Introduction
Hematopoietic stem cell transplantation (HSCT) is a
potentially effective strategy for treating several hematological
malignancies, such as leukemia, multiple lymphoma, other disorders
of blood and immune system (1,2).
Successful engraftment of hematopoietic stem cells (HSCs) in bone
marrow followed by expansion into mature hematopoietic lineages is
the prerequisite for effective HSCT (3,4). Delayed
or failed engraftment of HSCs (also called engraftment syndrome)
will significantly impede hematopoietic reconstitution and reduce
the treatment efficiency, resulting in higher incidence of
mortality and morbidity in patients after HSCT (5).
HSCs Engraftment is largely dependent on the bone
marrow environment, as HSCs reside in a highly specialized
microenvironment or niche in the bone marrow, which is consisted of
supporting cells, such as endothelial cells, lipocytes,
fibroblasts, reticulocytes, and osteoblasts (6,7). By
interacting with corresponding receptors expressed on HSCs, soluble
factors, molecules and ligands, which are produced from these
supporting cells, regulate the self-renewal, proliferation,
differentiation of HSCs (8). In the
settings of HSCT, bone marrow is severely damaged due to
pre-conditioning regimen treatment, leading to apoptosis of
supporting cells, which would activate the mononuclear phagocyte
system (MPS) comprising bone marrow progenitors, blood monocyte and
tissue macrophages, resulting in rapid clearance of these apoptotic
or dead cells (9).
Bone marrow microenvironment or niche is highly
complex and previous studies add to this complexity through
demonstrating a pivotal role of bone marrow mononuclear phagocytes
in promoting maintenance and retention of HSCs (10–12).
Using monocyte and macrophage conditional depletion models, Chow
et al demonstrated that decreased bone marrow mononuclear
phagocytes led to reduced bone marrow CXCL12 levels (CXCL12-CXCR4
is a critical niche retention signal), the selective downregulation
of HSC retention genes in Nestin+ niche cells, and
subsequent egress of HSCs/progenitors to the bloodstream (10). In addition, bone marrow macrophages
are also demonstrated to be required for the maintenance of
endosteal HSC niches in vivo and loss of macrophages
initiates a cellular cascade that ultimately leads to the
mobilization of functional HSCs with repopulating activity which
was associated with a decrease in transcripts of CXLC12,
angiopoietin 1 (Ang-1), stem cell factor (SCF) in both bone marrow
and endosteal stroma (12).
Furthermore, bone marrow monocytes and macrophages with high
expression of α-smooth muscle actin were capable to maintain
hematopoietic stem/progenitor cells and protect them from
exhaustion during alarm situations (13).
Our previous study demonstrated increased
infiltration of inflammatory cells (neutrophils and macrophages)
into bone marrow in mice following HSCT, accompanied with increased
secretion of several pro-inflammatory cytokines, including TNF-α,
IL-1β and IL-18 (14). Infiltrated
macrophages can not only exaggerate bone marrow inflammatory injury
through secretion inflammatory cytokines (15), but also can exert their function on
the clearance of these apoptotic or dead cells (16), providing space for transplanted HSCs.
Considering the importance of macrophage in bone marrow
microenvironment, whether macrophages affect hematopoietic
reconstitution after HSCT remains to be elucidated. In the present
study, we aimed to evaluate the role of macrophage in hematopoietic
reconstitution in mice after HSCT and found macrophage plays a
protective role in bone marrow inflammatory injury and promotes
hematopoietic reconstitution.
Materials and methods
Materials
Rabbit monoclonal to CD11b antibody was from Abcam
(Cambridge, MA, USA). PE-conjugated anti-mouse CD80 antibody was
purchased from eBioscience (San Diego, CA, USA). Alexa
Fluro488-conjugated anti-mouse CD206 antibody was purchased from
BioLegend (San Diego, CA, USA). PE-CY7-conjugated anti-mouse c-kit
and APC-conjugated anti-mouse Sca-1 antibody were from BD
Biosciences (San Jose, CA, USA). Clodronate Liposomes (Clo-Lipo)
and PBS Liposomes (PBS-Lipo) were purchased from Liposoma B.V.
(Amsterdam, The Netherlands). RS102895 was purchased from Tocris
Bioscience (Ellisville, MO, USA).
Animals and treatment
All experimental procedures involving animals were
approved by the Ethic Committee of Xuzhou Medical University
(Xuzhou, China).
C57BL/6 and BALB/c mice, aged 8–10 weeks and weighed
24–28 g, were purchased from SLAC Laboratory Animal Co., Ltd.
(Shanghai, China). The mice were housed in SPF grade environment in
the Experimental Animal Center of Xuzhou Medical University.
BALB/c mice were divided into five groups: HSCT
(n=16), HSCT+Clo-Lipo (n=20), HSCT+PBS-Lipo (n=20), HSCT+RS102895
(n=20) and HSCT+Vehicle (n=20). HSCT mice model was established as
previously described (14). Briefly,
BALB/c mice received lethal irradiation with 7.5 Gy followed by
infusion of 2×107 bone marrow mononuclear cells isolated
from the C57BL/6 mice. For injection of Clo-Lipo or PBS-Lipo, 200
µl Clo-Lipo or PBS-Lipo was administrated via tail vein on day 1
after HSCT with 100 µl injection per 3 days in the following
periods. Regarding injection of RS102895, 0.15 mg RS102895 was
intraperitoneally injected into mice after HSCT every other day.
Meanwhile, mice from Vehicle group received equal volume and
concentration of DMSO after HSCT. Normal health mice without any
treatment were served as a control.
H&E and immunohistochemical
staining
On D7, 14, 21, 28 and 35 after HSCT, mice were
sacrificed and bilateral femur and tibia were isolated, followed by
fixation in formaldehyde solution, dehydrated, waxed, and sliced
into 4 µm thickness. After that, H&E staining was performed and
the pathologic changes of bone marrow were evaluated by a light
microscope.
3% H2O2 was added into the
bone marrow slices and incubated at room temperature followed by
blockage by 5% goat serum. After that, the slices were incubated
with anti-mouse CD11b antibody and subsequent with HRP-conjugated
anti-rabbit secondary antibody. At the end point, color was
developed using 3, 3′-diaminobenzidine. The number of CD11b
positive cells in bone marrow was counted in 10 consecutive fields
under high-power fields (×40) and represented as cell number per
mm2.
Western blot analysis
Proteins were extracted from bone marrow cells of
mice, separated on 10% SDS-PAGE, and transferred to an NC membrane.
The membranes were incubated with rabbit anti-mouse CD11b antibody
and then incubated with HRP-conjugated anti-rabbit secondary
antibody. Membranes were visualized using enhanced
chemiluminescence. β-actin was used as a control. Protein
expression was quantified using Image J software. The results were
represented as a ratio to β-actin.
Measurement of HSCs and progenitor
cells
At indicated time point, bone marrow cells were
isolated to measure the number of cells with
c-kit+sca-1+, c-kit+ by flow
cytometry using anti-mouse c-kit+ and sca-1+
antibodies (17,18).
Statistical analysis
At least 3 independent experiments were performed
for each assay. Data were analyzed by GraphPad Prism software
(version 6.0; GraphPad Software, Inc., La Jolla, CA, USA) and
represented as mean ± SD. One-way ANOVA followed by Newman-Keuls
multiple comparison post hoc analysis was used for comparison of
one parameter at different time points after HSCT. Two-way ANOVA
followed by Bonferroni's post hoc test was performed for comparison
of differences between multiple groups over time. P<0.05 was
considered to indicate a statistically significant difference.
Results and Discussion
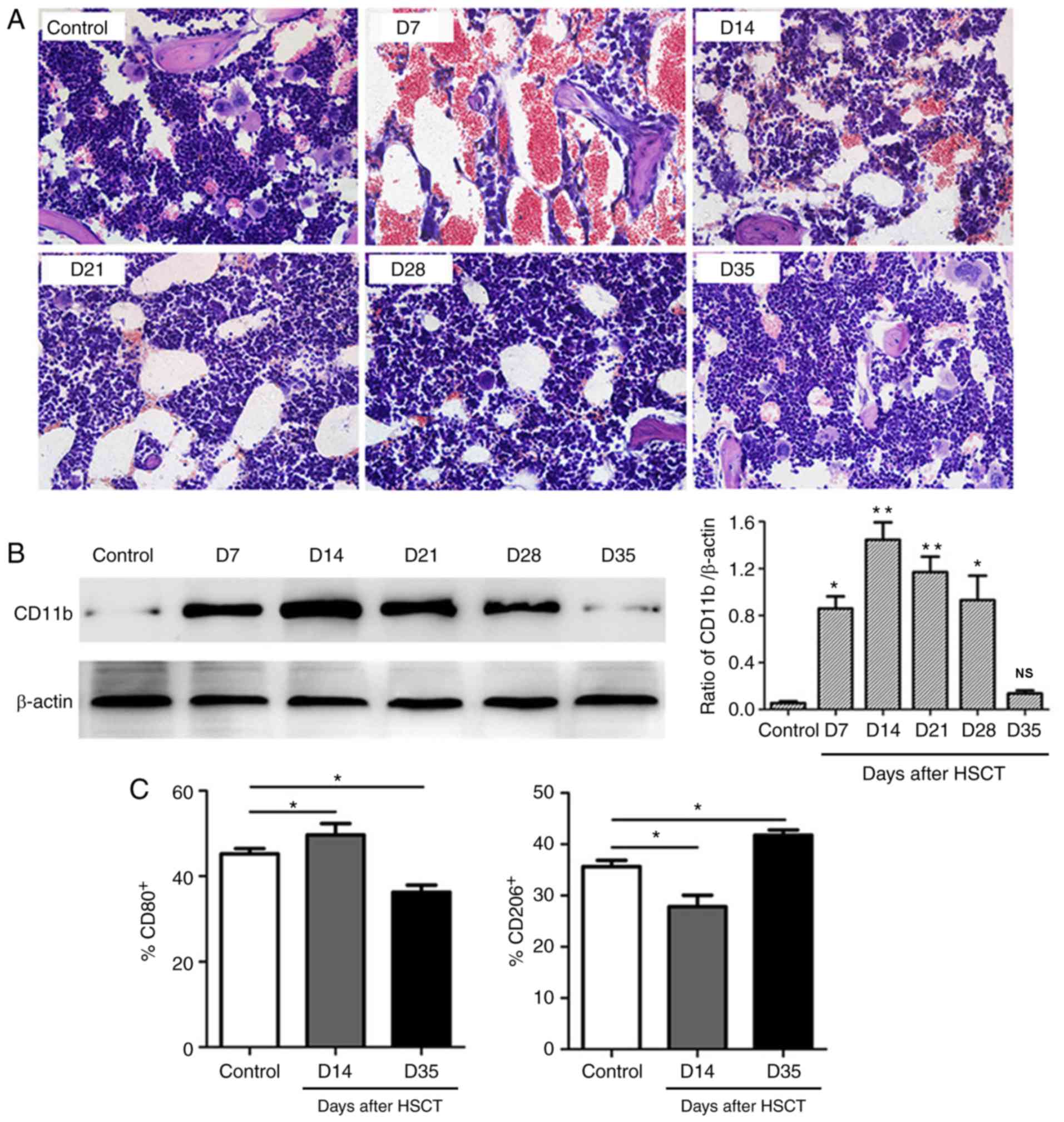
As shown in Fig. 1,
bone marrow inflammatory damage was observed after transplantation
with the most severe injury on D7 showing hemorrhage and cavity. On
D14, hemorrhage still existed with lots of inflammatory cells
infiltration into bone marrow. Afterwards, hemorrhage and cavity
were reduced from D21 with increased bone marrow cells and
ameliorated damage. The above pathological changes were almost
recovered to normal on D35. Western blot analysis showed numbers of
macrophages in bone marrow were increased after HSCT reaching a
peak on D14 followed by a gradual decrease in the following
periods, consistent with our previously study (14). In addition, infiltrated macrophage
polarization was also measured and showed significantly higher
number of M1 macrophage (%CD80+) and lower number of M2
macrophage (%CD206+) on D14 post transplantation.
However, the number of M1 macrophage decreased and M2 macrophage
increased on D35.
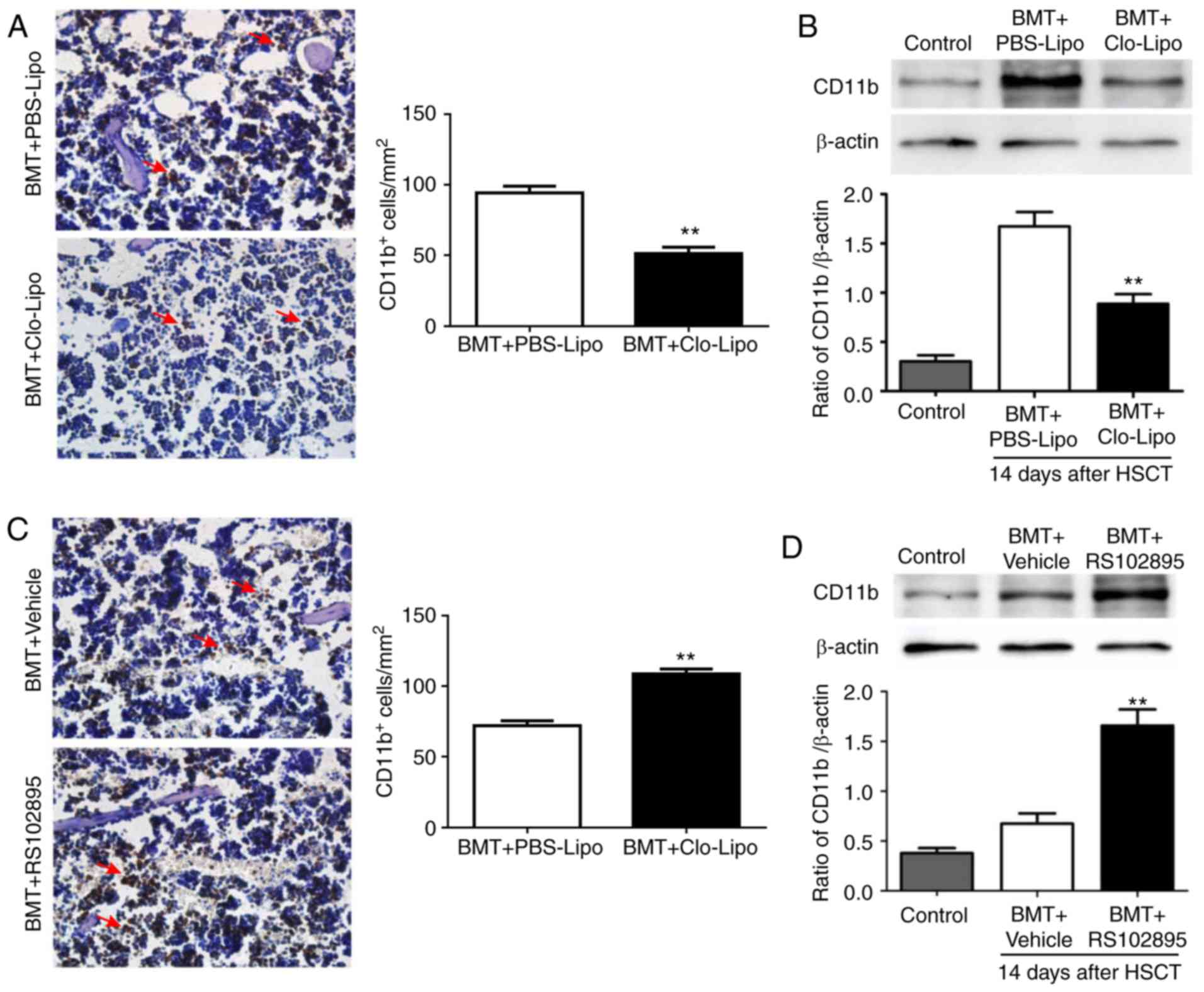
Considering increased number of macrophages in bone
marrow during bone marrow inflammation after transplantation,
Clo-Lipo was used to deplete macrophages (19) and RS102895 (inhibition chemotaxis of
macrophages to peripheral) was used to increase macrophages in bone
marrow to investigate the role of macrophages in HSCT. As seen in
Fig. 2A, number of macrophages in
bone marrow was dramatically reduced after Clo-Lipo treatment as
demonstrated by immunohistochemistry staining of bone marrow
compared with PBS-Lipo (P<0.01). Consistently, western blot
analysis also showed reduced CD11b (a pan-macrophage marker)
expression in bone marrow (Fig. 2B).
Meanwhile, after RS102895 treatment, number of macrophages in bone
marrow was increased as demonstrated by immunohistochemistry
staining (Fig. 2C) and western blot
(Fig. 2D) compared with Vehicle
(P<0.01).
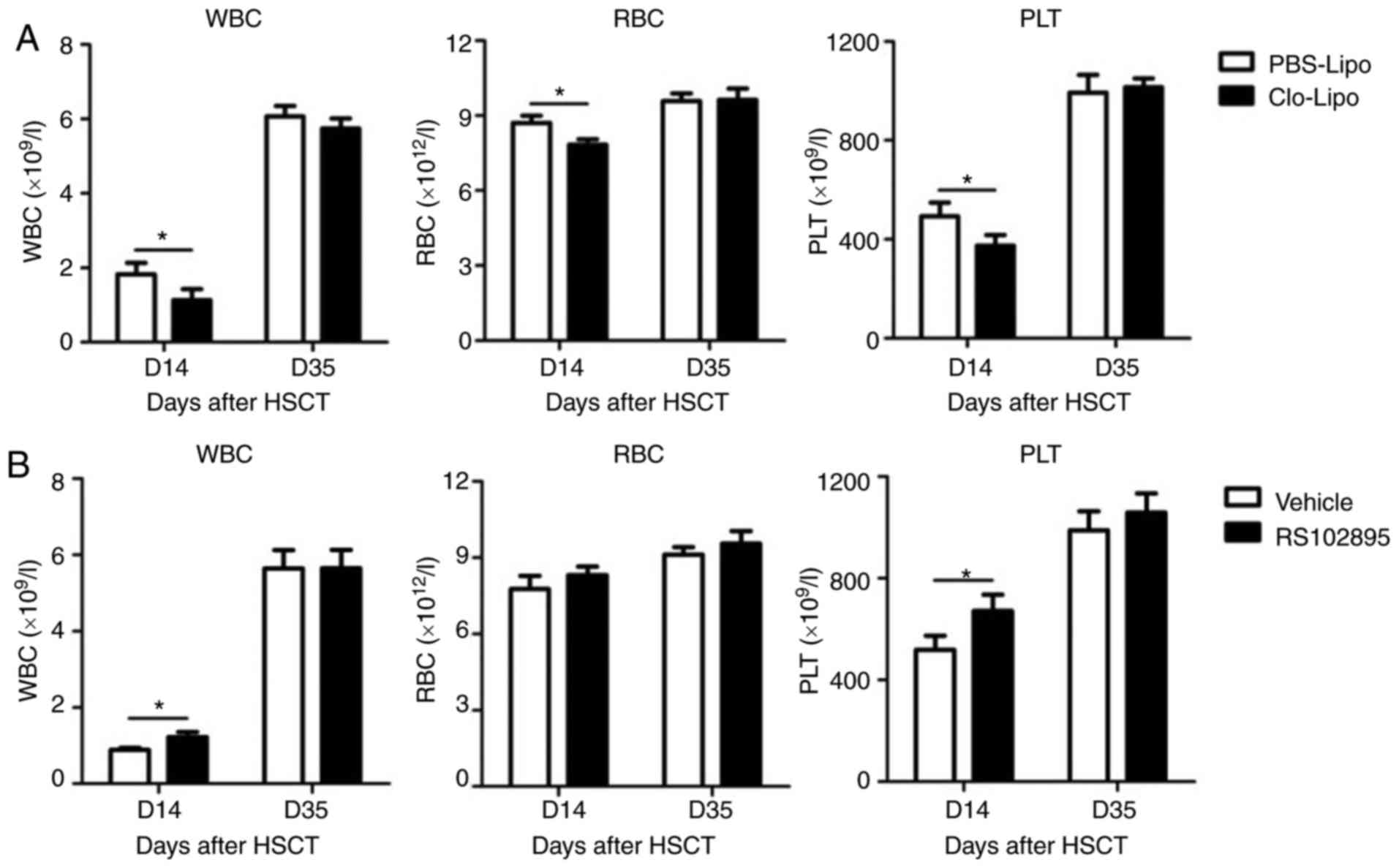
Peripheral blood was drawn from Clo-Lipo-treated
mice or control mice for analysis the effect of macrophages on
peripheral hematopoietic reconstitution. As seen in Fig. 3A, numbers of white blood cell, red
blood cell and platelet were significantly lower in mice treated
with Clo-Lipo compared with PBS-Lipo on D14 post transplantation.
However, no differences were observed on D35 between these two
groups. Different to Clo-Lipo-treated mice, mice after RS102895
treatment displayed accelerated hematopoietic reconstitution which
was demonstrated by higher number of white blood cells and
platelets on D14 post HSCT (Fig.
3B). Taken together, these data demonstrated that macrophages
might play an important role in promotion of peripheral
hematopoietic reconstitution after HSCT. However, the exact
mechanism by how macrophages promote peripheral hematopoiesis after
transplantation remains unclear and requires further
investigations.
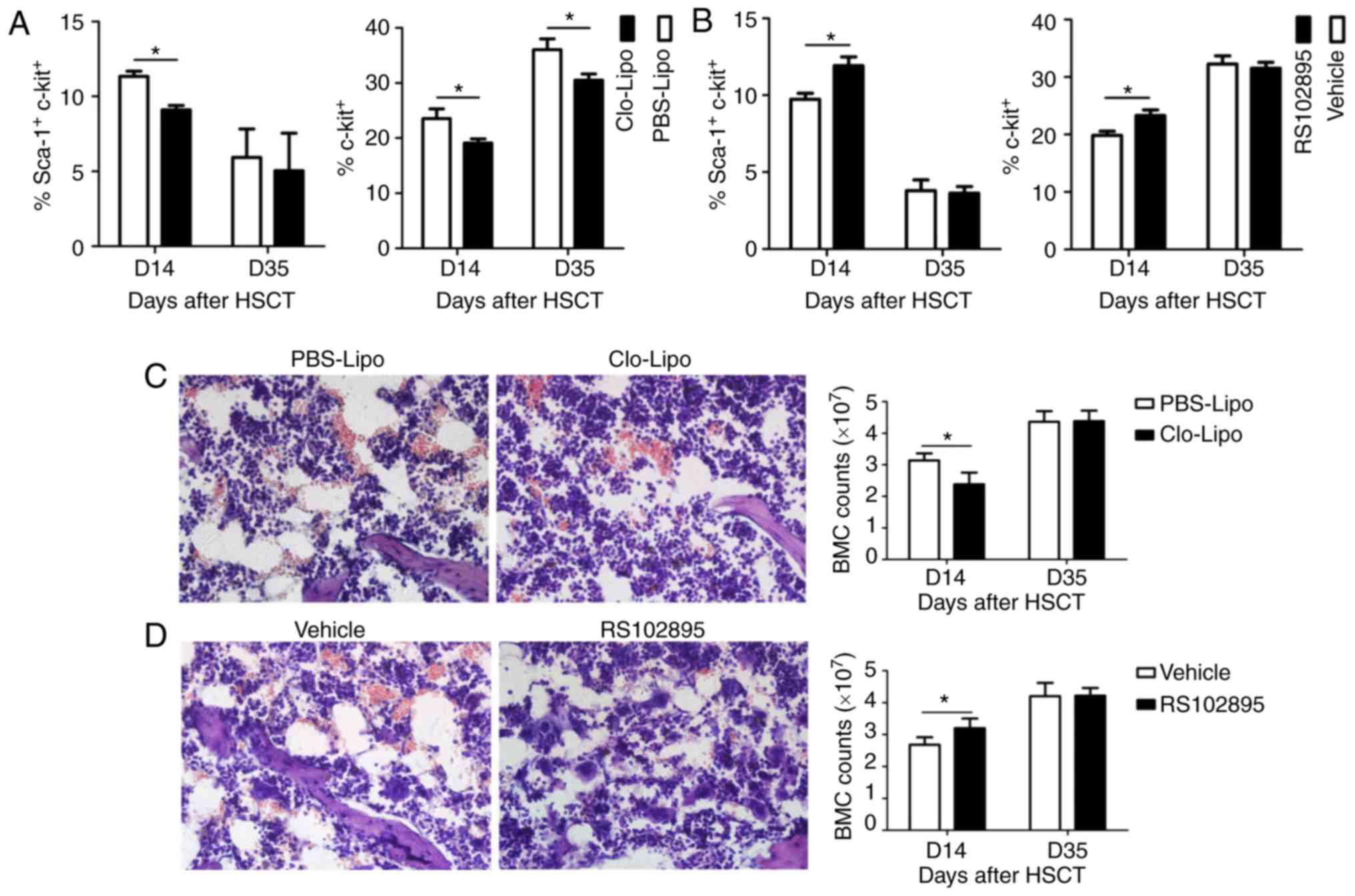
Given altered hematopoietic reconstitution after
manipulation of macrophages number, hematopoietic stem/progenitor
cells were also measured. Consistent with slower hematopoietic
recovery, numbers of hematopoietic stem and progenitor cells were
significantly lower in Clo-Lipo-treated mice on D14 compared with
PBS-Lipo (Fig. 4A). Even the number
of hematopoietic progenitor cells was increased on D35, it was
still lower in mice treated with Clo-Lipo than those treated with
PBS-Lipo (Fig. 4A). In terms of
RS102895-treated mice, numbers of hematopoietic stem/progenitor
cells were significantly higher on D14 compared with Vehicle
(P<0.05) (Fig. 4B). These data
indicated that altered numbers of hematopoietic stem and progenitor
cells might account for the different hematopoietic reconstitution
after manipulation of macrophages.
In order to evaluate the pathology changes of bone
marrow after manipulation of macrophages, H&E staining of bone
marrow was performed on D14 post HSCT. As seen in Fig. 4C, hemorrhage and cavity were observed
in Clo-Lipo and PBS-Lipo group with more severe in Clo-Lipo-treated
mice. In addition, number of bone marrow cells appeared to be lower
in mice treated with Clo-Lipo than PBS-Lipo. However, all the above
pathologic damages of bone marrow were ameliorated in mice after
RS102895 treatment (Fig. 4D) with
increased number of bone marrow cells. These data indicated that
macrophages might play a protective role during bone marrow injury
after transplantation. However, the exact mechanism by how
macrophages ameliorate bone marrow injury after HSCT remains
unclear and awaits investigation in the future.
The potential limitation of our study was that the
mechanism by which macrophages influence the hematopoietic cells
was not investigated, which might be through affecting bone marrow
microenvironment as demonstrated by ameliorated bone marrow injury
and increased bone marrow cell number after increasing macrophages
in bone marrow after HSCT (Fig. 4C and
D). We plan to investigate it in the future.
In conclusion, out study demonstrated that bone
marrow macrophages play a protective role in the inflammatory
injury of bone marrow and accelerate hematopoietic reconstitution
in mice after HSCT, suggesting manipulation of macrophages might be
a novel approach for improving the efficacy of HSCT.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural
Science Foundation of China (grant nos. 81370602, 81400082 and
81570096), the Natural Science Foundation of Jiangsu Province
(grant no. BK20140219) and the Jiangsu University Excellent Science
and Technology Innovation Team.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
JQ, LL, YX, WJ, PZ, YJ, ML, WL, LD, YW, KQ, and DL
performed the experiments and analyzed the data. XZ analyzed the
data. KX and LZ designed the study and co-wrote the manuscript.
Ethics approval and consent to
participate
All experimental procedures involving animals were
approved by the Ethics Committee of Xuzhou Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vyas P, Appelbaum FR and Craddock C:
Allogeneic hematopoietic cell transplantation for acute myeloid
leukemia. Biol Blood Marrow Transplant. 21:8–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Garcia IN: Role of hematopoietic stem cell
transplantation in multiple myeloma. Clin Lymphoma Myeloma Leuk.
15:86–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Danby R and Rocha V: Improving engraftment
and immune reconstitution in umbilical cord blood transplantation.
Front Immunol. 5:682014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Charbord P: Hemopoietic stem cells:
Analysis of some parameters critical for engraftment. Stem Cells.
12:545–562. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang L, Frame D, Braun T, Gatza E,
Hanauer DA, Zhao S, Magenau JM, Schultz K, Tokala H, Ferrara JL, et
al: Engraftment syndrome after allogeneic hematopoietic cell
transplantation predicts poor outcomes. Biol Blood Marrow
Transplant. 20:1407–1417. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morrison SJ and Scadden DT: The bone
marrow niche for haematopoietic stem cells. Nature. 505:327–334.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yin T and Li L: The stem cell niches in
bone. J Clin Invest. 116:1195–1201. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sison EA and Brown P: The bone marrow
microenvironment and leukemia: Biology and therapeutic targeting.
Expert Rev Hematol. 4:271–283. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hume DA: The mononuclear phagocyte system.
Curr Opin Immunol. 18:49–53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chow A, Lucas D, Hidalgo A, Méndez-Ferrer
S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C,
van Rooijen N, et al: Bone marrow CD169+ macrophages promote the
retention of hematopoietic stem and progenitor cells in the
mesenchymal stem cell niche. J Exp Med. 208:261–271. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Christopher MJ, Rao M, Liu F, Woloszynek
JR and Link DC: Expression of the G-CSF receptor in monocytic cells
is sufficient to mediate hematopoietic progenitor mobilization by
G-CSF in mice. J Exp Med. 208:251–260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Winkler IG, Sims NA, Pettit AR, Barbier V,
Nowlan B, Helwani F, Poulton IJ, van Rooijen N, Alexander KA,
Raggatt LJ and Lévesque JP: Bone marrow macrophages maintain
hematopoietic stem cell (HSC) niches and their depletion mobilizes
HSCs. Blood. 116:4815–4828. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ludin A, Itkin T, Gur-Cohen S, Mildner A,
Shezen E, Golan K, Kollet O, Kalinkovich A, Porat Z, D'Uva G, et
al: Monocytes-macrophages that express α-smooth muscle actin
preserve primitive hematopoietic cells in the bone marrow. Nat
Immunol. 13:1072–1082. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qiao J, Wu J, Li Y, Xia Y, Chu P, Qi K,
Yan Z, Yao H, Liu Y, Xu K and Zeng L: Blockage of caspase-1
activation ameliorates bone marrow inflammation in mice after
hematopoietic stem cell transplantation. Clin Immunol. 162:84–90.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arango Duque G and Descoteaux A:
Macrophage cytokines: Involvement in immunity and infectious
diseases. Front Immunol. 5:4912014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Poon IK, Lucas CD, Rossi AG and
Ravichandran KS: Apoptotic cell clearance: Basic biology and
therapeutic potential. Nat Rev Immunol. 14:166–180. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeng L, Ding S, Yan Z, Chen C, Sang W, Cao
J, Cheng H and Xu K: Irradiation induces homing of donor
endothelial progenitor cells in allogeneic hematopoietic stem cell
transplantation. Int J Hematol. 95:189–197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen W, Li M, Su G, Zang Y, Yan Z, Cheng
H, Pan B, Cao J, Wu Q, Zhao K, et al: Co-transplantation of
hematopoietic stem cells and Cxcr4 Gene-transduced mesenchymal stem
cells promotes hematopoiesis. Cell Biochem Biophys. 71:1579–1587.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Rooijen N and Hendrikx E: Liposomes
for specific depletion of macrophages from organs and tissues.
Methods Mol Biol. 605:189–203. 2010. View Article : Google Scholar : PubMed/NCBI
|