Introduction
Epilepsy is common among people of any age, region
and race. There are various manifestations of epilepsy, and status
epilepticus (SE) can occur in all kinds of epilepsy. Apoptosis is a
major way of neuron loss after SE (1). Some studies have confirmed that
epileptic seizures could cause damage of hippocampal neurons, and
this damage might be one of the most important factors in the
reconstruction of loop during recurrent seizures (2). The phosphatidyl inositol 3-kinase
(PI3K)/Akt pathway may be an important signal transduction pathway
for epileptic seizures. Bcl-2 and Bad belong to anti-apoptotic and
pro-apoptotic proteins respectively, which play important roles in
the mitochondrial apoptosis pathway. Recent studies have found that
erythropoietin (Epo) is not merely a hematopoietic factor in a
conventional way, but rather an important factor with multiple
functions. The results of many animal models with nervous system
damage have shown that Epo may reduce brain damage and improve
nervous system function by reducing apoptosis and promoting
survival (3–7). However, the anti-apoptotic mechanism of
Epo in animal models of SE is unclear. In this study, we used
pentylenetetrazol (PTZ) to induce epileptic seizures in rats to
establish the SE model. The effects of Epo on neuronal apoptosis
after epileptic seizures induced by PTZ, and on the expression of
mitochondrial-apoptosis-pathway-related regulator Bcl-2 and Bad
were observed, as well as whether the PI3K/Akt pathway was an
important approach of neuroprotective effect of Epo to reduce
apoptosis and promote survival.
Materials and methods
Experimental animals
A group of healthy adult rats (all male, 3–4 months)
of Sprague-Dawley (SD) strain from Hebei Experimental Animal Center
were selected. The range of initial weight was 220±20 g. Attempts
were made to minimize the number of animals used. Rats were
normally fed with food and water and group-housed in plastic cages
on a standard 12/12 h ligh/dark cycle. The temperature was
maintained within a normal range.
Main experimental reagents and
equipments
Dimethyl sulphoxide (DMSO), LY294002 and PTZ
(Sigma-Aldrich, St. Louis, MO, USA), recombinant human
erythropoietin (rHuEpo) (North China Pharmaceutical Group Jintan
Co., Ltd., Shijiazhuang, China), mouse monoclonal anti-rat
phospho-Thr308-Akt, rat Bcl-2, Bad polyclonal antibodies (cat. nos.
sc-377457, sc-56015, sc-8044; all from Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), in situ apoptosis detection kit
(Roche Diagnostics, Indianapolis, IN, USA), 7314F
electroencephalograph (Neurofax; Nihon Kohden Corporation, Tokyo,
Japan), SR-6N stereotaxic apparatus (Narishige, Tokyo, Japan),
microscopes (Olympus Corporation, Tokyo, Japan), pathology image
analysis software (Beijing University of Aeronautics and
Astronautics Image Center, Beijing, China), RT-PCR reverse
transcription and amplification reagents (Promega Corporation,
Madison, WI, USA), primers (Sangon Biotech Co., Ltd., Shanghai,
China), PCR instrument (Eppendorf, Hamburg, Germany), gel scanning
analysis system (Syngene Europe, Cambrige, UK).
SE model preparation and grouping
A total of 197 rats were used to prepare SE model
based on the accepted approach and dosage: at first, PTZ 20 mg/kg
was given by intraperitoneal injection. Then 10 mg/kg PTZ was
injected each time at the interval of 10 min until generalized
epileptic seizures and/or SE occurred. The performance of rats
after administration was evaluated according to Racine' scale.
Grade 0, no abnormal performance; grade I, facial muscle twitch
and/or rhythmic mastication; grade II, regular nodding or wet dog
shakes (WDS); grade III, clonus of a single forelimb; grade IV,
clonus of both forelimbs with standing posture; grade V, sudden
fall and/or generalized tonic-clonic convulsion. When grade IV or V
appeared, it was considered as a generalized seizure, and regarded
as SE when the duration of onset was 30 min or more. In order to
ensure experiment scale, rats of midway-death or unsuccessful
models were removed and randomly supplemented. The final 125
successful models were randomly divided into 5 groups: normal
control group [normal saline (NS)], model group (PTZ + NS), rHuEpo
intervention group (PTZ + rHuEpo), LY294002 intervention group (PTZ
+ rHuEpo + LY294002) and LY294002 control group (PTZ + rHuEpo +
DMSO), with 25 rats in each group.
Drug administration
Model group: An intraperitoneal injection (i.p.) of
2 ml of 0.9% sodium chloride solution at 30 min after SE was
induced with PTZ. rHuEpo intervention group: an intraperitoneal
injection of rHuEpo 5,000 U/kg at 30 min after SE was induced with
PTZ. LY294002 intervention group (with DMSO as solvent, LY294002
concentration at 10 µg/5 µl): an intraventricular injection of 5 µl
of LY294002 at 10 min after SE was induced with PTZ, followed by an
intraperitoneal injection of rHuEpo 5,000 U/kg at 30 min after SE.
LY294002 control group: an intraventricular injection of 5 µl of
DMSO at 10 min after SE was induced with PTZ, followed by an
intraperitoneal injection of rHuEpo 5,000 U/kg at 30 min after SE.
Normal control group: an intraperitoneal injection of 0.9% sodium
chloride solution of the same volume.
Stereotaxic localization of lateral
ventricle and administration in rats
The Rat Brain in Stereotaxic Coordinates was used as
the standard to locate the injection site (0.8 mm backward the
anterior fontanelle, 1.5 mm aside the sagittal slit and 3.8 mm
under the dura mater). First, SE was induced with PTZ. After 10
min, the LY294002 intervention group was injected with 5 µl of
LY294002, while the LY294002 control group was injected with 5 µl
of DMSO. The needles were not drawn until 5 min after injection,
while the behavior of the rat was observed.
Behavioral observation and
electroencephalogram (EEG) of rats
Continuous behavioral observation lasted for 2 h
after the drug administration. Whether there was seizure onset,
manifestation and duration were recorded in detail.
EEG: Five rats in each group were randomly selected
for EEG recording. An intraperitoneal injection of 4% chloral
hydrate (35 mg/kg) was given for anaesthesia, and the rat skull was
carefully exposed after fixed to the cephalostat. Eight leads were
connected by 4 electrodes on the left and right hippocampus and
cortex. The position of cortical electrode: 2 mm forward the
anterior fontanelle, 2 mm aside to the midline and 2 mm in depth.
The position of hippocampal electrode: 3.8 mm backward the anterior
fontanelle, 2.4 mm aside to the middle line and 3.8 mm in depth.
The electrodes were placed and fixed after drilling, with the
reference electrode placed at the tip of the rat's nose. EEG
machine parameters were set as follows: paper speed of 30 mm/sec,
sensitivity of 20 µV/mm, wave filtering of 15 Hz, time constant of
0.1. EEG was recorded after the rat awoke from anesthesia.
Observation of hippocampal histology
Specimen preparation
At 24 h after onset of SE, excessive anesthesia was
applied. After exposure of the heart, the aorta was intubated from
the left ventricle, and the opening of the right atrial appendage
made the perfusion fluid flow out smoothly. After connected to the
perfusion system, NS and 4% paraformaldehyde were injected
successively, each in the volume of 500 ml. Then the brain tissue
was quickly taken out after craniotomy and vertically transected at
the optic chiasma and mammillary bodies. The middle-segment brain
tissue containing hippocampus and intraventricular injection site
was fully soaked in the solution of sufficient polyformaldehyde for
24 h at 4°C followed by dehydration and embedding. The tissue was
continuously cut into slices all the way to the hippocampal level
at the thickness of 5 µm from coronal position. The sections were
prepared for TUNEL and immunohistochemical staining.
Histopathological observation of
hippocampus
i) TUNEL staining by TUNEL kit. After the sections
were deparaffinized and rehydrated, the proteinase K (which does
not contain deoxyribonuclease) was added for incubation. After full
washing with PBS, the conversion agent POD was added, followed by
the steps of color rendering, redyeing and covering. All the
specimens were processed into 3 sections according to the methods
above. In each of the hippocampal sections, 10 non-overlapping
fields were selected by random, and the number of cells with dyed
and visible nuclei was counted. The average value of 30 counts of
each sample was taken as final result, and the average counts of
samples in each group were obtained.
ii) Immunohistochemical staining: the
Streptavidin-Perosidase method was employed. After deparaffinized
and hydrated, the sections were washed with PBS 3 times, and 3%
H2O2 was used for quenching endogenous
peroxidase activity. Normal low-lethal serum was used as a blocking
buffer after PBS rinsing again. Mouse monoclonal
anti-phospho-Thr308-Akt antibody (diluted 1:200), anti-Bcl-2
antibody (diluted 1:200), anti-Bad antibody (diluted 1:150) were
added and placed at 4°C overnight, and biotinylated goat
anti-rabbit IgG was added at room temperature after PBS flushing.
Incubation, color rendering, redyeing and covering were
sequentially carried out. PBS was used to replace Bcl-2 and Bad
polyclonal antibodies in the control group. The observation target
was the expression of Bcl-2 and Bad in hippocampal tissues. All the
specimens were processed into 3 sections according to the method
above. In each of the hippocampal sections, 10 non-overlapping
fields were selected randomly, and the number of cells with dyed
and visible nuclei was counted. The average value of 30 counts of
each sample was taken as final result, and the average count of
samples in each group was obtained.
RT-PCR detection
Ten rats were randomly selected from each group and
the hippocampus was quickly taken out after death. The total RNA of
each rat's hippocampus was extracted according to the instruction
of TRIzol reagent, and the purity and concentration were measured
by ELISA microplate reader. A total of 2 µg of each sample was used
for reverse transcription into its complementary DNA, and 1 µg of
the complementary DNA was used as substrate for PCR amplification.
The primers were customized at Sangon Biotech Co., Ltd. A total of
5 µl of each reverse transcriptase PCR (RT-PCR) amplification
product was observed after electrophoresis on 2% agarose gel, and
the density of each band was analyzed on the gel image analysis
system to obtain semi-quantitative results. The statistical
analysis was carried out after 5 repeated average calculations. The
relative content of the product was exhibited by the ratio of
Bad/β-actin and Bcl-2/β-actin.
Western blotting
In each group, 10 experimental rats were randomly
selected. After rapid sacrifice, the hippocampal tissues were
rapidly removed. The total protein was extracted and the
concentration was determined (BCA method). After the protein were
denatured, electrically transferred and sealed, mouse monoclonal
anti-phospho-Thr308-Akt antibody (1:300), anti-Bcl-2 antibody
(1:200) and anti-Bad antibody (1:150) were added into 50 µg of
protein and placed at 4°C overnight. After TPBS bleaching, the
rabbit anti-mouse Igg monoclonal antibody (1:3,000; cat. no. 58802;
Cell Signaling Technology, Danvers, MA, USA) was added. After
incubation for 1 h, the target was rinsed with TPBS and PBS,
respectively. The target strip and the β-actin strip were scanned
and the unit density was measured. The ratio of the two was
calculated and analyzed statistically, then repeated 5 times to
calculate the mean value and carry out final statistical
analysis.
Statistical analysis
All the data collected in the experiment are
expressed as the mean ± standard deviation. The data were analyzed
by SPSS 21 software (IBM Corp., Armonk, NY, USA). One-way ANOVA
with Student-Newman-Keuls post hoc test was used for multiple
comparisons among the groups. P<0.05 was considered to indicate
a statistically significant difference.
Results
Behavioral changes in rats
A total of 197 rats were injected with PTZ. The
minimum dose and quantity distribution of convulsion were 30 mg/kg
(75), 40 mg/kg (77), and 50 mg/kg (45). The main manifestations of
convulsion are rhythmic head retraction and myoclonus. The
injection dose of PTZ increased rapidly with the time gradient,
rapidly triggering the twitching of the limbs of the rats. The
self-regulation ability of the body posture was quickly lost in the
stage of generalized tonic-clonic seizures. Severe contraction of
the extensors in the anterior and hind limbs of the rats were
observed and respiratory depression appeared. Hyperemia of eyeball
and blue around the lips were also observed. Within a few minutes,
generalized tonic-clonic convulsion occurred with the SE duration
of 30 min or more. Approximately 35% rats died during the process,
and 3 unsuccessful modeling rats were removed and randomly
supplemented. No behavioral changes were found in the normal
control group.
Changes of EEG
The EEG of the model group showed a large number of
paroxysmal high amplitude spike waves, sharp waves, sharp
slow/spike slow complex waves. The frequency and/or amplitude of
epileptic seizures in the rHuEpo intervention the LY294002
intervention and the LY294002 control groups were significantly
lower than those in the model group, which indicated the epileptic
discharge was significantly inhibited. Compared with the LY294002
intervention group, epileptic discharges in the rHuEpo intervention
group and the LY294002 control group decreased significantly.
TUNEL staining results (Table I)
Apoptotic cells are TUNEL-positive cells featured
with brown yellow nuclei under the light microscope. There was
significant difference for TUNEL-positive cells (F4, 45=19.19,
P<0.05) among the five groups. In the normal control group,
there were few apoptotic cells in brain tissue slices, while in the
other groups, apoptotic cells were distributed in hippocampal areas
to a different degree. The number of apoptotic cells in the normal
control group was significantly less than that in the other 4
groups (P<0.05). The frequency of apoptotic cells in the slices
of the model group was significantly increased, with the nuclei
fixed in a circular or irregular shape. Under high power
microscope, chromatin staining, aggregation, fragmentation and
other phenomena can be observed, which is consistent with the
morphological characteristics of apoptotic cells. The number of
apoptotic cells in the rHuEpo intervention, the LY294002
intervention and the LY294002 control groups was significantly less
than that in the model group (P<0.05). The number of apoptotic
cells in the rHuEpo intervention group and the LY294002 control
group was significantly less than that in the LY294002 intervention
group (P<0.05). The number of apoptotic cells in the LY294002
control group was more than that in the rHuEpo intervention group,
but there was no statistical difference (P>0.05).
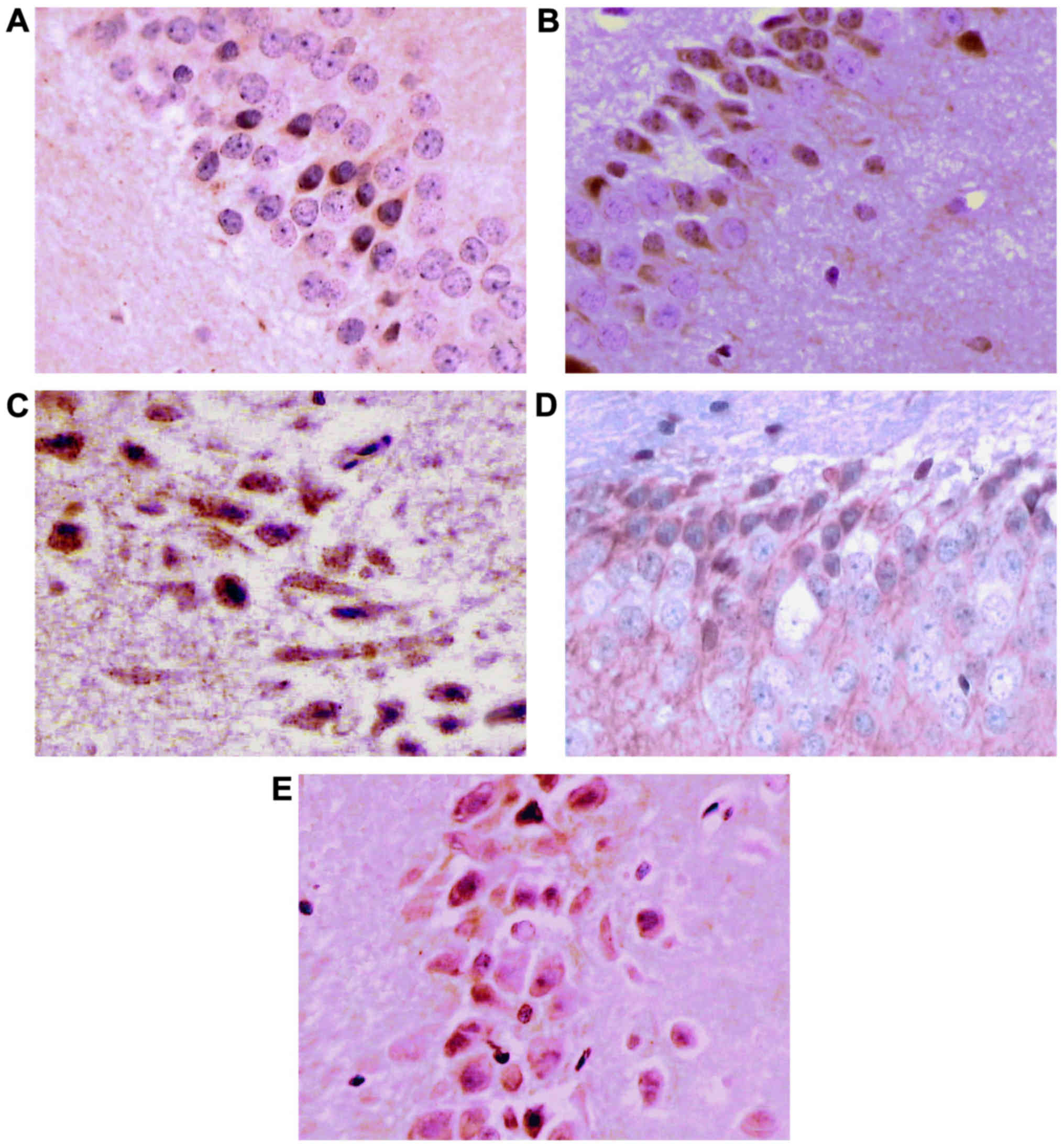
Immunohistochemical staining
results
The expression of p-Akt, Bcl-2 and Bad in
hippocampus (Tables I–III; Figs.
1 and 2).
 | Table III.The count of Bad positive cells, Bad
mRNA, Bad protein of rats hippocampus area in each group (mean ±
standard deviation, n=10). |
Table III.
The count of Bad positive cells, Bad
mRNA, Bad protein of rats hippocampus area in each group (mean ±
standard deviation, n=10).
| Groups | Bad-positive
cells | Bad mRNA | Bad |
|---|
| Control group | 16.931±1.158 | 0.350±0.041 | 0.291±0.033 |
| Model group |
43.804±4.386a |
0.728±0.076a |
0.668±0.045a |
| rHuEpo treated
group |
24.060±0.807a–c |
0.493±0.013a–c |
0.408±0.017a–c |
| LY294002 treated
group |
35.424±2.290a,b |
0.608±0.014a,b |
0.542±0.021a,b |
| LY294002 control
group |
24.308±0.677a–c |
0.495±0.013a–c |
0.454±0.026a–c |
| F-value | 21.00 | 13.12 | 22.07 |
| P-value | <0.05 | <0.05 | <0.05 |
Brown yellow is positive for staining, mainly
located in cytoplasm, with a small amount in nuclei and membranes.
There was significant difference for the number of p-Akt-IR cells
(F4, 45=28.77, P<0.05), Bcl-2-IR cells (F4, 45=19.27, P<0.05)
and Bad-IR cells (F4, 45=21.00, P<0.05) among the five groups.
In the cytoplasm of the normal control group, there were
occasionally scattered brown yellow particles in the cytoplasm.
Compared with the normal control group, the number of p-Akt-,
Bcl-2- and Bad-positive cells in the other 4 groups showed a
significant increase, showing statistical difference (P<0.05).
The number of p-Akt- and Bcl-2-positive cells in the rHuEpo
intervention group, the LY294002 intervention group and the
LY294002 control group was significantly more than that in the
model group, while the number of Bad-positive cells was
significantly less than that in the model group, showing
statistical difference (P<0.05). Compared with the LY294002
intervention group, the number of p-Akt- and Bcl-2-positive cells
in the rHuEpo intervention group and the LY294002 control group was
more, but the number of Bad-positive cells was less, showing
statistical difference (P<0.05). Compared with the LY294002
control group, the number of p-Akt and Bcl-2-positive cells in the
rHuEpo intervention group was more, but the number of Bad-positive
cells was less, showing no statistical difference (P>0.05).
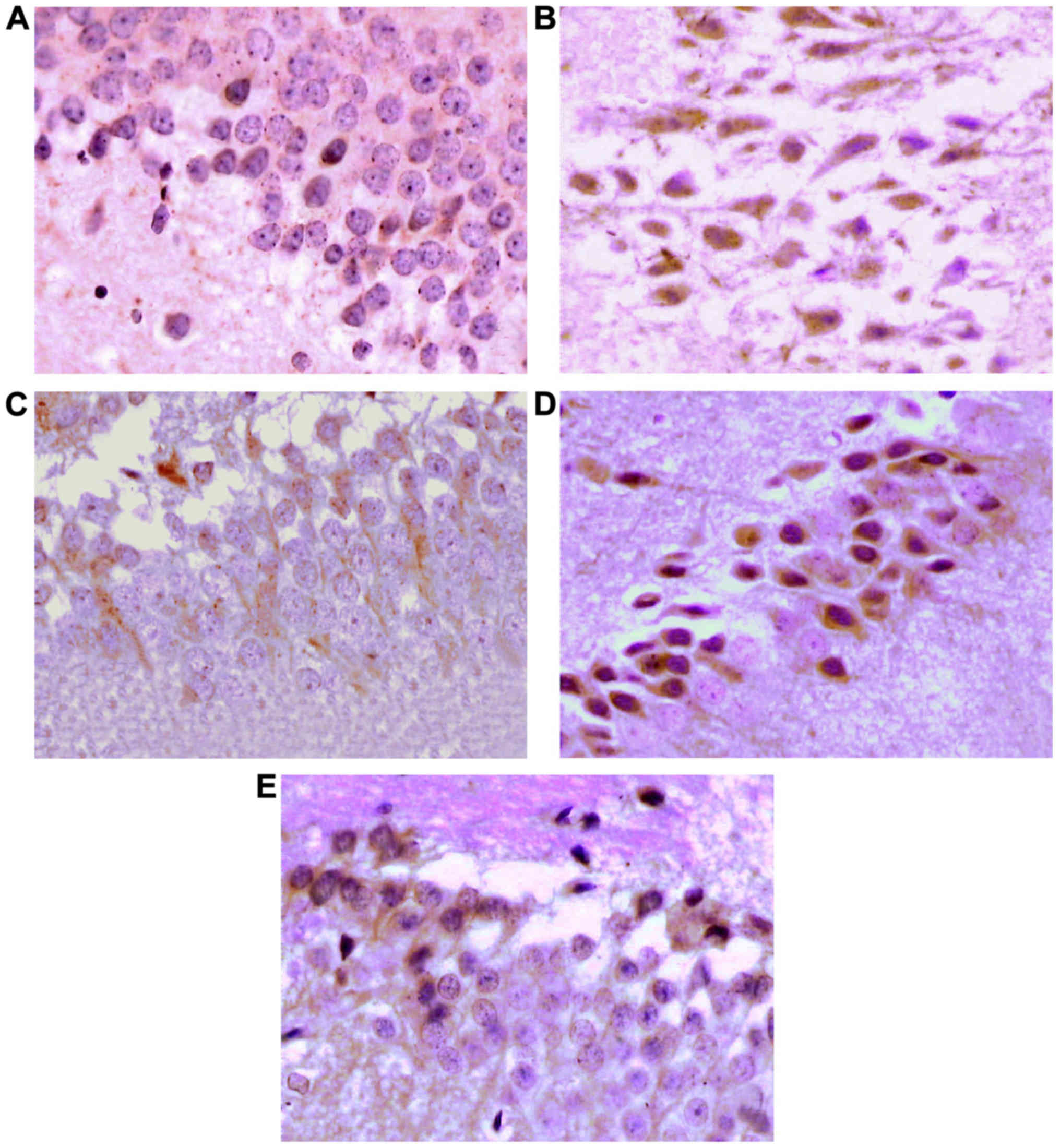
RT-PCR to determine the expression of
Bcl-2 mRNA and Bad mRNA (Tables II
and III; Figs. 3–5)
There was significant difference for Bcl-2 mRNA (F4,
45=27.95, P<0.05) and Bad mRNA (F4, 45=13.12, P<0.05) among
the five groups. There were weak expressions of Bcl-2 mRNA and Bad
mRNA in the control group. Compared with the control group, the
expression of Bcl-2 mRNA and Bad mRNA in the model, the rHuEpo
intervention, the LY294002 intervention and the LY294002 control
groups all increased significantly (P<0.05). The expression of
Bad mRNA in the model group was significantly higher than that in
the rHuEpo intervention, the LY294002 intervention and the LY294002
control groups, and the expression of Bcl-2 mRNA was significantly
decreased (P<0.05). Compared with the LY294002 intervention
group, the expression of Bad mRNA in the rHuEpo intervention group
and LY294002 control group decreased significantly, and the
expression of Bcl-2 mRNA increased significantly (P<0.05).
Compared with the LY294002 control group, the expression of Bad
mRNA in the rHuEpo intervention group decreased and the expression
of Bcl-2 mRNA increased, showing no statistical difference
(P>0.05).
Western blotting to determine the
expression of Akt, p-Akt, Bcl-2 and Bad (Tables I–III; Figs.
4–6)
The expression of Akt and p-Akt, Bcl-2 and Bad
protein in the hippocampus of rats was detected by western
blotting. There was significant difference for p-Akt (F4, 45=15.70,
P<0.01), Bcl-2 (F4, 45=18.07, P<0.05) and Bad (F4, 45=22.07,
P<0.05) among the five groups, while no significant difference
was detected for total AKT between each experimental groups (F4,
45=0.02, P>0.05). The results showed that compared with the
model group, rHuEpo intervention group, LY294002 intervention group
and LY294002 control group, the expression of p-Akt, Bcl-2 and Bad
in the control group increased significantly (P<0.05). Compared
with the rHuEpo intervention group, the LY294002 intervention group
and the LY294002 control group, the expression of p-Akt and Bcl-2
in the PTZ group was obviously decreased, and the expression of Bad
was significantly increased (P<0.05). Compared with the LY294002
intervention group, the expression of p-Akt and Bcl-2 in the rHuEpo
intervention group and the LY294002 control group increased
significantly, and the expression of Bad protein decreased
significantly, showing statistical difference (P<0.05). Although
compared with rHuEpo intervention group, the expression of p-Akt
and Bcl-2 in the LY294002 control group decreased, and the
expression of Bad increased, there was no statistical difference
(P>0.05). The intervention of rHuEpo, LY294002 and DMSO had no
significant effect on the expression of Akt, and there was no
statistical difference in the level of expression among groups
(P>0.05).
Discussion
The persistent state of epilepsy is an emergency of
the neurology department. If it is not treated in time, secondary
to brain edema, brain hernia, respiratory and circulation system
failure can cause persistent brain damage and cognitive impairment,
and the mortality rate is ~10-12% (8). In this study, it was used to improve
the concentration of extracellular K+, depolarize the
cell membrane and improve the excitatory PTZ as epileptogenic
agent. There was no other neurotoxicity in PTZ itself. So it is a
scientific choice to use PTZ as an epileptogenic agent to study
neuron apoptosis damage attributed to SE. EEG and Racine grading
were used to test the success of the SE model. Previous study shows
that the damage of neurons after SE seizures is a mixed state of
apoptosis, necrosis and coexistence of both, in which apoptosis is
a very important damage form (9),
but the mechanism of its occurrence is not clear. Some studies have
observed that apoptotic neurons and apoptotic bodies appear in
different degrees in the hippocampus of epileptic rats, while the
activity of apoptosis-related factors enhanced, and the apoptotic
cells with positive TUNEL staining appear in large numbers. In this
study, the changes of apoptotic cells were observed by TUNEL
staining in the PTZ kindled SE model rats. The results showed that
a mass of epileptiform discharges were found in the EEG of the
model group, and portion of the hippocampal neurons were necrosis
and apoptosis, and the number of apoptotic cells increased more
than that in the normal control group.
Mitochondria-dependent pathways play an important
role in three of the main apoptotic-dependent pathways including
mitochondria, death receptors and endoplasmic reticulum. The main
sites and effective sites of the Bcl-2 family proteins are all on
the mitochondrial membrane. The Bcl-2 family is the key to the
integration of the signal to the cell mitochondria, which is called
‘the doorman of apoptosis’. The members of Bcl-2 can form
dipolymers, including homologous dipolymers or hetero-dipolymers,
and these dipolymers play a vital role in the dynamic balance of
cell survival and death, and then determine the fate of cells. The
role of pro-apoptotic protein Bad is closely related to Bcl-2 and
Bcl-xL. Through the concentration-dependent form, Bad replaces the
apoptotic protein Bax in the Bcl-2/Bax, Bcl-xL/Bax dipolymer, which
promotes the formation of a large amount of homologous dipolymers.
When the homologous dipolymers content >80%, induced by
apoptotic stimulation signal, programmed-death/apoptosis appears in
the cell. The ratio of Bax homologous dipolymers and
hetero-dipolymers determine the survival of neurons, and Bad takes
effect on promoting apoptosis by regulating this ratio. When the
content of Bcl-xL/Bax and Bcl-2/Bax dipolymers in the cell is ≥50%,
the cells can resist apoptosis. Therefore, Bcl-2 and Bad are very
important factors in Bcl-2 family. The changes of Bcl-2 and Bad
were observed by immunohistochemistry, reverse transcription-PCR
and western blotting in our study. The experimental results showed
that the expressions of Bcl-2 and Bad immunoreactive cells, Bcl-2
mRNA, Bad mRNA and Bcl-2, Bad protein in the model group were
significantly upregulated than the normal control group, which
indicating PTZ activated Bad and Bcl-2 protein and mRNA increased
significantly in the hippocampus neurons. The study of Henshall
et al (10) and Meller et
al (11) also found that Bcl-2
and Bad proteins may be activated and involved in neuronal
apoptosis, which is consistent with our experimental results.
The in-depth study of pathophysiology of SE and the
development of new effective therapeutic drugs are still difficult
but are hot topics in world medical community now and even in the
future. Cortex, hippocampus, astrocytes and brain capillary
endothelial cells in brain tissue contain Epo receptors and can
also produce endogenous Epo (12).
Endogenous Epo needs full synthesis of protein and RNA
transcription in neurons. Endogenous Epo production is inadequate
in acute brain injury. The supply of exogenous Epo can reduce
neuron damage. In this experiment, rHuEpo was used as a
neuroprotective agent for intraperitoneal injection in the half
hour after SE. The results showed that the intervention of rHuEpo
could significantly reduce the occurrence of epileptic discharge,
upregulate the expression of Bcl-2 protein and mRNA in hippocampal
neurons, downregulate the expression of apoptotic protein Bad and
mRNA, suggesting that rHuEpo can regulate the expression level of
these mitochondria apoptotic pathway related factors then reduce
the necrosis and apoptosis of hippocampal neurons and play a
neuroprotective role. Based on the above research, how rHuEpo
regulates Bcl-2 and Bad, and then achieves neuroprotective effect
against apoptosis becomes our research direction.
The PI3K/Akt signal transduction pathway is a
classic signal transduction pathway that has biological effect. It
plays a multiple role in regulating cell growth, proliferation,
differentiation and survival. PI3K/Akt signaling pathway is
widespread in the nervous system, which can promote the survival of
neurons through regulation and management of apoptosis and
autophagy. Li et al (13)
found that the improvement of ginseng protein in Alzheimer's
disease was also mediated by activation of PI3K/Akt signaling
pathway. Uzüm et al (14)
found that Epo given through the abdominal cavity 24 h before the
seizure would reduce the intensity of tonic clonic seizure and
prolong the latent period of epileptic seizure. The activation of
Akt induced by Epo is the key to exert its antiapoptotic effect.
Akt is a direct downstream substrate of PI3K. The expression of
p-Akt can infer the activation of PI3K/Akt pathway. In our study,
we observed the changes of p-Akt by immunohistochemistry and
western blotting to study the activation of PI3K/Akt pathway in SE.
The results showed that rHuEpo increased the level of p-Akt in
hippocampal neurons significantly, which suggests the
neuroprotective effect of rHuEpo was closely related to the
activation of PI3K/Akt pathway.
The role of PI3K/Akt pathway in promoting cell
survival also have a series of important biological effects by
regulating its downstream apoptosis-related proteins. Among them,
the regulation of antiapoptotic protein Bcl-2 and proapoptotic
protein Bad in Bcl-2 family plays a significant role in nervous
system diseases. After ischemic/anoxic stimulation, PI3K can be
activated to phosphorylate Akt. P-Akt phosphorylates Bad in Ser136
and combines with chaperone 14–3-3 to form a complex. Bad is
dissociated from heterogeneous dipolymer, which indirectly
increases the expression of Bcl-2 and weakens the damage of
apoptosis. Fu et al (15)
found that PI3K/Akt/Bad signaling pathway played a role in
apoptosis of PC12 cells induced by taxol. The expression level of
Bcl-2 is regulated by p-Akt, thus exerting neuroprotective effect
(16). The results of knockout Akt
mice showed that the level of p-Akt decreased, the expression of
Bcl-2 protein in cytoplasm was reduced, and the damage of neurons
was aggravated. The results of Miao et al (17) showed that the level of Bcl-2
expression was upregulated and the occurrence of apoptosis
decreased, which might be achieved by enhancing PI3K/AKT signal
transduction. Kong et al (18) found that the use of PI3K inhibitor
LY294002 could prevent the protective effect due to increased p-Akt
level. Xiao et al (19)
studies showed that the expression of interleukin 1β in the induced
neurons could upregulate the expression of p-Akt, and the length of
the neuron dendrites could rapidly grow, and the expression of
p-Akt was downregulated after the pretreatment of LY294002. In this
experiment, we also carried out a more in-depth study to further
observe whether rHuEpo regulates the mitochondrial apoptosis
pathway related regulatory factors Bcl-2 and Bad via the PI3K/Akt
pathway in the PTZ induced SE rat model, and offers
neuroprotection. We used the PI3K inhibitor LY294002 to block it.
The results showed that the protective effect of rHuEpo was
obviously weakened when LY294002 was conducted before rHuEpo
intervention. There was an increase in the occurrence of epileptic
discharge in the LY294002 group and the apoptosis of the
hippocampal neurons compared with that in the rHuEpo group, and the
results of the immunodeficiency histochemistry and western blotting
showed a decreased number of p-Akt- and Bcl-2-positive cells, and
the expression of Bcl-2 mRNA and protein were also lower. The
number of Bad-positive cells and the expression of Bad mRNA and
protein were upregulated. It is further suggested that rHuEpo
activates the important survival passageway PI3K/Akt pathway, which
is related to improving the activity of Akt, and regulates the
regulatory factors of Bcl-2 and Bad of mitochondrial apoptosis
pathway through the PI3K/Akt signal transduction pathway to exert
neuroprotective effect on antiapoptosis.
There are still some limitations in this study: the
observation of large sample quantities without multi time-points.
There are cross series between the signal paths and the
complexities, and the PI3K/Akt path is not the only signal path of
the Epo. How it works with the other channels to carry out ‘cross
talk’ of neuroprotection is not clear. Thus more in-depth studies
are required.
In conclusion, Epo directly or indirectly regulates
the regulatory factors of Bcl-2 and Bad of mitochondrial apoptosis
pathway through PI3K/Akt signaling pathway, participates in the
different stages of apoptosis, and then exerts neuroprotective
effect on antiapoptosis and pro-survival. As a neuroprotective
agent, Epo is widely used in the basic research of all kinds of
nervous system diseases, such as closed brain injury, neonatal
hypoxic encephalopathy and Alzheimer's disease. But the safe dose
and treatment time of neuroprotection of Epo are different. The
safety, dose dependence and time selectivity of Epo have attracted
much attention. The study of Epo and formylated Epo is also being
carried out to provide an important laboratory basis for the search
for effective and feasible neuroprotective agents for the treatment
of epilepsy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY and ZS were responsible for specimen collection
and preparation. XS, YZ, BL and SW contributed to TUNEL and
immunohistochemical staining. LJ, BZ, MZ and XF performed PCR. KY
and WW helped with western blotting. All authors read and approved
the final study.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Second Hospital of Hebei Medical University (Hebei, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang C, Xie N, Wang Y, Li Y, Ge X and Wang
M: Role of the mitochondrial calcium uniporter in rat hippocampal
neuronal death after pilocarpine-induced status epilepticus.
Neurochem Res. 40:1739–1746. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buckmaster PS, Wen X, Toyoda I, Gulland FM
and van Bonn W: Hippocampal neuropathology of domoic acid-induced
epilepsy in California sea lions (Zalophus californianus). J Comp
Neurol. 522:1691–1706. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wassink G, Davidson JO, Dhillon SK, Fraser
M, Galinsky R, Bennet L and Gunn AJ: Partial white and grey matter
protection with prolonged infusion of recombinant human
erythropoietin after asphyxia in preterm fetal sheep. J Cereb Blood
Flow Metab. 37:1080–1094. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang M, Yan W, Liu Y, Hu H, Sun Q, Chen X,
Zang W and Chen L: Erythropoietin ameliorates diabetes-associated
cognitive dysfunction in vitro and in vivo. Sci Rep. 7:28012017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vinberg M, Miskowiak K, Hoejman P,
Pedersen M and Kessing LV: The effect of recombinant erythropoietin
on plasma brain derived neurotrophic factor levels in patients with
affective disorders: A randomised controlled study. PLoS One.
10:e01276292015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bahçekapılı N, Akgün-Dar K, Albeniz I,
Kapucu A, Kandil A, Yağız O and Üzüm G: Erythropoietin pretreatment
suppresses seizures and prevents the increase in inflammatory
mediators during pentylenetetrazole-induced generalized seizures.
Int J Neurosci. 124:762–770. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ugurluer G, Cebi A, Mert H, Mert N, Serin
M and Erkal HS: Neuroprotective effects of erythropoietin against
oxidant injury following brain irradiation: An experimental study.
Arch Med Sci. 12:1348–1353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu J, Si Z, Li S, Huang Z, He Y, Zhang T
and Wang A: The calcineurin inhibitor FK506 prevents cognitive
impairment by inhibiting reactive astrogliosis in
pilocarpine-induced status epilepticus rats. Front Cell Neurosci.
11:4282018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mikati MA, Abi-Habib RJ, El Sabban ME,
Dbaibo GS, Kurdi RM, Kobeissi M, Farhat F and Asaad W: Hippocampal
programmed cell death after status epilepticus: Evidence for
NMDA-receptor and ceramide-mediated mechanisms. Epilepsia.
44:282–291. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Henshall DC, Araki T, Schindler CK, Lan
J-Q, Tiekoter KL, Taki W and Simon RP: Activation of
Bcl-2-associated death protein and counter-response of Akt within
cell populations during seizure-induced neuronal death. J Neurosci.
22:8458–8465. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meller R, Schindler CK, Chu XP, Xiong ZG,
Cameron JA, Simon RP and Henshall DC: Seizure-like activity leads
to the release of BAD from 14-3-3 protein and cell death in
hippocampal neurons in vitro. Cell Death Differ. 10:539–547. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Genc S, Koroglu TF and Genc K:
Erythropoietin as a novel neuroprotectant. Restor Neurol Neurosci.
22:105–119. 2004.PubMed/NCBI
|
|
13
|
Li H, Kang T, Qi B, Kong L, Jiao Y, Cao Y,
Zhang J and Yang J: Neuroprotective effects of ginseng protein on
PI3K/Akt signaling pathway in the hippocampus of D-galactose/AlCl3
inducing rats model of Alzheimer's disease. J Ethnopharmacol.
179:162–169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Uzüm G, Sarper Diler A, Bahçekapili N and
Ziya Ziylan Y: Erythropoietin prevents the increase in blood-brain
barrier permeability during pentylentetrazol induced seizures. Life
Sci. 78:2571–2576. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fu Z, Yang J, Wei Y and Li J: Effects of
piceatannol and pterostilbene against β-amyloid-induced apoptosis
on the PI3K/Akt/Bad signaling pathway in PC12 cells. Food Funct.
7:1014–1023. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang C, Wang Z, Zhang X, Zhang X, Dong L,
Xing Y, Li Y, Liu Z, Chen L, Qiao H, et al: Protection by silibinin
against experimental ischemic stroke: Up-regulated pAkt, pmTOR,
HIF-1α and Bcl-2, down-regulated Bax, NF-κB expression. Neurosci
Lett. 529:45–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miao J, Wang L, Zhang X, Zhu C, Cui L, Ji
H, Liu Y and Wang X: Protective effect of aliskiren in experimental
ischemic stroke: Up-regulated p-PI3K, p-AKT, Bcl-2 expression,
Attenuated Bax Expression. Neurochem Res. 41:2300–2310. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kong J, Ren G, Jia N, Wang Y, Zhang H,
Zhang W, Chen B and Cao Y: Effects of nicorandil in neuroprotective
activation of PI3K/AKT pathways in a cellular model of Alzheimer's
disease. Eur Neurol. 70:233–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiao Z, Peng J, Yang L, Kong H and Yin F:
Interleukin-1β plays a role in the pathogenesis of mesial temporal
lobe epilepsy through the PI3K/Akt/mTOR signaling pathway in
hippocampal neurons. J Neuroimmunol. 282:110–117. 2015. View Article : Google Scholar : PubMed/NCBI
|