OLP, OFH was considered as a negative control
for oral malignancy (proliferative benign lesion), OD was regarded
as a standard of pre-malignant lesion and CLP was used as the skin
counterpart of OLP with no tendency to malignancy thus also a
negative control. The present results provide indications of the
possible mechanisms associated with the nature of OLP and the
association of p16 and Ki-67 expression with OLP.
In the present study, the criteria for selecting the
groups was defined by separating lesions with a low risk of
malignant transformation of dysplastic epithelium lesions and those
with malignant potential and these were compared with OLP. Although
OD is not considered a lesion, it is the most well-known factor
that has the potential for malignant transformation (5).
p16, a protein identified as a part of the
retinoblastoma (Rb) pathway, has a key role in cell cycle
regulation and binds to CDK4 and 6, which prevents them from
binding to cyclin D1 (19). This
inhibits Rb protein from being phosphorylated and induces cell
cycle arrest (19). An initial event
in the development of oral carcinomas is a change in the 9p21
locus, which results in the suppression of p16 (19). This suggests that the presence of p16
may be protective. Paradoxically, some OSCCs have demonstrated
overexpression of p16, resembling what is typically observed in
cervical intraepithelial neoplasia and cervical cancer (20). This may be explained by the fact that
human papillomavirus (HPV) inhibits Rb, which leads to a high
expression of p16 due to negative feedback regulation (21).
There are conflicting results in studies evaluating
p16 expression in oral pre-malignant lesions (22,23),
which may be due to the complexity of the mechanism involved. In
the present study, the PI of p16 was extremely high in the CLP
group, whereas the OD group exhibited the lowest PI (P<0.002).
This is consistent with the protective characteristics of p16,
considering that CLP reportedly does not have malignant potential
(8). To the best of our knowledge,
the high expression index of p16 in CLP has not been previously
reported. Furthermore, the OLP group revelaed the second-to-highest
expression index for p16. Therefore, the present study classified
cytoplasmic staining as p16-positive in order to include all cell
mechanisms associated with p16 deregulated expression (20,21).
Oral cancer has multiple etiologies and the major
contributing factors include tobacco and alcohol intake (5). The presence of HPV was not evaluated in
the present study as previous studies have demonstrated that the
majority of OSCC and associated lesions were unrelated to HPV
infection (19–21). However, recent findings have
indicated a higher prevalence of HPV in associated OSCC lesions
indicating a the possibility that some OLP lesions are positive for
HPV and also associated with a high p16 expression (22).
However, there is a lack of consistency regarding
how to evaluate the expression of p16 in OLP. Distinct criteria
impacts the results and the translation of these results in
clinical practice for the positive expression of the p16 marker,
increasing the variability of the results. For example, in some
cases the PI result is dichotomized into positive or negative p16
with different margins in various studies. Of these, certain
studies have considered a positive result for cases that have a
mark >70% (12,23). Only one study demonstrated a high
prevalence of HPV in 20 samples of eroOLP using two different
molecular techniques. However, the expression of p16 was not
assessed (24). In the present
study, 20.65% of cells observed in OLP lesions were positive for
p16, suggesting that HPV may be present in OLP.
In the present study, PI can be considered as a
robust method as it is standardized, feasible and reproducible for
the evaluation of immunoexpression. Future studies aiming to assess
the expression p16 in HPV-infected tissues should evaluate such an
association. The present results indicated a difference in p16
expression between OLP subtypes, particularly in eroOLP. eroOLP is
associated with a higher risk of transformation to OSCC. Notably, a
previous study has suggested a correlation between HPV and eroOLP
(24). The present findings
indicated the difference between OLP subtypes was not statistically
significant. However, further investigation into this area is
required, taking into account the wide data dispersion compared
with other clinical lichen types.
Ki-67 is a nuclear antigen correlated with cellular
proliferation and is an extensively explored marker (25). Ki-67 has been evaluated as a
predictor of metastasis and as an indicator for the prognosis and
recurrence of OSCC (25,26). Furthermore, a positive correlation
between Ki-67 expression rate and histological grade has been
indicated in studies with non-malignant, pre-malignant and
cancerous oral lesions, which suggests its potential as an indirect
measure of malignant transformation risk and histologic grading
(26). In the present study, Ki-67
expression among the studied lesion groups was the lowest for OFH,
followed by CLP, OLP and OD. As expected, OD lesions exhibited the
highest PI for this marker. However, OLP lesions exhibited the
second-highest PI (11.6%), which was similar to the expression rate
described in previous studies (13 and 13.8%) (10,11).
Notably, eight of the 30 OLP samples presented a Ki-67 expression
above the median of that associated with OD (14,4%). This may
suggest that only specific OLP lesions exhibited malignant
potential, which may explain the controversies of current evidence
regarding OLP as a premalignant lesion. Furthermore, the expression
of Ki-67 was significantly lower between OLP and OFH (P<0.005),
and between OD and OFH (P=0.012), supporting that OFH has a reduced
risk of transformation, when compared with OD and OLP.
As a member of the mitotic checkpoint complex
proteins, BUB3 has an essential role in coupling with other
proteins from the same family to prevent cells entering anaphase
prematurely (13). The absence of
BUB3 has been associated with tumorigenesis in knockout animal
models, with widespread aneuploidy and high proliferative potential
(27). Similarly, various studies in
humans have indicated the association of deregulated expression and
mutations in BUB3 with specific types of cancer, including
colorectal and lung tumors (14,15).
Consistent with this, the results from the present study indicated
differential BUB3 expression among the lesions, with the lowest
expression in OD and the highest in CLP. These differences were
marginally statistically significant, which suggests this marker
may be less sensitive in the present assay compared with the two
previously discussed markers. This may convey the limited clinical
value of this marker on its own. However, further studies are
required to investigate the differential expression of BUB3 among
different histologic grades of dysplasia.
SOX4 is a member of the SOX transcription factor
family (17). Previous findings have
suggested that the overexpression of this marker may be associated
with cancer development and progression (17). Furthermore, SOX4 has been correlated
with the prognosis of breast cancer and recurrence of colorectal
cancer (28). However, the role of
SOX4 in cancer development has also been debated in the literature.
Previous results have identified SOX4 as an oncogene and potential
target for cancer vaccinations, suggesting that the loss of SOX4 is
associated with loss of tissue viability, whereas its
overexpression is associated with immortalized cells (17). However, these conclusions were drawn
from in vitro experiments alone. Alternatively, findings
have indicated that SOX4 has a role in cell cycle arrest and
apoptosis by activating p53, which suggests SOX4 may be an indirect
marker of carcinogenesis (28). In
this way, upregulated SOX4 expression may be considered an indirect
indicator of cell cycle deregulation. The clinical relevance of
these hypotheses translates into the usefulness of SOX4 as a
biomarker for cancer in soft tissues, particularly in epithelial
tissues (29).
In the present study, the expression of SOX4 was
high in all groups, particularly in CLP. This finding was
unexpected as low expression was anticipated in the CLP group.
Additionally, to the best of our knowledge, this is the first time
that high SOX4 expression was indicated in CLP. It was hypothesized
that the proliferation pattern of epithelium compared with oral
mucosa cells varies in lichen planus. In this regard, although the
increased expression of SOX4 is typically associated with
malignancy (28,29), the present study results suggests
that SOX4 overexpression may have a protective role in CLP from
malignancies including skin carcinoma. In addition, the durability
of SOX4 in CLP may be extremely high, which may lead to an abnormal
accumulation within the cell that is not associated with malignancy
(29). However, further
investigation is required to detect the cause for increased SOX4
expression and whether this may clarify the different clinical
course of OLP compared with CLP.
In the present study, the lowest level of SOX4
expression was indicated in OLP, followed by OD. Although no
statistically significant difference was detected between these
groups in the pairwise comparison, the data distribution suggests
it is possible that the various clinicopathological characteristics
of OLP may be associated with different expressions of SOX4 in each
form of OLP. As with p16, this irregular expression may be
associated with OLP-specific lesions that may have either a higher
or lower likelihood to undergo malignant transformation. Further
studies with large and varied populations may aid to elucidate the
role of SOX4 in oral carcinogenesis.
The present study did exhibit some limitations.
Notably, this was a retrospective study where there was an
imbalance in the distribution of the biopsy sites and the clinical
lesion subtypes, specifically in the eroOLP group and the grades of
dysplasia. This may have affected the results, as there are
potential differences between mild and severe dysplasia and between
retOLP and eroOLP in the underlying pathophysiology. In addition to
the study design, subjects and lesions cannot be followed through
time to detect actual malignant transformation of lesions, which
would add value in the clinical setting. Furthermore, procedures
did not include HPV or double antigen detection, which may provide
further useful information. However, double antigen detection is a
more technically demanding procedure compared with the single
antigen detection, which suggests this may be less feasible in the
primary care clinical setting (30).
However, for the more equipped facilities, there are promising
results on the double-marking of lesions combined with polymerase
chain reaction techniques for detection of high-risk oral lesions
(30). Furthermore, it was
acknowledged that a larger sample size would further support the
present findings, particularly considering the variability in the
location and type of lesions among some of the studied groups. In
addition, the samples used in the present study did not include
OSCC biopsies. However, the objective was to evaluate the
expression profiles of potentially premalignant lesions as opposed
to detecting cancer.
In conclusion, the present study indicated that p16
and Ki-67 expression may suggest that some OLP lesions have an
intermediate malignant potential and should be more carefully
followed up. The intense SOX4 staining in CLP indicates a different
proliferation pattern of epithelium compared with oral mucosa cells
and may also be associated with the clinical course in lichen
planus. Additional studies are warranted to improve the
understanding of the role of BUB3 and SOX4 in OLP.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
EAR and AMH made substantial contributions to the
conception and design of the study, as well as the acquisition,
analysis, and interpretation of all data. They also contributed in
drafting the manuscript. DPF made substantial contributions to the
design of the study and the analysis of general data, and was
responsible for the statistical analysis. LEARF, FFCN and HM made
substantial contributions to the design of the study and the
analysis of immunohistochemical data. VT and EMK made substantial
contributions to the design of the study and the acquisition of
oral biopsy data. FPG made substantial contributions to the design
of the study and the acquisition of skin biopsy data. RFBA made
substantial contributions to the conception and design of the
study. DPF, LEARF, FFCN, VT, FPG, EMK, HM and RFBA helped to
critically revise the manuscript for important intellectual
content. All authors approved the final manuscript and agree to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
All procedures were reviewed and approved by the
Institutional Review Board (IRB) from the School of Medicine,
University of Brasília (Brasília, Brazil; approval number, CEPFM
042/2010) and conducted in accordance with the principles of the
Declaration of Helsinki. Informed consent was obtained in the
majority of cases; however, some patients were not available
following several contact attempts or had experienced mortality.
Therefore, the IRB approved not having the formal consent in these
cases.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dias GS and Almeida AP: A histological and
clinical study on oral cancer: Descriptive analyses of 365 cases.
Med Oral Patol Oral Cir Bucal. 12:E474–E478. 2007.PubMed/NCBI
|
|
3
|
Amagasa T, Yamashiro M and Uzawa N: Oral
premalignant lesions: From a clinical perspective. Int J Clin
Oncol. 16:5–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Omal P, Jacob V, Prathap A and Thomas NG:
Prevalence of oral, skin, and oral and skin lesions of lichen
planus in patients visiting a dental school in southern India.
Indian J Dermatol. 57:107–109. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barnes L, Eveson JW, Reichart P and
Sidransky D: Pathology and genetics of head and neck tumours. WHO
Classif Tumour. 163–75. 2005.
|
|
6
|
van der Meij EH, Mast H and van der Waal
I: The possible premalignant character of oral lichen planus and
oral lichenoid lesions: A prospective five-year follow-up study of
192 patients. Oral Oncol. 43:742–748. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xue JL, Fan MW, Wang SZ, Chen XM, Li Y and
Wang L: A clinical study of 674 patients with oral lichen planus in
China. J Oral Pathol Med. 34:467–472. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eisen D: The clinical features, malignant
potential, and systemic associations of oral lichen planus: A study
of 723 patients. J Am Acad Dermatol. 46:207–214. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rosa EA, Lia EN, Macedo SB and Amorim RF:
In situ carcinoma developed over oral lichen planus: A case report
with analysis of BUB3, p16, p53, Ki67 and SOX4 expression. J Appl
Oral Sci. 23:442–447. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Acay RR, Felizzola CR, de Araújo N and de
Sousa SOM: Evaluation of proliferative potential in oral lichen
planus and oral lichenoid lesions using immunohistochemical
expression of p53 and Ki67. Oral Oncol. 42:475–480. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zargaran M, Jamshidi S, Eshghyar N and
Moghimbeigi A: Suitability/unsuitability of cell proliferation as
an indicator of malignant potential in oral lichen planus: An
immunohistochemical study. Asian Pac J Cancer Prev. 14:6979–6983.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nankivell P, Williams H, Webster K,
Pearson D, High A, MacLennan K, Senguven B, McConkey C, Rabbitts P
and Mehanna H: Investigation of p16(INK4a) as a prognostic
biomarker in oral epithelial dysplasia. J Oral Pathol Med.
43:245–249. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han JS, Vitre B, Fachinetti D and
Cleveland DW: Bimodal activation of BubR1 by Bub3 sustains mitotic
checkpoint signaling. Proc Natl Acad Sci USA. 111:E4185–E4193.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mukherjee A, Joseph C, Craze M,
Chrysanthou E and Ellis IO: The role of BUB and CDC proteins in
low-grade breast cancers. Lancet. 385 Suppl 1:S722015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Voer RM, van Kessel Geurts A, Weren RD,
Ligtenberg MJ, Smeets D, Fu L, Vreede L, Kamping EJ, Verwiel ET,
Hahn MM, et al: Germline mutations in the spindle assembly
checkpoint genes BUB1 and BUB3 are risk factors for colorectal
cancer. Gastroenterology. 145:544–547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bangur CS, Switzer A, Fan L, Marton MJ,
Meyer MR and Wang T: Identification of genes over-expressed in
small cell lung carcinoma using suppression subtractive
hybridization and cDNA microarray expression analysis. Oncogene.
21:3814–3825. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Friedman RS, Bangur CS, Zasloff EJ, Fan L,
Wang T, Watanabe Y and Kalos M: Molecular and immunological
evaluation of the transcription factor SOX-4 as a lung tumor
vaccine antigen. J Immunol. 172:3319–3327. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van der Meij EH and van der Waal I: Lack
of clinicopathologic correlation in the diagnosis of oral lichen
planus based on the presently available diagnostic criteria and
suggestions for modifications. J Oral Pathol Med. 32:507–512. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ai L, Stephenson KK, Ling W, Zuo C,
Mukunyadzi P, Suen JY, Hanna E and Fan CY: The p16 (CDKN2a/INK4a)
tumor-suppressor gene in head and neck squamous cell carcinoma: A
promoter methylation and protein expression study in 100 cases. Mod
Pathol. 16:944–950. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abrahao AC, Bonelli BV, Nunes FD, Dias EP
and Cabral MG: Immunohistochemical expression of p53, p16 and hTERT
in oral squamous cell carcinoma and potentially malignant
disorders. Braz Oral Res. 25:34–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Murphy N, Ring M, Heffron CC, King B,
Killalea AG, Hughes C, Martin CM, McGuinness E, Sheils O and
O'Leary JJ: p16INK4A, CDC6, and MCM5: Predictive biomarkers in
cervical preinvasive neoplasia and cervical cancer. J Clin Pathol.
58:525–534. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Broglie MA, Jochum W, Förbs D, Schönegg R
and Stoeckli SJ: Brush cytology for the detection of high-risk HPV
infection in oropharyngeal squamous cell carcinoma. Cancer
Cytopathol. 123:732–738. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Salehinejad J, Sharifi N, Amirchaghmaghi
M, Ghazi N, Shakeri MT and Ghazi A: Immunohistochemical expression
of p16 protein in oral squamous cell carcinoma and lichen planus.
Ann Diagn Pathol. 18:210–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jontell M, Watts S, Wallström M, Levin L
and Sloberg K: Human papilloma virus in erosive oral lichen planus.
J Oral Pathol Med Off Publ Int Assoc Oral Pathol Am Acad Oral
Pathol. 19:273–277. 1990.
|
|
25
|
Perisanidis C, Perisanidis B, Wrba F,
Brandstetter A, El Gazzar S, Papadogeorgakis N, Seemann R, Ewers R,
Kyzas PA and Filipits M: Evaluation of immunohistochemical
expression of p53, p21, p27, cyclin D1, and Ki67 in oral and
oropharyngeal squamous cell carcinoma. J Oral Pathol Med. 41:40–46.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Birajdar SS, Radhika M, Paremala K,
Sudhakara M, Soumya M and Gadivan M: Expression of Ki-67 in normal
oral epithelium, leukoplakic oral epithelium and oral squamous cell
carcinoma. J Oral Maxillofac Pathol. 18:169–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
da Silva Morais S, Moutinho-Santos T and
Sunkel CE: A tumor suppressor role of the Bub3 spindle checkpoint
protein after apoptosis inhibition. J Cell Biol. 201:385–393. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pan X, Zhao J, Zhang WN, Li HY, Mu R, Zhou
T, Zhang HY, Gong WL, Yu M, Man JH, et al: Induction of SOX4 by DNA
damage is critical for p53 stabilization and function. Proc Natl
Acad Sci USA. 106:3788–3793. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Watanabe M, Ohnishi Y, Wato M, Tanaka A
and Kakudo K: SOX4 expression is closely associated with
differentiation and lymph node metastasis in oral squamous cell
carcinoma. Med Mol Morphol. 47:150–155. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Linxweiler M, Bochen F, Wemmert S, Lerner
C, Hasenfus A, Bohle RM, Al-Kadah B, Takacs ZF, Smola S and Schick
B: Combination of p16INK4a/Ki67 immunocytology and hpv polymerase
chain reaction for the noninvasive analysis of HPV involvement in
head and neck cancer. Cancer Cytopathol. 123:219–229. 2015.
View Article : Google Scholar : PubMed/NCBI
|