Introduction
Dual antiplatelet therapy (DAPT) has been considered
of high value in relieving the hypercoagulable states in patients
with acute coronary syndrome (ACS) (1,2).
However, for patients scheduled for off-pump coronary artery bypass
grafting (CABG), DAPT poses strong risk for perioperative bleeding
in patients with CABG (3). Moreover,
consumption of coagulation factor during cardiopulmonary bypass,
dilution of fibrinogen and platelets, combined with other risk
factors such as heparin and decreased body temperature, all result
in depression on coagulation function, thus increasing the risk of
perioperative bleeding, blood transfusion, postoperative mortality
and rate of complication (4).
There has been no consensus on the timing of ACS
patients receiving DAPT treatment. Previous findings showed that
there was no significant difference of bleeding event between ACS
patients withholding clopidogrel ≤5 or >5 days before PCI
(5). While according to class I
recommendation by American College of Cardiology
Foundation/American Heart Association (ACCF/AHA), patients should
withhold clopidogrel for 5 days before CABG in order to restore the
platelet function (6). Whereas,
Class IIb recommendation by expert team on blood conservation of
Society of Thoracic Surgeons (STS) suggested that platelet function
test should be performed, based on which the surgical timing will
be determined for patients receiving DAPT treatment (7). Therefore, in the present study,
platelet function was measured using a TEG5000 thrombelastograph
analyzer and the surgical timing was determined based on the
measured maximum amplitude of adenosine diphosphate
(MAADP) values.
Materials and methods
Clinical material
In total, 90 subjects (including 47 males and 43
females; aged 42 to 80 years; average age, 61.2±10.2 years) with
ACS treated from February 2014 to December 2016 in cardiothoracic
surgery department of Henan Provincial People's Hospital
(Zhengzhou, China) were recruited. The patients received DAPT and
were scheduled for CABG. All the patients received regular DAPT
treatment before surgery of aspirin (100 mg/day) and clopidogrel
(75 mg/day) (aspirin was purchased from Chifeng Wanze
Pharmaceutical Co., Ltd. (Chifeng, China; NMPN. H10940218);
clopidogrel was purchased from Wuhan Wuyao Pharmaceutical Co. Ltd.
(Wuhan, China; NMPN. H20123155). Subjects were randomly allocated
into two groups, thrombelastography (TEG) group (n=45) and non-TEG
group (n=45), respectively in order of admission, and 45 patients
(hereinafter referred to as TEG group/study group) withheld
medications 24 h before surgery and received TEG examination. The
TEG group included 23 males and 22 females, aged 43 to 79 years
(average age, 61.3±9.8 years). The other 45 patients (including 24
males and 21 females; aged 42–80 years; average age, 61.2±11.3
years) received surgery after 5–7 days of medication withdrawal.
For the TEG group, based on MAADP, subjects were further
grouped into 3 sub-groups with MAADP <35 mm, 35–50
mm, and >50 mm, respectively, then accordingly, received CABG
within 1 day, 3–5 days and 5 days after withdrawal. The patients
were treated with off-pump CABG under general anesthesia. Heparin
was given routinely for antithrombotic use.
Exclusion criteria for the study were: Patients that
received emergency surgery after failing percutaneous coronary
intervention; patients in need of either valve repair or valve
replacement; patients with hematocrit (PCV) <30%; patients with
coagulation disorders, renal insufficiency (creatinine clearance
rate <30 ml/min), or viral hepatitis.
The study protocol was reviewed and approved by the
ethics committee of Henan Provincial People's Hospital. The
patients and families were fully informed of the study and signed
the informed consent. No statistically significant difference was
found between the two groups regarding age, sex ratio, prevalence
of hypertension, diabetes, medical history of myocardial infarction
(MI), preoperative ejection fraction and preoperative drug use
(P>0.05). The general preoperative clinical data of the two
groups of patients are presented in Table I.
 | Table I.General preoperative condition of the
two groups of patients undergoing CABG. |
Table I.
General preoperative condition of the
two groups of patients undergoing CABG.
| Items | Total (n=90) | TEG group (n=45) | Non-TEG group
(n=45) | χ2/t | P-value |
|---|
| Age (years, mean ±
SD) | 60.7±9.8 | 61.2±9.9 | 60.3±10.1 | 0.945 | 0.31 |
| Sex, n (%) | 47 (52.22) | 23 (51.11) | 24 (53.33) | 0.845 | 0.34 |
| Hypertension, n
(%) | 71 (78.89) | 35 (77.78) | 36 (80.0) | 0.942 | 0.33 |
| Diabetics, n (%) | 38 (42.22) | 19 (42.22) | 20 (44.44) | 0.415 | 0.55 |
| MI history, n
(%) | 29 (32.22) | 15 (33.33) | 14 (31.11) | 0.428 | 0.52 |
| Preoperative ACS, n
(%) | 37 (41.11) | 19 (42.22) | 18 (40.0) | 0.275 | 0.74 |
| Ejection fraction (%,
mean) | 52.3±12.1 | 51.3±12.2 | 53.4±12.1 | 0.439 | 0.54 |
| Unfractionated
heparin (24 h before surgery), n (%) | 31 (34.44) | 15 (33.33) | 16 (57.78) | 0.117 | 0.92 |
| ACEIs/ARBs, n
(%) | 62 (68.89) | 30 (66.67) | 32 (71.11) | 0.029 | 0.86 |
| β-blocker, n (%) | 68 (75.56) | 33 (73.33) | 35 (77.77) | 0.569 | 0.51 |
| Statins, n (%) | 9 (10.0) | 4 (8.89) | 5 (11.11) | 0.023 | 0.48 |
| PPIs, n (%) | 9 (10.0) | 4 (8.89) | 5 (11.11) | 0.339 | 0.75 |
| Pre-operative
INR | 0.91±0.18 | 0.93±0.21 | 0.94±0.15 | 0.328 | 0.78 |
| APTT (sec) | 29.42±2.13 | 28.72±2.51 | 28.62±2.25 | 0.143 | 0.89 |
Examination methods
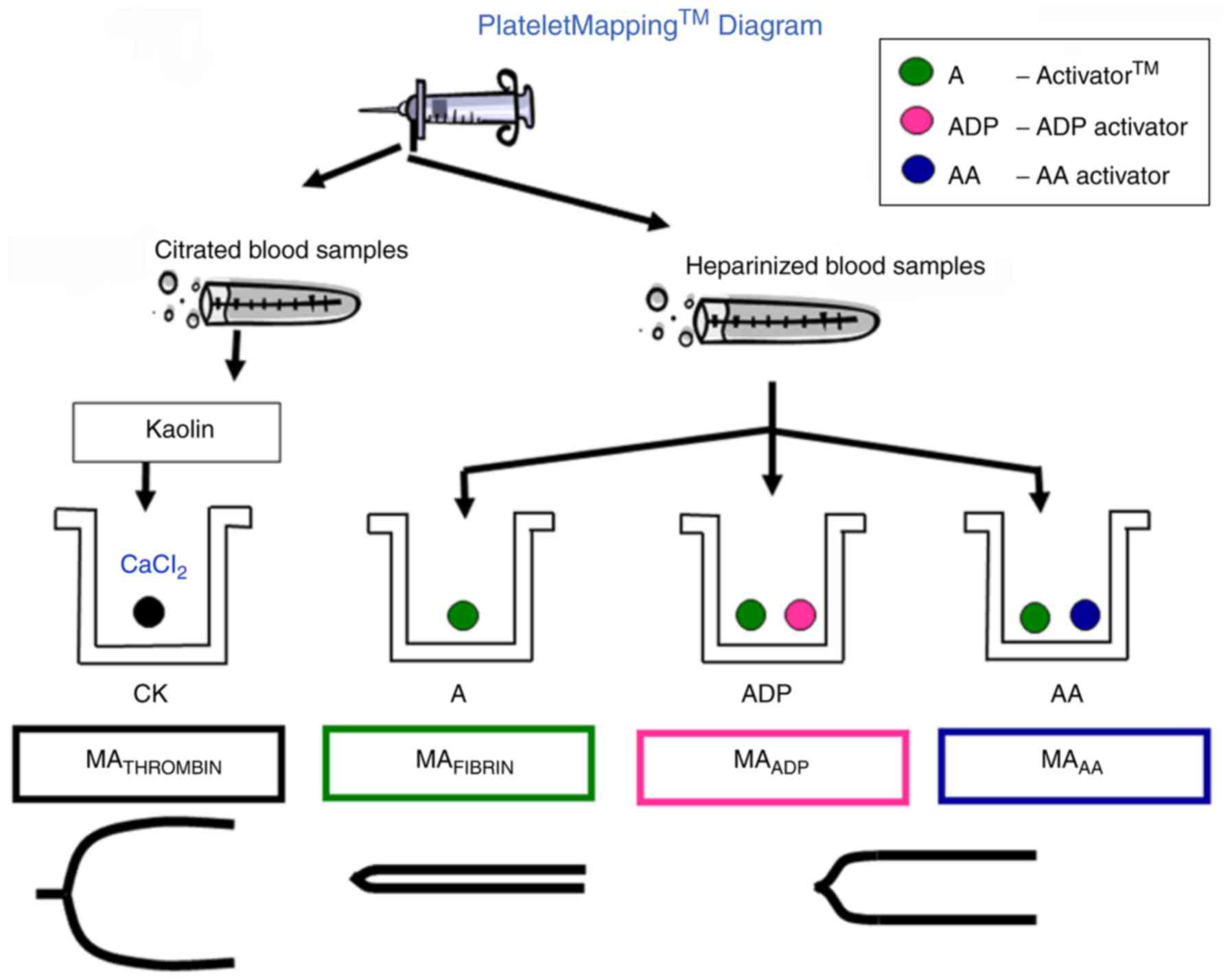
A TEG 5000 thrombelastograph analyzer (Haemoscope
Corporation, Niles, IL, USA) was used for the examination with ADP
as inducer. The analyzer was operated under normal conditions, and
relevant reagents and the instrument were operated as per
instructions (Fig. 1). For the
heparinized blood samples, at least 3 ml peripheral blood was
collected with heparinized anticoagulant tube followed by adequate
stirring. Samples were sent for testing immediately. Examination
were completed within 3 h after sample collection. For the TEG test
results, MAADP, was recorded. Patients of TEG group were
tested after 24 h of medication withdrawal. Based on the screening
results of MAADP, patients of TEG group were further
grouped into three sub-groups with MAADP <35 mm
(patients with platelet hyperreactivity), 35–50 mm (patients with
medium platelet activity), and >50 mm (patients with platelet
hyporeactivity), respectively. Accordingly, subjects of the 3
sub-groups received CABG within 1 day, 3–5 days and 5 days after
withdrawal. Patients in the control group commonly received surgery
5–7 days after medication withdrawal. Platelet inhibition rate
induced by AA and ADP was calculated by computer software. At the
same time, MA-Thrombin, MA induced by AA (MA-AA), MA induced by ADP
(MAADP) were recorded.
Observation parameter
Chest drainage volume within 24 h (primary endpoint)
and red blood cell transfusion in the perioperative period
(secondary endpoint) were compared. Incubation period, intensive
care unit (ICU) length of stay (LOS), hospital stay, incidence of
30-day adverse events, the amount of bleeding and readmission rate
of the subjects in the two groups were reported.
Statistical analysis
IBM SPSS Statistics 21 software (IBM Corp., Armonk,
NY, USA) was used to analyze the data. Quantitative data are
described with difference between the two groups as mean ± standard
deviation, and assessed using t-test. Numeration data variables
were expressed as frequencies and percentages and performed with
χ2 test to compare the difference. The one-way analysis
of variance (ANOVA) with the least significant difference test was
used to correct the variables. Count data were expressed by
percentage (%) and assessed with χ2 test. Difference
with P<0.05 was considered statistically significant.
Results
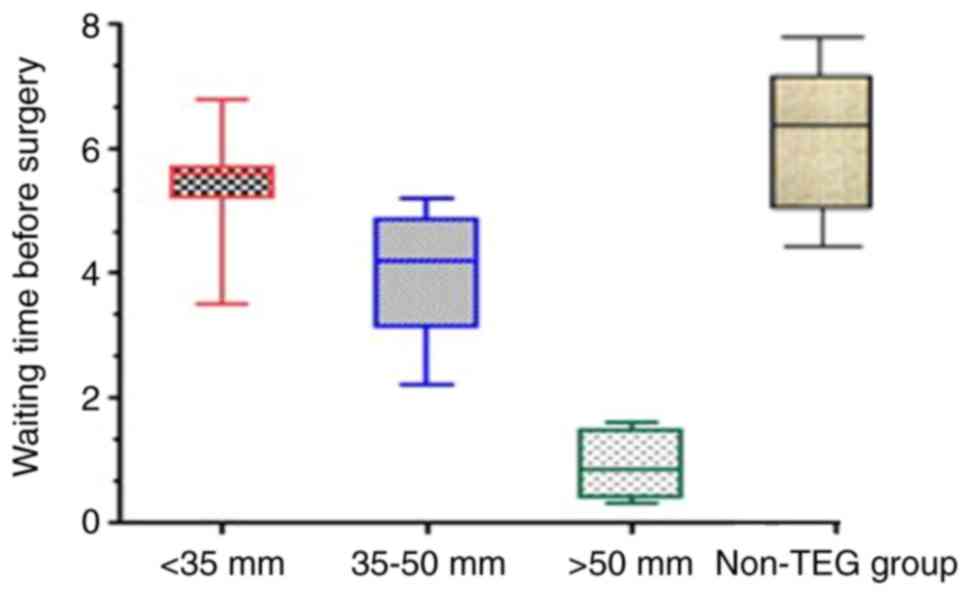
Waiting time before surgery for TEG
group
Based on screening results of MAADP,
patients of the TEG group for elective CABG were grouped into three
sub-groups with MAADP <35 mm, 35–50 mm, and >50
mm, respectively. Accordingly, subjects of 3 sub-groups received
CABG within 1 day, 3–5 days and 5 days after withdrawal. Patients
in the control group commonly received surgery 5–7 days after
medication withdrawal. The average waiting time of patients in TEG
group before CABG was 3.2 days. The detailed screening results of
MAADP and waiting time prior surgery of the 3 sub-groups
of patients are shown in Fig. 2.
Among patients of TEG group with MAADP <35 mm, one
patient received surgery earlier than scheduled. The matching rate
between scheduled and actual waiting time was 86.67% (13/15). Among
patients in TEG group with MAADP of 35–50 mm scheduled
to receive elective CABG within 3–5 days, two received surgery
earlier than scheduled, resulting in a matching rate between
scheduled and actual waiting time of 90.47% (19/21). Among patients
with MAADP >50 mm and scheduled to receive CABG
within 1 day, four patients received surgery after the scheduled
time, resulting in a matching rate between scheduled and actual
waiting time of 44.44% (4/9).
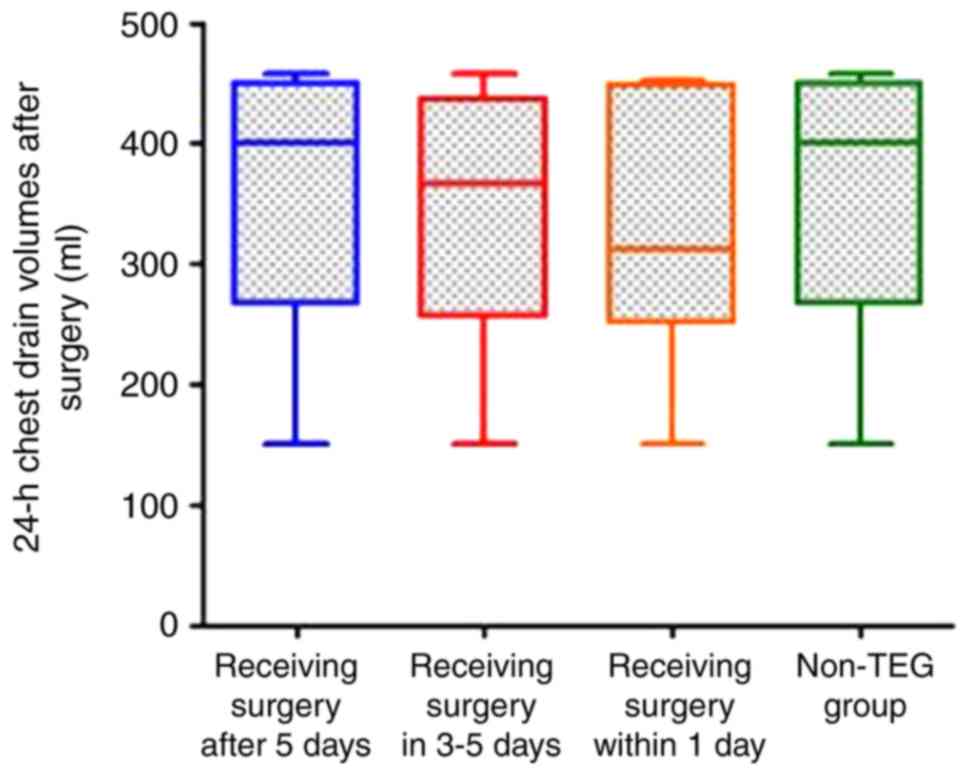
Comparison of the 24-h chest drains
after surgery between the TEG and non-TEG groups
Statistical analysis was conducted with
ANOVA-corrected variables and the results showed that there was no
significant difference of the median 24-h chest drain volumes of
patients between the TEG and non-TEG groups (435 and 488 ml for TEG
and non-TEG group, respectively; P=0.287). Further analysis
revealed that among patients of the three MAADP-based
sub-groups of the TEG group, the 24-h postoperative chest drain
volume of patients receiving surgery after 5 days was higher than
those receiving CABG in 3–5 days, which was higher than those
received within 1 day. However, no statistical difference was
detected by comparing the chest drains of the three sub-groups
(P=0.532). Detailed results are presented in Fig. 3.
Comparison of red cell transfusion
during perioperative period between patients in TEG and non-TEG
groups
No statistically significant difference was detected
between TEG and non-TEG groups by comparing the average red blood
cell transfusion volumes during perioperative period, which were
494.7 and 554.2 ml, respectively (P=0.23). With variables being
corrected with ANOVA test, the average red blood cell transfusion
volumes during perioperative period of TEG and non-TEG groups were
495.2 and 555.3 ml, respectively. No statistically significant
difference was detected with bilateral U-test (P=0.06).
Comparison of clinical endpoint events
between patients in TEG and non-TEG groups
The median hospital stay of patients in TEG group
was 24 days, significantly shorter than that in non-TEG group
(P=0.037). The bleeding amount of patients in TEG group was
220.16±80.56 ml, which was significantly lower than that of non-TEG
group (435.29±90.16). The difference was statistically significant
(P=0.032). No statistically significant differences were found by
comparing the perioperative chest drains, incubation period, ICU
LOS, 30-day mortality, 30-day readmission rate (P>0.05).
Detailed results are listed in Table
II.
 | Table II.Comparison of clinical endpoint events
between patients in TEG and non-TEG groups. |
Table II.
Comparison of clinical endpoint events
between patients in TEG and non-TEG groups.
| Item | TEG group (n=45) | Non-TEG group
(n=45) | χ2/t value | P-value |
|---|
| Perioperative chest
drain (ml, median) | 438 | 489 | 1.037 | 0.29 |
| Incubation period
(hour, median) | 21 | 22 | 0.835 | 0.46 |
| Hospital stay (day,
median) | 24 | 32a | 2.076 | 0.04 |
| ICU length of stay
(day, median) | 2 | 2 | 0.843 | 0.47 |
| Second thoracotomy,
n (%) | 0 (0) | 0 (0) | 0.081 | 1.00 |
| 30-day mortality, n
(%) | 0 (0) | 0 (0) | 0.081 | 1.00 |
| 30-day readmission
rate, n (%) | 7 (15.56) | 8 (17.78) | 0.337 | 0.75 |
| Amount of
bleeding | 220.16±80.56 | 435.29±90.16a | 2.213 | 0.03 |
Discussion
Perioperative use of antiplatelet therapy is
important for patients undergoing CABG. DAPT combining two
medications of different mechanisms, aspirin with clopidogrel, is
the most common antiplatelet therapy for patients with ACS.
Specifically, with aspirin mainly inhibiting COX-1 (cyclooxygenase
1) by inhibiting the platelet thrombus formation and clopidogrel
inhibiting the P2Y receptor on platelets, both medications
irreversibly inhibit the platelet function (8,9).
Therefore, for ACS patients undergoing CABG surgery, reasonable
selection of surgical timing and monitoring of platelet function is
important to reduce perioperative bleeding, postoperative morbidity
and mortality. Latest comparison of guidelines showed that patients
taking clopidogrel are mostly recommended to have the surgery after
5 days of medication withdrawal. However, considering the
individual difference in clopidogrel metabolism, platelet function
in some patients may have already restored within 5 days of
medication withdrawal. With such fact taken into consideration,
preoperative monitoring of platelet function provides significant
clinical value for selecting surgical time points, which is also
considered as a safer approach (10,11).
TEG analyzer is a monitor recording dynamic
processes including blood coagulation, platelet aggregation,
coagulation and fibrinolysis. It has become an important indicator
of blood coagulation function during liver transplantation, and
bypass surgery. It has been widely used in the assessment of
antithrombotic therapy for coronary heart disease, platelet
activity and antiplatelet effect in the world. But there are few
studies in China. It is used in this study to evaluate the
inhibition of platelets after treatment. Inhibition of platelet
aggregation can be achieved by inhibition of cyclooxygenase pathway
reducing the formation of TXA2, or inhibiting ADP receptor pathway.
TEG platelet aggregation AA and ADP induced pathway is based on
this. It was found that for PCI patients with high platelet
reactivity detected by TEG, cTnI, CK-MB level increased
significantly after PCI surgery. The incidence of clinical ischemia
was increased 6 months after surgery, indicating that TEG is
reliable for determining the high platelet reactivity caused by
drug resistance after the operation.
Platelets play important roles in blood coagulation
and determine approximately 80% of the strength of the clot.
According to the response to antiplatelet therapy, patients can be
categorized as platelet hyperreactivity (bleeding tendency), medium
platelet activity, and platelet hyporeactivity (resistance to
antiplatelet therapy) in clinic (12). Fitchett et al (13) performed blood platelet aggregation
test using optical microscopy with varying inducers. Hyporeactivity
to aspirin (aspirin resistance) was defined as platelet aggregation
rate when taking aspirin ≥50% with AA as inducer, and
hyporeactivity to clopidogrel (clopidogrel resistance) was defined
as platelet aggregation of ≥70% with ADP as inducer. According to
Fitchett et al, the MAADP value was defined as 35
mm and 50 mm as transition point, TEG 5000 thrombelastograph
analyzer was used to measure the platelet function and evaluate the
medication-induced platelet inhibition. Based on the preoperative
screening results of MAADP, patients in TEG group were
allocated in to three sub-groups with MAADP <35 mm,
35–50 mm, and >50 mm, respectively. Accordingly, patients of
these three sub-groups received CABG within 1 day of medication
withdrawal, after 3–5 days of medication withdrawal, and after 5
days of withdrawal, respectively. Patients of the TEG group waited
for an average of 3.2 days before CABG, 36% shorter than 5 days as
recommended by the guideline. Major parameters tested by
TEG-measured platelet thrombelastogram included platelet inhibition
rate (AA/ADP inhibition rate), MAADP and MACK
(14). Platelet inhibition rate is a
reference index of efficiency of antiplatelet drugs. Usually,
inhibition of AA platelet stimulation <50% or ADP% inhibition
<30% suggested inadequate antiplatelet efficacy; inhibition rate
>76% indicated relatively high platelet inhibition and clinical
attention should be paid to potential risk of bleeding (15,16). For
patients taking antiplatelet medication before surgery, surgical
timing can be selected according to the inhibition rate so as to
better prevent the preoperative thrombus formation and
intraoperative massive hemorrhage. MAADP provides
significant value by guiding the selection of surgical timing.
Typically, for elective CABG, patients with MAADP <35
mm should wait for more than 5 days before surgery; patients with
MAADP ranged 35 mm-50 mm should wait for 3–5 days prior
to surgery; and patients with MAADP >50 mm are
allowed to receive the surgery on the same day as medication
withdrawal (4,17). In the present study, no significant
difference was observed when comparing the postoperative 24-h chest
drains among the three TEG sub-groups. In addition, no
statistically significant difference was found by comparing the
24-h chest drains, perioperative average red blood cell transfusion
volume, incubation period, ICU LOS, 30-day mortality, and 30-day
readmission rate. These results showed that TEG-measured platelet
function successfully guided the appropriate surgical timing, which
resulted in comparable outcome and adverse event rates and reduced
waiting time compared with patients received CABG 5 days after
medication withdrawal. More importantly, TEG-based platelet
function measurement is featured with easy operation, high
repeatability, and stable performance. Only trace amount of whole
blood was required without any sample treatment. Results can be
achieved within short test time down to 30 min. Simultaneous
categorized detection is possible for combined medication,
providing test results free from effect of heparin drugs (18,19).
MAADP value, MAAA, MACK and
platelet inhibition rate measured by TEG are all objective
parameters adequately reflecting the drug-induced platelet
inhibition, which provides important value for patients in medical
and surgical departments (20).
Physicians may also benefit from the above-mentioned parameters by
adjusting dosage to reduce risk of bleeding and thrombosis.
Surgeons can guide the surgical and postoperative examination
according to the indexes above so as to reduce the risk of
perioperative bleeding and incidence of complications.
In summary, TEG-based platelet function evaluation
for patient elective for CABG can reasonably guide the surgeons in
surgical timing and reduce the waiting time prior to operation,
without significant increase of postoperative bleeding volume and
blood transfusion volume, as well as postoperative incidence rate
of adverse events.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZY collected and analyzed the general data. ZX
performed TEG test. XP and XQ recorded MA-thrombin, MA induced by
AA, MADP. ZY and DF recorded and interpreted incubation period,
intensive care unit (ICU) length of stay (LOS), hospital stay,
incidence of 30-day adverse events, the amount of bleeding and
readmission rate. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study protocol was reviewed and approved by the
Ethics Committee of Henan Provincial People's Hospital. The
patients and families were fully informed of the study and signed
the informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Roffi M, Gencer B, Storey RF, Andreotti F
and Patrono C: Clinical perspectives and pearls from the 2015 ESC
NSTE-ACS guidelines. Curr Cardiol Rep. 18:482016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yanagawa B, Ruel M, Bonneau C, Lee MM,
Chung J, Al Shouli S, Fagan A, Al Khalifa A, White CW, Yamashita
MH, et al: Dual antiplatelet therapy use by Canadian cardiac
surgeons. J Thorac Cardiovasc Surg. 150:1548–1554.e3. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barbieri L, Pergolini P, Verdoia M, Rolla
R, Nardin M, Marino P, Bellomo G, Suryapranata H and de Luca G;
Novara Atherosclerosis Study Group (NAS): Platelet reactivity in
patients with impaired renal function receiving dual antiplatelet
therapy with clopidogrel or ticagrelor. Vascul Pharmacol. 79:11–15.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Janssen PWA, Claassens DMF, Willemsen LM,
Bergmeijer TO, Klein P and Ten Berg JM: Perioperative management of
antiplatelet treatment in patients undergoing isolated coronary
artery bypass grafting in Dutch cardiothoracic centres. Neth Heart
J. 25:482–489. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pilgrim T and Windecker S: Antiplatelet
therapy for secondary prevention of coronary artery disease. Heart.
100:1750–1756. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Levine GN, Bates ER, Bittl JA, Brindis RG,
Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, et
al: 2016 ACC/AHA guideline focused update on duration of dual
antiplatelet therapy in patients with coronary artery disease: A
report of the American College of Cardiology/American Heart
Association Task Force on Clinical Practice Guidelines. J Thorac
Cardiovasc Surg. 152:1243–1275. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van Diepen S, Fuster V, Verma S, Hamza TH,
Siami FS, Goodman SG and Farkouh ME: Dual antiplatelet therapy
versus aspirin monotherapy in diabetics with multivessel disease
undergoing CABG: FREEDOM insights. J Am Coll Cardiol. 69:119–127.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Verma S, Goodman SG, Mehta SR, Latter DA,
Ruel M, Gupta M, Yanagawa B, Al-Omran M, Gupta N, Teoh H, et al:
Should dual antiplatelet therapy be used in patients following
coronary artery bypass surgery? A meta-analysis of randomized
controlled trials. BMC Surg. 15:1122015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng JW: Ticagrelor: Oral reversible
P2Y(12) receptor antagonist for the management of acute coronary
syndromes. Clin Ther. 34:1209–1220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Karon BS, Tolan NV, Koch CD, Wockenfus AM,
Miller RS, Lingineni RK, Pruthi RK, Chen D and Jaffe AS: Precision
and reliability of 5 platelet function tests in healthy volunteers
and donors on daily antiplatelet agent therapy. Clin Chem.
60:1524–1531. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tantry US, Bonello L, Aradi D, Price MJ,
Jeong YH, Angiolillo DJ, Stone GW, Curzen N, Geisler T, Ten Berg J,
et al: Consensus and update on the definition of on-treatment
platelet reactivity to adenosine diphosphate associated with
ischemia and bleeding. J Am Coll Cardiol. 62:2261–2273. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bomb R, Oliphant CS and Khouzam RN: Dual
antiplatelet therapy after coronary artery bypass grafting in the
setting of acute coronary syndrome. Am J Cardiol. 116:148–154.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fitchett D, Eikelboom J, Fremes S, Mazer
D, Singh S, Bittira B, Brister S, Graham J, Gupta M, Karkouti K, et
al: Dual antiplatelet therapy in patients requiring urgent coronary
artery bypass grafting surgery: A position statement of the
Canadian Cardiovascular Society. Can J Cardiol. 25:683–689. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dias JD, Norem K, Doorneweerd DD, Thurer
RL, Popovsky MA and Omert LA: Use of thromboelastography (TEG) for
detection of new oral anticoagulants. Arch Pathol Lab Med.
139:665–673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
James K, Bertoja E, O'Beirne J and Mallett
S: Use of thromboelastography PlateletMapping to monitor
antithrombotic therapy in a patient with Budd-Chiari syndrome.
Liver Transpl. 16:38–41. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ramakrishna H, Gutsche JT, Patel PA, Evans
AS, Weiner M, Morozowich ST, Gordon EK, Riha H, Bracker J, Ghadimi
K, et al: The year in cardiothoracic and vascular anesthesia:
Selected highlights from 2016. J Cardiothorac Vasc Anesth. 31:1–13.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hansson EC, Malm CJ, Hesse C, Hornestam B,
Dellborg M, Rexius H and Jeppsson A: Platelet function recovery
after ticagrelor withdrawal in patients awaiting urgent coronary
surgery. Eur J Cardiothorac Surg. 51:633–637. 2017.PubMed/NCBI
|
|
18
|
Tang XF, Han YL, Zhang JH, Wang J, Zhang
Y, Xu B, Gao Z, Qiao SB, Chen J, Wu Y, et al: Comparing of light
transmittance aggregometry and modified thrombelastograph in
predicting clinical outcomes in Chinese patients undergoing
coronary stenting with clopidogrel. Chin Med J (Engl). 128:774–779.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yao Y, Zhang JH, Tang XF, He C, Ma YL, Xu
JJ, Song Y, Liu R, Meng XM, Song L, et al: Head to head comparison
of two point-of-care platelet function tests used for assessment of
on-clopidogrel platelet reactivity in Chinese acute myocardial
infarction patients undergoing percutaneous coronary intervention.
Chin Med J (Engl). 129:2269–2274. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bochsen L, Wiinberg B, Kjelgaard-Hansen M,
Steinbrüchel DA and Johansson PI: Evaluation of the TEG platelet
mapping assay in blood donors. Thromb J. 5:32007. View Article : Google Scholar : PubMed/NCBI
|