Introduction
The treatment of refractory ulcers in the feet of
patients with diabetes is a major challenge (1). Patients with diabetic foot ulcers often
also experience peripheral vascular lesions that cause ischemia,
further worsening the ulcers. Such cases may eventually require
amputation or result in the mortality of patients (2). Although platelet derived growth factor
gel is effective at treating non-ischemic ulcers, it is ineffective
at treating ischemic ulcers (3).
Therefore, novel therapeutic strategies to treat life-threatening
ischemic diabetic ulcers are urgently required.
Previous studies have indicated that transplantation
with embryonic artery cluster of differentiation cluster of
differentiation (CD)133+ cells (EACCs) may promote the
healing of diabetic ulcers (4).
EACCs release vascular endothelial growth factor A (VEGFA) and
interleukin-8 (IL-8), which promote the proliferation, migration
and angiogenesis of endothelial cells via a paracrine mechanism
(5). However, glucose levels in the
ulcer region are high, resulting in the inhibition of EACC
viability, function and survival; thus, the pathological
environment of the ulcer region may limit the efficacy of EACCs in
the treatment of diabetic ulcers (6,7).
Therefore, it is important to identify methods to effectively
enhance the survival and biological function of EACCs in the ulcer
area to improve the treatment of diabetic ulcers.
It has been demonstrated that sirtuin (Sirt) family
proteins serve an important role in maintaining cell survival and
biological activity. The sirtuin family is a family of highly
conserved NAD+ dependent deacetylases, Sirt1 is the most
widely studied sirtuin protein at present and a popular drug design
target (8). Sirt1 is able to
interact with a variety of signal transduction proteins, induce the
deacetylation of histone lysine residues and transcription factors,
and regulate neuroprotection, cell senescence, apoptosis, lipid
metabolism, insulin secretion, inflammation, oxidative stress
response and angiogenesis (9,10). Due
to the effect of Sirt1 on biomedical regulation and in order to
effectively apply Sirt1 in the treatment of diabetes,
cardiovascular disease, metabolic syndrome and aging-associated
diseases; several Sirt1 agonists have been identified and studied
(11,12). Among various Sirt1 agonists, SRT1720
was revealed to be the most effective at activating Sirt1 (11–14).
Therefore, it has been suggested that Sirt1 may be
used to enhance the survival rate and function of EACCs in the
ulcer region. In the current study, poly(lactic-co-glycolic acid)
(PLGA), collagen and silk were mixed with SRT1720 to form the
composite material PCSS using electrospinning technology and EACCs
were seeded onto the PCSS to construct the novel dressing to treat
patients with diabetic ulcers.
The current study investigated whether PCSS was able
to release SRT1720 slowly over a period of 15 days. Furthermore, it
was assessed whether EACCs are able to grow well on the PCSS and
whether SRT1720 is able to effectively promote the secretion of
vascular endothelial growth factor A (VEGFA), interleukin 8 (IL-8)
and basic fibroblast growth factor (bFGF) and inhibit the secretion
of tumor necrosis factor α (TNF-α) by EACCs. The results of the
current study demonstrated that this novel dressing markedly
increased the survival rate of EACCs in diabetic ulcers and
promoted angiogenesis, thus promoting the healing of diabetic
ulcers. Therefore, the PLGA-SRT1720-EACCs composite dressing
assessed in the current study may be used as a novel and effective
treatment for diabetic ulcers.
Materials and methods
Cell separation and culture
C57 mice (n=10; 5–8 weeks old; weighing 20±4 g; sex
ratio, 1:1) were purchased from the Experimental Animal Center of
the Third Military Medical University (Chongqing, China). The mice
were housed in the specific-pathogen free environment with a
temperature of 24–28°C, relative humidity of 50–60% and natural
light cycle. The mice were given sterilized food, and water with
bacitracin (4 g/l) and neomycin (4 g/l) ad libitum. All
procedures performed in animals were approved by the Animal Care
and Use Committee of the Third Military Medical University. The
adult healthy mice mated and the vaginal plug was observed at 8:00
in the morning, the day that vaginal plug was identified was
recorded as gestational age 0 day. After 15–20 days of pregnancy,
the fetal aorta-derived vascular CD133+ cells were
obtained from aortas of the mouse embryos following a previously
reported protocol (15). Briefly,
1×106 cells were separated from the aorta tissue using
EDTA (5 mM), centrifuged at 1,000 × g for 3 min at room temperature
and incubated with magnetic microbeads (Miltenyi Biotec, Inc.,
Cambridge, MA, USA) conjugated to the anti-CD133 antibody (cat. no.
ab19898; 1:1,000; Abcam, Cambridge, MA, USA) for 30 min at 4°C.
CD133+ cells were separated using the Quadro
MACS™ Separation Unit (Miltenyi Biotec, Inc.).
CD133+ cells were then cultured in Dulbecco's modified
Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS;
both Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 2 mM
L-glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin. In
order to simulate the environment of high blood sugar levels in
vitro, human umbilical vein endothelial cells (HUVECs), which
was purchased from American Type Culture Collection (Manassas, VA,
USA), were cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 30 mM glucose, 10% FBS, 2 mM
L-glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin. All
cells were maintained at 37°C in 5% CO2.
Synthesis of composited material
Briefly, 80% polylactic acid, 10% collagen protein
and 10% silk (PCS) were dissolved to prepare a mixed solution; the
concentration of PCS was adjusted by adding distilled water and the
final concentration was 5%. Subsequently, 100 mM SRT1720 (Shanghai
BetterBioChem Co., Ltd., Suzhou, China) was added to the mixture to
form PCSS. An FM-1205 electrospinning device was purchased from
Beijing Future Material Sci-tech Co., Ltd. (Beijing, China). Under
an operating voltage of 220 kV/m, the mixed solution was ejected
from the nozzle of the electro spinner and collected. The surface
of the biological material was photographed using an S-3400N-II
scanning electron microscope (Hitachi, Ltd., Tokyo, Japan).
SRT1720 release detection
The PCSS composite material was dissolved in the PBS
at 37°C for 15 days to determine the release of SRT1720. The
release of the sample was detected using a UV-VIS spectrometer
(Nicolet Evolution 300; Thermo Fisher Scientific, Inc.) at a
wavelength of 428 nm and the release ratio was calculated using
Beer's law (16).
Growth of EACCs on composite
material
The cell was seeded on composite materials, then
cell bioactivity was evaluated; the proliferation and adhesion of
the cells were the important indexes in the evaluation of cell
bioactivity (17). To measure the
growth of EACCs, after three passages, 5×105 EACCs were
seeded onto the surface of PCS composite material (PCS-EACCs) or
PCSS composite material (PCSS-EACCs) following immersion in DMEM
supplemented with 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin
and 100 mg/ml streptomycin at 37°C in 5% CO2. Cells were
then incubated for 72 h and cell growth was determined using a LSM
780 NLO laser scanning microscope (Carl Zeiss AG, Oberkochen,
Germany).
Enzyme-linked immunosorbent assay
(ELISA)
Quantitative analysis of the cytokines secreted by
EACCs following different treatments was performed using ELISA.
TNF-α (cat. no. PT512; Beyotime Institute of Biotechnology,
Beijing, China), VEGFA (cat. no. EK0541; Wuhan Boster Biological
Technology, Ltd., Wuhan, China), IL-8 (cat. no. EMC104.48) and bFGF
(cat. no. EHC130.48; both Neobioscience Technology Company,
Shenzhen, China) ELISA kits were used to determine the
concentrations of TNF-α, VEGFA, IL-8 and bFGF according to the
manufacturer's protocol.
Cell proliferation
The cell proliferation ratio was detected using the
Cell-Light™ EdU DNA Cell Proliferation kit (Guangzhou
RiboBio Co., Ltd., Guangzhou, China), following the manufacturer's
protocol.
Cell migration assay
Cell migration was measured by performing a wound
assay. Briefly, 5×106 HUVECs were seeded in the 6-well
plate and cultured in RPMI-1640 medium supplemented with glucose,
10% FBS, 2 mM L-glutamine, 100 U/ml penicillin and 100 mg/ml
streptomycin, and incubated overnight at 37°C in 5% CO2.
A wound was created in each well and the plate was washed 3 times
with RPMI 1640. Then 2 ml RPMI 1640 medium with 10% FBS was added
into each well; after 24 h, the scar areas of each well were
observed and photographed using an Olympus BX50 microscope (Olympus
Corporation, Tokyo, Japan; magnification, ×200) and were quantified
using Image-Pro Plus software (version 6.0; Media Cybernetics,
Inc., Rockville, MD, USA).
Cell culture media collection
EACCs (5×106) were seeded in 6-well
plates, 30 nM glucose was added into DMEM supplemented with 10%
FBS, 2 mM L-glutamine, 100 U/ml penicillin and 100 mg/ml
streptomycin at 37°C in 5% CO2 and incubated at 37°C in
5% CO2 overnight. Following the adherence of the cells
to the wells, the cells were treated with dimethyl sulfoxide
(0.1%), PCS (50 mM), SRT170 (10 mM) or PCSS (50 mM). After 36 h of
incubation, the cell culture media were collected and stored at
−20°C.
Cell invasion assay
Cell invasion was measured using a Transwell assay.
Transwell chambers were purchased from Corning, Inc. (Corning, NY,
USA). Chambers inserted in the lower chamber were coated with
diluted Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). A total
of 5×105 HUVECs in RPMI 1640 medium were incubated in
the upper chamber; the lower chambers were filled with 500 µl
RPMI-1640 medium with 10% FBS. Subsequently, the collected cell
culture media (100 µl) were added to the lower chambers. Following
12 h incubation, insert membranes were collected. Cells were
stained with 0.5% crystal violet at room temperature for 30 min,
photographed and counted using an Olympus BX50 microscope
(magnification, ×200).
Animal experiments
A total of 120 C57 mice (5–8 weeks old; weighing
20±4 g; sex ratio, 1:1) were used in the current study to establish
the diabetes model. A total of 100 5–8 week old C57 mice weighing
20±4 g received an intraperitoneal injection of 40 mg/kg
streptozotocin (STZ) everyday following 12 h fasting over a period
of 5 days to establish the diabetes model. The remaining 20 mice
were with injected intraperitoneally with 1 ml PBS and used as
controls. After 5 days of continuous STZ injections, fasting blood
glucose levels were detected using the ACCU-CHEK Active meter
(Roche Applied Science, Rotkreuz, Switzerland). The glucose levels
>16.7 mM indicated that the diabetes model was successfully
established; the results indicated that all 100 mice were
successfully induced as diabetes models and the control mice were
normal. Mice with diabetes were anesthetized with 30 mg/kg sodium
pentobarbital (10 mg/ml) via intraperitoneal injection.
Subsequently, the terminal branches of the femoral artery were
ligated and the skin tissue was excised at an area of 6×6 mm at the
lateral thigh to establish the ischemic diabetic ulcer.
Subsequently, ulcers were covered by EACCs, EACCs grown on the PCS
materials (PCS-EACCs), EACCs pretreated with SRT1720
(SRT1720-EACCs), PCSS and EACCs grown on the PCSS materials
(PCSS-EACCs; all n=20). C57 mice in the control group (n=20)
underwent the same ulcer surgery and the ulcers were left
untreated. Breathable medical dressings were used to bandage the
wounds.
Histological assessments
Bandages were removed 2 days following treatment.
Wound recovery was recorded and photographed using a D90 camera
(Nikon Corporation, Tokyo, Japan) on days 3, 7 and 14. Wound areas
were measured and calculated using Image-Pro Plus 6.0 software
(Media Cybernetics, Inc.) to measure wound recovery. On day 14, the
tissues surrounding the wounds were collected, fixed with 4%
paraformaldehyde at room temperature for 24 h and embedded in
paraffin. Tissues were cut into 5-µm-thick sections, and
hematoxylin and eosin (H&E) staining was performed using the
Hematoxylin and Eosin Staining Kit (cat. no. C0105; Beyotime
Institute of Biotechnology, Beijing, China) according to the
manufacturer's protocol, then the results were observed by an
Olympus BX50 microscope (magnification, ×200). In order to further
detect the vascular density, the vascular endothelial cell marker
CD31 was selected for immunofluorescence staining. The sections
were permeabilized with 0.1% triton X-100 for 15 min at room
temperature, then incubated by 100% goat serum (cat. no. C0265;
Beyotime Institute of Biotechnology) for 30 min at 37°C. The
sections were then incubated with anti-CD31 antibodies (cat. no.
ab28364; 1:300; Abcam) diluted in 100% goat serum at 4°C overnight,
washed 3 times with PBS, incubated with Alexa Fluor®
680-conjugated donkey anti-rabbit IgG antibodies (cat. no. A10043;
1:500; Thermo Fisher Scientific, Inc.) diluted in 100% goat serum
at 37°C for 2 h and washed 3 times with PBS. The images of the
sections were observed through a laser scanning confocal microscope
(TCS-SP5; Leica Microsystems GmbH; magnification, ×400) and then
the size of endothelium was assessed.
Statistical analysis
All data are presented as the mean ± standard
deviation. The nonparametric Mann-Whitney rank-sum test was used to
estimate differences between two samples. Intergroup comparisons
were performed to assess differences among >2 groups using
one-way analysis of variance followed by Bonferroni's correction.
P<0.05 was determined to indicate a statistically significant
difference.
Results
The characteristics of PCSS-EACCs
To improve the uniformity of the matrix material,
electrospinning technology was used to form the PCS (80% polylactic
acid, 10% collagen protein and 10% silk) and PCSS (100 nM SRT172
was added to the PCS solution to form the PCSS). Scanning electron
microscopy was used to determine the uniformity of the materials
and it was identified that the silk itself and the gaps between the
silk were uniform, and that the structure of the material was also
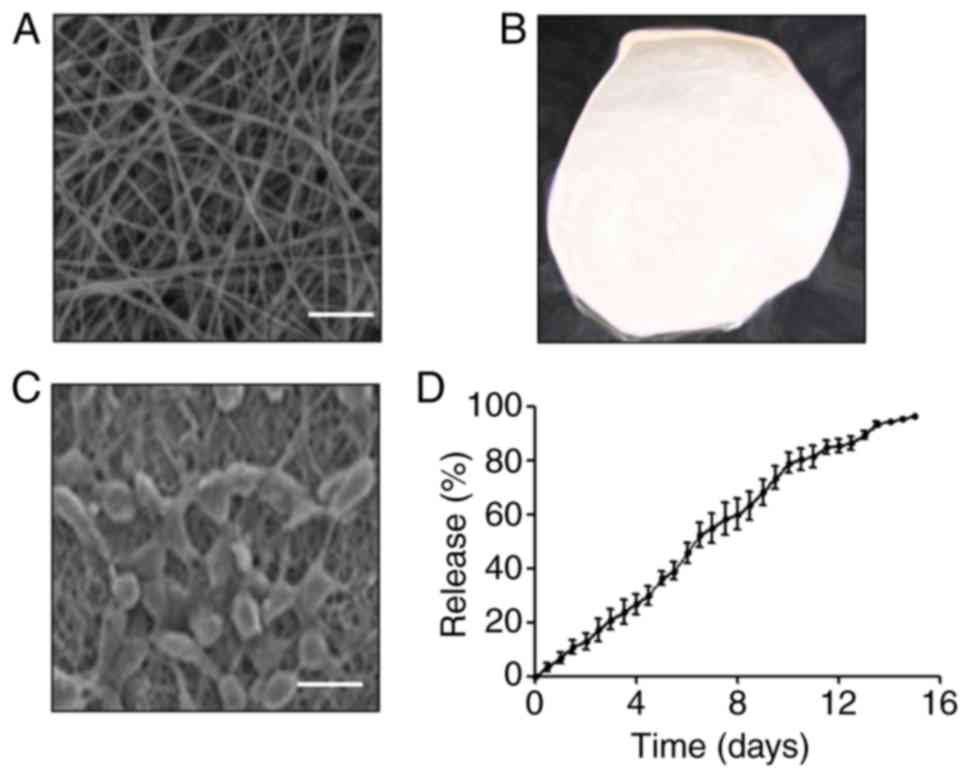
uniform (Fig. 1A). The materials
were regular and uniform and there was no difference in thickness
(Fig. 1B). To detect the growth of
EACCs on the PCSS, EACCs were seeded on the PCSS and observed using
a scanning electron microscope. The results indicated that EACCs
were able to grow well on the PCSS, indicating that PCSS
effectively promote the growth and proliferation of EACCs (Fig. 1C). To detect the release ability of
PCSS for SRT1720, a release experiment was performed and the
results indicated that the PCSS is able to release SRT1720 slowly
and steadily over a period of 15 days (Fig. 1D). These results indicate that the
materials designed in the current study not only promote the growth
of EACCs but may also be used to promote the steady release of
SRT1720 over a prolonged period.
SRT1720 promotes the biological
function of EACCs
As Sirt1 is closely associated with the biological
function of cells, the PCSS used in the current study contained the
Sirt1 agonist SRT1720. A number of experiments were conducted to
determine the effect of SRT1720 on the biological function of
EACCs. To detect cell proliferation, an EdU kit was used. The
results demonstrated that SRT1720 significantly promotes the
proliferation of EACCs in a high glucose environment (Fig. 2A and B). ELISA experiments were also
performed to determine the effects of SRT1720 and PCSS on EACCs in
a high glucose environment. TNF-α is an important cytokine that
causes cell death (18). In a high
glucose environment, the secretion of TNF-α by EACCs increased
significantly; however, the secretion of TNF-α by EACCs was
significantly decreased following treatment with SRT1720 and PCSS
(Fig. 2C). VEGFA induces
angiogenesis and promotes cell migration (19). In a high glucose environment, the
secretion of VEGFA by EACCs was significantly inhibited; however,
treatment with SRT1720 and PCSS significantly increased the
secretion of VEGFA by EACCs (Fig.
2D). IL-8 is a multifunctional factor; it is able to stimulate
the migration of neutrophils into inflammatory tissue and activate
inflammatory cells and is also able to promote fibroblast
proliferation. Additionally, IL-8 is a chemotactic cytokine that
can promote inflammatory cell chemotaxis and induce cell
proliferation (20). The main role
of IL-8 is to attract and activate neutrophils, promote the
lysosomal enzyme activity of neutrophils and phagocytosis, and have
chemotactic effect on basophils and T cells; IL-8 can induce
endothelial cell migration and proliferation, further promoting
vascular proliferation (20). As
angiogenesis facilitates the healing of diabetic ulcers, levels of
IL-8 secreted by EACCs in a high glucose environment were measured.
The secretion of IL-8 by EACCs was significantly inhibited in a
high glucose environment; however, following treatment with SRT1720
and PCSS treatment, levels of secreted IL-8 were significantly
increased; furthermore, these levels were markedly higher than in
normal EACCs (Fig. 2E). bFGF is a
fibroblast growth factor and fibroblasts effectively promote the
formation of scars, thereby promoting wound recovery (21). It was demonstrated that the secretion
of bFGF by EACCs was significantly inhibited in high glucose;
however, following treatment with SRT1720 and PCSS, the secretion
of bFGF was restored (Fig. 2F).
Notably, the effect of PCSS on bFGF secretion was greater than that
of SRT1720. Taken together, these results demonstrate that,
although EACCs treated with SRT1720 and PCSS normalize secretion of
the four cytokines, PCSS was more effective than SRT1720 at
normalizing cytokine secretion. This may be due to the stable
release of SRT1720 by PCSS; SRT1720 is released from PCSS at a rate
of ~7.14%/day for 2 weeks.
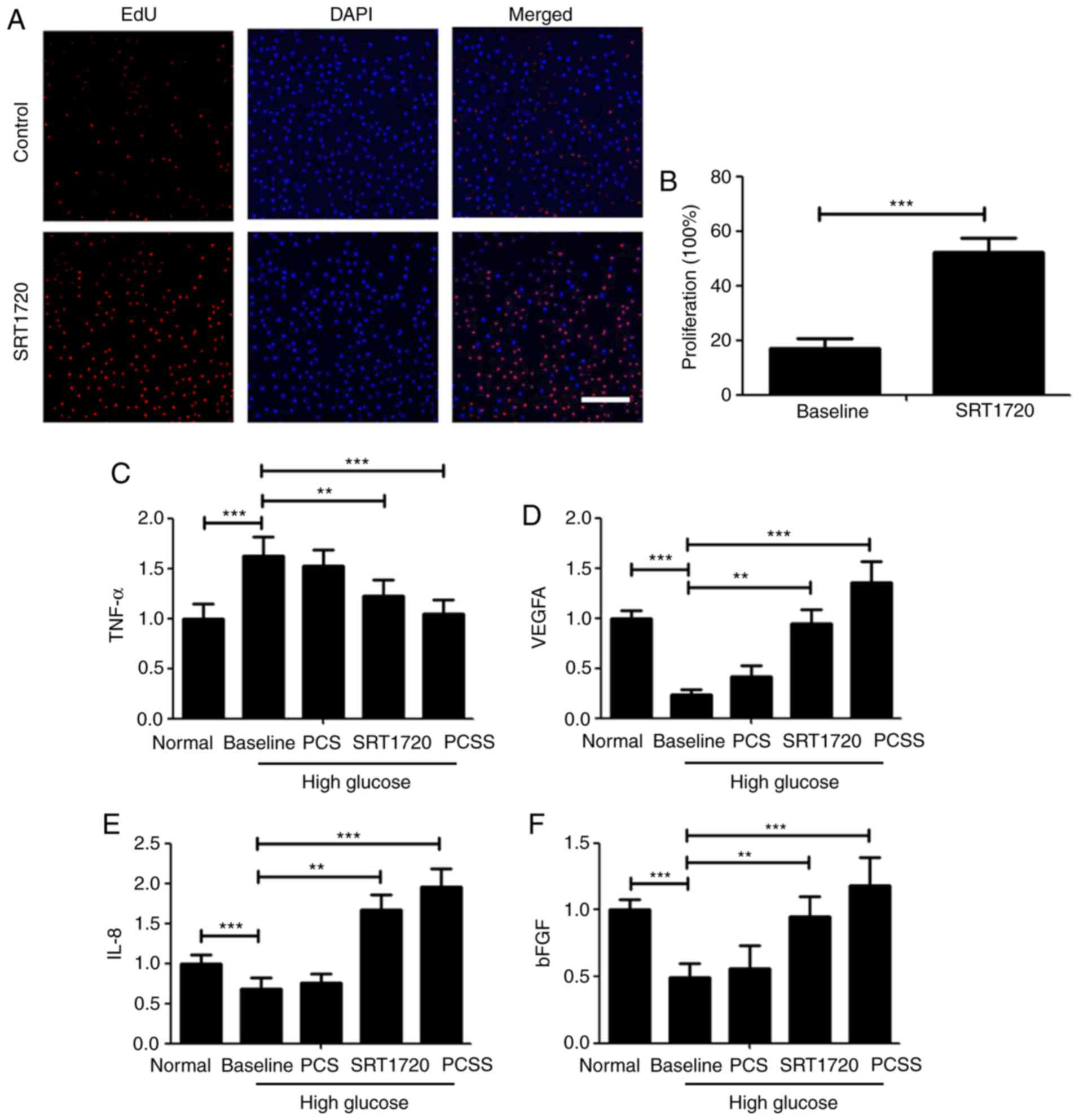 | Figure 2.SRT1720 promotes the biological
function of EACCs. (A) EACCs were treated with 10 mM SRT1720 and
cell proliferation was detected using an EdU kit. Scale bar=200 µm.
(B) Quantification of cell proliferation rates. In the normal
group, EACCs were cultured in normal medium and this medium was
collected for further detection; in the baseline group, EACCs were
cultured in high glucose medium and the conditional medium was
collected for further detection; EACCs were also co-cultured in
high glucose medium and PCS, SRT1720 or PCSS, and the conditional
medium was collected for further detection. Enzyme-linked
immunosorbent assays were conducted on conditional media from all
groups to measure. (C) TNF-α, (D) VEGFA, (E) IL-8 and (F) bFGF
levels. **P<0.01 and ***P<0.005. EACCs, embryonic artery
cluster of differentiation 133+ cells; VEGFA, vascular
endothelial growth factor A; IL-8, interleukin-8; PCS, polylactic
acid mixed with collagen protein and silk; PCSS, PCS mixed with
SRT1720; TNF-α, tumor necrosis factor α; bFGF, basic fibroblast
growth factor. |
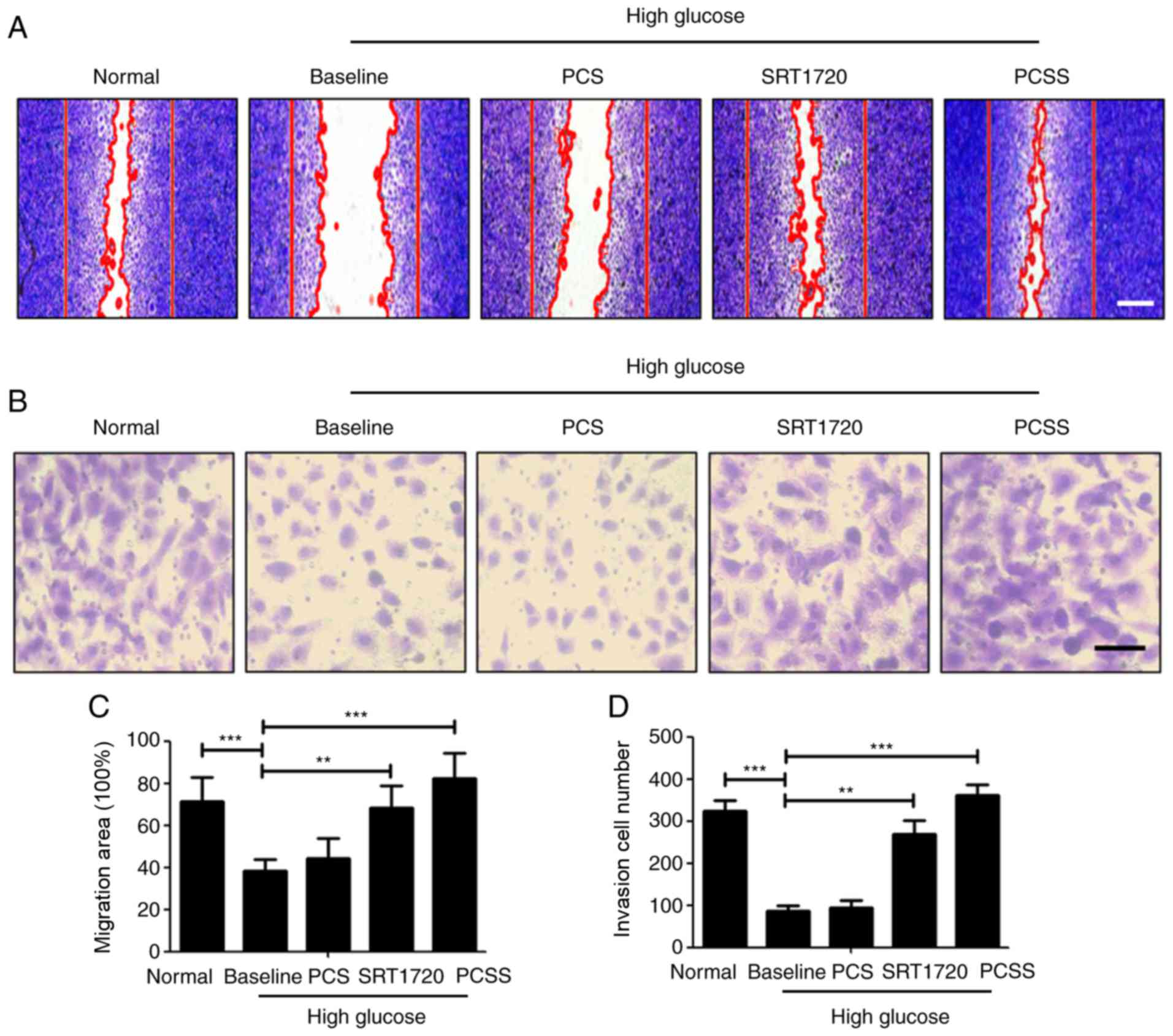
PCSS-treated EACCs promote the
proliferation and migration of HUVECs
To verify whether EACCs are able to promote the
proliferation and migration of vascular endothelial cells
paracrine, EACCs were cultured in high glucose medium (Baseline),
or co-cultured in high-glucose medium along with PCS, SRT1720 or
PCSS. Subsequently, the conditioned media were collected, and the
medium from EACCs cultured in normal medium was collected as a
normal control. Collected conditioned media were used to treat
HUVECs and determine the effect on the proliferation and migration
of HUVECs (Fig. 3). The results of
the scratch wound assay indicated that, although HUVECs in the
Baseline group underwent migration to a certain degree, the
migration rate was much lower than in the normal group. However,
treatment of HUVECs with the conditioned media from SRT1720- or
PCSS-treated EACCs, the migration of HUVECs increased
significantly. HUVECs treated with conditioned medium from
PCSS-treated EACCs exhibited the highest rate of migration
(Fig. 3A and C). To further examine
the invasion of HUVECs following treatments with different
conditioned media, a Transwell invasion assay was conducted. The
results of this assay were consistent with the results of scratch
wound assay; conditioned media from SRT1720- or PCSS-treated EACCs
significantly promoted the invasion of HUVECs and the effect of the
medium from PCSS-treated EACCs was better (Fig. 3B and D). These results indicated that
PCSS significantly promoted the paracrine action of EACCs, thus
significantly increasing the migration and invasion of HUVECs.
Treatment with conditioned medium from SRT1720-treated EACCs also
significantly increased the migration and invasion of HUVECs, but
to a lesser extent than PCSS.
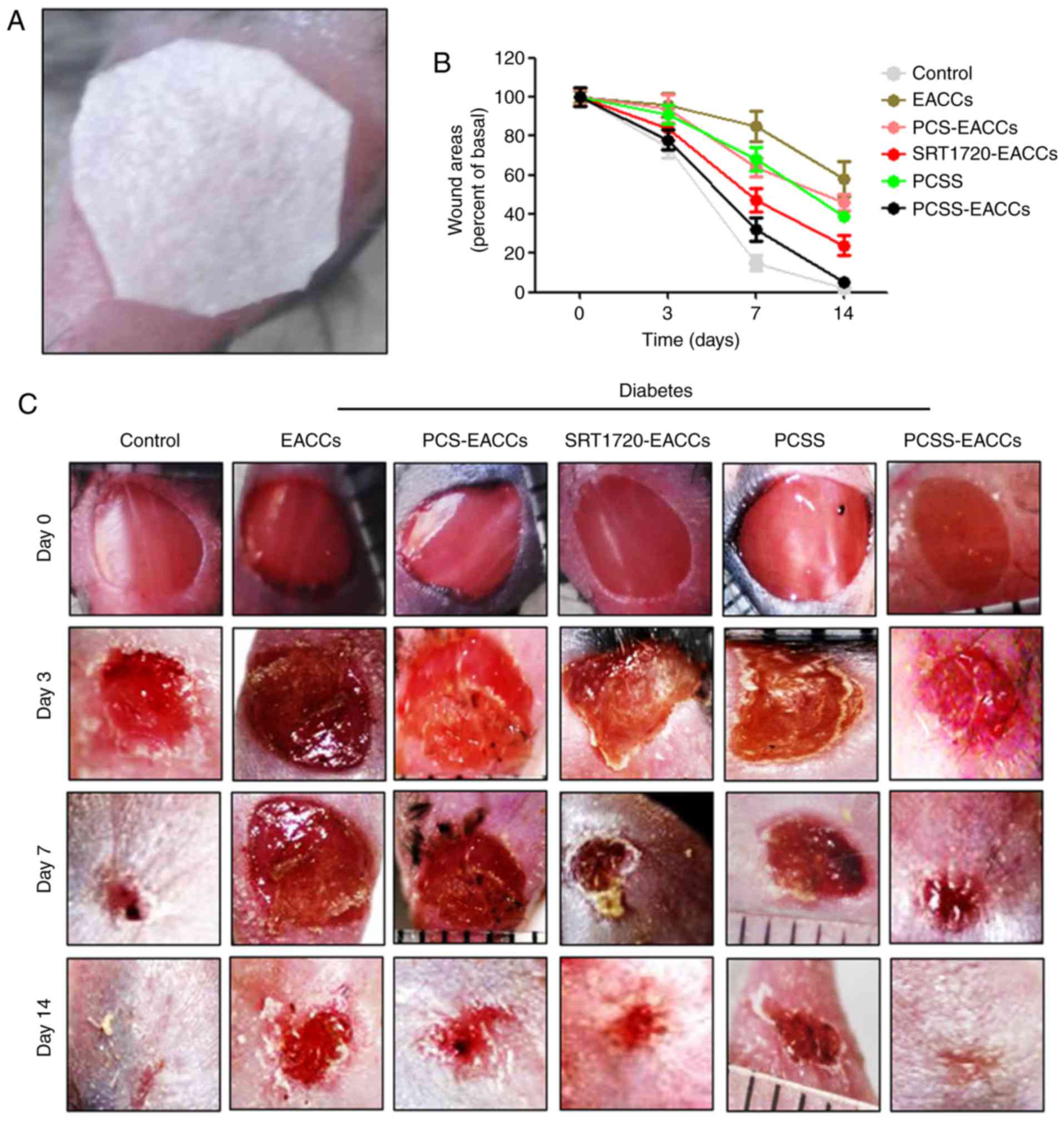
PCSS-EACCs promote the healing of
diabetic ischemic ulcers
To clarify whether PCSS-treated-EACCs promote the
recovery of diabetic ischemic ulcers; a model of diabetic ischemic
ulcers was established by performing STZ injections in C57 mice;
subsequently, the material containing EACCs was used to cover the
wound (Fig. 4A). To investigate the
effect of PCSS-treated-EACCs, the C57 mice were split into 6
different groups (all, n=20). At 0, 3, 7 and 14 days following
transplant, wound healing was recorded and photographed. The
results indicated that the wounds of the normal control mice had
completely recovered by day 7. The wound healing process in the
mice with diabetes was slower; the wound healing process in mice
from the EACC group was markedly inhibited compared with the
control group. However, in the PCS-EACCs group, the wound healing
process was quicker compared with the EACCs group. Furthermore,
SRT1720-EACCs had a more beneficial effect on the wound healing
process than PCS-EACCs. The effect of PCSS on wound healing was
almost the same as that of PCSS-EACCs. Treatment with PCSS-EACCs
had the best effect on the healing time of ischemic ulcers in
diabetic mice and the recovery rate of the mice in this group was
similar to that of mice in the control group (Fig. 4B and C). These results may be due to
the stable release of SRT1720 by PCSS over a prolonged period of
time and that SRT1720 is able to promote the biological function of
EACCs. These results further verify the beneficial effect of
PCSS-EACCs on the healing of diabetic ischemic ulcers.
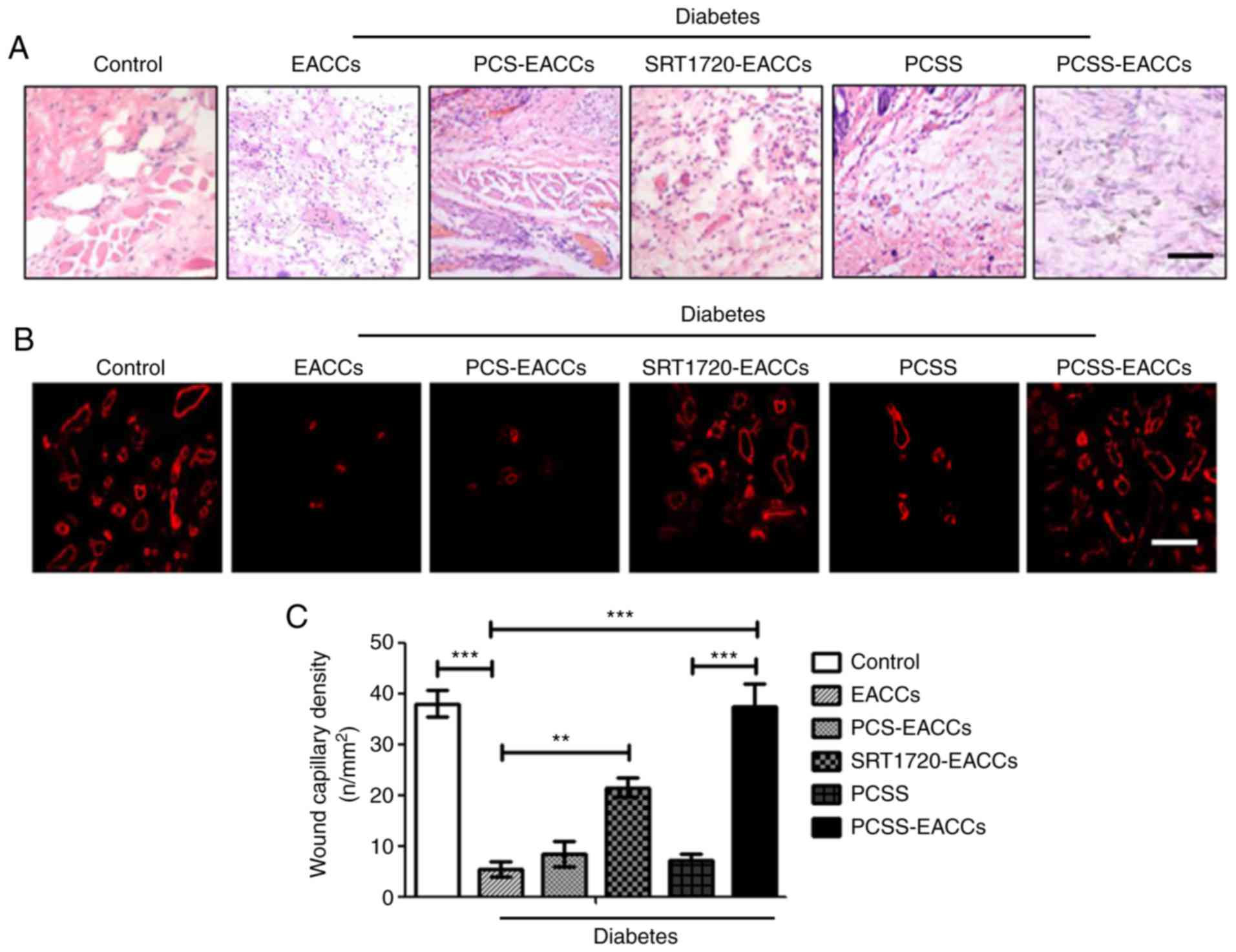
PCSS-EACCs promote the angiogenesis of
capillaries in the healing of diabetic ischemic ulcers
To further evaluate the effect of PCSS-EACCs on the
repair of diabetic ischemic ulcers, ulcer tissues from each group
of mice were collected and stained with H&E. The results
indicated that the immune response was effectively suppressed
following PCSS-EACCs treatment; additionally, the amount of
vascularization of capillaries in ulcer tissues was markedly
improved following PCSS-EACCs treatment (Fig. 5A). In order to further detect the
effect of PCSS-EACCs on angiogenesis in diabetic ischemic ulcers,
immunofluorescence staining was performed to detect CD31
expression, which is a marker of vascular endothelial cell
(Fig. 5B). The results suggested
that SRT1720-EACCs could effectively promote the proliferation of
vascular endothelial cells and therefore angiogenesis. The effect
of PCSS-EACCs on angiogenesis was better compared with that of
SRT1720-EACCs, due to the stable release of SRT1720 by PCSS. Wound
capillary density was measured using a microscope (Fig. 5C). The results indicated that
angiogenesis was significantly promoted following transplantation
with PCSS-EACCs, restoring blood supply and promoting the healing
of the diabetic ischemic ulcer.
Discussion
Diabetic ulcers primarily occur in patients with
early diabetes that do not exhibit peripheral neuropathy and
peripheral vascular disease, but experience foot infections,
suppuration or ulcers caused by paronychia or beriberi; the primary
symptoms of nerve and vascular diseases (22,23).
Diabetic ulcers may be classified as diabetic neuropathic ulcers,
diabetic ischemic ulcers and diabetic mixed ulcers; diabetic
ischemic ulcers account for ~36% of all diabetic ulcers (24,25). At
present dressings seeded with growth factors may be an effective
method of treating diabetic neuropathic ulcers; however, there are
currently no effective methods of treating diabetic ischemic ulcers
(26).
Stem cell therapy may be an effective method of
treating diabetic ischemic ulcers (27). Previous studies have demonstrated
that EACCs markedly improve the treatment of diabetic ischemic
ulcers. However, the survival, growth and biological function of
EACCs were inhibited due to the high glucose environment of the
ulcers (4–7). Therefore, promoting the survival and
function of EACCs in a high glucose environment may improve the
therapeutic effect of EACCs against diabetic ischemic ulcers.
The Sirt family serves an important role in
regulating the survival and biological functions of cells; the 7
members of the Sirt family regulate cell proliferation,
differentiation, senescence, apoptosis and metabolism by
interacting with the P53, forkhead box protein 0, Ku70 and PGC-la
proteins (28). Sirt1 interacts with
PGC-la, which is the cofactor of peroxisome proliferator-activated
receptor (PPAR)-γ and, along with NAD, regulates
gluconeogenesis-related gene transcription. Furthermore, Sirt1
reduces cellular apoptosis and senescence, and increases the
survival rate of cells under oxidative stress through the
inhibition of pro-apoptotic factor p53 (29,30). It
has been demonstrated that SRT1720 effectively activates the
expression of Sirt1 (13). As an
agonist of Sirt1, SRT1720 increases metabolism and mitochondrial
biogenesis via the transcriptional activation of PGC-1a and PPAR
family members, which are peroxisome proliferator-activated
receptors. SRT1720 inhibits the immune response and increases
insulin sensitivity in type II diabetes; it also inhibits acute
oxidant injury and protects mitochondrial function (31). Furthermore, it has been demonstrated
that SRT1720 stimulates cell survival and inhibits cellular
apoptosis (31,32).
Although treatment with EACCs induces a beneficial
effect on diabetic ischemic ulcers, the high glucose environment of
the ulcers inhibits the therapeutic effect of EACCs. To determine
the effect of Sirt1 on the biological activity of cells, the
composite material PCSS was designed using the PLGA collagen
protein, silk and SRT1720. PCSS is able to induce the steady
release of SRT1720 and also promotes the growth and biological
function of EACCs following the seeding of EACCs thus further
promoting the proliferation and migration of HUVECs. The results of
the animal experiments performed in the current study indicated
that transplantation with PCSS-EACCs effectively promotes the
healing of diabetic ischemic ulcers. The mechanism of this effect
is the release of SRT1720 by PCSS, which activates Sirt1 in EACCs,
promotes the growth and paracrine secretion of EACCs and promotes
angiogenesis in the local ulcer tissue, thus restoring blood supply
and promoting the healing of diabetic ischemic ulcers.
Many people are still suffering from diabetic ulcers
and there are currently no effective treatments for diabetic
ischemic ulcers (1,2). The current study used Sirt1 as a target
molecule. The Sirt1 agonist SRT1720 was selected as a therapeutic
drug and the cell growth matrix (PCSS) was designed by combining
PLGA, collagen, silk and SRT1720 via electrospinning, which allowed
the slow and steady release of SRT1720 over a prolonged period of
time. Subsequently, EACCs were seeded onto the matrix (PCSS-EACCs)
and transplanted into diabetic ischemic ulcers in mice. The results
of these experiments indicated that the angiogenesis in ulcer
tissue was markedly improved following the transplantation of
PCSS-EACCs, thus improving the healing of diabetic ischemic ulcers.
Therefore, the results of the current study suggest that PCSS-EACCs
may be developed as a novel and effective method of treating
diabetic ischemic ulcers.
Acknowledgements
The authors are very thankful to Professor Qiang
Huang (Department of Orthopaedics, Traditional Chinese Medicine
Hospital, China) for their help in the design of the study and
synthesize the complex materials, and Dr Yang Wang (Department of
Anatomy, Third Military Medical University, China) for their help
in the establishment of the diabetic ischemic ulcers model and for
helping the authors with the animal experiments.
Funding
The present study was supported by the National
Science Foundation of China (grant no. 31470046).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
PKC and CLH contributed to the conception, design,
writing and revision of the manuscript, PKC and XLC contributed to
the acquisition of data, and XXS and XJS contributed to the
analysis and interpretation of data.
Ethics approval and consent to
participate
All procedures performed in animals were approved by
the Animal Care and Use Committee of the Third Military Medical
University (Chongqing, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EACCs
|
embryonic artery cluster of
differentiation 133+ cells
|
|
VEGFA
|
vascular endothelial growth factor
A
|
|
IL-8
|
interleukin-8
|
|
PCS
|
polylactic acid mixed with collagen
protein and silk
|
|
PCSS
|
PCS mixed with SRT1720
|
|
STZ
|
streptozotocin
|
|
TNF-α
|
tumor necrosis factor α
|
|
bFGF
|
basic fibroblast growth factor
|
References
|
1
|
Murad MH, Haydour Q and Benkhadra K: ACP
journal club. Review: In patients with chronic diabetic foot
ulcers, hyperbaric oxygen reduces major amputations. Ann Intern
Med. 159:JC92013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kolossváry E, Bánsághi Z, Szabó GV, Járai
Z and Farkas K: Ischemic origin of diabetic foot disease.
Epidemiology, difficulties of diagnosis, options for prevention and
revascularization. Orv Hetil. 158:203–211. 2017.(In Hungarian).
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang S, Geng Z, Ma K, Sun X and Fu X:
Efficacy of topical recombinant human epidermal growth factor for
treatment of diabetic foot ulcer: A systematic review and
meta-analysis. Int J Low Extrem Wounds. 15:120–125. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao WN, Xu SQ, Liang JF, Peng L, Liu HL,
Wang Z, Fang Q, Wang M, Yin WQ, Zhang WJ and Lou JN: Endothelial
progenitor cells from human fetal aorta cure diabetic foot in a rat
model. Metabolism. 65:1755–1767. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barcelos LS, Duplaa C, Kränkel N, Graiani
G, Invernici G, Katare R, Siragusa M, Meloni M, Campesi I, Monica
M, et al: Human CD133+ progenitor cells promote the healing of
diabetic ischemic ulcers by paracrine stimulation of angiogenesis
and activation of Wnt signaling. Circ Res. 104:1095–1102. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lawall H and Diehm C: Diabetic foot
syndrome from the perspective of angiology and diabetology.
Orthopade. 38:1149–1159. 2009.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ascione R and Madeddu P: Risk and benefit
of CD133+ progenitors. Circ Res. 105:e22009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kitada M, Kume S, Kanasaki K,
Takeda-Watanabe A and Koya D: Sirtuins as possible drug targets in
type 2 diabetes. Curr Drug Targets. 14:622–636. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kume S, Kitada M, Kanasaki K, Maegawa H
and Koya D: Anti-aging molecule, Sirt1: A novel therapeutic target
for diabetic nephropathy. Arch Pharm Res. 36:230–236. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nimmagadda VK, Makar TK, Chandrasekaran K,
Sagi AR, Ray J, Russell JW and Bever CT Jr: SIRT1 and NAD+
precursors: Therapeutic targets in multiple sclerosis a review. J
Neuroimmunol. 304:29–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumar A and Chauhan S: How much successful
are the medicinal chemists in modulation of SIRT1: A critical
review. Eur J Med Chem. 119:45–69. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Milner J: Cellular regulation of SIRT1.
Curr Pharm Des. 15:39–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang HC and Guarente L: SIRT1 and other
sirtuins in metabolism. Trends Endocrinol Metab. 25:138–145. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cao Y, Jiang X, Ma H, Wang Y, Xue P and
Liu Y: SIRT1 and insulin resistance. J Diabetes Complications.
30:178–183. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Y, Zhang F, Tsai Y, Yang X, Yang L,
Duan S, Wang X, Keng P and Lee SO: IL-6 signaling promotes DNA
repair and prevents apoptosis in CD133+ stem-like cells of lung
cancer after radiation. Radiat Oncol. 10:2272015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abitan H, Bohr H and Buchhave P:
Correction to the beer-lambert-bouguer law for optical absorption.
Appl Opt. 47:5354–5357. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seliktar D: Designing cell-compatible
hydrogels for biomedical applications. Science. 336:1124–1128.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wallach D: The TNF cytokine family: One
track in a road paved by many. Cytokine. 63:225–229. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McFee RM and Cupp AS: Vascular
contributions to early ovarian development: Potential roles of
VEGFA isoforms. Reprod Fertil Dev. 25:333–342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lan CC, Wu CS, Huang SM, Wu IH and Chen
GS: High-glucose environment enhanced oxidative stress and
increased interleukin-8 secretion from keratinocytes: New insights
into impaired diabetic wound healing. Diabetes. 62:2530–2538. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakamizo S, Egawa G, Doi H, Natsuaki Y,
Miyachi Y and Kabashima K: Topical treatment with basic fibroblast
growth factor promotes wound healing and barrier recovery induced
by skin abrasion. Skin Pharmacol Physiol. 26:22–29. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bus SA: The role of pressure offloading on
diabetic foot ulcer healing and prevention of recurrence. Plast
Reconstr Surg. 138 Suppl 3:179S–187S. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boulton AJ, Vileikyte L,
Ragnarson-Tennvall G and Apelqvist J: The global burden of diabetic
foot disease. Lancet. 366:1719–1724. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Futrega K, King M, Lott WB and Doran MR:
Treating the whole not the hole: Necessary coupling of technologies
for diabetic foot ulcer treatment. Trends Mol Med. 20:137–142.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fiordaliso F, Clerici G, Maggioni S,
Caminiti M, Bisighini C, Novelli D, Minnella D, Corbelli A, Morisi
R, De Iaco A and Faglia E: Prospective study on microangiopathy in
type 2 diabetic foot ulcer. Diabetologia. 59:1542–1548. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ndip A, Ebah L and Mbako A: Neuropathic
diabetic foot ulcers-evidence-to-practice. Int J Gen Med.
5:129–134. 2012.PubMed/NCBI
|
|
27
|
Şener LT and Albeniz I: Challenge of
mesenchymal stem cells against diabetic foot ulcer. Curr Stem Cell
Res Ther. 10:530–534. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bheda P, Jing H, Wolberger C and Lin H:
The substrate specificity of sirtuins. Annu Rev Biochem.
85:405–429. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kwon HS and Ott M: The ups and downs of
SIRT1. Trends Biochem Sci. 33:517–525. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Imai S and Guarente L: NAD+ and sirtuins
in aging and disease. Trends Cell Biol. 24:464–471. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huber JL, McBurney MW, Distefano PS and
McDonagh T: SIRT1-independent mechanisms of the putative sirtuin
enzyme activators SRT1720 and SRT2183. Future Med Chem.
2:1751–1759. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Villalba JM, de Cabo R and Alcain FJ: A
patent review of sirtuin activators: An update. Expert Opin Ther
Pat. 22:355–367. 2012. View Article : Google Scholar : PubMed/NCBI
|