Introduction
Successful treatment with chemotherapeutic agents is
largely dependent on their ability to trigger cell apoptosisin
tumor cells (1). Many studies
demonstrate that certain phytochemicals found in medicinal plants
exert anti-tumor activity by inducing apoptosis in cancer cells
(2,3). Trillium tschonoskii Maxim, also
named ‘a pearl on head’, mainly distributed in mid-western China,
has been in folk used to cure headache, hypertension, neurasthenia,
giddiness, cancer, eliminating carbuncles and relieving the pains
for at least one thousand years (4,5). A
Initial studies have demonstrated that variety of bioactive
compounds including steroidal saponins and steroidal glycosides
have been identified in plants belonging to the Trillium
genus, such as Trillium erectum (6,7),
Trillium kamtschaticum (8,9) and
Trillium tschonoskii Maxim (10). Active chemical components, including
saponin polysaccharide, derived from Trillium tschonoskii
Maxim exhibit anti-bacterial and anti-cancer effects (11,12). In
a previous study (12), it was also
determined that pennogenin 3-O-α-L-rhamnopyranosyl-(1→2)
[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside (TTB2) suppressed
the proliferation of the Ewing sarcoma cell line and arrested cells
in the G2/M and S phases of the cell cycle in a dose- and
time-dependent fashion (12).
Furthermore, the phosphorylation of extracellular signal-regulated
kinase which serves an important role in tumorigenesis (12). However, it remains unclear which
types of cancer cells TTB2 induces death induced by TTB2. In a
previous study, it was demonstrated that TTB2, a steroidal saponin
from the n-butanol extracts of Trillium tschonoskii Maxim,
inhibits cell growth, induces apoptosis of Ewing sarcoma cells
(12). Additionally, the present
study indicated that TTB2 could trigger apoptosis protein cascades,
decrease the mitochondrial membrane potential (MMP) and alter the
expression of bax and bcl-2. The results suggest that TTB2 may
execute its anticancer activity by inducing apoptosis and may be
developed as a new class of chemotherapy drug.
Materials and methods
Reagents
Bovine serum albumin (BSA), the Annexin V-FITC
Apoptosis Test kit and chloromethyl-X-rosamine (CMX-Ros) were all
obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). RPMI
1640 culture media and fetal bovine serum were purchased from
Gibco, Thermo Fisher Scientific, Inc. (Waltham, MA, USA). BCA
protein assay kit and ECL chemiluminescence system were supplied by
PIERCE Biotechnology Inc. (Rockford, IL, USA). Rabbit polyclonal
anti-caspase 3 and 9 (cat. nos. 9661 and 7237, respectively) were
purchased from Cell Signaling, Inc. (Danvers, MA, USA). Mouse
monoclonal anti-cytochrome c (cat. no. sc-65396), bax (cat. no.
sc-7480), bcl-2 (cat. no. sc-7382), and tubulin (cat. no. sc-8035)
were from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Goat
anti-Rabbit IgG secondary antibody (cat. no. A32731) and Goat
anti-Mouse IgG secondary antibody (cat. no. A32723) were from
Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Other chemicals used in the current study were special grade
commercial products.
Plant material, extraction and
isolation of TTB2
The rhizomes (6.4 kg) of Trillium
tschonoskiiMaxim were picked from Muyu, a town in the
Shennongjia Forest District of Hubei Province, China. The compound
TTB2 was extracted and isolated from Trillium tschonoskii
Maxim following a previously published method (12). The TTB22 mg) powder was dissolved in
deionized water 60 µl). To prevent cell contamination, the TTB2
stock solution was filtered with 0.22 um-sized filtering membrane
and separated into individual aliquots, which were kept at −20°C
until further use.
Cell culture
Rh1 cells, derived from mesenchymal tissue, are a
type of human rhabdomyosarcoma cell, and were provided by St. Jude
Children's Research Hospital, Memphis, TN, USA were cultured in
antibiotic-free RPMI-1640 medium containing 10% FBS at 37°C and 5%
CO2 for 24 h as demonstrated in a previous study
(12).
Annexin V-FITC/PI cytometric
analysis
To determine the mechanism by which TTB2-induced
death of Rh1 cells, Annexin V and PI double staining were analyzed
by Flow Cytometry (FCM, BD Biosciences, Franklin Lakes, NJ, USA).
Rh1 cells (2×105) were seeded and incubated for 24 h at
37°C. RPMI medium was replaced with an equal volume of fresh medium
containing different concentrations (5 and 10 µM) of TTB2. PBS was
used as the negative control. Following supplementation with TTB2
for 24 h all the cells containing attached and nonattached cell
were collected and washed twice with PBS. Subsequently, the
harvested cells were added to 400 µl 1× binding buffer (obtained
from the Annexin V/PI staining kit), 5 µl Annexin V and 10 µl PI
staining, and kept in the dark for 15 min. Apoptotic, necrotic and
mechanically damaged cells were determined using flow cytometry
software (BD FACSuite™; v1.0.5; BD Biosciences) with the single
beam at a 488 nm wave length.
Assessment of mitochondrial membrane
potential
CMX-Ros (cat. no. 40741ES50; Shanghai yisheng
Biotechnology Co., Ltd., Qingdao, China) was used to analyze MMP.
Rh1 cells (2×105) for each sample were seeded and
incubated for 24 h at 37°C. RPMI medium was replaced with an equal
volume of fresh medium containing different concentrations of TTB2
(5 and 10 µM). PBS was used as the negative control. Following
supplementation with TTB2 for 24 h, all living cells were harvested
and washed twice with PBS. MitoTracker Red CMXRos powder (50 µg;
cat. no. 40741ES50; Shanghai Yisheng Biotechnology Co., Ltd.) was
dissolved in 94 µl of dimethylsulfoxide to be used as a storage
solution (1 mM Mitotracker Red CMXRos), which was stored at −20°C.
Storage solution (1 µl) was eventually diluted in 2 ml PBS, which
became the working solution with a final concentration of 500 nM
CMX-Ros. Cells were resuspended in 100 µl of PBS containing 500 nM
MitoTrackerCMX-Ros and incubated for 45 min at 37°C. Cells were
washed with PBS and resuspended in PBS supplemented with 0.2% BSA
and then placed on ice prior to flow cytometric (FACSVerse; BD
Biosciences, Franklin Lakes, NJ, USA) analysis with a single
excitation beam at 488 nm. MitoTracker Red CMXRos, an oxidized red
fluorescent dye, travel across the cell membrane and gather in
active mitochondria. Thus, once the mitochondria were stained, the
probe remained within the cell. The stained cell was then directly
analyzed using flow cytometry. Results were analyzed using BD
FACSuite™ software v1.0.5 (cat. no. 657658; BD Biosciences).
Western blot analysis
Analysis of relative protein expression analysis was
conducted by western blot method. Briefly, Rh1 cells
(2×105 per well) were seeded in 6-well plates and
cultured until an 80% confluence was reached. Cells were then
treated with indicated concentrations (0, 5 µM) of TTB2 for 24 h at
37°C and then harvested and washed with cold PBS. Cell pellets were
lysed in 40 µl lysis buffer (20 mM HEPES/NaOH, pH 7.5, 250 mM
sucrose, 10 mM KCl, 2 mM MgCl2, 1 mM EDTA, 1 mM DTT,
protease inhibitor cocktail (cat. no. S8820-2TAB; Sigma-Aldrich;
Merck KGaA) for 20 min on ice. The lysis solution was centrifuged
at 25,000 × g for 10 min at 4°C and protein contents in the
supernatant were measured using a DC protein assay kit (cat. no.
500-0119; Bio-Rad, Laboratories, Inc., Hercules, CA, USA). Proteins
(25 µg) were separated using 12% SDS-PAGE and transferred to
polyvinylidene difluoride membranes. Membranes were then blocked
with 5% skimmed milk dissolved in Tris-buffered saline (TBS; 2 mM
Tris-HCl, 140 mM NaCl; pH 7.6) with Tween-20 for 2 h at room
temperature. Following five washes (each, 5 min), membranes were
incubated with corresponding antibodies against cytochrome c
(cat. no. sc-65396), bax (cat. no. sc-7480), bcl-2 (cat. no.
sc-7382), caspase-3 (cat. no. 9661), caspase 9 (cat. no. 7237) and
tubulin (cat. no. sc-8035; each 1:200) at 4°C overnight. Following
five washes with TBS with Tween-20, membranes were incubated with
corresponding horseradish peroxidase-conjugated secondary
antibodies obtained from Santa Cruz Biotechnology, Inc. (1:5,000)
for 50 min at room temperature. The bands were visualized using the
ECL chemiluminescence system (Bioshine ChemiQ 4800 mini; Hangzhou
Yaxu Biotechnology Co., Ltd., Shanghai of China) and subjected to
densitometric analysis using Image-J2 software (National Institutes
of Health, Bethesda, MD, USA) to semi-quantify the protein
expression of TTB2 treated groups against the control group
(tubulin).
Statistical analysis
All data were presented as mean ± standard
deviation. Data were processed with the SPSS 19.0 for Windows
software package (IBM Corp., Armonk, NY, USA). One-way analysis of
variance was used for statistical evaluations. Multiple comparisons
between the groups were performed using Newman-Keuls test.
P<0.05 was considered to indicate a significant difference.
Results
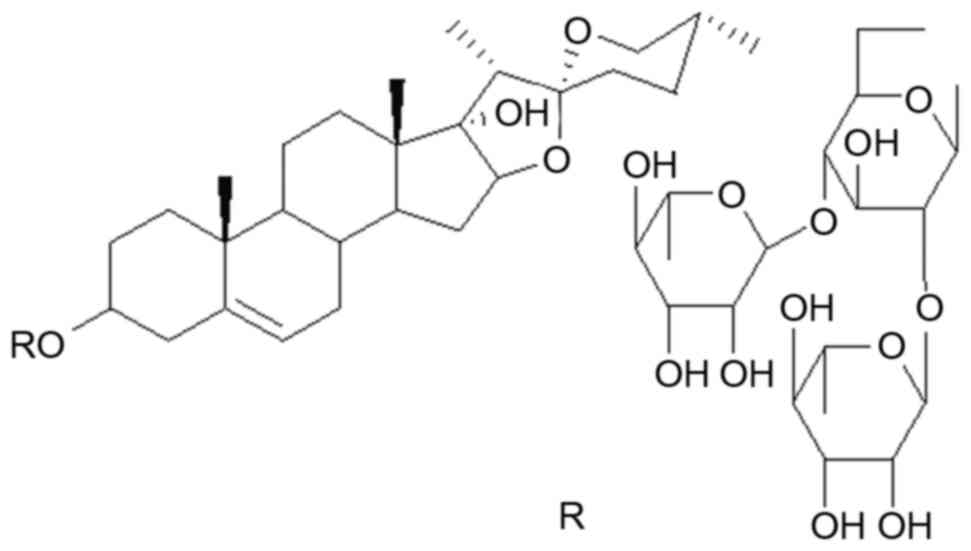
Chemical structure of TTB2
The structure of TTB2is presented in Fig. 1. Its full chemical name is pennogenin
3-O-α-L-rhamnopyranosyl-(1→2)
[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside.
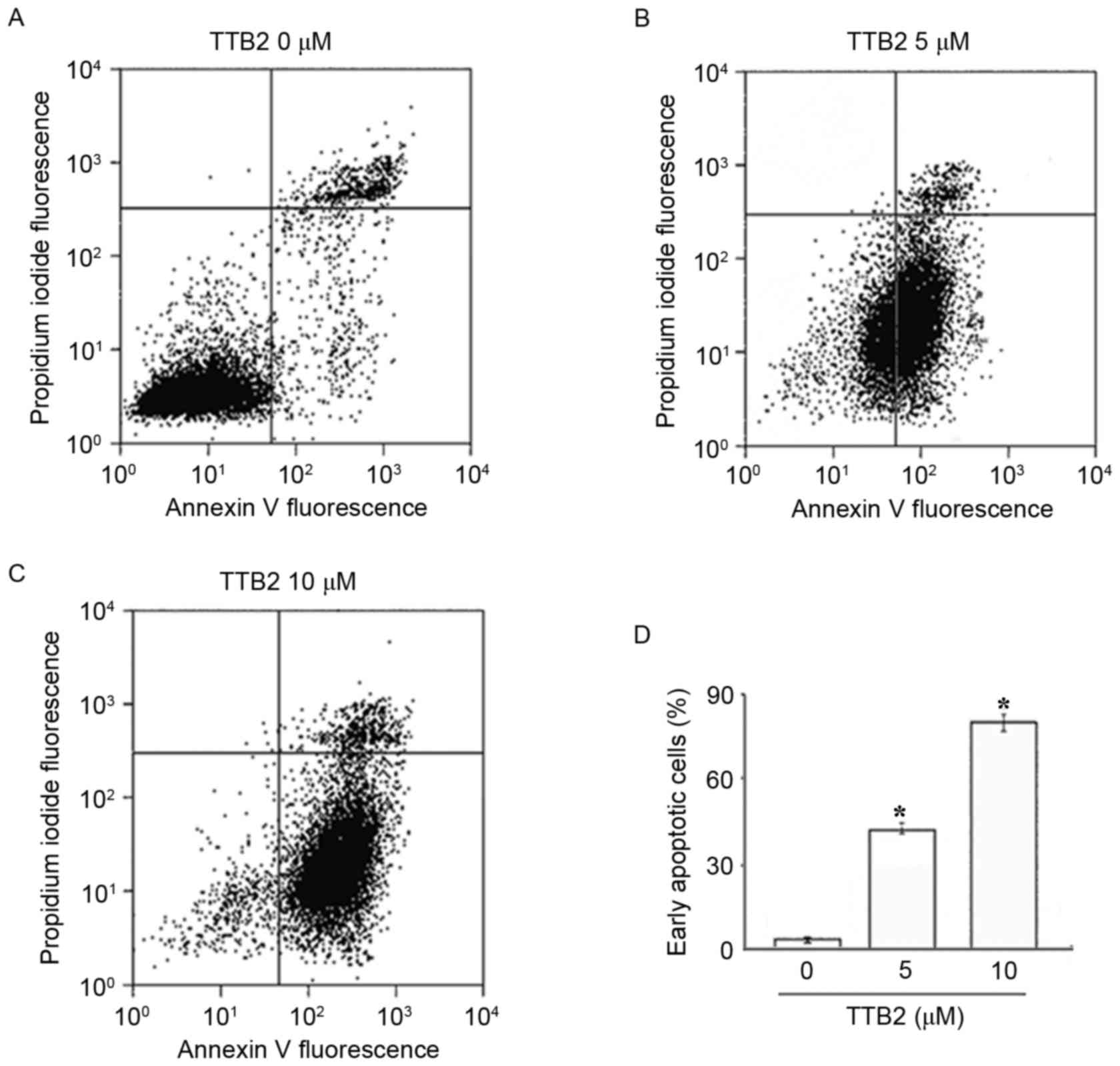
TTB2 induces apoptosis in Rh1
cells
To identify the nature of cell death, the early
marker of apoptosis Annexin V and the dead cell marker propidium
iodide were used to evaluate the cytotoxic effects of TTB2 on Rh1
cells. As presented in Fig. 2,
following treatment with different concentrations of TTB2, the
numbers of early apoptotic and late apoptotic/necrotic cells were
determined. Treatment with 5 and 10 µM TTB2 for 24 h resulted in
45.48 and 82.65% of Rh1 cells undergoing early apoptosis,
respectively compared with 2.44% of cells in the control (Fig. 2A-C). These results indicate that the
antitumor action of TTB2 is associated with the induction of potent
apoptosis in a dose-dependent manner (Fig. 2D).
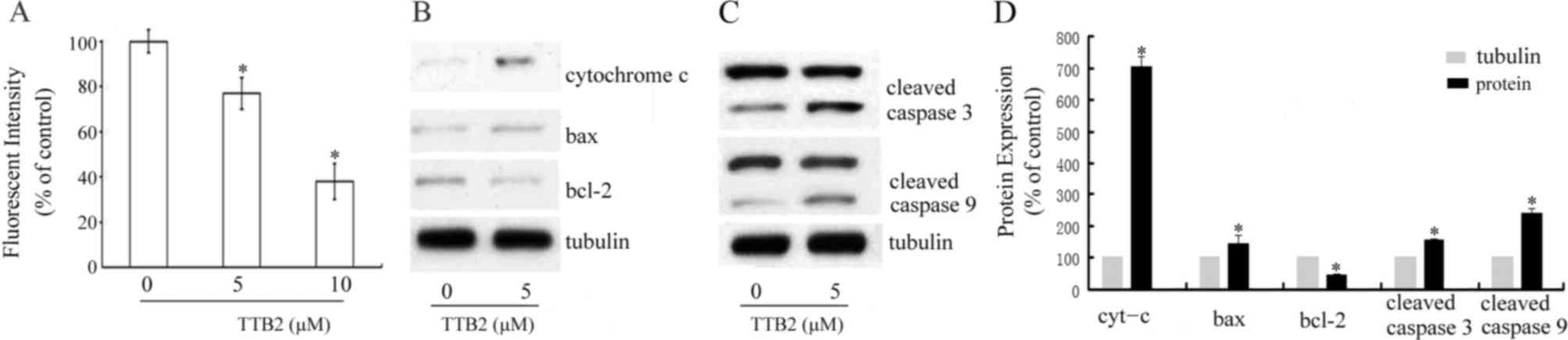
TTB2 treatment affects mitochondrial
function
To determine whether changes in the MMP were
implicated in the apoptosis induced by TTB2, cells were stained
with CMX-Ros and analyzed using flow cytometry. Fig. 3 demonstrates that, compared with the
control, treatment with 5 and 10 µM TTB2 significantly decreased
the MMP in Rh1 cells (P<0.05; Fig.
3A). Additionally, the release of cytochrome c following
TTB2 treatment was evaluated using western blotting. The results
demonstrated that there was a significant increase in cytochrome
c levels following treatment with 5 µM TTB2 (P<0.05;
Fig. 3B and D). To determine the
molecular mechanisms of apoptosis induced by TTB2, the levels of
the anti-apoptotic protein bcl-2 and the pro-apoptotic protein bax
were measured. Levels of bax significantly increased and levels of
bcl-2 significantly decreased following treatment with TTB2 (both
P<0.05; Fig. 3D). These results
indicate that TTB2 induces apoptosis in Rh1 cells via the intrinsic
mitochondrial pathway.
TTB2 treatment affects the levels of
apoptosis proteins
Western blotting was used to determine whether TTB2
treatment altered the levels of the cleaved apoptotic proteins
caspases-3 and −9. As presented in Fig.
3C and D, it was determined that TTB2 treatment activated
caspases-3 and −9. Compared with controls, TTB2 treatment
significantly increased levels of cleaved caspases-3 and −9
(P<0.05; Fig. 3D).
Discussion
Cancer is a type of disease with poor patient
prognosis. The incidence of most types of cancer continues to
increase annually and the mortality rates generally remain high
(13). Most tumors develop drug
resistant following exposure to drug over a prolonged period of
time (14,15). Thus, it's imperative to develop novel
medicines to reduce the morbidity and mortality of cancer.
Steroidal saponins have been isolated from many types of medicinal
plants. They have numerous pharmacological functions and exhibit
biological activities, including cytotoxic, antifungal,
enzyme-inhibitory, anti-inflammatory and antithrombotic activity
(16–18). Compound TTB2, pennogenin
3-O-α-L-rhamnopyranosyl-(1→2)
[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside, is a steroidal
saponin extracted from Trillium kamtschaticum (9) and Paris polyphylla var.
Yunnanensis (19). However,
there have been few studies investigating its bioactivity (9). It has been demonstrated that some
pennogenin steroid analogues from other plants exerted different
bioactivity (19). For example,
pennogenin
3-O-α-L-rhamnopyranosyl-(1→2)[α-L-arabinofuranosyl-(1→4)]-β-D-glucopyranoside
from Paris polyphylla var. yunnanensis protects the stomach
from damage induced by ethanol and indomethacin (20). Furthermore, certain analogues
including pennogenin
3-O-alpha-l-rhamnopyranosyl-(1->2)-[alpha-l-rhamnopyranosyl-(1->3)]-[6-O-acetyl]-beta-d-glucopyranoside
from Dracaena (21),
pennogenin
3-O-R-L-rhamnopyranosyl-(1→2)-[R-L-rhamnopyranosyl-(1→3)]-β-D-glucopyranosidefrom
D. deisteliana (20) and
pennogenin 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside from
Paris vietnamensis (22) were
induce cytotoxicityin cancer cells. And we ever reported that TTB2
from the rhizomes of Trillium tschonoskii Maxim has the
cytotoxicity on the Ewing sarcoma cell line and attenuate the cells
in the G2/M and S phases (12).
When cells undergo apoptosis, phosphatidyl serine
shifts from the inner side of the plasma membrane to its outer
leaflet (23). Under this
circumstance phosphatidyl serine is able to bind to Annexin V
(24) and this property means that
it is relatively easy to investigate apoptosis in all cells. The
current study demonstrated the TTB2 potently induced apoptosis in
Rh1 cells in a dose-dependent manner. Indeed, to the best of our
knowledge, the current study is the first indicate that TTB2, a
pennogenin steroid isolated from TrilliumtschonoskiiMaxim,
could induce cancer cell line apoptosis. Mitochondrial dysfunction
may trigger intrinsic pathway of apoptosis and is associated with
numerous cell processes (25,26). It
has been reported that some stimuli such as radiation and toxins
may stimulate the opening of mitochondrial permeability transition
pores which then increase the permeability of the mitochondrial
inner membrane, leading to disruption of ionic homeostasis, the
collapse of MMP and cytochrome c release (27). Furthermore, procaspase-9 and released
cytochrome c may stimulate production of the apoptosome that
drives the activation of caspase-3 and eventually leads to the
apoptosis (28). When cells are
exposed to pathogens or mild inflammatory factors, the
pro-apoptotic member bax shifts from the cytosol to mitochondrial
outer membrane, which breaks the mitochondrial stability and
facilitates the mitochondrial permeability transition and release
of cytochrome c (29). And
anti-apoptotic protein bcl-2 is primarily located on mitochondrial
outer membranes where it takes part in blocking membrane
permeabilization (30). The results
of the current study indicate that these molecular mechanisms
involved in TTB2-treated Rh1 cells. Western blot analysis
demonstrated that treatment with TTB2 provoked a marked decrease in
the expression of bcl-2 and an increase in bax expression in
TTB2-treated cells. Additionally, it was determined that TTB2
elevated the level of cytosolic cytochrome c, which serves a
critical role in the formation of apoptosome as well as the caspase
activation cascades that ultimately activates caspase-3. Combined
with the results of previous study, which indicated that the
TTB2inhibits the phosphorylation of extracellular signal-regulated
kinase and promotes cell cycle arrest (12), the present study determined the
procedures that have been closely implicated in the apoptosis of
Rh1 cells caused by TTB2.
In conclusion, the results of the current study
identified that the effects of TTB2 on Rh1 cells rely
onTTB2-induced apoptosis, which is closely associated with
mitochondrial signaling pathways. The current study suggested that
TTB2 isolated from Trillium tschonoskii Maxim may be
developed as novel anti-cancer drugs in the future.
Acknowledgements
Not applicable.
Funding
This study was financially supported by the National
Natural Science Foundation of China (grant nos. 31070313 and
21272136), Hubei Province Natural Science Foundation (grant no.
2016CFB444) and Yichang Science and Technology Research and
Development Project (grant no. A201230234) and CTGU Talent
Scientific Research Initial Foundation (grant no. KJ2012B063).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ and WH designed and completed the experiments,
and analyzed the results together. WH was a major contributor in
writing the manuscript. Both authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pistritto G, Trisciuoqlio Ceci C, Garufi A
and D'Orazi G: Apoptosis as anticancer mechanism: Function and
dysfunction of its modulators and targeted therapeutic strategies.
Age (Albany NY). 8:603–619. 2016.
|
|
2
|
Millimouno FM, Dong J, Yang L, Li J and Li
X: Targeting apotosis pathway in cancer and perspectives with
natural compounds from mother nature. Cancer Prev Res (Phila).
7:1081–1107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang LJ and Chen Y: New targets for the
antitumor activity of gambogic acid in hematologic malignancies.
Acta Pharmacol Sin. 34:191–198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fu L: Plant Red Book of China: Rare
threatened plant. Science Publishing House Press; Beijing; 1992
|
|
5
|
Li Q, Xiao M, Guo L, Wang L, Tang L, Xu Y,
Yan F and Chen F: Genetic diversity and genetic structure of an
endangered species, Trilliumtschonoskii. Biochem Genet. 43:445–458.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hayes PY, Lehmann R, Penman K, Kitching W
and de Voss JJ: Steroidal saponins from the roots of
Trilliumerectum (Beth root). Phytochemistry. 70:105–113. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yokosuka A and Mimaki Y: Steroidal
glycosides from the underground parts of Trilliumerectum and their
cytotoxic activity. Phytochemistry. 69:2724–2730. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ono M, Sugita F, Shigematsu S, Takamura C,
Yoshimitsu H, Miyashita H, Ikeda T and Nohara T: Three new steroid
glycosides from the underground parts of Trilliumkamtschaticum.
Chem Pharm Bull (Tokyo). 55:1093–1096. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nohara T, Miyahara K and Kawasaki T:
Steroid saponins and sapogenins of underground parts of
Trilliumkamtschaticum Pall II. Pennogenin- and Kryptogenin
3-O-Glycosides and related compounds. Chem Pharm Bull (Tokyo).
23:872–885. 1975. View Article : Google Scholar
|
|
10
|
Nohara T, Kumamoto F, Miyahara K and
Kawasaki T: Steroid saponins of aerial parts of Paris tetraphylla
A. Gray and of underground parts of Trillium tschonoskii Maxim.
Chem Pharm Bull (Tokyo). 23:1158–1160. 1975. View Article : Google Scholar
|
|
11
|
Zhao W, Gao W, Wei J, Wang Y, Huang L and
Xiao PG: Steroid Saponins and other constituents from the rhizome
of Trillium tschonoskii Maxim and their cytotoxic activity. Latin
Am J Pharmacy. 30:1702–1708. 2011.
|
|
12
|
Huang W and Zou K: Cytotoxicity of the
saponin TTB2 on Ewing sarcoma cells. Exp Ther Med. 10:625–628.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kohler BA, Sherman RL, Howlader N, Jemal
A, Ryerson AB, Henry KA, Boscoe FP, Cronin KA, Lake A, Noone AM, et
al: Annual report to the nation on the status of cancer, 1975–2011,
featuring incidence of breast cancer subtypes by race/ethnicity,
poverty, and state. J Natl Cancer Inst. 107:djv0482015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Turke AB, Zejnullahu K, Wu YL, Song Y,
Dias-Santagata D, Lifshits E, Toschi L, Rogers A, Mok T, Sequist L,
et al: Preexistence and clonal selection of MET amplification in
EGFR mutant NSCLC. Cancer Cell. 17:77–88. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Su KY, Chen HY, Li KC, Kuo ML, Yang JC,
Chan WK, Ho BC, Chang GC, Shih JY, Yu SL and Yang PC: Pretreatment
epidermal growth factor receptor (EGFK) T790M mutation predicts
shorter EGFR tyrosine Kinase inhibitor response duration in
patients with non-small-cell lung cancer. J Clin Oncol. 30:433–440.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Güçlü-Ustündağ O and Mazza G: Saponins:
Properties, applications and processing. Crit Rev Food Sci Nutr.
47:231–258. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang SL, Liu XK, Wu H, Wang HB and Qing C:
Steroidal saponins and cytoxicity of the wild edible
vegetable-Smilacina atropurpurea. Steroids. 74:7–12. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nordin N, Majid NA, Mohan S, Dehghan F,
Karimian H, Rahman MA, Ali HM and Hashim NM: Cleistopholine
isolated from Enicosanthellum pulchrum exhibits apoptogenic
properties in human ovarian cancer cells. Phytomedicine.
23:406–416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen CX, Zhou J, Zhang YT and Zhao Y:
Steroid saponins of aerial parts of paris polyphylla var.
Yunnanensis. Acta Bot Yunnan. 12:323–329. 1990.
|
|
20
|
Matsuda H, Pongpiriyadacha Y, Morikawa T,
Kishi A, Kataoka S and Yoshikawa M: Protective effects of steroid
saponins from Paris polyphylla var. yunnanensis on ethanol- or
indomethacin-induced gastric mucosal lesions in rats: Structural
requirement for activity and mode of action. Bioorg Med Chem Lett.
13:1101–1106. 2003.
|
|
21
|
Kougan GB, Miyamoto T, Tanaka C, Paululat
T, Mirjolet JF, Duchamp O, Sondengam BL and Lacaille-Dubois MA:
Steroidal saponins from two species of Dracaena. J Nat Prod.
73:1266–1270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang Y, Cui LJ, Wang Q and Ye WC:
Separation and identification of active constituents of Paris
vietnamensis. Yao Xue Xue Bao. 41:361–364. 2006.(In Chinese).
PubMed/NCBI
|
|
23
|
Martínez MC and Freyssinet JM: Deciphering
the plasma membrane hallmarks of apoptotic cells:
Phosphatidylserine transverse redistribution and calcium entry. BMC
Cell Biol. 2:202001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Demchenko AP: The change of cellular
membranes on apoptosis: Fluorescence detection. Exp Oncol.
34:263–268. 2012.PubMed/NCBI
|
|
25
|
Galluzzi L, Kepp O and Kroemer G:
Mitochondria: Master regulators of danger signaling. Nat Rev Mol
Cell Biol. 13:780–788. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Galluzzi L, Kepp O, Trojel-Hansen C and
Kroemer G: Mitochondria control of cellular life, stress, and
death. Circ Res. 111:1198–1207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zamzami N, Larochette N and Kroemer G:
Mitochondrial permeability transition in apoptosis and necrosis.
Cell Death Differ. 12(Suppl 2): S1478–S1480. 2005. View Article : Google Scholar
|
|
28
|
Brentnall M, Rodriguez-Menocal L, de
Guevara RL, Cepero E and Boise LH: Caspase-9, caspase-3 and
caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell
Biol. 14:322013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morales-Cano D, Calviño E, Rubio V,
Herráez A, Sancho P, Tejedor MC and Diez JC: Apoptosis induced by
paclitaxel via Bcl-2, Bax and caspases 3 and 9 activation in NB4
human leukaemia cells is not modulated by ERK inhibition. Exp
Toxicol Pathol. 65:1101–1108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hardwick JM and Soane L: Multiple
functions of BCL-2 family proteins. Cold Spring Harb Perspect Biol.
5(pii): a0087222013.PubMed/NCBI
|