Introduction
Mammalian oocytes are physiologically arrested in
the prophase of the first meiotic division. It is well established
that meiosis is regulated by the levels of cyclic adenosine
monophosphate (cAMP) within the oocyte (1,2). The
downstream pathway by which high cAMP levels prevent meiotic
maturation has remained to be fully elucidated. Numerous studies on
starfish, fish, amphibians and mammals support the hypothesis that
protein kinase A (PKA) has major roles in the maintenance of
meiotic arrest (3–5).
Although increases in cAMP and activation of PKA are
crucial for the induction and maintenance of meiotic arrest, the
precise targets and downstream effectors of cAMP signaling have not
been fully defined (1,6). Initially, all effects of cAMP were
attributed to the activation of PKA. The identification of a novel
class of cAMP-binding proteins termed guanine nucleotide exchange
factors (cAMP-GEFs) provided a means by which changes in cAMP yield
actions that are independent of PKA (7,8).
Recently, the importance of PKA-independent signaling pathways in
the regulation of oocyte maturation has been demonstrated (9,10).
Exchange protein directly activated by cAMP (Epac)
was discovered during a database search for proteins that may
explain for the insensitivity to cAMP-induced activation of the
small GTPase Ras-related protein-1 (Rap1) (7,8). To
date, two isoforms of Epac (Epac1 and Epac2) have been identified.
Epac1 and Epac2 exhibit distinct expression patterns in mature and
developing tissues. In particular, Epac1 mRNA is expressed
ubiquitously, whereas Epac2 mRNA is predominantly expressed in the
brain (8). Accordingly, the present
study focused on the expression pattern of Epac1. Epac is a
Rap1-specific cAMP-GEF and activates Rap1 directly as an effector
molecule of cAMP, with no involvement of PKA. The cAMP/Epac/Rap1
pathway has been demonstrated and implicated in numerous cell
systems (11–13). It is involved in a variety of
cellular processes, including secretion (14), cell adhesion (15), intercellular junction formation
(16), apoptosis (17), cell proliferation (18) and cell differentiation (19). The objectives of the present study
were to investigate whether a cAMP/Epac/Rap1 pathway exists in
oocyte maturation as a cAMP-dependent but PKA-independent
factor.
Materials and methods
Animals
Animal care and handling was performed in accordance
with the Animal Research Committee guidelines of the Institute of
Zoology, Chinese Academy of Sciences. Unless otherwise stated,
ovaries and ooctyes were obtained from a total of 20, 5-week-old
B6D2F1 strain mice (C57BL/6 × DBA/2J) weighing 18–20 g. C57BL/6
female mice (n=10) and DAB/2 male (n=5) mice were purchased from
Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China).
Animals were housed at a controlled temperature (22–24°C) and were
subjected to a 12-h light/dark cycle (lights on from 6:00
until-18:00). Animals received ad libitum access to food and
tap water.
Immunohistochemistry
Ovaries from 5-week-old B6D2F1 strain mice (C57BL/6
× DBA/2J) were fixed overnight in Bouin's solution and then
processed into paraffin wax blocks. Sections were cut at a
thickness of 5 µm and mounted on poly-l-lysine-coated glass slides.
Sections were de-paraffinized in xylene, rehydrated in a graded
ethanol series and washed in deionized water for 5 min. The
sections were next heated in citrate buffer (pH 9.0) for 30 min in
a water bath at 96°C. The samples were treated with 0.3% hydrogen
peroxide in absolute methanol for 10 min at room temperature (RT)
to inhibit endogenous peroxidase activity. Non-specific protein
binding was blocked by incubation with 3% normal donkey and goat
serum for Epac and Rap1, respectively, in PBS for 10 min at RT.
Working solutions of primary antibodies 91:100 dilution) for
anti-Epac1 (cat. no. sc-8879) and anti-Rap1 (cat. no. sc-65; both
from Santa Cruz Biotechnology, Inc., Dallas, TX, USA) were applied
to the sections overnight at 4°C. Subsequently, two
peroxidase-conjugated donkey anti-goat (cat. no. sc-2059) and goat
anti-rabbit immunoglobulin G (IgG) secondary antibodies (cat. no.
sc-2054; both Santa Cruz Biotechnology, Inc.), diluted 1:2,000 in
PBS containing 1.5% bovine serum albumin (BSA), were applied for 1
h at 37°C. Labeling was visualized using 3,3′-diaminobenzidine and
counterstained with hematoxylin at RT for 5 min. After each step,
the sections were rinsed with PBS. The control sections were
incubated with PBS only, without the primary antibody. The
experiments were performed at least three times for each antibody
staining.
Oocyte and embryo retrieval
All oocytes and zygotes were obtained from the
5-week-old B6D2F1 female mice (C57BL/6 females × DBA/2J males).
The culture medium was G1 medium (Vitrolife,
Göteborg, Sweden) containing 4 mg/ml BSA (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany). Oocytes were cultured in an incubator at
37°C with 6% CO2. Germinal vesicle (GV) oocytes were
isolated from ovaries into G1 medium containing dibutyryl cAMP (100
µg/ml; Sigma-Aldrich; Merck KGaA) to prevent resumption of meiosis.
Metaphase II (MII) stage oocytes were recovered from ampullae from
mice superovulated by intraperitoneal injection of 7.5 IU of
pregnant mare serum gonadotropin followed by 7.5 IU of human
chorionic gonatropin (hCG) 48 h later. Cumulus cells were removed
with 0.1 mg/ml hyaluronidase (Sigma-Aldrich; Merck KGaA) by
pipetting. To collect synchronized embryos, superovulated females
were caged 13 h after hCG injection with male mice for 1.5 h and
vaginal plugs were checked the next morning. Zygotes (1-cell
embryos) were collected from the oviducts 18–20 h after mating.
Early 2-cell, 4-cell and 8-cell embryos were collected at 44, 54
and 68 h after mating, respectively.
Immunofluorescene
Mouse oocytes were fixed with 2% formaldehyde for 15
min at 37°C and permeabilized with 0.02% Triton X-100 for 1 h at
37°C. To determine the expression of Epac and Rap1, the fixed
oocytes were incubated with goat anti-Epac1 (sc-8879; Santa Cruz
Biotechnology, Inc.) polyclonal antibodies and rabbit anti-Rap1
(sc-65; Santa Cruz Biotechnology, Inc.) polyclonal antibodies for 1
h at 37°C. After being washed with PBS, oocytes were transferred to
a solution with secondary antibodies: Cy3-conjugated anti-goat IgG
(1:500; cat. no. 705–165-003; Jackson ImmunoResearch Laboratoris,
Inc., West Grove, PA, USA) and Cy3-conjugated anti-rabbit IgG
(1:500; cat. no. 111-165-003; Jackson ImmunoResearch Laboratoris,
Inc.) for 1 h at 37°C. All of the secondary antibodies were
purchased from Jackson Laboratories (Bar Harbor, ME, USA). DAPI
(0.2 µg/ml, DA0001, Sigma-Aldrich) was used to stain the nuclei for
5 min at RT. Slides were examined using a laser-scanning confocal
microscope with a 63 × oil immersion objective lens and a Leica SP2
krypton-argon ion laser (Leica Microsystems, Wetzlar, Germany) for
the simultaneous excitation of fluorescence for proteins and DAPI
for DNA.
Reverse transcription polymerase chain
reaction (RT-PCR) analysis
mRNA was prepared from 100 MII-stage oocytes using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer's instructions.
Standard complentary DNA synthesis by reverse transcription of the
RNA was then performed using random primers and SuperScript™ III
RNase H-Reverse Transcriptase (Invitrogen; Thermo Fisher
Scientific, Inc.). Epac1 and Rap1a mRNA were detected by RT-PCR
with mRNA primer pairs using hot start Taq DNA polymerase (Takara
Bio Inc., Shiga, Japan). PCR was performed in 96-well plates with 2
l 10 × Taq Buffer, 10 mM deoxy-ribonucleoside triphosphate, 25 mM
MgCL2, primers at a final concentration of 0.2 µm and a
cDNA sample derived from 5 ng total RNA in a total volume of 20 µl.
Following 2 min incubation at 50°C followed by 10 min incubation at
95°C, the samples were subjected to 40 cycles of amplification
(95°C for 15 sec, 60°C for 1 min) and dissociation (95°C for 15
sec, 60°C for 1 min and 95°C for 15 sec). All the test reactions
were accompanied by negative controls and were performed with at
least two independent preparations. The PCR primers were
non-dimerizing under the conditions employed and were typically
intron-flanking. They were designed according to the mouse Epac1
mRNA sequence with the GenBank accession no. NM_144850.1: Sense,
5′-TCCCCTCCTGTCATCCCC-3′ and antisense, 5′-GCCATCATCCGCATCTTCTC-3′
[product length, 116 base pairs (bp)] and Rap1a mRNA sequence with
the accession no. NM_145541.4: Sense, 5′-TTCTGCAAAGTCAAAGATCAACG-3′
and antisense, 5′-TTTTAGGCTTCTTCTTTTCCACTG-3′ (product length, 95
bp). As a positive control, the mRNA for GAPDH was amplified using
the following primers (based on accession no. NM_001001303): Sense,
5′-TGACGTGCCGCCTGGAGAAA-3′ and antisense,
5′-AGTGTAGCCCAAGATGC-CCTTCAG-3′ (product length, 98 bp). The PCR
products were separated using 1.0% agarose gel electrophoresis,
ethidium bromide staining and UV transillumination followed by
capture with a charge-coupled device camera. Fragment sizes were
estimated using a 100 bp DNA ladder (M106R; Genscript Biotech
Corporation, Nanjing, China).
Results
Expression of Epac1 and Rap1a mRNA in
MII-stage oocytes
The expression of Epac1 and Rap1a mRNAs was detected
in the MII-stage oocytes using RT-PCR analysis. This result
demonstrated Epac1 and Rap1a mRNA is expressed in MII-stage mouse
oocytes (Fig. 1).
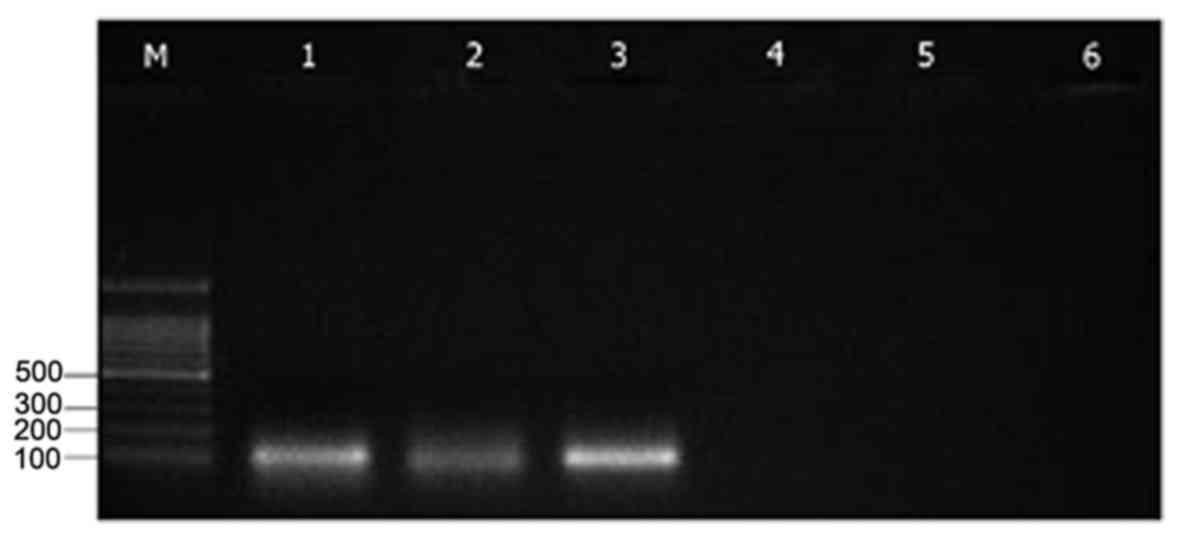 | Figure 1.Reverse transcription polymerase chain
reaction performed on 100 MII-stage mouse oocytes with primers
specific for Epac1 (product length, 116 bp), Rap1 (product length,
95 bp) and GAPDH (product length, 98 bp). Lanes: M, 100-bp DNA
ladder; 1–3, amplification products for Epac1, Rap1 and GAPDH,
respectively; 4–6, negative controls without reverse transcriptase,
RNA and complementary DNA in the reaction tube, respectively. Rap1,
Ras-related protein-1; Epac, exchange proteins directly activated
by cyclic adenosine monophosphate. |
Localization of Epac1 and Rap1a in the
adult mouse ovary
The existence of Epac1 in the human ovary has been
demonstrated by northern blotting (8). In addition, the cAMP/Epac1/Rap1a
pathway has been indicated to participate in the proliferation of
immature rat granulosa cells and the regulation of progesterone
secretion by luteinizing human granulose cells (20,21).
However, data regarding the specific expression patterns of these
two genes in the female germline are limited. To address this, the
sections of mouse ovaries were stained to analyze the distribution
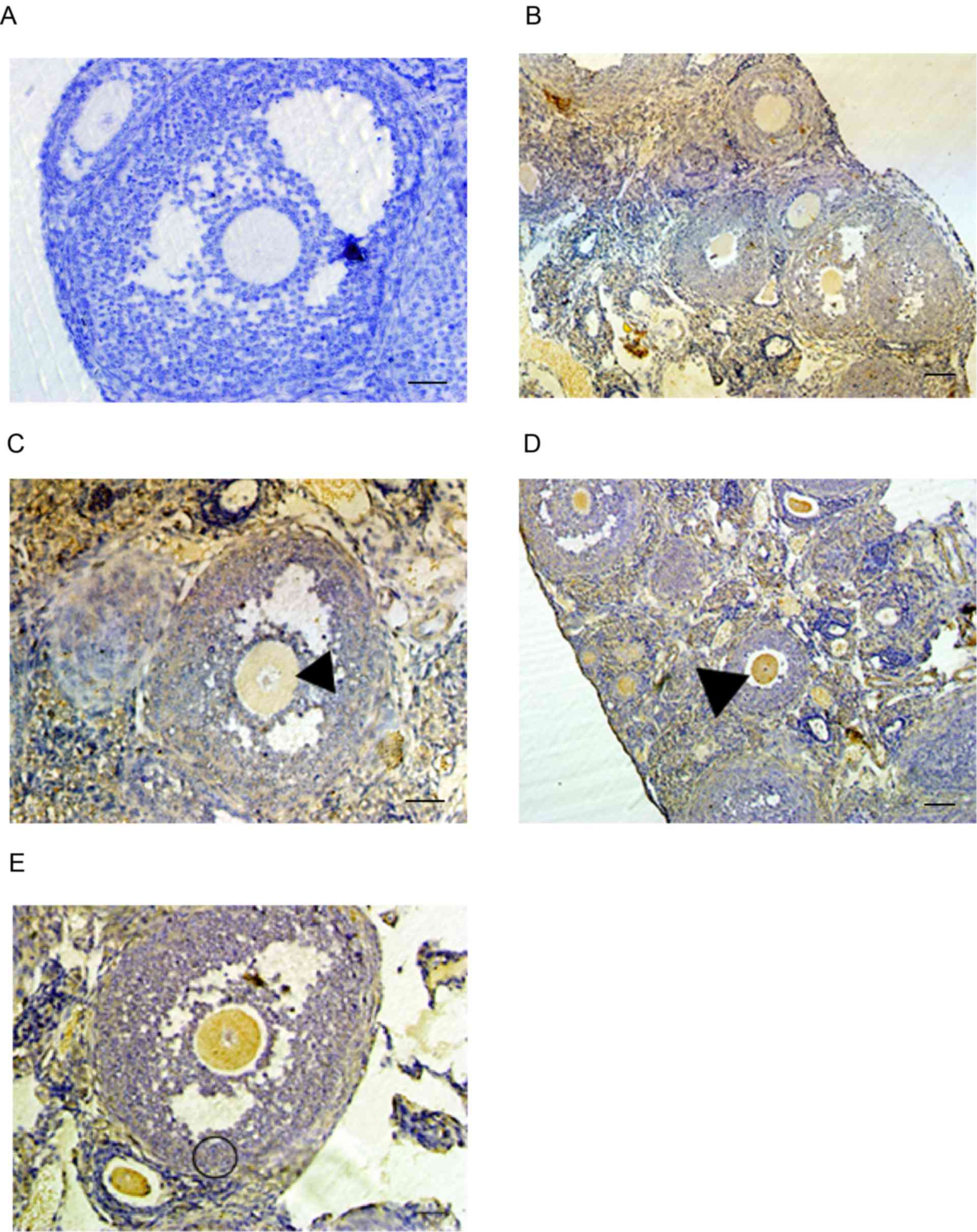
of Epac1 and Rap1a proteins throughout oogenesis. Immunostaining
indicated that the two proteins were expressed throughout the
entire ovary (Fig. 2B-E). The two
proteins were detected in either germ or follicle cell tissues
throughout the different stages of oocyte development, from
primordial follicles to Graafian follicles and were distributed
throughout the cytoplasm. Epac1 immunostaining in oocyte and
granulosa cells appeared uniform (Fig.
2B and C). By contrast, the immunostaining for Rap1a in oocytes
appeared stronger than that in granulosa cells (Fig. 2D and E).
Localization of Epac during meiotic
maturation of mouse oocytes
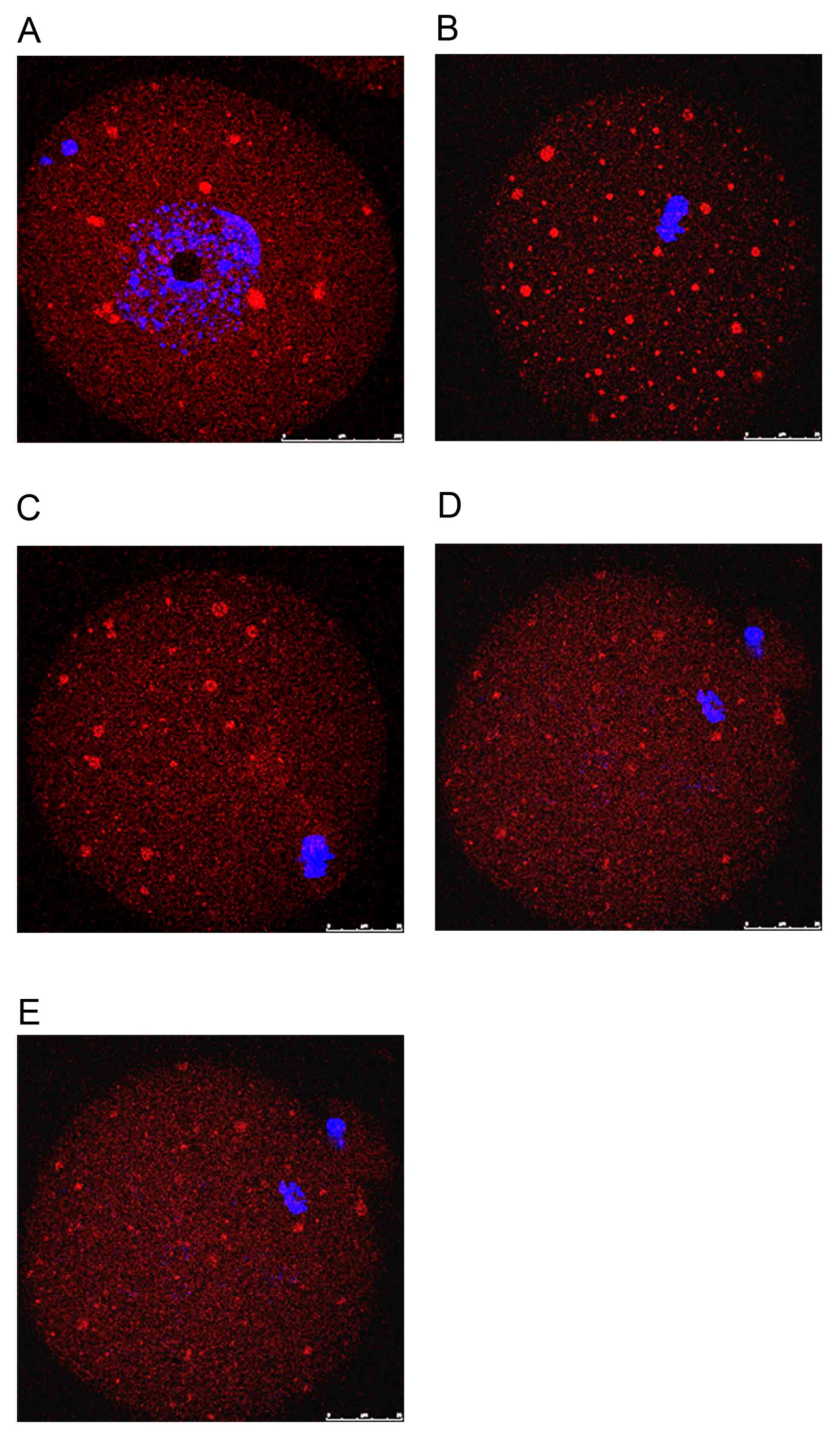
The present study examined the dynamic localization
of Epac1 in mouse oocytes using immunocytochemistry and confocal
microscopy. At the GV stage, Epac was first seen as small
accumulations scattered in the cytoplasm (Fig. 3A). After GV breakdown (GVBD), the
chromatin condensed towards the center of the oocyte. A network of
small clusters, distributed uniformly throughout the cytoplasm,
then became evident (Fig. 3B).
Following the migration of the condensed meiotic chromosomes to the
cortex of the oocyte, small clusters became polarized and
concentrated at opposite ends (Fig.
3C). However, at the MII stage, when the first polar body is
extruded and the MII spindle is already established, the
accumulations appeared to become reduced and spread out in the
ooplasm (Fig. 3D). When the MII
oocyte had been fertilized and developed into a one-cell stage
zygote, the network of clusters almost disappeared and Epac became
uniformly distributed throughout the embryo (Fig. 3E).
Differential expression of Rap1a
protein in oocytes and embryos
As a member of the Ras-like small GTPase
superfamily, Rap1 has been implicated in the regulation of a
variety of cellular processes. However, its role in oocyte
maturation and early embryonic development has remained to be
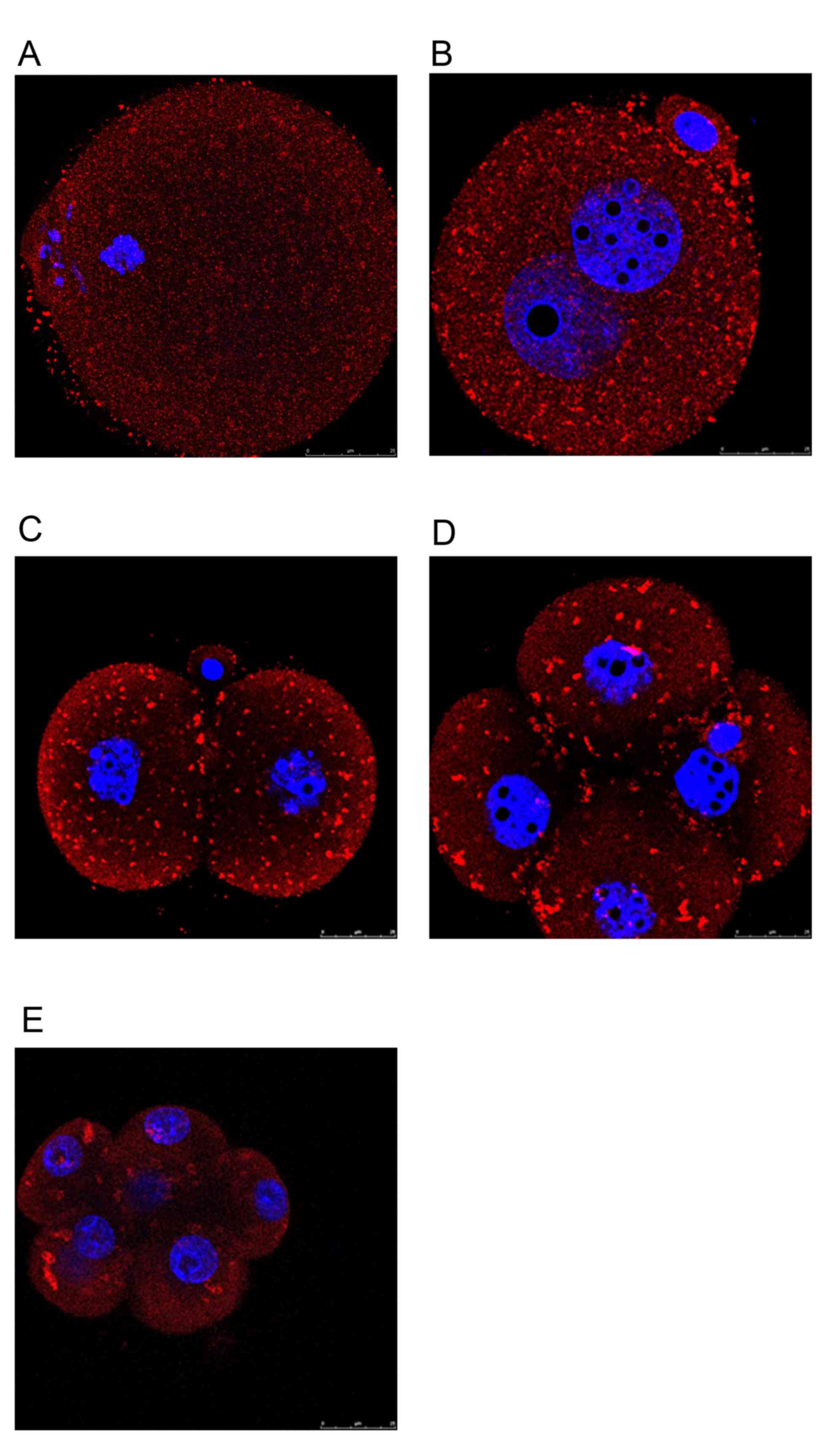
elucidated. Using immunocytochemistry, it was evidenced that Rap1a
expression patterns differed between mouse oocytes and early
preimplantation embryos. During different stages of oocyte
maturation, Rap1a was dispersed uniformly as small punctuations
throughout the cytoplasm (Fig. 4A).
When oocytes were fertilized and allowed to develop into 1-cell
zygotes, the small punctuations aggregated into visible clusters of
particles that mainly occupied the cortical region of blastomeres
(Fig. 4B). This phenomenon was
evident even in 2-cell and 4-cell stage embryos (Fig. 4C and D) and persisted in subsequent
early preimplantation embryos (Fig.
4E). In general, the localization of Rap1a is variable and
depends on the cell type (22,23). The
activation of Rap1a and the subsequent response are determined by
the local environment. In addition, the spatial and temporal
localization of Rap1 is associated with different cellular
functions (24). Therefore, it is
speculated that Rap1a may have a different function in oocyte
maturation from that in early embryonic development.
Discussion
The present study indicated a mutually exclusive
dynamic expression of Epac1 and Rap1a in oocytes and embryos. Epac1
has a unique distribution during oocyte maturation, which
disappeared upon entering the MII stages. Rap1a exhibited its
typical expression only beginning from pronuclear-stage embryos and
persisting in subsequent early preimplantation embryos (Fig. 4A-E). As the limitation of our
experimental research method, the 8-cell stage embryo showed in
Fig. 4E may appear the cell <8
for the embryo was squeezed on a glass slide. The different and
dynamic localizations of Epac1 and Rap1a are likely to be crucial
determinants of the functions of these proteins. It is anticipated
that, as with PKA, the multiple functions of this pathway may
depend on the localization of these two proteins (25).
It is thought that a high level of cAMP in the
oocyte maintains putative initiator proteins in a phosphorylated
(inactive) state, inhibiting oocyte maturation and that the
activation of initiator proteins by dephosphorylation via
mechanisms that remain elusive induces maturation-promoting factor
(MPF) activity (26,27). MPF is activated upon GVBD and
increases until it reaches a plateau at the end of the first
meiotic M-phase (28,29). MPF is rapidly reactivated to enter
meiosis II and is maintained at a high level during the MII phase
arrest. The discrete sets of steps through which cAMP activates or
inactivates MPF are still under investigation. There is a consensus
that PKA has major roles in this process. Thus, the change of cAMP
levels within the oocyte leads to the activation of MPF in a
PKA-dependent manner. However, the inhibitory role of cAMP on
meiosis contradicts the fact that maturation is stimulated by a
surge of the gonadotrophins, luteinizing hormone and follicle
stimulating hormone (30), both of
which stimulate cAMP production in the somatic cells of follicles
and increase the level of cAMP in oocytes via gap junctions
(31). Studies have attempted to
explain this apparent paradoxical action of cAMP concerning the
spatial localization of the downstream signaling proteins involving
the cAMP-PKA pathway (32). However,
it is also possible that PKA-independent pathways (e.g., the
cAMP/Epac/Rap1 pathway) are involved.
Epac has emerged as an important target of cAMP in a
variety of processes. The isomers contain a cAMP-binding domain
with significant sequence homology to the R subunits of PKA and a
GEF domain that functions to exchange GTP for GDP (7,8). Epac
was first demonstrated to be an exchange factor for the small
GTPase Rap1. GTP-bound Rap1 causes the activation and initiation of
a cascade of protein kinases of the mitogen-activated protein
kinase (MAPK) signal transduction pathway (21,33,34).
Numerous protein kinases and phosphatases have been suggested to
participate in the process of meiotic arrest and progression in
mice. The MAPK extracellular signal-regulated kinase 1/2 is one of
the protein kinases that may participate directly and indirectly in
the regulation of meiotic resumption of oocytes (35,36). The
MAPK pathway has a crucial role in the reactivation of MPF after
meiosis I and thereafter in the maintenance of MII arrest (37). It may be speculated that the
cAMP/Epac/Rap1 pathway regulates the inhibition and progression of
mouse oocyte maturation through activating the MAPK pathway. Of
course, further experiments should be performed to test this
hypothesis.
In most cases, the involvement of Epac compensates
for the role of PKA and it is frequently intrinsically linked to
the cAMP/PKA pathway (15). Dual
control involving PKA and Epac may enhance the dynamic range of
cAMP signaling (22). PKA activity
per se is not sufficient, and it is rather the complex cAMP
signaling pathways that regulate meiotic arrest and
progression.
In conclusion, the present study was the first, to
the best of our knowledge, to demonstrate that Epac and Rap1
display dynamic and characteristic expression patterns in mouse
oocytes and embryos. These results offer a basis for further
research to elucidate the function of the cAMP/Epac/Rap1 pathway in
oocytes and embryos.
Acknowledgements
Not applicable.
Funding
This research was funded by the Key Project of the
Health Industry in Tianjin (‘Functional Research of Cryopreserved
Human Ovary Tissue’, 2012–2015; grant no. 12ZK101). It was also
supported by a grant from the Tianjin Health and Family Planning
Commission (‘Research on miRNA/SIRT1 regulating oocyte apoptosis in
premature ovary failure’, 2015–2018; grant no. 15KG141).
Availability of data and materials
The analysed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
JW was involved in project development and wrote the
manuscript. YG made substantial contributions to the acquisition,
analysis and interpretation of data. YH was involved in data
collection and analysis. HL was involved in designing the
experiment and revising the manuscript, and agreed to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of any part of the work are
appropriately investigated and resolved. YZ was involved in the
design and execution of the study, as well as data analysis.
Ethical approval and consent to
participate
The study protocol was approved by the ethics
committee of the Center for Reproductive Medicine, Tianjin Central
Hospital of Obstetrics and Gynaecology (Tianjin, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Conti M, Andersen CB, Richard F, Mehats C,
Chun SY, Homer K, Jin C and Tsafriri A: Role of cyclic nucleotide
signaling in oocyte maturation. Mol Cell Endocrinol. 187:153–159.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eppig JJ, Vivieros MM, Marin-Bivens C and
de La Fuente R: 2004, The Ovary. Leung PCK: &. Adashi EY:
Elsevier, Academic Press; pp. 113–129, 2004. View Article : Google Scholar
|
|
3
|
Meijer L, Dostmann W, Genieser HG, Butt E
and Jastorff B: Starfish oocyte maturation: Evidence for a cyclic
AMP-dependent inhibitory pathway. Dev Biol. 133:58–66. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jalabert G, Fostier A, Breton B and Weil
Cin: Vertebrate Endocrinology: Fundamentals and Biomedical
Implications. (Pang PKT &. Schreibman MP: 4A. New York;
Academic Press; pp. 113–129. 1991
|
|
5
|
Chaube SK and Haider S: J Exp Zool.
277:166–170. 1997. View Article : Google Scholar
|
|
6
|
Josefberg LB and Dekel N: Translational
and post-translational modifications in meiosis of the mammalian
oocyte. Mol Cell Endocrinol. 187:161–171. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Rooij J, Zwartkruis FJ, Verheijen MH,
Cool RH, Nijman SM, Wittinghofer A and Bos JL: Epac is a Rap1
guanine-nucleotide-exchange factor directly activated by cyclic
AMP. Nature. 396:474–477. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kawasaki H, Springett GM, Mochizuki N,
Toki S, Nakay M, Matsuda M, Houseman DE and Graybiel AM: A family
of cAMP-binding proteins that directly activate Rap1. Science.
282:2275–2279. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schmitt A and Nebreda AR: Inhibition of
xenopus oocyte meiotic maturation by catalytically inactive protein
kinase a. Proc Natl Acad Sci USA. 99:4361–4366. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pace MC and Thomas P: Steroid-induced
oocyte maturation in Atlantic croaker (Micropogonias undulatus) is
dependent on activation of the phosphatidylinositol 3-kinase/Akt
signal transduction pathway. Biol Reprod. 73:988–996. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brennesvik EO, Ktori C, Ruzzin J, Jebens
E, Shepherd PR and Jensen J: Adrenaline potentiates
insulin-stimulated PKB activation via cAMP and Epac: Implications
for cross talk between insulin and adrenaline. Cell Signal.
17:1551–1559. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hucho TB, Dina OA and Levine JD: Epac
mediates a cAMP-to-PKC signaling in inflammatory pain: An isolectin
B4(+) neuron-specific mechanism. J Neurosci. 25:6119–6126. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Branham MT, Mayorga LS and Tomes CN:
Calcium-induced acrosomal exocytosis requires cAMP acting through a
protein kinase A-independent, Epac-mediated pathway. J Biol Chem.
281:8656–8666. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Robert S: Regulation of the amyloid
precursor protein ectodomain shedding by the 5-HT4 receptor and
Epac. FEBS Lett. 579:1136–1142. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rangarajan S, Enserink JM, Kuiperij HB, de
Rooij J, Price LS, Schwede F and Bos JL: Cyclic AMP induces
integrin-mediated cell adhesion through Epac and Rap1 upon
stimulation of the beta 2-adrenergic receptor. J Cell Biol.
160:487–493. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fukuhara S, Sakurai A, Sano H, Yamagishi
A, Somekawa S, Takakura N, Saito Y, Kangawa K and Mochizuki N:
Cyclic AMP potentiates vascular endothelial cadherin-mediated
cell-cell contact to enhance endothelial barrier function through
an Epac-Rap1 signaling pathway. Mol Cell Biol. 25:136–146. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kwon G, Pappan KL, Marshall CA, Schaffer
JE and McDaniel ML: cAMP Dose-dependently prevents
palmitate-induced apoptosis by both protein kinase A- and
cAMP-guanine nucleotide exchange factor-dependent pathways in
beta-cells. J Biol Chem. 279:8938–8945. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Misra UK and Pizzo SV: Coordinate
regulation of forskolin-induced cellular proliferation in
macrophages by protein kinase A/cAMP-response element-binding
protein (CREB) and Epac1-Rap1 signaling: Effects of silencing CREB
gene expression on Akt activation. J Biol Chem. 280:38276–38289.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bryn T, Mahic M, Enserink JM, Schwede F,
Aandahl EM and Tasken K: The cyclic AMP-Epac1-Rap1 pathway is
dissociated from regulation of effector functions in monocytes but
acquires immunoregulatory function in mature macrophages. J
Immunol. 176:7361–7370. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gonzalez-Robayna IJ, Falender AE, Ochsner
S, Firestone GL and Richards JS: Follicle-Stimulating hormone (FSH)
stimulates phosphorylation and activation of protein kinase B
(PKB/Akt) and serum and glucocorticoid-lnduced kinase (Sgk):
Evidence for A kinase-independent signaling by FSH in granulosa
cells. Mol Endocrinol. 14:1283–1300. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chin EC and Abayasekara DR: Progesterone
secretion by luteinizing human granulosa cells: A possible
cAMP-dependent but PKA-independent mechanism involved in its
regulation. J Endocrinol. 183:51–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Beranger F, Goud B, Tavitian A and de
Gunzburg J: Association of the Ras-antagonistic Rap1/Krev-1
proteins with the Golgi complex. Proc Natl Acad Sci. 88:1606–1610.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maridonneau-Parini I and de Gunzburg J:
Association of rap1 and rap2 proteins with the specific granules of
human neutrophils Translocation to the plasma membrane during cell
activation. J Biol Chem. 267:6396–6402. 1992.PubMed/NCBI
|
|
24
|
Zwartkruis FJ and Bos JL: Ras and Rap1:
Two highly related small GTPases with distinct function. Exp Cell
Res. 253:157–165. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dodge-Kafka KL, Soughayer J, Pare GC,
Carlisle Michel JJ, Langeberg LK, Kapiloff MS and Scott JD: The
protein kinase A anchoring protein mAKAP coordinates two integrated
cAMP effector pathways. Nature. 437:574–578. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dekel N: Protein
phosphorylation/dephosphorylation in the meiotic cell cycle of
mammalian oocytes. Rev Reprod. 1:82–88. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Duckworth BC, Weaver JS and Ruderman JV:
G2 arrest in Xenopus oocytes depends on phosphorylation of cdc25 by
protein kinase A. Proc Natl Acad Sci USA. 99:16794–16799. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Choi T, Aoki F, Mori M, Yamashita M,
Nagahama Y and Kohomoto K: Activation of p34cdc2 protein kinase
activity in meiotic and mitotic cell cycles in mouse oocytes and
embryos. Development. 113:789–795. 1991.PubMed/NCBI
|
|
29
|
Verlhac MH, Kubiak JZ, Clarke HJ and Maro
B: Microtubule and chromatin behavior follow MAP kinase activity
but not MPF activity during meiosis in mouse oocytes. Development.
120:1017–1025. 1994.PubMed/NCBI
|
|
30
|
Pincus G and Enzmann EV: The comparative
behavior of mammalian eggs in vivo and in vitro: I. the activation
of ovarian eggs. J Exp Med. 62:655–675. 1935.
|
|
31
|
Webb RJ, Marshall F, Swann K and Carroll
J: Follicle-stimulating hormone induces a gap junction-dependent
dynamic change in [cAMP] and protein kinase a in mammalian oocytes.
Dev Biol. 246:441–451. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Webb RJ, Tinworth L, Thomas GM, Zaccolo M
and Carroll J: Developmentally acquired PKA localisation in mouse
oocytes and embryos. Dev Biol. 317:36–45. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vossler MR, Yao H, York RD, Pan MG, Rim CS
and Stork PJ: cAMP activates MAP kinase and Elk-1 through a B-Raf-
and Rap1-dependent pathway. Cell. 89:73–82. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
York RD, Yao H, Dillon T, Ellig CL, Eckert
SP, McCleskey EW and Stork PJ: Rap1 mediates sustained MAP kinase
activation induced by nerve growth factor. Nature. 392:622–626.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun QY, Breitbart H and Schatten H: Role
of the MAPK cascade in mammalian germ cells. Reprod Fertil Dev.
11:443–450. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fan HY and Sun QY: Involvement of
mitogen-activated protein kinase cascade during oocyte maturation
and fertilization in mammals. Biol Reprod. 70:535–547. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Araki K, Naito K, Haraguchi S, Suzuki R,
Yokoyama M, Inoue M, Aizawa S, Toyoda Y and Sato E: Meiotic
abnormalities of c-mos knockout mouse oocytes: Activation after
first meiosis or entrance into third meiotic metaphase. Biol
Reprod. 55:1315–1324. 1996. View Article : Google Scholar : PubMed/NCBI
|