Introduction
Proteins with a nucleotide-binding oligomerization
domain (NOD) and leucine-rich repeats (LRRs) are referred to as
nod-like receptors (NLRs) and are involved in inflammation and
apoptosis (1,2). Most NLRs have critical functions in
sensing pathogenic organisms or other inducers of inflammation.
Indeed, Nod1 and Nod2 promote NF-κB activation by interacting with
RICK/RIP2 (3). CARD12, NALP1, and
PYPAF promote NF-κB activation, caspase-1-associated interleukin
(IL)-1β synthesis, and/or apoptosis (4). On the other hand, PAN2 binds IKKα to
reduce tumor necrosis factor (TNF)-α and IL-1β-related NF-κB
activation (5). And PYPAF3 abrogates
the secretion of IL-1β by macrophages (6).
Recent years, several studies have reported a new
NLR-like protein with anti-inflammatory effects (7), comprising pyrin domain (PYD) and NOD
sequences but lacking LRRs; it was termed PYNOD (also named NLRP10
and NALP10) (8). Previous studies
revealed that PYNOD binds to an adaptor protein,
apoptosis-associated speck-like protein containing a CARD (ASC),
and inhibits ASC-mediated NF-κB activation and apoptosis; PYNOD
also suppresses caspase-1-mediated IL-1β maturation and secretion;
and the effects are dose-dependent (8,9). PYNOD
has been shown to be essential to the functions of immunocompetent
cells such as dendritic cells (10–13),
macrophages and neutrophils (8,9).
Microglia are immunocompetent cells of the brain and
the first line effector cells responsible for immune reactions in
the central nervous system (CNS) (14–16).
Activation of microglia causes the secretion of multiple immune
effectors, including nitric oxide (NO) via the inducible nitric
oxide synthase (iNOS) and pro-inflammatory cytokines, e.g., IL-1β,
IL-6, and TNF-α, which restore CNS homeostasis by cell and debris
removal (17–19). Microglia can activate the NF-κB
pathway during neuroinflammation (20–22).
Taken together, microglia activation results in chronic
neuroinflammation and increased neuronal injury, and eventually
neuronal death. The death of neurons usually follows microglia
activation in multiple CNS diseases such as Alzheimer's disease
(AD) (23), Parkinson's disease (PD)
(24,25), and amyotrophic lateral sclerosis
(ALS) (26). Microglia-related
neurotoxicity represents an important mechanism for
neurodegeneration and reduced neuron function. Therefore,
suppressing microglia induction is considered a therapeutic tool in
neurodegenerative diseases (27).
A previous study showed that the degradation of
PYNOD in the cytoplasm of mixed glial cells allowed the formation
of the inflammasome, while exogenous addition of recombinant PYNOD
to the glial cultures could reduce Aβ-induced caspase-1 activation
and IL-1β release (28). So far,
studies had only examined mixed glial cells and there is no study
focusing on the immunoregulatory effects of PYNOD specifically in
microglia. Whether PYNOD suppresses microglia activation under
inflammatory conditions remains unknown.
Therefore, this study aimed to explore how PYNOD
regulates the inflammatory signaling in microglia upon
lipopolysaccharides (LPS) stimulation and how it affects
microglia-induced neurotoxicity, using BV-2 cell line
(raf/myc-immortalised murine neonatal microglia) as a substitute
for primary microglia (29) and
human neuroblastoma cell line SK-N-SH for neuronal cells (30).
Materials and methods
Cell culture and grouping
Murine microglial cell line BV-2 and human
neuroblastoma cell line SK-N-SH were obtained from the Chinese
Academy of Sciences (Beijing, China) and cultured in Dulbecco's
modified Eagle's medium, containing 10% fetal bovine serum (FBS),
100 U/ml penicillin, and 100 U/ml streptomycin (all from Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), at 37°C in a
humid environment containing 5% CO2.
BV-2 cells cultured in 24-well plates
(6×104 cells/ml) or on glass coverslips (for
immunofluorescent staining) were subjected to transfection of
pEGFP-C2-PYNOD (0, 0.5, 2.5, and 5.0 µg/ml; Takara Biotechnology
Co., Ltd., Dalian, China) for 24 h, followed by incubation with or
without LPS (1 µg/ml; Sigma-Aldrich, St. Louis, MO, USA) for
another 24 h; the only exception was that to detect the protein
expression and distribution of NF-κB p65, only 1 h was taken for
LPS treatment). Then cell viability was determined; the levels of
NO, IL-1β and caspase-1 in supernatant of cell culture were
measured; and the protein expressions of NF-κB p65 and iNOS were
detected by immunofluorescent staining and/or western blot
analysis.
And a co-culture in transwell plates was performed
to assess the effects of microglia on neuronal cells in
vitro, according to a previous study (31). BV-2 cells were grown in culture
inserts and were subjected to transfection of pEGFP-C2-PYNOD (0 or
5.0 µg/ml) for 24 h, followed by incubation with or without LPS (1
µg/ml) for another 24 h. SK-N-SH cells were then added into the
wells. The co-culture lasted for 24 h. Then cell viability and
apoptosis of SK-N-SH cells were determined after co-culture.
Cell viability
Cell viability was quantified using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay. After removal of the medium, 0.5 mg/ml of MTT solution in
medium was added for 3 h. Then, the supernatants were carefully
aspirated, and formazan crystals were dissolved with DMSO;
absorbance was measured at 540 nm.
Analysis of cell apoptosis using flow
cytometry
Detection of apoptotic cells was performed using the
terminal deoxynucleotidyl transferase-mediated dUTP nick end
labeling (TUNEL) assay using an Annexin V-FITC/PI apoptosis
detection kit (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Briefly, cells were
incubated with fluorescein isothiocyanate (FITC)-conjugated Annexin
V and propidium iodide (PI) for 15 min at 4°C in the dark. Cell
cycle distribution was quantified by flow cytometry.
NO level measurement
The supernatant of BV-2 cell culture was obtained,
and NO amounts were determined by the Griess method. The
supernatants were added with equal amounts of Griess reagent
(Promega Corporation, Madison, WI, USA) and kept at room
temperature for 10 min. Optical density was measured at 540 nm. A
standard curve was obtained with sodium nitrite at 10–100 M.
Enzyme-linked immunosorbent assay
(ELISA)
The supernatant of BV-2 cell culture was obtained,
and IL-1β (cat. no. PMLB00C; R&D Systems, Inc., Minneapolis,
MN, USA) and caspase-1 (cat. no. K111-25; BioVision, Inc.,
Milpitas, CA, USA) amounts were measured by specific ELISA kits
according to the manufacturer's instructions. Optical density was
measured at 450 nm. According to the manufacturer, the IL-1β kit
had a sensitivity of 2.31 pg/ml, an intra-assay coefficient of
variation (CV) of 3.0–7.5% and an inter-assay CV of 5.7–8.4%.
Immunofluorescent staining
BV-2 cells were fixed with 3.7% paraformaldehyde.
After permeabilization (0.2% Triton X-100) and blocking (2% bovine
serum albumin; Sigma-Aldrich), the samples were treated with rabbit
polyclonal anti-NF-κB p65 primary antibodies (1:1,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and Cy5-labelled goat
anti-rabbit secondary antibodies (1:5,000; Sigma-Aldrich). The
co-staining of nucleus by Hoechst 33258 was performed. Analysis was
carried out under a laser scanning confocal microscope (Carl Zeiss
AG, Oberkochen, Germany).
Western blot analysis
Cytoplasmic and nuclear proteins were extracted with
NE-PER™ nuclear and cytoplasmic extraction reagents
(Thermo Fisher Scientific, Inc.). The BV-2 cells were lysed with
radioimmunoprecipitation assay (RIPA) buffer (Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
Total protein amounts were determined. Electrophoresis was
performed with 25 µg of proteins loaded in each lane, followed by
electro-transfer onto polyvinylidene difluoride (PVDF) membranes.
After blocking in 5% nonfat milk in Tris-buffered saline (TBS)/0.1%
Tween-20, the samples were incubated with primary antibodies
(rabbit anti-mouse iNOS, p65, and GAPDH polyclonal antibodies; all
at 1:1,000) overnight at 4°C in blocking buffer. After thorough
washing with phosphate buffered saline Tween-20 (PBST), horseradish
peroxidase (HRP)-conjugated secondary goat anti-mouse antibodies
(Cell Signaling Technology, Inc., Danvers, MA, USA) were added for
1 h at room temperature. Immunoreaction was detected using an
enhanced chemiluminescence detection system. The amounts of
objective proteins were normalized to that of GAPDH.
Statistical analysis
All statistical analyses were performed using SPSS
13.0 (SPSS, Inc., Chicago, IL, USA). The normal distribution of the
data was tested using the Kolmogorov-Smirnov test and all data were
found to be normally distributed. Data were presented as mean ±
standard deviation of three independent experiments. Statistical
analysis was done using one-way analysis of variance followed by
Tukey's post hoc test. P<0.05 indicated statistically
significant differences.
Results
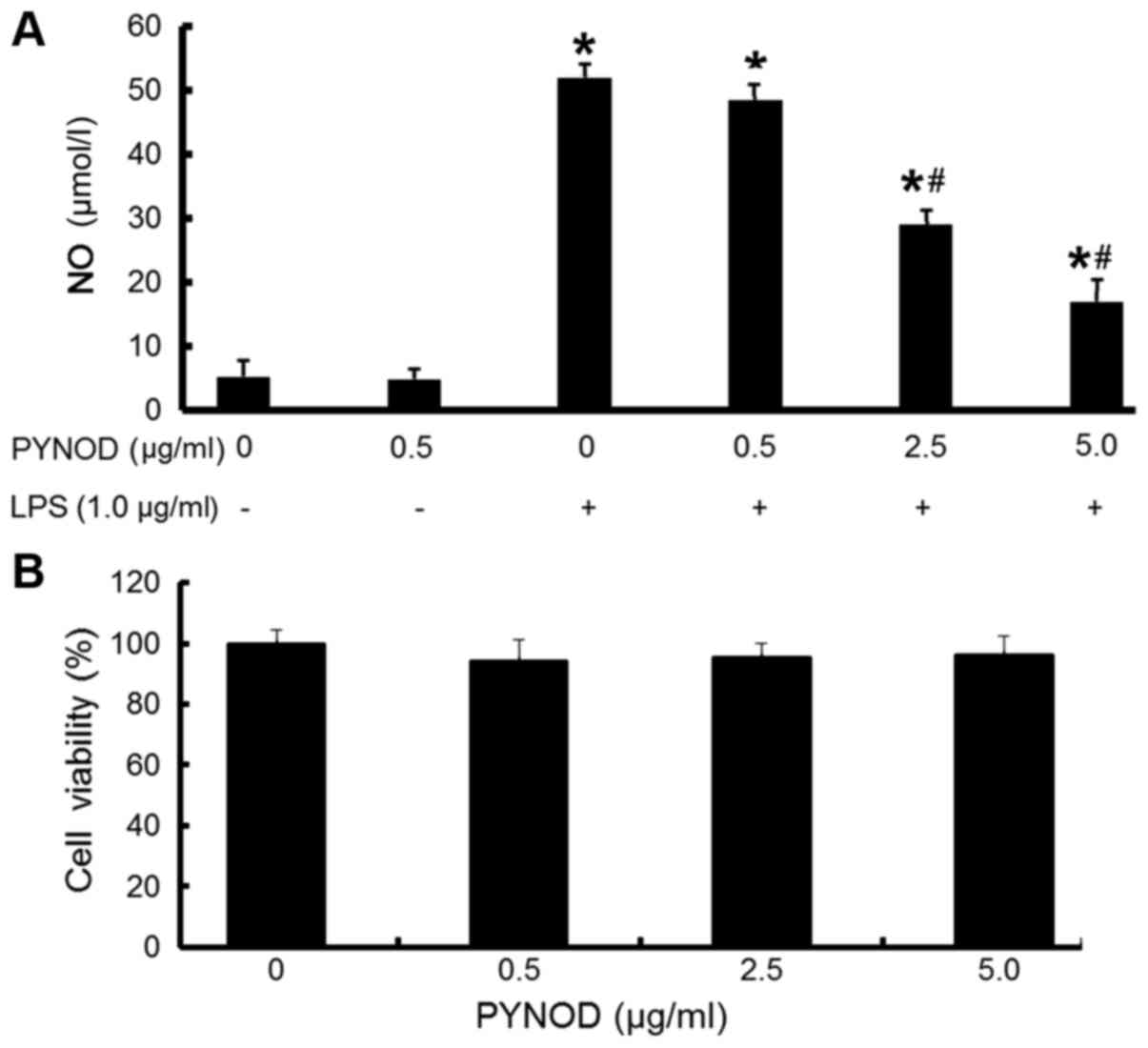
PYNOD concentration-dependently
decreases NO production without cytotoxicity in BV-2 microglial
cells upon LPS stimulation
To explore how PYNOD affects the NO production in
microglia under inflammatory conditions, BV-2 cells were
overexpressed with pEGFP-C2-PYNOD plasmid, followed by LPS
stimulation. PYNOD concentration-dependently reduced the NO
secretion from BV-2 cells induced by LPS, with statistically
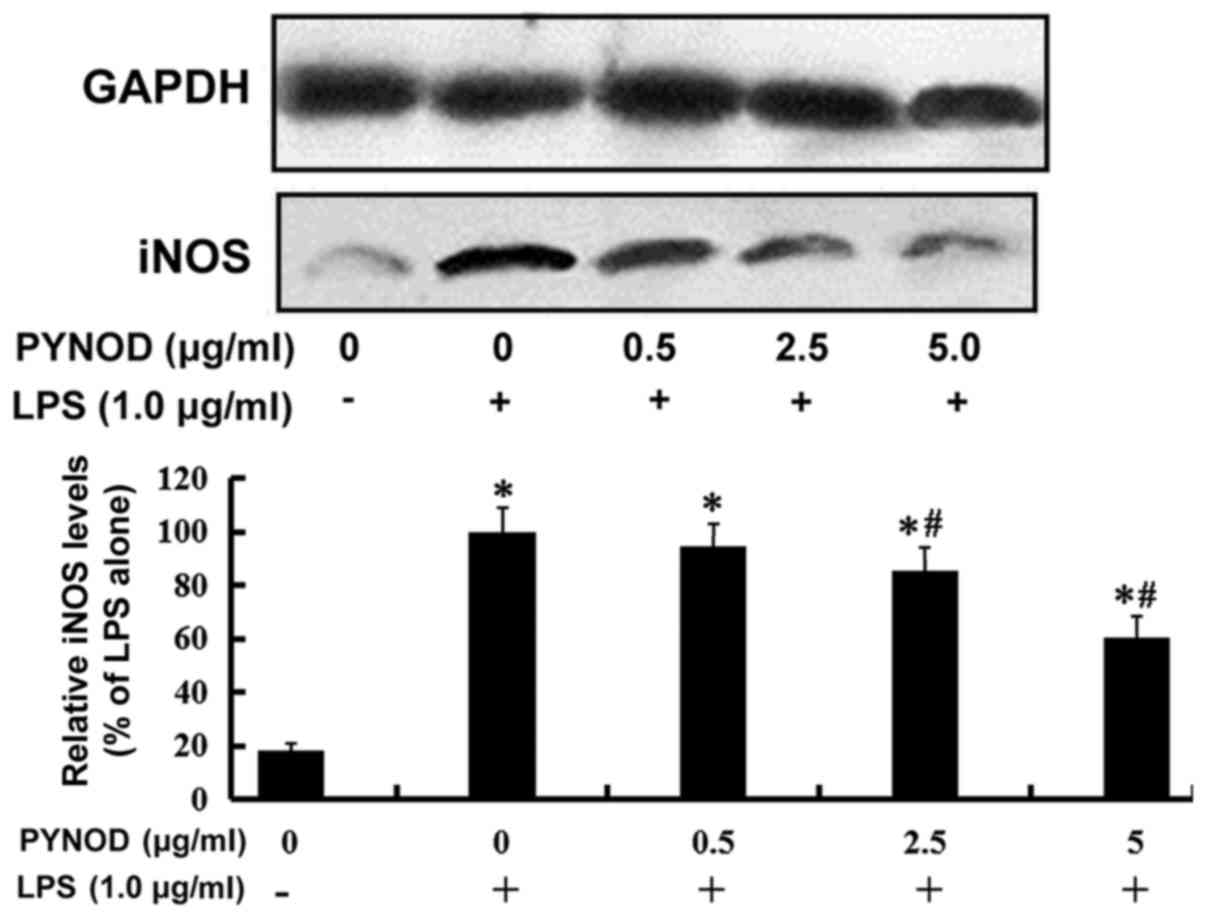
significant differences (both P<0.05; Fig. 1A). Furthermore, it was found that LPS
stimulation increased the iNOS protein expression in BV-2 cells,
which could be suppressed by PYNOD-overexpression, also in a
dose-dependent manner (both P<0.05; Fig. 2). Whether such inhibitory effect of
PYNOD resulted from cytotoxicity was then examined.
PYNOD-overexpression lead to no changes in the growth of BV-2 cells
(Fig. 1B), suggesting that PYNOD
reduced the NO production independently of cytotoxicity in BV-2
microglial cells upon LPS stimulation.
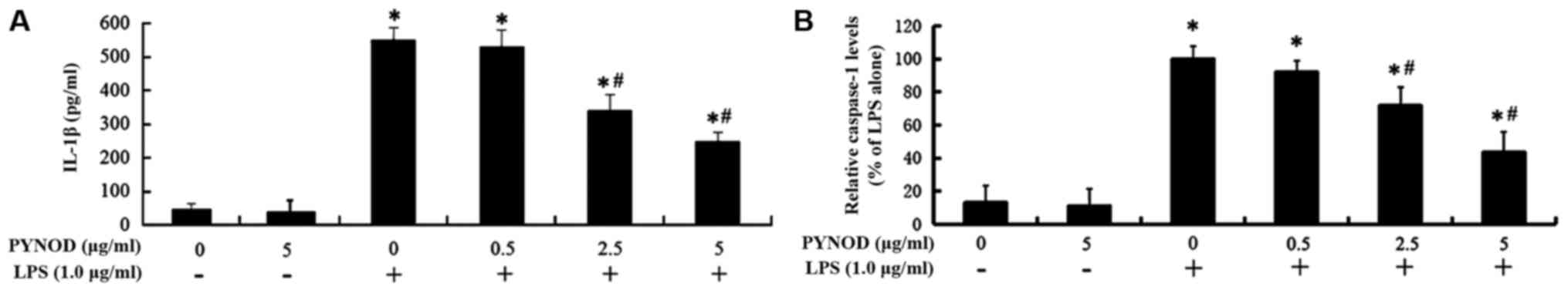
PYNOD concentration-dependently
reduces the secretion of IL-1β and caspase-1 from BV-2 microglial
cells upon LPS stimulation
IL-1β secretion from BV-2 microglial cells was
significantly higher (551.5 pg/ml) after LPS stimulation
(P<0.05, Fig. 3A); however, the
increase of IL-1β secretion was inhibited by PYNOD overexpression
(both P<0.05, Fig. 3A), declining
to 340.6 pg/ml (2.5 µg/ml pEGFP-C2-PYNOD transfection) or 250.3
pg/ml (5.0 µg/ml pEGFP-C2-PYNOD transfection). Caspase-1 amount in
the supernatant of BV-2 cell culture was 7.62-fold higher after LPS
stimulation (P<0.05; Fig. 3B),
while it was significantly suppressed by PYNOD overexpression to
lower level, about 5.47-fold or 3.32-fold of the un-stimulated
control (2.5 or 5.0 µg/ml pEGFP-C2-PYNOD transfection) (both
P<0.05; Fig. 3B).
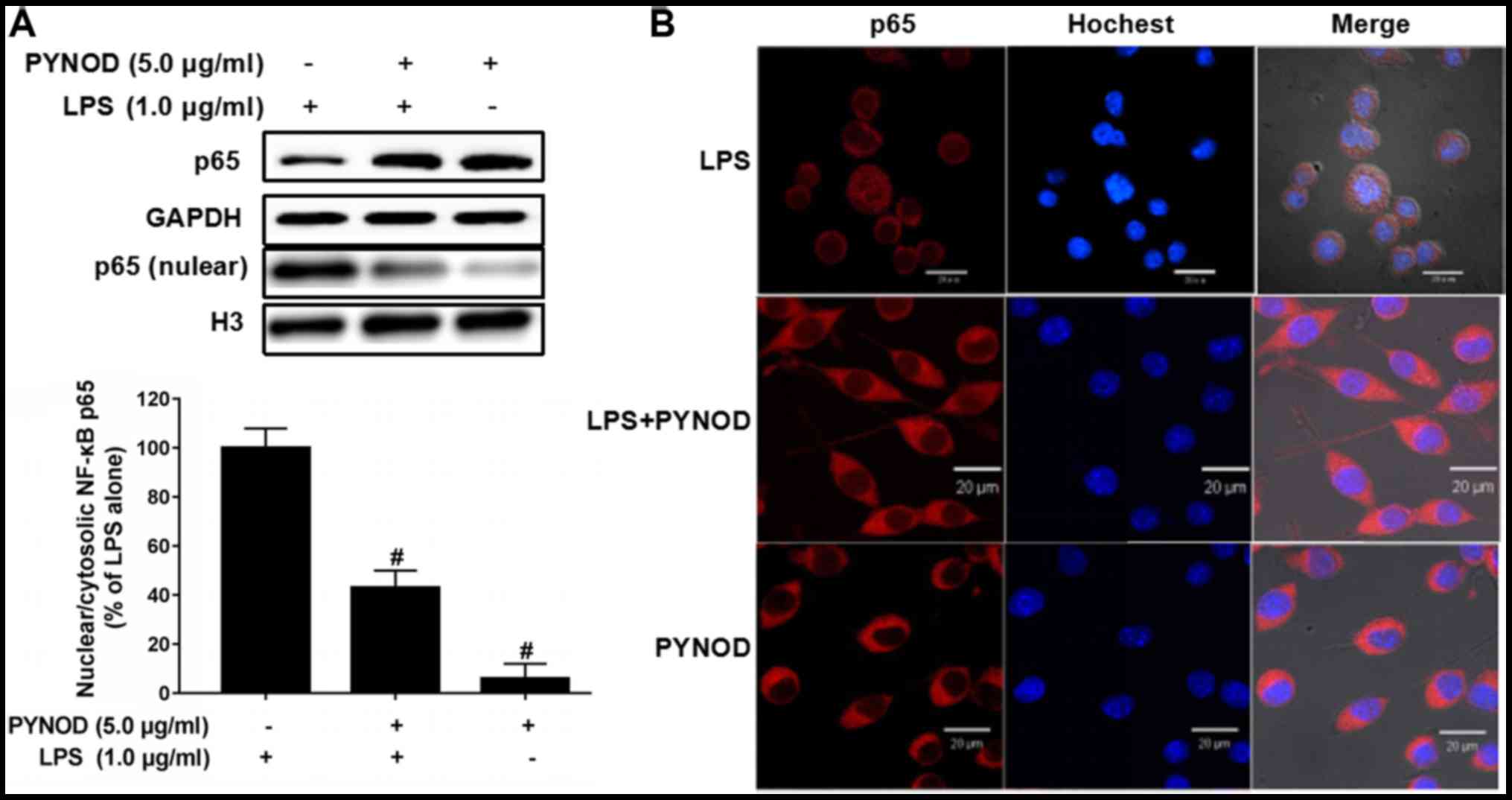
PYNOD prevent the nuclear
translocation of NF-κB in BV-2 microglial cells upon LPS
stimulation
The protein amount of NF-κB p65 in nucleus of BV-2
cells was high after LPS stimulation (Fig. 4A), which was significantly decreased
by PYNOD overexpression (P<0.05; Fig.
4A). And immunofluorescent staining confirmed that the
intracellular distribution of NF-κB p65 was restricted in nuclear
or perinuclear regions (Fig. 4B);
however, PYNOD overexpression resulted in cytoplasmic distribution
of NF-κB p65. The results suggested that PYNOD might prevent the
nuclear translocation of NF-κB p65 in BV-2 cells upon LPS
stimulation, thus inhibiting the generation of inflammatory
singling.
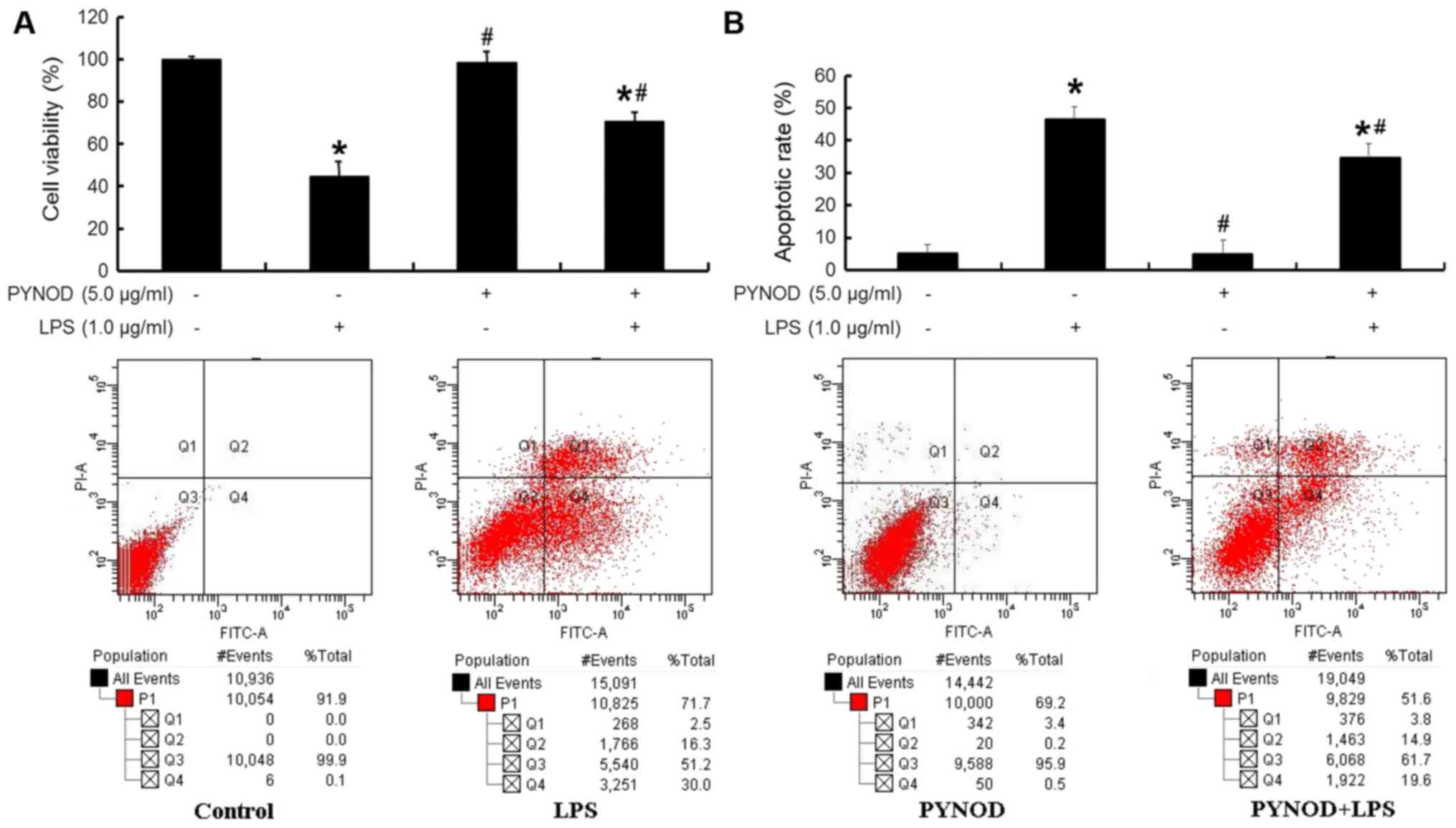
PYNOD alleviated the neurotoxic
effects of microglia upon LPS stimulation
There was a decrease of cell viability (P<0.05;
Fig. 5A) and an increase of
apoptosis (P<0.05; Fig. 5B) in
human neuroblastoma SK-N-SH cells when the co-cultured BV-2 cells
had been subjected to LPS stimulation. However, these changes in
SK-N-SH cells were significantly alleviated (both P<0.05;
Fig. 5A and B) if the co-cultured
BV-2 cells had been overexpressed with PYNOD before LPS
stimulation. Notably, PYNOD overexpression in BV-2 cells without
LPS stimulation would never affect the cell viability or cell
apoptosis of co-cultured SK-N-SH cells.
Discussion
This study aimed to explore the roles of PYNOD
involved in the LPS-induced microglial inflammation and consequent
neurotoxicity. The results showed that PYNOD overexpression
inhibited NO release and iNOS protein expression induced by LPS in
BV-2 cells, independently of cytotoxicity. It also reduced the
secretion of IL-1β and caspase-1 from BV-2 cells upon LPS
stimulation. The effects were dose-dependent. PYNOD overexpression
prevented LPS-induced nuclear translocation of NF-κB p65 in BV-2
cells. The growth-inhibitory and apoptosis-promoting effects of
BV-2 cells towards SK-N-SH cells were alleviated as a result of
PYNOD overexpression.
Increased amounts of NO produced by iNOS in induced
microglia are considered to be cytotoxic in CNS inflammation, and
related to neurodegenerative disease etiology (32). Indeed, agents inhibiting iNOS
expression carry potential benefits in the treatment of
high-NO-related inflammatory disorders (33). Pro-inflammatory cytokines, like
IL-1β, are produced in large amounts by microglia after stimulation
by molecules such as IFN-γ and LPS during the inflammatory process,
constituting potential factors causing neurological diseases
(34). Caspase-1 is known to cleave
pro-IL-1β for activation, a process critical to IL-1β secretion. A
previous study showed in that treating mixed glial cells with
recombinant PYNOD led to reduced caspase-1 activation and IL-1β
secretion (28). The present study
was carried out using microglial cells (which are included among
the mixed glial cells) and showed that PYNOD reduced LPS-induced
production of IL-1β and caspase-1 in a dose-dependent manner. The
present study implied that PYNOD modulates the secretion of the
pro-inflammatory cytokine IL-1β after microglia activation through
caspase-1 inhibition.
NF-κB plays key roles in multiple pathologies and
represents a main drug target for various diseases (35,36). The
promoter region of the mouse gene encoding iNOS harbors an NF-κB
binding site, and NF-κB induction is key in NO and iNOS production
in LPS-stimulated microglia (37).
As shown above, PYNOD prevented the nuclear translocation of
LPS-stimulated p65 in LPS-treated BV-2 microglial cells, revealing
a potential role for NF-κB in PYNOD's suppressive effects on
inflammatory effectors such as NO and IL-1β.
Microglial activation is deleterious to neurons,
causing apoptosis (19). Neurotoxic
microglial-neuronal interactions are considered to be important in
the development of neurological diseases (38). Our results showed that neurons
co-cultured with LPS-treated BV-2 microglial cells showed enhanced
cell death and apoptosis. Nevertheless, transfection with PYNOD in
LPS-stimulated co-cultures resulted in higher cell viability and
less apoptotic cells. These results suggested a beneficial effect
of PYNOD on microglia neurotoxicity in co-cultures. Thus,
neuroprotection by PYNOD might result from its effects on
microglia. Nevertheless, other cells in the brain may also be
affected by PYNOD. Indeed, PYNOD is expressed in neurons and its
expression is increased in injured neurons (39). NLRs (including PYNOD) play important
roles in the cerebral endothelial cells and the maintenance of the
brain-blood barrier under immune and inflammatory processes
(40). NLRs (again including PYNOD)
also play roles in the non-canonical inflammasone activation in
pericytes (41). Taken together,
these results strongly suggest that PYNOD is involved in the
neuroinflammatory process of many cell types in the CNS. Additional
studies are necessary to determine its exact roles in the brain and
how these different cell types interact together in
neuroinflammation. However this protein was recently discovered and
none of the inhibitors against it, so activators/inhibitors of
downstream signalling molecules (such as IL-1β, NF-κB) will need to
be demonstrate the specific effects of PYNOD. In addition,
knock-out models and PYNOD-transgenic mice will be necessary to
delineate the exact roles of PYNOD.
Taken together, the present study revealed that
PYNOD could mitigate microglial inflammation and consequent
neurotoxicity. Therefore, PYNOD might be therapeutically used as an
anti-inflammatory drug target suppressing excessive microglia
activation associated with exacerbated neuronal cell death under
brain injury conditions.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81441046),
Science Foundation of Jiangxi Province (grant no. 20142BAB215055),
and Science and Technology Commission of Jiangxi Province Health
Department (grant no. 20143124).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QZ contributed to the study design, data acquisition
and analysis and drafted the manuscript; CH was involved in data
acquisition and revision of the manuscript; RQ was involved in data
acquisition and analysis; DL worked on aspects of the study design,
data acquisition and analysis and revised the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interests
The authors declare that they have no competing
interests.
References
|
1
|
Pérez-Figueroa E, Torres J, Sánchez-Zauco
N, Contreras-Ramos A, Alvarez-Arellano L and Maldonado-Bernal C:
Activation of NLRP3 inflammasome in human neutrophils by
Helicobacter pylori infection. Innate Immun. 22:103–112. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zambetti LP, Laudisi F, Licandro G,
Ricciardi-Castagnoli P and Mortellaro A: The rhapsody of NLRPs:
Master players of inflammation…and a lot more. Immunol Res.
53:78–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Caruso R, Warner N, Inohara N and Núñez G:
NOD1 and NOD2: Signaling, host defense and inflammatory disease.
Immunity. 41:898–908. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chavarría-Smith J and Vance RE: The NLRP1
inflammasomes. Immunol Rev. 265:22–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garbarino-Pico E, Niu S, Rollag MD,
Strayer CA, Besharse JC and Green CB: Immediate early response of
the circadian polyA ribonuclease nocturnin to two extracellular
stimuli. RNA. 13:745–755. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kinoshita T, Wang Y, Hasegawa M, Imamura R
and Suda T: PYPAF3, a PYRIN-containing APAF-1-like protein, is a
feedback regulator of caspase-1-dependent interleukin-1beta
secretion. J Biol Chem. 280:21720–21725. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zeng Q, Lu D, Tang Q, Tian L, Wang H, Tang
S and Hu C: Functional characterization of the p53 binding site in
the human PYNOD promoter. Hum Immunol. 73:355–363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Hasegawa M, Imamura R, Kinoshita
T, Kondo C, Konaka K and Suda T: PYNOD, a novel Apaf-1/CED4-like
protein is an inhibitor of ASC and caspase-1. Int Immunol.
16:777–786. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Imamura R, Wang Y, Kinoshita T, Suzuki M,
Noda T, Sagara J, Taniguchi S, Okamoto H and Suda T:
Anti-inflammatory activity of PYNOD and its mechanism in humans and
mice. J Immunol. 184:5874–5884. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eisenbarth SC, Williams A, Colegio OR,
Meng H, Strowig T, Rongvaux A, Henao-Mejia J, Thaiss CA, Joly S,
Gonzalez DG, et al: NLRP10 is a NOD-like receptor essential to
initiate adaptive immunity by dendritic cells. Nature. 484:510–513.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu D, Rhebergen AM and Eisenbarth SC:
Licensing adaptive immunity by NOD-like receptors. Front Immunol.
4:4862013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krishnaswamy JK, Singh A, Gowthaman U, Wu
R, Gorrepati P, Nascimento Sales M, Gallman A, Liu D, Rhebergen AM,
Calabro S, et al: Coincidental loss of DOCK8 function in
NLRP10-deficient and C3H/HeJ mice results in defective dendritic
cell migration. Proc Natl Acad Sci USA. 112:3056–3061. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vacca M, Böhme J, Zambetti LP, Khameneh
HJ, Paleja BS, Laudisi F, Ho AWS, Neo K, Leong KWK, Marzuki M, et
al: NLRP10 enhances CD4+ T-cell-mediated IFNγ response via
regulation of dendritic cell-derived IL-12 release. Front Immunol.
8:14622017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Imai K, Kotani T, Tsuda H, Mano Y, Nakano
T, Ushida T, Li H, Miki R, Sumigama S, Iwase A, et al:
Neuroprotective potential of molecular hydrogen against perinatal
brain injury via suppression of activated microglia. Free Radic
Biol Med. 91:154–163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zindler E and Zipp F: Neuronal injury in
chronic CNS inflammation. Best Pract Res Clin Anaesthesiol.
24:551–562. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baby N, Patnala R, Ling EA and Dheen ST:
Nanomedicine and its application in treatment of microglia-mediated
neuroinflammation. Curr Med Chem. 21:4215–4226. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang LL, Zhou Y, Tian WD, Li HJ,
Kang-Chu-Li, Miao X, An GZ, Wang XW, Guo GZ and Ding GR:
Electromagnetic pulse activated brain microglia via the p38 MAPK
pathway. Neurotoxicology. 52:144–149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Papageorgiou IE, Lewen A, Galow LV,
Cesetti T, Scheffel J, Regen T, Hanisch UK and Kann O:
TLR4-activated microglia require IFN-γ to induce severe neuronal
dysfunction and death in situ. Proc Natl Acad Sci USA. 113:212–217.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gu Y, Yang DK, Spinas E, Kritas SK,
Saggini A, Caraffa A, Antinolfi P, Saggini R and Conti P: Role of
TNF in mast cell neuroinflammation and pain. J Biol Regul Homeost
Agents. 29:787–791. 2015.PubMed/NCBI
|
|
20
|
Shih RH, Wang CY and Yang CM: NF-kappaB
signaling pathways in neurological inflammation: A mini review.
Front Mol Neurosci. 8:772015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Frakes AE, Ferraiuolo L, Haidet-Phillips
AM, Schmelzer L, Braun L, Miranda CJ, Ladner KJ, Bevan AK, Foust
KD, Godbout JP, et al: Microglia induce motor neuron death via the
classical NF-κB pathway in amyotrophic lateral sclerosis. Neuron.
81:1009–1023. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Owens R, Grabert K, Davies CL, Alfieri A,
Antel JP, Healy LM and McColl BW: Divergent neuroinflammatory
regulation of microglial TREM expression and involvement of NF-κB.
Front Cell Neurosci. 11:562017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xiong JY, Li SC, Sun YX, Zhang XS, Dong
ZZ, Zhong P and Sun XR: Long-term treadmill exercise improves
spatial memory of male APPswe/PS1dE9 mice by regulation of BDNF
expression and microglia activation. Biol Sport. 32:295–300. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li JY, Ma SS, Huang QY and Li MT: The
function of neuroinflammation in parkinson disease. Sheng Li Ke Xue
Jin Zhan. 46:175–179. 2015.(In Chinese). PubMed/NCBI
|
|
25
|
Rangarajan P, Karthikeyan A, Lu J, Ling EA
and Dheen ST: Sirtuin 3 regulates Foxo3a-mediated antioxidant
pathway in microglia. Neuroscience. 311:398–414. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gravel M, Béland LC, Soucy G, Abdelhamid
E, Rahimian R, Gravel C and Kriz J: IL-10 controls early microglial
phenotypes and disease onset in ALS caused by misfolded superoxide
dismutase 1. J Neurosci. 36:1031–1048. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gendelman HE and Mosley RL: A perspective
on roles played by innate and adaptive immunity in the pathobiology
of neurodegenerative disorders. J Neuroimmune Pharmacol.
10:645–650. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Murphy N, Grehan B and Lynch MA: Glial
uptake of amyloid beta induces NLRP3 inflammasome formation via
cathepsin-dependent degradation of NLRP10. Neuromolecular Med.
16:205–215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Henn A, Lund S, Hedtjärn M, Schrattenholz
A, Pörzgen P and Leist M: The suitability of BV2 cells as
alternative model system for primary microglia cultures or for
animal experiments examining brain inflammation. ALTEX. 26:83–94.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Blasko I, Apochal A, Boeck G, Hartmann T,
Grubeck-Loebenstein B and Ransmayr G: Ibuprofen decreases
cytokine-induced amyloid beta production in neuronal cells.
Neurobiol Dis. 8:1094–1101. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kiyota T, Ingraham KL, Swan RJ, Jacobsen
MT, Andrews SJ and Ikezu T: AAV serotype 2/1-mediated gene delivery
of anti-inflammatory interleukin-10 enhances neurogenesis and
cognitive function in APP+PS1 mice. Gene Ther. 19:724–733. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
White PA, Oliveira RC, Oliveira AP,
Serafini MR, Araújo AA, Gelain DP, Moreira JC, Almeida JR, Quintans
JS, Quintans-Junior LJ and Santos MR: Antioxidant activity and
mechanisms of action of natural compounds isolated from lichens: A
systematic review. Molecules. 19:14496–14527. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McCarthy RC, Lu DY, Alkhateeb A, Gardeck
AM, Lee CH and Wessling-Resnick M: Characterization of a novel
adult murine immortalized microglial cell line and its activation
by amyloid-beta. J Neuroinflammation. 13:212016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang WY, Tan MS, Yu JT and Tan L: Role of
pro-inflammatory cytokines released from microglia in Alzheimer's
disease. Ann Transl Med. 3:1362015.PubMed/NCBI
|
|
35
|
Muriel P: NF-kappaB in liver diseases: A
target for drug therapy. J Appl Toxicol. 29:91–100. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu L, Li L, Medeiros LJ and Young KH:
NF-κB signaling pathway and its potential as a target for therapy
in lymphoid neoplasms. Blood Rev. 31:77–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Habashi Abbasi S, Sabouni F, Moghimi A and
Majd Ansari S: Modulation of lipopolysaccharide stimulated nuclear
factor kappa B mediated iNOS/NO production by bromelain in rat
primary microglial cells. Iran Biomed J. 20:33–40. 2016.PubMed/NCBI
|
|
38
|
Bi W, Zhu L, Jing X, Zeng Z, Liang Y, Xu
A, Liu J, Xiao S, Yang L, Shi Q, et al: Rifampicin improves
neuronal apoptosis in LPS-stimulated co-cultured BV2 cells through
inhibition of the TLR-4 pathway. Mol Med Rep. 10:1793–1799. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lo Frederick C, Ning X, Gonzales C and
Ozenberger BA: Induced expression of death domain genes NALP1 and
NALP5 following neuronal injury. Biochem Biophys Res Commun.
366:664–669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nagyőszi P, Nyúl-Tóth Á, Fazakas C,
Wilhelm I, Kozma M, Molnár J, Haskó J and Krizbai IA: Regulation of
NOD-like receptors and inflammasome activation in cerebral
endothelial cells. J Neurochem. 135:551–564. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nyúl-Tóth Á, Kozma M, Nagyőszi P, Nagy K,
Fazakas C, Haskó J, Molnár K, Farkas AE, Végh AG, Váró G, et al:
Expression of pattern recognition receptors and activation of the
non-canonical inflammasome pathway in brain pericytes. Brain Behav
Immun. 64:220–231. 2017. View Article : Google Scholar : PubMed/NCBI
|