Introduction
Hepatic perfusion disorder (HPD) refers to the
difference in perfusion between a number of different liver
segments and sub-segments induced by numerous causes, reflecting
the partial liver microcirculation hemodynamic status (1). In 1997, Gryspeerdt et al
(2) analyzed the phenomenon of
hepatic perfusion differences identified in dual-phase spiral
computed tomography (CT) scan, and first nominated the disorder as
HPD. Following this identification of HPD, several studies
concerning HPD have been published (3,4).
Etiopathogenesis of HPD is associcated with the
certain imaging characteristics of HPD focal lesions, which are
primarily categorized into three types: Diffuse type, liver
lobe-liver segment type and wedge-sheet type (5). These diffuse types describe the
multiple forms and various sizes of abnormal perfusion regions
associated with HPD in the liver parenchyma. HPD is commonly
observed in portal vein thrombosis and compression caused by
malignant tumors (6). HPD liver
lobe-liver segment type may be identified according to abnormally
enhanced signals in the liver segment or liver lobe in the hepatic
arterial phase and istypically observed in liver tumors or tumors
of adjacent organs, post-liver surgery, interventional treatment or
inflammatory lesions (4). The
wedge-sheet type HPD is characterized by a wedge or triangular
shape, which are commonly observed at the edge of the liver or
liver lesions and are caused by liver cancer, cirrhosis, or
abnormal physiological perfusion (7).
In China, the prevalence of liver disease is high,
particularly those associated with viral hepatitis (predominantly
hepatitis B virus), alcoholic liver disease and non alcoholic fatty
liver disease, which affects ~300 million people (8). These diseases may progress into hepatic
cirrhosis and liver cancer, which may result in HPD (9). Therefore, evaluation of HPD may help
elucidate the hepatic pathological changes that occur in patients
with HPD.
Following the extensive application of multislice
computed tomography (MSCT), CT evaluations of HPD have previously
been reported (10). However, few
MRI evaluations of HPD have been reported, which is likely due to
the fact that the use of MRI in imaging for evaulating HPD was
introduced after CT and MRI is not used as widely as CT in imaging
of HPD (11). However, the features
of HPD in MRI imaging have been described in the literature
(1), and it has been reported that
MRI imaging maybe used to indicate the characteristics of different
hepatic diseases that cause HPD (3).
As the use of MRI has developed, it has been accepted in the
medical community that MRI is superior to MSCT (10,12,13).
Although the scanning speed of CT is fast, its soft-tissue
resolution is lower than MRI (14).
In addition, CT cannot be repeatedly performed in a the short term
due to accumulation of radiation, whereas MRI does not harm the
human body, thus repeated MRI may be deemed as safe to patients
(15). MRI liver imaging can be
completed in a short time period; regular enhanced MRI scanning of
the liver can be done in 14 sec for each phase. In partitcular
cases, the scanning speed of MRI can be shortened to several sec
for each phase, and multi-phase scanning has been applied in
evaluations of liver diseases (16).
Furthermore, when assessing the liver and other abdominal lesions,
more perfusion disorders may be identified using MRI scanning
(3). In the present study, a
retrospective analysis of 35 patients diagnosed with HPD through
MRI in between January 2010 and January 2013 has been completed.
The literature has been reviewed in order to enhance the awareness
of HPD, improve the diagnosis of liver diseases and identify
potential novel liver diseases (17).
Materials and methods
General information
A total of 35 patients with variation of HPD: 6
patients exhibtied diffuse type; 7 patients presented with liver
lobe or liver segment type; and 22 patients had wedge or flaky
type. Patients were diagnosed by unenhanced and dynamic
contrast-enhanced MRI scanning in the Affiliated Hospital of
Binzhou Medical College (Yantai, China) between January 2010 and
January 2013 were selected for the current study. Among the 35 HPD
patients, 26 were male and 9 were female; and the age of patients
ranged from 41 to 82 years, with an average age of 59 years. Among
these cases, there were 16 cases of liver cancer, 1 case of
pancreatic cancer with hepatic metastasis, 1 case of hepatic
hemangioma, 5 cases of hepatocellular carcinoma following
interventional treatment, 1 case of liver cancer following surgery,
1 case of liver metastasis of colon cancer following radio
frequency ablation, 1 case of gastric cancer, 3 cases of liver
cirrhosis, 1 case of liver abscess, 1 case of cholecystitis and 1
case of pancreatitis. Primary lesions were not identified in 3
cases of radiological examination. All patients underwent plain MRI
scanning, dynamic triple-phase enhanced scanning and ultrasound
examination. Part of those cases underwent CT (7 cases), digital
subtraction angiography (DSA, 5 cases), or needle biopsy (performed
in 2 cases). DSA and needle biopsies were conducted according to
previously described methods (18,19).
MRI examination
A Siemens MAGNETOM® Avanto 1.5T MR
instrument (Siemens AG, Munich, Germany) was used to conduct
examinations in the present study. A body phased-array coil was
also used during examinations, where the phased array body coil was
placed on the upper abdomen and close to the abdominal wall of the
patient. All 35 patients underwent conventional T1 weighted image
(WI), T2WI, fat saturation (FS) T2WI, FS T1WI, diffusion weighted
images (DWI), dynamic contrast-enhanced imaging and delayed
imaging. MRI imaging was performed as described previously
(20). Patients were maintained in
the supine position, in which the head entered the MRI machine
first. High-resolution MRI imaging sequences include: T1WI
repetition time (TR) 170 msec, echo time (TE) 4.8 msec, T2WI TR
1,200 msec, TE 92.0 msec; DWI b value was set to 50 and 600
sec/mm2; FS T2WI TR 4,352 ms, TE 90 ms; FS T1WI1
acquisition matrix 512×512, field of view 380 mm, slice thickness 3
mm. The dynamic enhanced MRI scanning contrast agent was 20–25 ml
gadolinium-diethylenetruamune pentaacetic acid. A scanning rate of
2 m/sec was used and the imaging time was as follows: Arterial
phase 20–25 sec, portal venous phase 50–60 sec, delayed phase
120–180 sec. A total of 25 patients had a 5 min delay in imaging
due to image changes.
MRI image viewing
Images were assessed by two experienced and
specialized physicians in a blinded retrospective method. The
occurrence of HPD was evaluated according to unenhanced and dynamic
contrast-enhanced MRI imaging results at the same time, lesion
size, and location, using the same enhancement pattern. The
association between the HPD and lesions in the liver and abdomen
was recorded, whether or not these were accompanied by an
arterioportal shunt (APS). MRI, CT, DSA and related image data,
laboratory tests, surgery and pathology results associated with the
patient's primary lesions were comprehensively analyzed for the
purpose of clarifying the causes of the diseases and provide an
insight into the development mechanism of HPD.
MRI criteria that determined HPD were as follows:
Abnormal perfusion regions in the arterial phase revealed a wedge
or irregular hyperintense signal; the portal venous phase area
revealed isointense or slightly hyperintense signals; the delayed
phase imaging revealed isointense or hyperintense signals.
According to its performance characteristics in the liver
parenchyma, HPD may be classified as diffuse type, liver lobe-liver
segment type, and wedges or flaky type.
The direct signs of APS to identify the patients
with APS were as follows: i) Dynamic enhanced imaging in the
arterial phase revealed intrahepatic portal vein branches, but the
main portal vein did not appear; ii) Portal vein branches and trunk
imaging appeared, but the superior mesenteric vein and splenic vein
did not appear; iii) Signals within the proximal branches of the
portal vein were markedly higher than within the distal
branches.
Results
Patient information
Of all 35 HPD cases, 26 were tumor-related;
accounting for 74.3% of the total number of cases assessed, which
included 16 cases of primary liver cancer, 1 case of liver
metastases, 1 case of hepatic hemangioma, 1 case of gastric cancer,
6 cases of hepatocellular carcinoma following surgery and
interventional treatment and 1 case of liver metastases following
treatment intervention. There were 6 non-tumor associated cases,
accounting for 17.1% of total HPD cases including 3 cases of
inflammatory lesions and 3 cases of liver cirrhosis while 10 cases
of APS HPD were identified. Therefore, tumor-associated lesions
account for a relatively large amount of HPD cases in the group in
the present study. Primary liver cancer is a common malignant tumor
that seriously threatens human health globally, as the sixth most
lethal malignant tumor (21) and is
the most common disease that causes HPD. Furthermore, inflammatory
lesions and non-neoplastic causes of cirrhosis are common causes of
HPD (22,23). Transcatheter arterial
chemoembolization (TACE) is the primary measurement approach of
interventional treatment for liver cancer (24). Interventional therapy typically leads
to HPD (25). Clear primary lesions
were not identified in three of the cases presented in the current
study, which were considered to physiologically cause HPD;
accounting for 8.6% of HPD cases. Pathogenic causes and the
different types of HPD are presented in Table I.
 | Table I.Pathogenic causes and different types
of hepatic perfusion disorders of 35 patients. |
Table I.
Pathogenic causes and different types
of hepatic perfusion disorders of 35 patients.
| Pathogenic
cause | Diffuse type | Liver lobe and
segment type | Wedge-shaped
type | Total |
|---|
| Liver cancer | 3 | 1 | 12 | 16 |
| Following liver
cancer treatment | 1 | 2 | 4 | 7 |
| Hepatic
hemangioma |
|
| 1 | 1 |
| Hepatic
metastasis | 1 |
|
| 1 |
| Gastric cancer |
| 1 |
| 1 |
| Cirrhosis |
| 1 | 2 | 3 |
| Liver abscess |
| 1 |
| 1 |
| Cholecystitis |
| 1 |
| 1 |
| Pancreatitis | 1 |
|
| 1 |
| Physiology |
|
| 3 | 3 |
| Total | 6 | 7 | 22 | 35 |
Comparison of MRI and CT in HPD
imaging
Of the 35 HPD cases assessed, 7 also underwent plain
CT and 3 underwent enhanced scan imaging. Furthermore, 6 of the 35
cases underwent CT scan imaging, which revealed abnormal perfusion
regions with a marked enhancement in the arterial phase and signals
in balanced and delayed phases that appeared near the liver
parenchyma; the performance of the CT scanning images appear to be
similar to MRI enhanced imaging. Atypical signs were observed in 1
case of HPD that underwent CT scanning. This may be associated with
the rate of contrast agents and different imaging modalities. As
noted previously, MRI has a number of advantages in displaying
HPD.
MRI imaging of patients
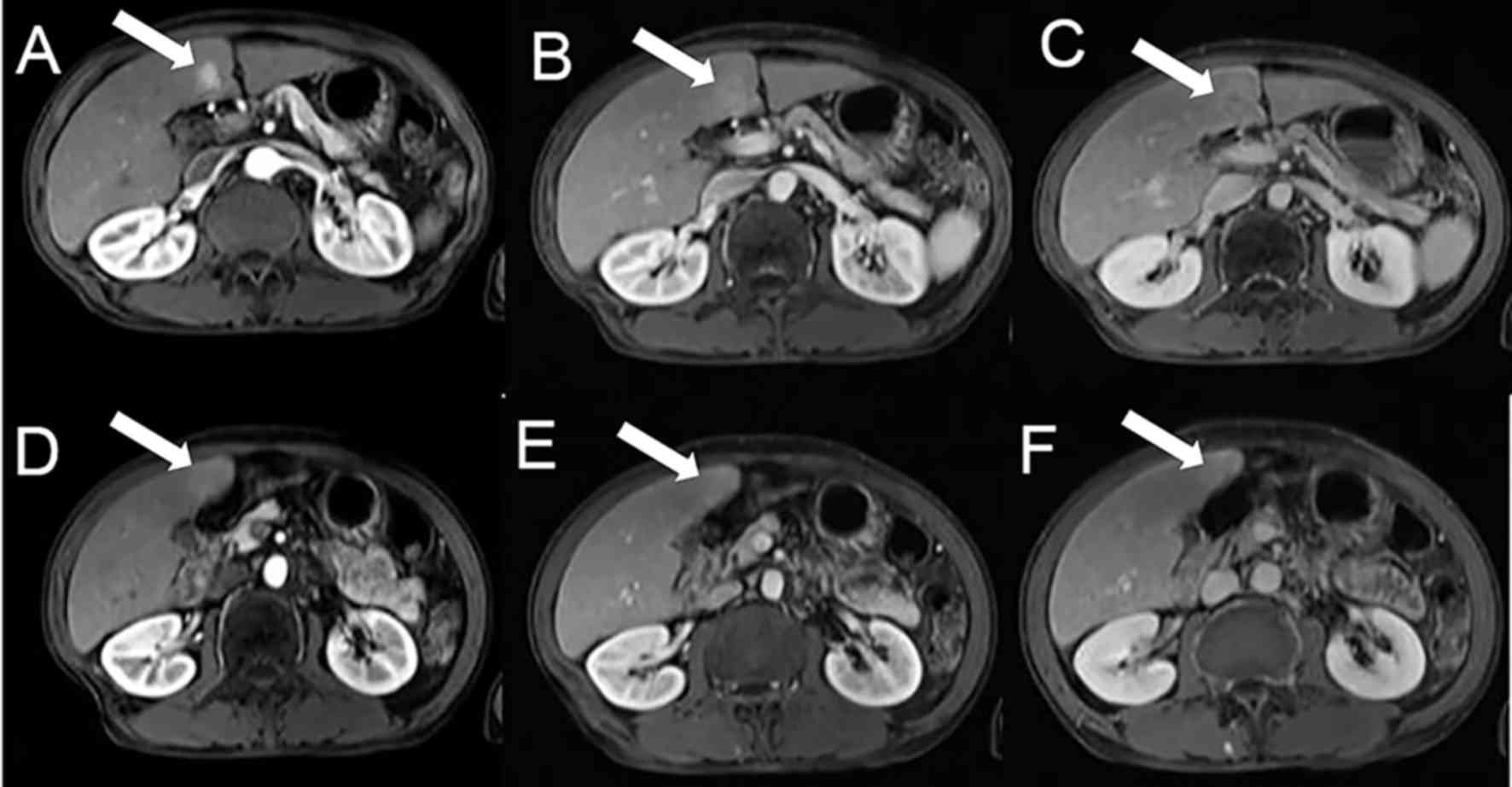
Fig. 1 indicated the
MRI imaging of a patient (male, with a history of hepatitis B) with
liver cell adenocarcinoma revealed by biopsy. Fig. 1A-C demonstrated the imaging of the
tumor. Fig. 1A indicated the tumor
in the arterial phase was markedly enhanced. Fig. 1B demonstrated that the signal of the
tumor in the venous phase was slightly hyperintense, while in the
delayed phase the tumor displayed hypointense signals (Fig. 1C). Fig.
1D-F demonstrated the imaging of abnormal perfusion in the
liver inferior to the adjacent region. Abnormal perfusion in the
arterial phase was hyperintense (Fig.
1D). Furthermore, abnormal perfusion in the venous phase and
the delayed phase was also slightly hyperintense (Fig. 1E and F, respectively).
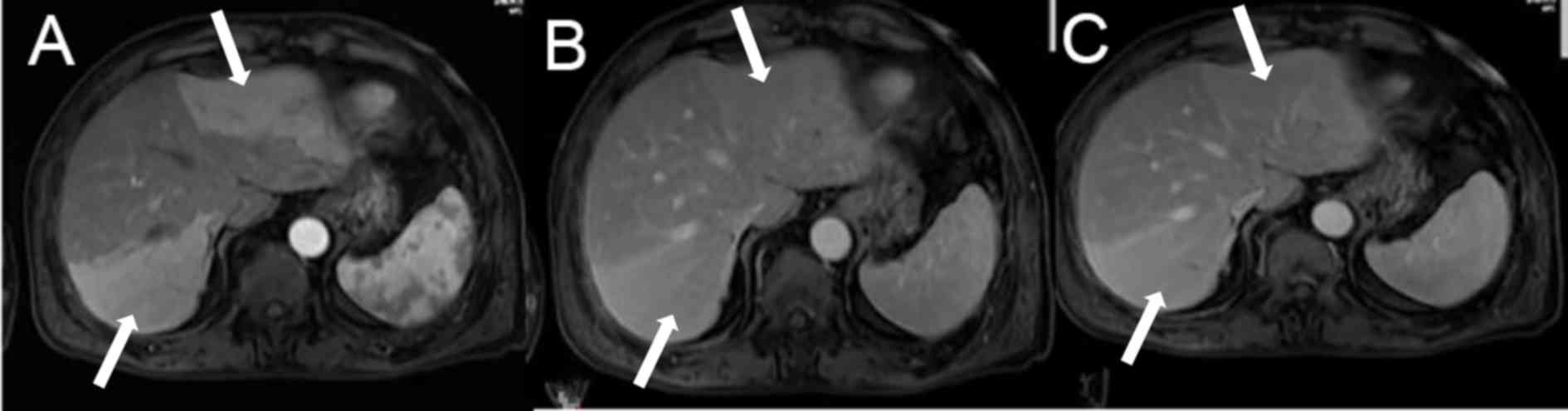
Fig. 2 indicated the
liver enhanced MRI imaging of a patient with cholecystitis. Imaging
in the arterial phase demosntrated multiple abnormal enhancements
in the left and right lobe of the liver with clear boundaries
(Fig. 2A). In the portal phase,
abnormalities in the left and right lobe of the liver were slightly
hyperintense (Fig. 2B). Notably,
abnormalities in the left and right lobe of the liver in the
delayed phase were also slightly hyperintense (Fig. 2C).
Discussion
MRI scanning of HPD primarily indicates isointense
T1 and T2 signals. A previous study reported that HPD may exhibit
long T1 and T2 signals (17). In all
35 cases, the plain MRI scan revealed isointense-T1 and
isointense-T2 signals, as well as isointense-DWI signals; when
cases underwent an enhanced scan. HPD primarily appeared as a
transient wedge, triangular, oval-shaped or an abnormal enhancement
zone in the arterial phase. Signals were isointense with sharp
edges, which demonstrate a clear narrow transition zone with the
surrounding liver tissue. Furthermore, there was no vasculature
displacement, blood vessel signals were observed within
high-perfusion abnormal signals and isointense or slightly
hyperintense signals were observed in the portal venous phase and
delayed phase. The segment and sub-segment of the liver and liver
lobe may be affected and can be single or multiple sites, which are
primarily located in the peripheral region of the liver (26).
According to its performance characteristics in the
liver parenchyma, HPD may be divided into three types (5): i) The diffuse type: Presents with
multiple forms, numerous sizes of abnormal perfusion regions in the
liver parenchyma; exhibits irregular hyperintense abnormal
enhancement signals, patchy or round in the arterial phase. In the
present study, 6 cases were determined to be this type, which are
common in malignant tumor portal vein thrombosis and compression.
ii) Liver lobe-liver segment type: Exhibits abnormal enhanced
signals in the liver segment or liver lobe in the hepatic arterial
phase (27). In the present study, 7
cases were identified to be this type, which are commonly observed
in liver tumors or adjacent organ tumors (28), post-liver surgery or interventional
treatment (4). or in inflammatory
lesions (29). iii) Wedge-sheet
type: Abnormal perfusion regions present a wedge or sheet shape,
which are commonly observed at the edge of the liver or liver
lesions. In the present study, 22 cases belonged to this type,
which are commonly observed in liver cancer, cirrhosis and abnormal
physiological perfusion (30,31).
Danet et al (32) reported
that in 16 cases of pancreatic cancer liver metastasis enhanced MRI
scans, 8 cases (50%, 8/16) revealed a wedge enhancement. In the
present study, in the 16 patients with liver cancer, 12 cases (75%)
indicated wedge-sheet type HPD, which higher than the rate
indicated in the study by Danet et al (32), indicating that the present results
were consistent with these previous findings. Furthermore, these
findings suggest that the wedge-sheet typemay be a typical
characteristic for HPD caused by liver cancer.
HPD with atypical performance often requires the
identification of hepatocellular carcinoma, liver metastases, liver
hemangioma, focal nodular hyperplasia, hepatic adenoma and hepatic
tumor recurrence following the treatment of differentiated lesions
(33). Hepatocellular cancer
primarily manifests as long T1 and T2 signals, while DWI
demonstrates high signals in enhanced scanning imaging (34). High signals were observed in the
arterial phase, while the delayed phase exhibited low signals
(35). Metastatic tumors demonstrate
complex signal changes, while typical liver metastases exhibit a
T2WI ‘target sign’ or ‘bull's-eye’ sign (36). In the portal phase, enhanced scanning
revealed a loop-shaped enhancement. Hypointense T1WI signals and
hyperintense T2WI signals primarily demonstrate hemangiomas, while
enhanced scans revealed progressive enhancements. The presence of
focal nodular hyperplasia was determined by isointense or slightly
hypointense T1WI signals and slightly hyperintense or isointense
T2WI signals. In the central scar, T2WI hyperintense signals were
observed. In the early stage of enhanced scanning, significantly
enhanced signals were observed and slightly hyperintense signals
were observed in the late stage. In the central scar, delayed
enhancement was demonstrated. Solitary round shapes were primarily
observed in the liver adenoma, T1WI signals may be slightly
hyperintense or hypointense and T2WI revealed slightly hyperintense
signals. In enhanced scans, a significant enhancement was
demonstrated in the arterial phase, while the portal and delayed
phases revealed isointense and hypointense signals or isointense
and hyperintense signals. The recurrence of tumors following
treatment typically indicates long T1 and T2 signals (37). In enhanced scans, enhancement signals
are observed in the arterial phase, while decreased signals are
observed in the delayed phase. DWI presents with hyperintense
signals and thus, these were identified as HPD (26). In DWI, by evaluating the microscopic
motion of water molecules within living tissues, it is possible to
detect functional changes in tumor tissues following TACE surgery
in the early stages of cancer (38).
Previous studies have indicated that DWI is expected to become an
effective means of determining efficacy, as well as the
post-surgery follow-up assessment of liver cancer TACE therapy
(39). In order to understand the
effect of interventional therapy, important reference information
for the follow-up treatment of patients is required (40).
Anatomic variations of hepatic artery constitutes a
‘third hepatic inflow tract’ that primarily occurs in: The
gallbladder fossa, the attachment point of the ligament, front side
of the porta hepatis, the front margin of the II segment, front and
rear margins of the III segment and specific parts of the liver
subcapsular region (41). In the
early arterial phase of liver CT and MRI enhanced scans, the
contrast agent and reflux of the portal vein through the spleen and
gastrointestinal tract have not been fully and uniformly mixed;
thus, abnormal physiological hypoperfusion of the liver may occur
(42).
Mechanisms of abnormal pathological hyper perfusion
are complex and are summarized as follows (2): i) This may occur due to trauma, a
number of different invasive procedures., including after liver
tumor interventional therapy, or following liver transplantation
(43). ii) Tumors, including benign
and malignant liver tumors (26),
the incidence of HPD in primary liver cancer is 6.4 to 21%
(44) and Kim et al (45) reported that the incidence of HPD in
liver hemangiomas was 25.7%. iii) Inflammation; inflammatory
diseases in the liver and adjacent organs such as liver abscess,
cholangitis and cholecystitis (23);
abnormal perfusion phenomenon of round lesions or near the liver
parenchyma associated with liver abscess has been reported
(46) and is considered an important
diagnostic indication of liver abscess (47). iv) Compressed or blocked blood
vessels within the liver: The hepatic portal vain is the most
commonly involved (42); portal vein
obstruction, portal vein tumor thrombus or direct compression of
the portal vein by tumors that lead to reduced blood flow and blood
supply via the hepatic artery is increased to compensate (25).
The most common cause for this is a compressed or
blocked liver artery, hemodynamic balance between hepatic artery
and portal vein changes and reduction in perfusion of the affected
liver or hepatic artery (22); thus,
inducing liver pathologic hypoperfusion abnormalities (48).
HPD in specific regions and other parts of the
gallbladder fossa, when other reasons are excluded, may suggest the
presence of a third inflow tract without prompting any pathological
significance. HPD in simple imaging should not be considered as a
simple hemodynamic change and should lead to clinical attention and
regular follow-ups, if necessary. Biopsy should be conducted to
exclude or diagnose the true disease and to avoid misdiagnosis or
missed diagnosis.
The difference between APS dynamic portal pressure
observed in the patients may be relatively large, and portal vein
branches that present early may be observed in the arterial phase.
Certain dynamic portal pressure differences are relatively small,
and it is not easy to identify portal vein branches that present
early. The presence or absence of APS is significant for
determining an intervention treatment plan. Liver cirrhosis may
lead to reduced portal vein perfusion and increased compensatory
hepatic artery perfusion; thus, causing HPDs (49). APS is commonly identified in
cirrhosis, with an angiographic detection rate of 13% (42). However, the MRI detection rate is
relatively low and definite APS was not detected in three cases of
cirrhosis in the current study.
HPD may be an indirect sign of true lesions, since
early intrahepatic metastasis affects small liver blood vessel
branches. Imaging cannot exhibit direct metastatic lesions, and may
only present as HPD. Over time, metastasis may develop and larger
lesions may form. Thus, early intrahepatic metastasis in patients
with cancer who exhibit signs of HPD away from the main tumor foci
should be given attention.
Certain extra-hepatic adjacent organs such as the
stomach, pancreas, kidneys or retroperitoneal tumors may induce
hemodynamic changes in the portal vein and hepatic artery when they
violate extra-hepatic portal vein branches, celiac or the inferior
vena cava; which lead to liver perfusion differences and present
HPD.
In conclusion, HPD induced by liver cancer and other
causes, including cirrhosis, hemangioma, inflammation and adjacent
viscera lesions, is not uncommon. HPD does not have a
characteristic diagnostic value for liver lesions, but it may
indicate the presence of abnormal hepatic arterial blood flow,
portal vein obstruction or artery-portal vein shunt and other
potential pathological changes. Dynamic contrast-enhanced MRI
imaging is sensitive to HPD and is able to detect small liver
lesions and artery-portal vein diversion, as well as in detecting
occult lesions. However, further studies are required to establish
suitable methods for the detection and evaluation of HPD using MRI
imaging.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (grant nos. 81171303 and 30470518),
Development Project of Health and Science and Technology (grant
nos. 2011WSB29009 and 2011HW067) and the Department of Shandong
Province Outstanding Academic Leaders Fund of the Health System of
Shandong Province (grant no. 2006-39).
References
|
1
|
Tian JL and Zhang JS: Hepatic perfusion
disorders: Etiopathogenesis and related diseases. World J
Gastroenterol. 12:3265–3270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gryspeerdt S, Van Hoe L, Marchal G and
Baert AL: Evaluation of hepatic perfusion disorders with
double-phase spiral CT. Radiographics. 17:337–348. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lupescu IG, Grasu M, Capsa R, Pitrop A and
Georgescu SA: Hepatic perfusion disorders: Computer-tomographic and
magnetic resonance imaging. J Gastrointestin Liver Dis. 15:273–279.
2006.PubMed/NCBI
|
|
4
|
Quiroga S, Sebastià C, Pallisa E, Castellà
E, Pérez-Lafuente M and Alvarez-Castells A: Improved diagnosis of
hepatic perfusion disorders: Value of hepatic arterial phase
imaging during helical CT. Radiographics. 21:65–81, Questionnaire
288–294. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Colagrande S, Centi N, La Villa G and
Villari N: Transient hepatic attenuation differences. AJR Am J
Roentgenol. 183:459–464. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou X, Luo Y, Peng YL, Cai W, Lu Q, Lin
L, Sha XX, Li YZ and Zhu M: Hepatic perfusion disorder associated
with focal liver lesions: Contrast-enhanced US patterns-correlation
study with contrast-enhanced CT. Radiology. 260:274–281. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Colagrande S, Centi N, Carmignani L,
Politi Salvatore L and Villari N: Meaning and etiopathogenesis of
sectorial transient hepatic attenuation differences (THAD). Radiol
Med. 105:180–187. 2003.(In Italian). PubMed/NCBI
|
|
8
|
Wang FS, Fan JG, Zhang Z, Gao B and Wang
HY: The global burden of liver disease: The major impact of China.
Hepatology. 60:2099–2108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Byun JH, Kim TK, Lee CW, Lee JK, Kim AY,
Kim PN, Ha HK and Lee MG: Arterioportal shunt: Prevalence in small
hemangiomas versus that in hepatocellular carcinomas 3 cm or
smaller at two-phase helical CT. Radiology. 232:354–360. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hwang J, Kim SH, Lee MW and Lee JY: Small
(≤2 cm) hepatocellular carcinoma in patients with chronic liver
disease: Comparison of gadoxetic acid-enhanced 3.0 T MRI and
multiphasic 64-multirow detector CT. Br J Radiol. 85:e314–e322.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Imai Y, Katayama K, Hori M, Yakushijin T,
Fujimoto K, Itoh T, Igura T, Sakakibara M, Takamura M, Tsurusaki M,
et al: Prospective comparison of Gd-EOB-DTPA-enhanced MRI with
dynamic CT for detecting recurrence of HCC after radiofrequency
ablation. Liver Cancer. 6:349–359. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park MJ, Kim YK, Lee MW, Lee WJ, Kim YS,
Kim SH, Choi D and Rhim H: Small hepatocellular carcinomas:
Improved sensitivity by combining gadoxetic acid-enhanced and
diffusion-weighted MR imaging patterns. Radiology. 264:761–770.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang JZ, Huo YJ, Zhang Y, Yuan J, Yang FY
and Zhao YQ: Experience of 3.0T MRI detection and diagnosis of
<1.0 cm small hepatocellular cancer. J Pract Radiol. 30:2014.(In
Chinese).
|
|
14
|
Tang A, Cruite I and Sirlin CB: Toward a
standardized system for hepatocellular carcinoma diagnosis using
computed tomography and MRI. Expert Rev Gastroenterol Hepatol.
7:269–279. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mirsadraee S and van Beek EJ: Functional
imaging: Computed tomography and MRI. Clin Chest Med. 36(349–363):
x2015.
|
|
16
|
Marolf AJ: Computed tomography and MRI of
the hepatobiliary system and pancreas. Vet Clin North Am Small Anim
Pract. 46(481–497): vi2016.
|
|
17
|
Chen SL, Cao LR, Ji SZ and Liu M: The MRI
manifestations of hepatic perfusion disorders in focal liver
lesions. J Pract Radiol. 2009.(In Chinese).
|
|
18
|
Yan SX, Liang TB, Fujii M, Kawamitsu H,
Sugimura K and Zheng SS: Modified magnetic resonance angiography of
the liver using sensitivity encoding in comparison with digital
subtraction angiography and CT arterial portography. Hepatobiliary
Pancreat Dis Int. 4:185–191. 2005.PubMed/NCBI
|
|
19
|
Amédée-Manesme O, Furr HC and Olson JA:
The correlation between liver vitamin A concentrations in
micro-(needle biopsy) and macrosamples of human liver specimens
obtained at autopsy. Am J Clin Nutr. 39:315–319. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rofsky NM, Lee VS, Laub G, Pollack MA,
Krinsky GA, Thomasson D, Ambrosino MM and Weinreb JC: Abdominal MR
imaging with a volumetric interpolated breath-hold examination.
Radiology. 212:876–884. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dong S, Ye XD, Yuan Z, Xu LC and Xiao XS:
Relationship of apparent diffusion coefficient to survival for
patients with unresectable primary hepatocellular carcinoma after
chemoembolization. Eur J Radiol. 81:472–477. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang BZ and Yang HX: CT findings and
formation mechanism of hepatic abnormal perfusion. Inner Mongolia
Med J. 2:32011.
|
|
23
|
Luo GZ, Huang LM and Zou W: Liver
transient perfusion abnormalities in spiral CT diagnosis of acute
cholecystitis. Hunan Med. 25:32014.(In Chinese).
|
|
24
|
Vilgrain V: Advancement in HCC imaging:
diagnosis, staging and treatment efficacy assessments:
Hepatocellular carcinoma: Imaging in assessing treatment efficacy.
J Hepatobiliary Pancreat Sci. 17:374–379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim HJ, Kim AY, Kim TK, Byun JH, Won HJ,
Kim KW, Shin YM, Kim PN, Ha HK and Lee MG: Transient hepatic
attenuation differences in focal hepatic lesions: Dynamic CT
features. AJR Am J Roentgenol. 184:83–90. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou KG and Chen ZW: Body magnetic
resonance imaging. Shanghai Med Univ Press; 4. 2009
|
|
27
|
Tan Y and Yang ZH: Multislice spiral CT
evaluation for anormal peripheral perfusion of hepatic hemangioma.
J Clin Radiol. 2008.(In Chinese).
|
|
28
|
Itai Y and Matsui O: Blood flow and liver
imaging. Radiology. 202:306–314. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Arai K, Kawai K, Kohda W, Tatsu H, Matsui
O and Nakahama T: Dynamic CT of acute cholangitis: Early
inhomogeneous enhancement of the liver. AJR Am J Roentgenol.
181:115–118. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu YM, Cui Y and Wang YX: Preliminary
application of 128-slice 4D CT whole-liver perfusion imaging in
hepatocellular carcinoma. Chin Imaging J Integr Tradit West Med.
9:32011.(In Chinese).
|
|
31
|
Li XH, Mo HM and Guo PX: Relationship
between liver perfusion imaging of CT and severity of hepatic
cirrhosis. Chin Imaging J Integr Tradit West Med. 8:32010.(In
Chinese).
|
|
32
|
Danet IM, Semelka RC, Nagase LL, Woosely
JT, Leonardou P and Armao D: Liver metastases from pancreatic
adenocarcinoma: MR imaging characteristics. J Magn Reson Imaging.
18:181–188. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Van Beers BE, Leconte I, Materne R, Smith
AM, Jamart J and Horsmans Y: Hepatic perfusion parameters in
chronic liver disease: Dynamic CT measurements correlated with
disease severity. AJR Am J Roentgenol. 176:667–673. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee MJ, Saini S, Compton CC and Malt RA:
MR demonstration of edema adjacent to a liver metastasis:
Pathologic correlation. AJR Am J Roentgenol. 157:499–501. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brodsky EK, Bultman EM, Johnson KM, Horng
DE, Schelman WR, Block WF and Reeder SB: High-spatial and
high-temporal resolution dynamic contrast-enhanced perfusion
imaging of the liver with time-resolved three-dimensional radial
MRI. Magn Reson Med. 71:934–941. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ferrucci JT: Liver tumor imaging: Current
concepts. AJR Am J Roentgenol. 155:473–484. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Harned RK II, Chezmar JL and Nelson RC:
Imaging of patients with potentially resectable hepatic neoplasms.
AJR Am J Roentgenol. 159:1191–1194. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen CY, Li CW, Kuo YT, Jaw TS, Wu DK, Jao
JC, Hsu JS and Liu GC: Early response of hepatocellular carcinoma
to transcatheter arterial chemoembolization: Choline levels and MR
diffusion constants-initial experience. Radiology. 239:448–456.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ma XH and Zhou C: Progress of
diffusion-weighted imaging and perfusion-weighted imaging in
evaluation on therapeutic efficiency of transcatheter arterial
chemoembolization of hepatocellular carcinoma. J Int Med Radiol.
36:344–348. 2013.(In Chinese).
|
|
40
|
Kim HH, Kim JC, Park EK, Hur YH, Koh YS,
Cho CK, Kim HS and Kim HJ: Undifferentiated embryonal sarcoma of
the liver presenting as a hemorrhagic cystic tumor in an adult.
Hepatobiliary Pancreat Dis Int. 10:657–660. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ikeda O, Tamura Y, Nakasone Y, Shiraishi
S, Kawanaka K, Tomiguchi S, Yamashita Y, Takamori H, Kanemitsu K
and Baba H: Comparison of intrahepatic and pancreatic perfusion on
fusion images using a combined SPECT/CT system and assessment of
efficacy of combined continuous arterial infusion and systemic
chemotherapy in advanced pancreatic carcinoma. Cardiovasc Intervent
Radiol. 30:912–921. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Colagrande S, Carmignani L, Pagliari A,
Capaccioli L and Villari N: Transient hepatic attenuation
differences (THAD) not connected to focal lesions. Radiol Med.
104:25–43. 2002.(In English, Italian). PubMed/NCBI
|
|
43
|
Wang J, He BJ, Liao BH, Jiang ZB, Luo L,
Zhang YQ, Chen GH, Yang Y and Shan H: MRI diagnosis of liver grafts
complications after liver transplantation. Chin Med Imaging
Technol. 27:997–1000. 2011.(In Chinese).
|
|
44
|
Giovagnoni A, Terilli F, Ercolani P, Paci
E and Piga A: MR imaging of hepatic masses: Diagnostic significance
of wedge-shaped areas of increased signal intensity surrounding the
lesion. AJR Am J Roentgenol. 163:1093–1097. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim KW, Kim TK, Han JK, Kim AY, Lee HJ and
Choi BI: Hepatic hemangiomas with arterioportal shunt: Findings at
two-phase CT. Radiology. 219:707–711. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Balci NC, Semelka RC, Noone TC, Siegelman
ES, de Beeck BO, Brown JJ and Lee MG: Pyogenic hepatic abscesses:
MRI findings on T1- and T2-weighted and serial gadolinium-enhanced
gradient-echo images. J Magn Reson Imaging. 9:285–290. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Colagrande S, Centi N, Galdiero R and
Ragozzino A: Transient hepatic intensity differences: Part 1, Those
associated with focal lesions. AJR Am J Roentgenol. 188:154–159.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Qin GD: Liver perfusion abnormalities
imaging findings and mechanisms. Chin Contemp Med. 34:2012.(In
Chinese).
|
|
49
|
Choi BI, Lee KH, Han JK and Lee JM:
Hepatic arterioportal shunts: Dynamic CT and MR features. Korean J
Radiol. 3:1–15. 2002. View Article : Google Scholar : PubMed/NCBI
|