Introduction
Left ventricular hypertrophy is a common
complication, even in mild hypertension, and is detected in up to
36% of patients globally (1). Left
ventricular hypertrophy is generally regarded as an adaptive
response to chronic pressure overload. It is also a gateway to
pathogenesis and heart failure. Concentric hypertrophy is an early
remodeling event that often precedes myocardial dilatation that can
progress to eccentric hypertrophy and global pump failure.
Preventing and reversing the development of cardiac hypertrophy may
be an effective therapeutic strategy for the treatment of
hypertension with heart failure, resulting in an improved prognosis
(2).
The renin-angiotensin-aldosterone system (RAS),
especially angiotensin-converting enzyme 1 (ACE1) and angiotensin
II (Ang II), plays an important role in the pathophysiology of left
ventricular remodeling. Under physiological conditions, ACE1
expression in the heart is relatively low and mainly localized in
the endothelium of the coronary artery and myocardial endometrium
(3). The expression of ACE1 is
upregulated in models of heart damage that is induced by stress
overload, volume overload, myocardial infarction, and heart failure
(4). Under pathological conditions,
both cardiomyocytes and fibroblasts express ACE1 protein. Although
the RAS plays a supportive role in hemodynamics, sustained
activation of the RAS can lead to heart disease, including heart
deterioration and failure (5).
Angiotensin-converting enzyme inhibitors and angiotensin receptor
blockers are applied clinically to manage pathological cardiac
hypertrophy (6).
Naringenin (NGN) is a flavonoid that is found in
citrus fruits (e.g., grapefruit) and Chinese herbs (e.g., C.
aurantium) (7), and its
cardioprotective effect has been recently reported. Epidemiological
studies have found a correlation between higher flavonoid intake
from fruits and vegetables and a lower risk of developing
cardiovascular disease (8). NGN has
been shown to alleviate myocardial ischemia/reperfusion injury and
protect against doxorubicin-induced cardiac toxicity (9,10).
However, remaining unclear is whether NGN mitigates the development
of pressure overload-induced cardiac hypertrophy in its early stage
and delays the progression of heart failure.
In the present study, NG
-nitro-L-arginine methyl ester (L-NAME) was used to induce cardiac
hypertrophy in Sprague-Dawley rats. We sought to determine whether
NGN is able to exert a protective effect against left ventricular
hypertrophy. We also sought to identify the possible mechanisms
that underlie these effects. Additionally, we investigated whether
NGN influences activation of the RAS, which is overactivated by
pressure overload.
Materials and methods
Animal care and use
All of the studies followed the guidelines of the
Animal Care and Use Committee of Peking University. The animal
protocol was approved by the Animal Ethical and Welfare Committee
at China-Japan Friendship Hospital (Beijing, China). Male
Sprague-Dawley rats (160–180 g) were purchased from Beijing Vital
River Laboratory Animal Technology Company (Beijing, China) and
maintained in a specific pathogen-free facility under a 12/12 h
light/dark cycle with controlled room temperature and free access
to standard chow and water. Three groups of rats (n=10 per
group) received the following by gavage daily for 8 weeks: L-NAME
(50 mg/kg) + NGN (100 mg/kg), L-NAME (50 mg/kg) + saline, or saline
+ saline. L-NAME and NGN were purchased from Sigma-Aldrich; Merck
KGaA (Darmstadt, Germany).
Blood pressure measurement
Arterial systolic blood pressure and heart rate were
measured in conscious, restrained rats using a noninvasive
computerized tail-cuff system (Beijing Softron Biotechnology Co.,
Ltd, Beijing, China). Measurements were performed every week,
beginning 1 day before intragastric administration. Blood pressure
was calculated for each rat as the average of three separate
measurements in each session.
Echocardiography
Transthoracic echocardiography (Vevo 2100 image
system, FUJIFILM VisualSonics, Toronto, Canada) was performed
before the rats were sacrificed. Under anesthesia by an
intraperitoneal injection of 45 mg/kg sodium pentobarbital, the
rats were fixed on their backs. Chest fur was shaved, and the skin
was cleaned. The rats were examined on day 56 after drug
administration to monitor IVSth and LVPWth using a miniature M-mode
ultrasound probe.
Histology and
immunohistochemistry
Paraformaldehyde-fixed tissue was embedded in
paraffin and processed, and 6-µm sections were stained with
hematoxylin-eosin or Masson's Trichrome. Additional sections were
immunostained using an indirect horseradish peroxidase
immunoperoxidase method and specific antibody for the antigen of
ACE1 (Abcam, Cambridge, MA, USA). Negative controls for
immunostaining consisted of replacing each of the primary
antibodies with an equivalent concentration of irrelevant rabbit
polyclonal antibody. At least three different specimens were
analyzed. The myocardial tissue staining results were analyzed
using ImageJ image analysis software (National Institutes of
Health, Bethesda, MD, USA).
Enzyme-linked immunosorbent assay
The concentrations of Ang II in blood samples and
myocardial tissues were analyzed using a rat Ang II ELISA kit
(Cusabio Biotech Co., Ltd., Wuhan, China) according to the
manufacture's instructions. Absorbance at 450 nm was read using a
Multi-Mode Microplate Reader (SpectraMax M2; MTX Lab Systems,
Bradenton, FL, USA).
Reverse-transcription-quantitative
polymerase chain reaction
Total RNA from the left ventricular was isolated
using Trizol reagent (Applygen Technologies, Inc., Beijing, China).
RNA then was quantified by Nanodrop (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and samples were included when the 260/280
nm ratio was >1.6. cDNA was reverse-transcribed using a reverse
transcription system (Promega Corporation, Madison, WI, USA).
Real-time polymerase chain reaction (PCR) was performed using
TransStart Green qPCR Supermix (TransGen Biotech, Beijing, China)
and all samples were amplified in duplicate. The PCR reaction were
carried out using the ABI Prism 7500 Sequence Detector (Applied
Biosystems; Thermo Fisher Scientific Inc.). All of the
amplification reactions underwent 35 cycles (initial stage at 95°C
for 5 min, followed by a three-step cycle at 95°C for 30 sec, 58°C
for 30 sec, and 72°C for 30 sec). The accuracy of the PCR products
was confirmed by sequencing the amplicons. Fold changes in
expression were calculated using 2−(ΔCt1−ΔCt2), where
ΔCt represents the difference between the threshold cycle (Ct) for
each target gene and housekeeping β-actin mRNA transcript
levels.
The primers used were as follows: AGT (forward,
ACACCCCTGCTACAGTCCAC; reverse, TTTTCTGGGCAGCAAGAACT), ACE1
(forward, CAGGGGTCCAAGTTCCACGTT; reverse, GCCACTGCTTACTGTAGCCCAA),
ACE2 (forward, GAATGCGACCATCAAGCG; reverse, CAAGCCCAGAGCCTACGA),
AT1R (forward, GGAAACAGCTTGGTGGTGAT; reverse,
CACACTGGCGTAGAGGTTGA), β-actin (forward, GAGACCTTCAACACCCCAGCC;
reverse, TCGGGGCATCGGAACCGCTCA).
Western blot analysis
Extracts of rat tissue were collected using tissue
lysis buffer (Beyotime Institute of Biotechnology, Haimen, China)
plus 1 mM phenylmethylsulfonyl fluoride. Total protein was
quantified using a bicinchoninic acid protein assay (Pierce; Thermo
Fisher Scientific, Inc.). Proteins were separated by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and
electrophoretically transferred to nitrocellulose membranes. The
membranes were blocked with PBS and Tween 20 containing 5% non-fat
dry milk to prevent nonspecific antibody binding. Equal protein
loading was determined using specific antibodies for the antigens
of GAPDH (CWBiotech, Beijing, China), ACE1, AT1R and AT2R (Abcam,
Cambridge, MA, USA), washed, and incubated with an appropriate
IRDye800-conjugated secondary antibody (Rockland, Gilbertsville,
PA, USA). Specific immunofluorescence bands were detected and
analyzed using the Odyssey infrared imaging system and software
(LI-COR Biosciences, Lincoln, NE, USA).
Statistical analysis
All of the results are expressed as the mean ± SEM
or representative original data for 10 animals per treatment group.
The statistical analysis was performed using Prism v.5.0 software
(GraphPad Software, Inc., La Jolla, CA, USA). Significant
differences were assessed using one-way analysis of variance
followed by the Student-Newman-Keuls post hoc test or two-way
analysis of variance followed by the Bonferroni post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Comparison of systolic blood pressure
in L-NAME alone, L-NAME + NGN, and control groups
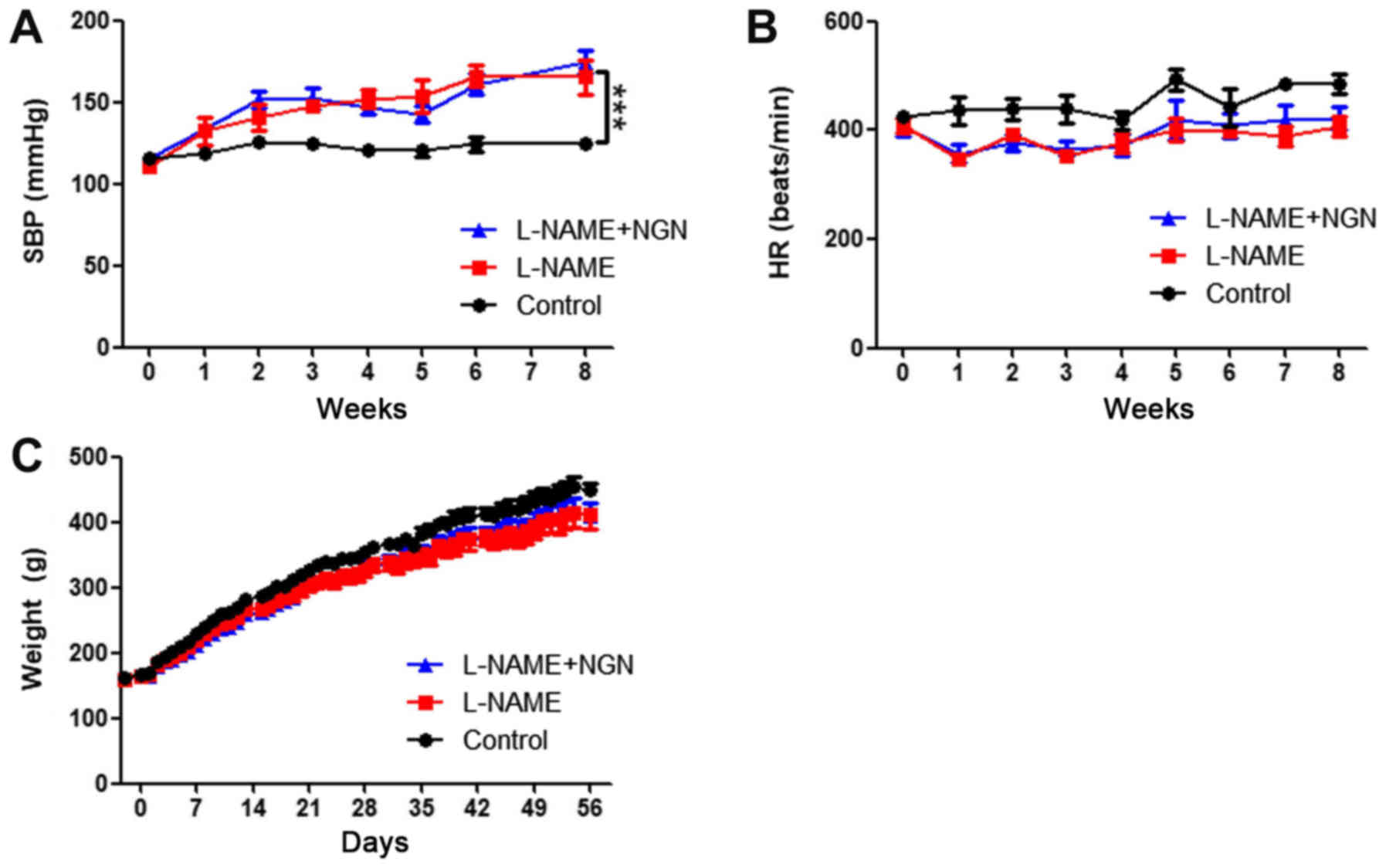
To investigate the protective effect of NGN against
cardiac hypertrophy, we first developed a rat model of hypertension
by administering 50 mg/kg L-NAME by gavage. As shown in Fig. 1A, L-NAME produced modest effects on
blood pressure. Systolic blood pressure increased beginning in the
third week after L-NAME administration and reached 175.0±6.9 mmHg
at 8 weeks compared with 125.2±1.9 mmHg in the control group
(P<0.001). No significant difference in systolic blood pressure
was found between the L-NAME and L-NAME + NGN groups. NGN and
L-NAME had no significant effects on body weight or heart rate
(Fig. 1B and C).
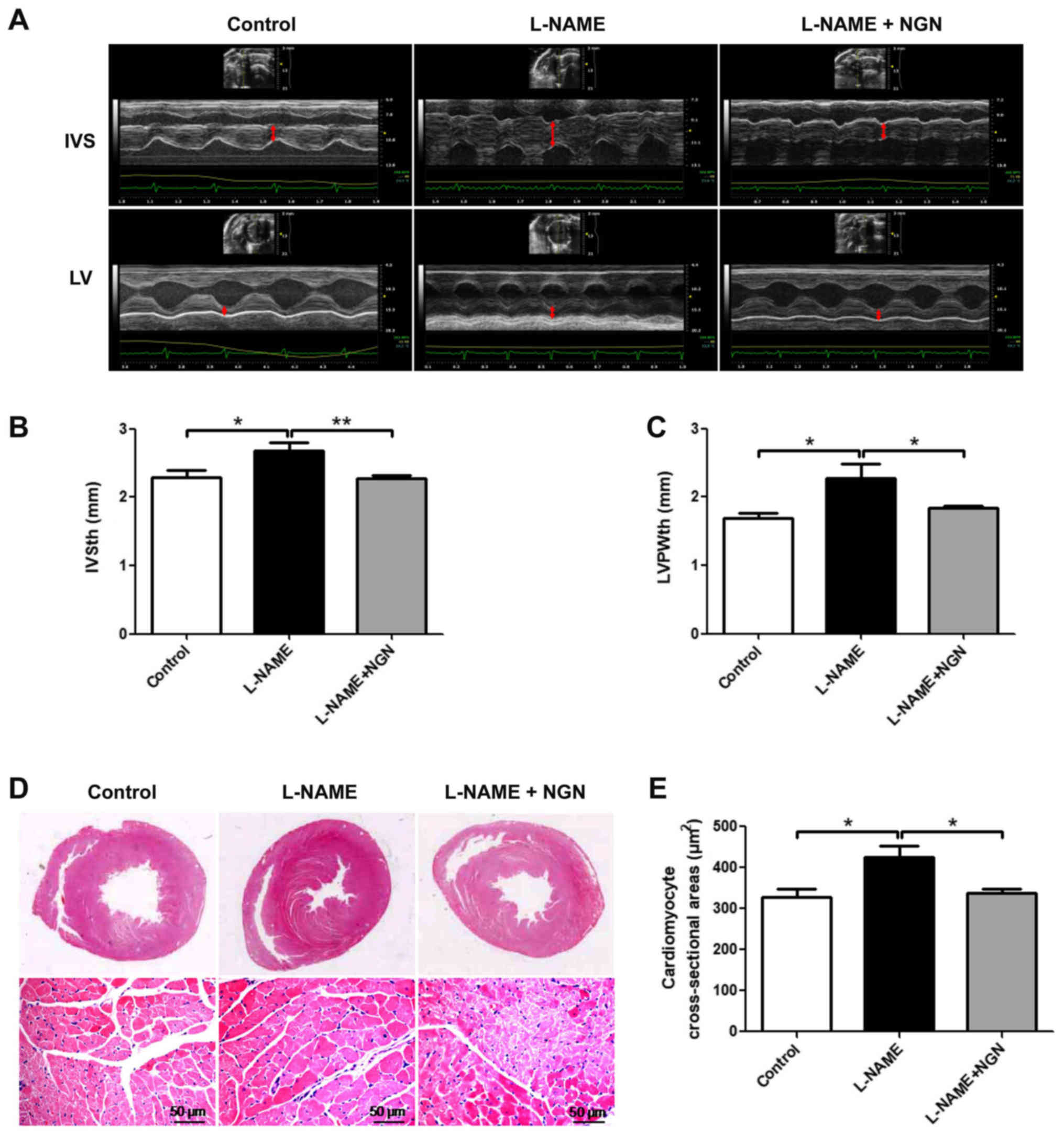
Effects of NGN on indices of cardiac
hypertrophy in rats
To evaluate the effect of NGN on cardiac remodeling,
the wall thickness of the left ventricular was assessed by
echocardiography, and morphological changes in myocardial tissue
were assessed by hematoxylin-eosin staining. As shown in Fig. 2, the L-NAME group exhibited increases
in interventricular septal thickness (IVSth), left ventricular
posterior wall thickness (LVPWth), cardiomyocyte cross-sectional
area (all P<0.05), and concentric hypertrophy compared with the
control group. These effects were partially attenuated in the
L-NAME + NGN group. No significant differences in myocardial
fibrosis were observed among the three groups (data not shown).
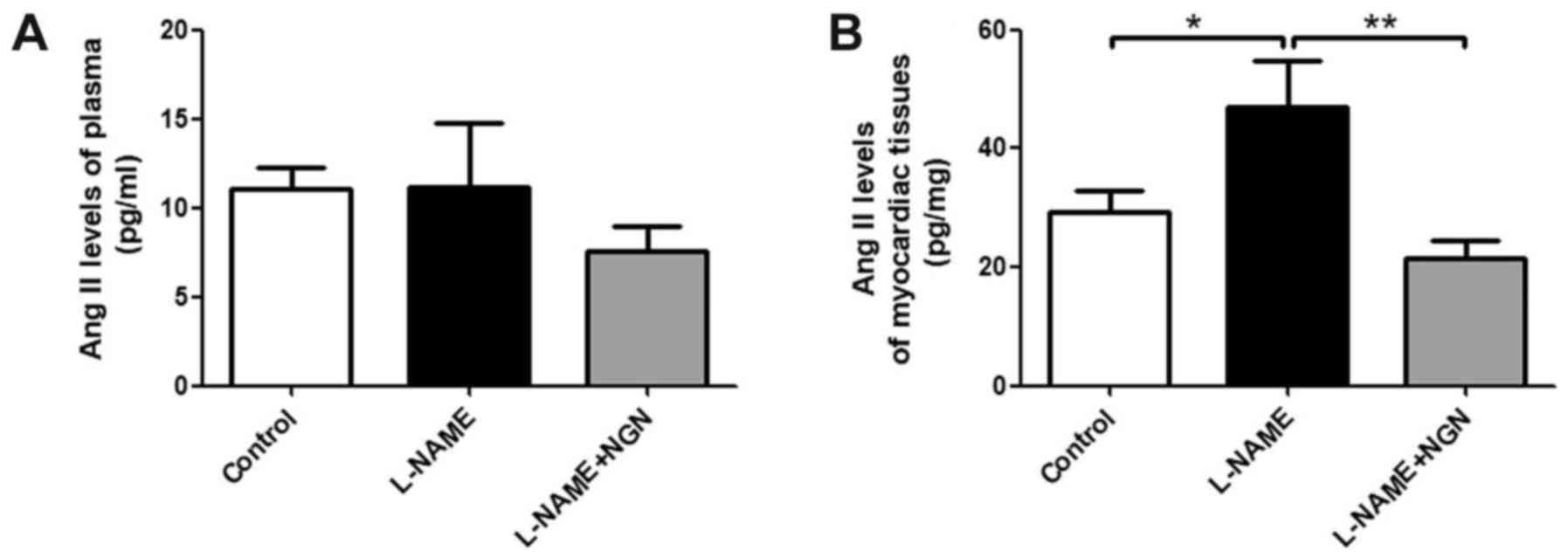
Effects of NGN on Ang II levels in
plasma and myocardial tissue in rats
Ang II is a key molecule in myocardial remodeling.
We detected Ang II levels in plasma and myocardial tissue in the
three groups of rats by enzyme-linked immunosorbent assay (ELISA).
Myocardial Ang II levels were significantly elevated in the L-NAME
group compared with the control group (P<0.05; Fig. 3A). NGN reversed the L-NAME-induced
elevation of Ang II levels in hypertensive cardiac tissue
(P<0.05; Fig. 3A). No significant
differences in serum Ang II levels were found among the three
groups (Fig. 3B).
Comparison of ACE1 expression in
myocardial tissue
To clarify the mechanism of the elevation of Ang II
levels, ACE1 mRNA and protein were detected in myocardial tissue in
the L-NAME, L-NAME + NGN, and control groups by real-time
polymerase chain reaction (PCR), Western blot, and
immunohistochemistry. No significant difference in ACE1 mRNA
expression was found among the three groups (Fig. 4A). The protein expression of ACE1 in
the L-NAME group was significantly higher than in the control group
(P<0.05). Compared with the L-NAME group, ACE1 protein
expression significantly decreased in the L-NAME + NGN group
(P<0.05; Fig. 4B and C).
Immunohistochemistry showed that ACE1 protein levels were
significantly higher in the subendocardial and epicardial
myocardium in the L-NAME group than in the control and L-NAME + NGN
groups (Fig. 4D and E). No
significant difference in angiotensinogen, ACE2, AT1R and AT2R mRNA
or protein expression was found among the three groups (Fig. 4A-C).
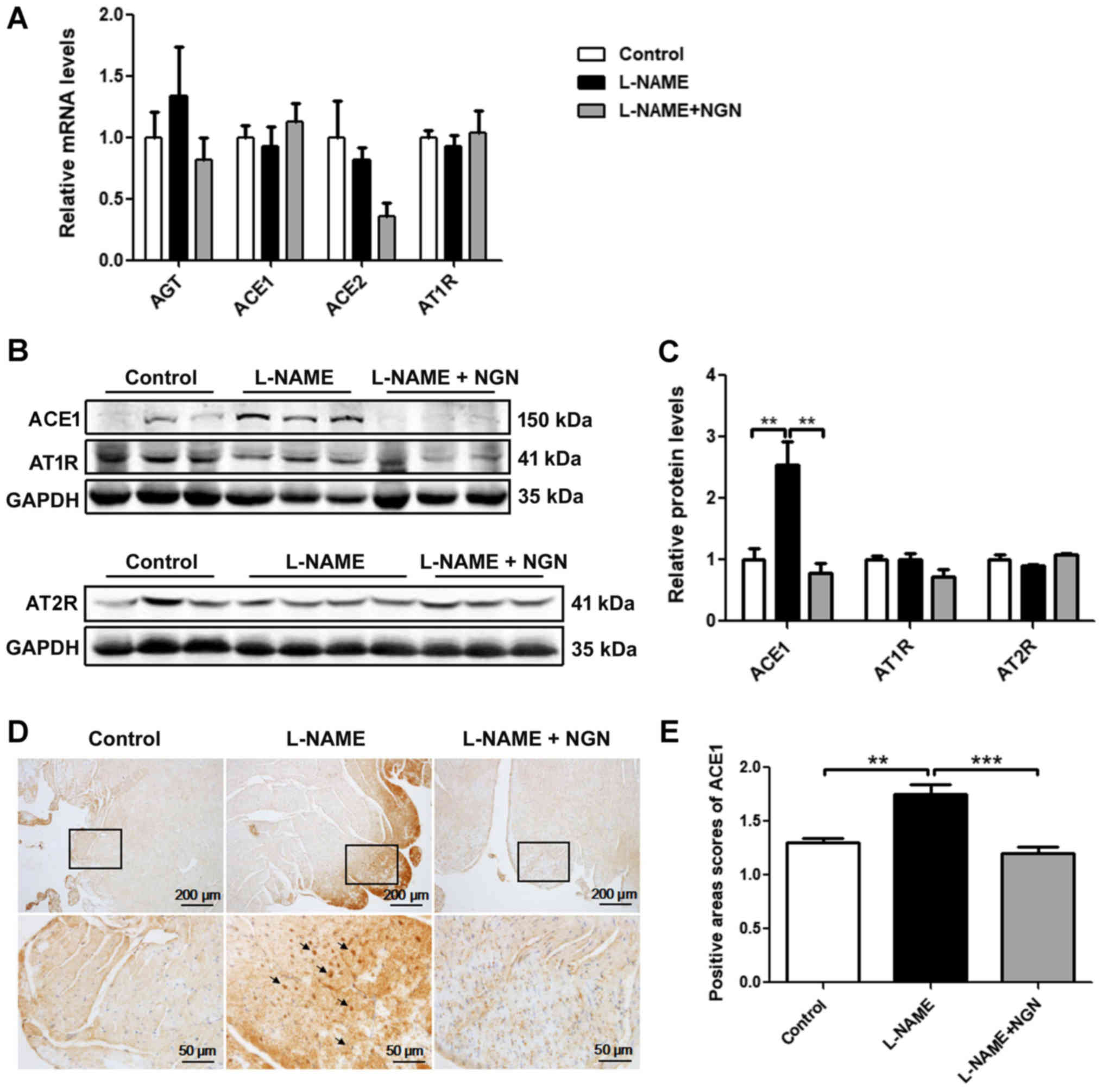 | Figure 4.NGN inhibited ACE1 protein expression
in myocardial tissue in rats with L-NAME-induced hypertension. (A)
Analysis of angiotensinogen, ACE1, ACE2 and AT1R mRNA expression by
reverse transcription-quantitative polymerase chain reaction in
myocardial tissue from rats treated with vehicle (control), L-NAME,
or L-NAME + NGN for 8 weeks. (B) Representative western blots of
ACE1, AT1R and AT2R in myocardial tissue from rats in the control,
L-NAME, and L-NAME + NGN groups 8 weeks after L-NAME
administration. (C) Analysis of ACE1, AT1R and AT2R protein
expression by western blot analysis. (D) Immunohistochemistry was
used to detect ACE1 in serial paraffin sections of myocardial
tissue from rats with L-NAME-induced hypertension. The insets show
high-magnification images of the rectangular areas in each panel.
The black arrowheads indicate strong ACE1 expression. (E) Analysis
of ACE1 protein expression by immunohistochemistry n=10. The
data are expressed as mean ± standard error of the mean.
**P<0.01, ***P<0.001. NGN, naringenin; ACE,
angiotensin-converting enzyme; AGT, angiotensinogen; AT1R,
angiotensin II receptor type 1; AT2R, angiotensin II receptor type
2; L-NAME, NG-nitro-L-arginine methyl ester. |
Discussion
The present study investigated the cardioprotective
effect of NGN against pressure overload-induced left ventricular
hypertrophy in rats and the underlying mechanism. The results
showed that NGN had a protective effect on the heart by inhibiting
the abnormal enlargement of cardiomyocytes and concentric
myocardial hypertrophy that were induced by L-NAME. NGN also
decreased ACE1 expression and Ang II content in cardiac tissue that
were induced by pressure overload, suggesting that the inhibition
of RAS overactivation in myocardial tissue might be related to the
cardioprotective effect of NGN.
NGN is widely used in traditional Chinese medicine
for its multiple pharmacological effects, including
antiinflammatory and antioxidative actions (8,11,12). NGN
has several purported beneficial cardiovascular effects and has
been shown to prevent the growth of atherosclerotic plaques,
improve vascular reactivity, inhibit platelet aggregation, decrease
plasma lipids and lipoproteins, alleviate myocardial
ischemia/reperfusion injury, and protect against
doxorubicin-induced cardiac toxicity (8–10,13,14).
A previous study reported that NGN supplementation improved
hypertension in high-carbohydrate/high-fat-diet-fed obese rats
(15). However, in the present
study, NGN did not significantly lower the elevation of systolic
blood pressure that was induced by L-NAME.
Left ventricular hypertrophy is characterized by an
abnormal increase in left ventricular mass and the enlargement of
cardiomyocytes, originating from an increase in myocyte size
(16). Numerous clinical studies
have indicated that left ventricular hypertrophy is an independent
risk factor for coronary events, stroke, heart failure, peripheral
arterial disease, and cardiovascular mortality in patients with
hypertension (17,18). In the present study, we found that
NGN treatment in rats with L-NAME-induced hypertension
significantly inhibited cardiomyocyte enlargement and concentric
cardiac hypertrophy.
Sustained RAS overactivation can lead to
cardiovascular disease progression and deterioration, although the
RAS exerts a supportive effect on hemodynamic blood (5). Furthermore, the RAS in tissues and
organs plays an important role in disease development.
Angiotensin-converting enzyme and Ang II play a pivotal role in the
RAS, and their expression in the heart is relatively low under
physiological conditions (3). In a
rat model of cardiac remodeling that was triggered by myocardial
infarction, cardiac metabolism was improved by low-dose ACE
inhibitor treatment, which did not prevent hypertension (19). In the present study, Ang II levels
and ACE1 expression in left ventricular tissue were upregulated in
the L-NAME-treated group, and NGN inhibited this upregulation in
cardiac tissues. This suggests one mechanism by which NGN protects
against left ventricular remodeling. NGN had no significant effect
on plasma Ang II levels, which was consistent with its effect on
blood pressure.
In the present study, hypertensive cardiac
hypertrophy was still in the compensatory phase after 8 weeks of
L-NAME administration, with intermediate hypertension, centripetal
left ventricular wall thickening, a smaller end-diastolic diameter,
and no serious myocardial fibrosis. Our animal model simulates
patients who are in the early stage of hypertensive heart damage
that is caused by long-term mild-to-moderate hypertension. Zhang
et al (20), reported that
NGN attenuated cardiac hypertrophy and interstitial fibrosis that
were induced by aortic banding in mice, which was consistent with
the present results in rats. However, blood pressure was acute and
malignant increased, and myocardial damage appeared to be serious
in the aortic-banding mouse model. Zhang et al (20), found that the cardioprotective effect
of NGN may be associated with inhibition of the
phosphatidylinositol-4,5-bisphosphate 3-kinase/Akt, extracellular
signal-regulated kinase, and c-Jun N-terminal kinase signaling
pathways. Our results suggest that NGN plays a protective role
against cardiac remodeling by decreasing the expression of ACE1 and
Ang II in heart tissue.
In summary, the present study revealed a novel
mechanism by which NGN exerts cardioprotective effects, namely by
negatively regulating Ang II and ACE1 expression in cardiac
tissues. NGN protected against left ventricular hypertrophy after
long-term mild to moderate hypertension that was induced by L-NAME
administration in rats. Increasing the intake of NGN-rich citrus
fruits and vegetables may be beneficial for preventing hypertensive
cardiac hypertrophy, and pharmacological NGN analogues may be
applied clinically for the treatment of hypertensive cardiac
hypertrophy.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant nos. 81500326, 91639110) and
Beijing Natural Science Foundation (grant no. 7172195).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YG was involved in drafting the manuscript. YG and
JZ designed the study. YG, ZW, YZ, YL, SW, WS, JG and CY collected
and analyzed the data. YG, YW, WK and JZ interpreted the data,
collected the funding for this study and gave final approval of the
version to be published. All authors reviewed the initial
manuscript and revised it critically for important intellectual
content.
Ethics approval and consent to
participate
The animal protocol was approved by the Animal
Ethical and Welfare Committee at China-Japan Friendship Hospital
(Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
L-NAME
|
NG-nitro-L-arginine methyl
ester
|
|
ACE
|
angiotensin-converting enzyme
|
|
Ang II
|
angiotensin II
|
|
RAS
|
renin-angiotensin-aldosterone
system
|
|
IVSth
|
interventricular septal thickness
|
|
LVPWth
|
left ventricular posterior wall
thickness
|
|
NGN
|
narigenin
|
References
|
1
|
Boonpeng H and Yusoff K: The utility of
copy number variation (CNV) in studies of hypertension-related left
ventricular hypertrophy (LVH): Rationale, potential and challenges.
Mol Cytogenet. 6:82013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nayor M, Enserro DM, Vasan RS and
Xanthakis V: Cardiovascular health status and incidence of heart
failure in the framingham offspring study. Circ Heart Fail.
9:e0024162016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamada H, Fabris B, Allen AM, Jackson B,
Johnston CI and Mendelsohn AO: Localization of angiotensin
converting enzyme in rat heart. Circ Res. 68:141–149. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hirsch AT, Talsness CE, Schunkert H, Paul
M and Dzau VJ: Tissue-specific activation of cardiac angiotensin
converting enzyme in experimental heart failure. Circ Res.
69:475–482. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Borghi C; SIIA Task Force and Rossi F; SIF
Task Force, : Role of the renin-angiotensin-aldosterone system and
its pharmacological inhibitors in cardiovascular diseases: Complex
and critical issues. High Blood Press Cardiovasc Prev. 22:429–444.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hellawell JL and Margulies KB: Myocardial
reverse remodeling. Cardiovasc Ther. 30:172–181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu Y, Zhang C, Bucheli P and Wei D: Citrus
flavonoids in fruit and traditional Chinese medicinal food
ingredients in China. Plant Foods Hum Nutr. 61:57–65. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mulvihill EE, Burke AC and Huff MW: Citrus
flavonoids as regulators of lipoprotein metabolism and
atherosclerosis. Annu Rev Nutr. 36:275–299. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Testai L, Martelli A, Cristofaro M,
Breschi MC and Calderone V: Cardioprotective effects of different
flavonoids against myocardial ischaemia/reperfusion injury in
Langendorff-perfused rat hearts. J Pharm Pharmacol. 65:750–756.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Subburaman S, Ganesan K and Ramachandran
M: Protective role of naringenin against doxorubicin-induced
cardiotoxicity in a rat model: Histopathology and mRNA expression
profile studies. J Environ Pathol Toxicol Oncol. 33:363–376. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu X, Wang N, Fan S, Zheng X, Yang Y, Zhu
Y, Lu Y, Chen Q, Zhou H and Zheng J: The citrus flavonoid
naringenin confers protection in a murine endotoxaemia model
through AMPK-ATF3-dependent negative regulation of the TLR4
signalling pathway. Sci Rep. 6:397352016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang K, Chen Z, Huang L, Meng B, Zhou X,
Wen X and Ren D: Naringenin reduces oxidative stress and improves
mitochondrial dysfunction via activation of the Nrf2/ARE signaling
pathway in neurons. Int J Mol Med. 40:1582–1590. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren B, Qin W, Wu F, Wang S, Pan C, Wang L,
Zeng B, Ma S and Liang J: Apigenin and naringenin regulate glucose
and lipid metabolism, and ameliorate vascular dysfunction in type 2
diabetic rats. Eur J Pharmacol. 773:13–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zaragozá C, Monserrat J, Mantecón C,
Villaescusa L, Zaragozá F and Álvarez-Mon M: Antiplatelet activity
of flavonoid and coumarin drugs. Vascul Pharmacol. 87:139–149.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alam MA, Kauter K and Brown L: Naringin
improves diet-induced cardiovascular dysfunction and obesity in
high carbohydrate, high fat diet-fed rats. Nutrients. 5:637–650.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Samak M, Fatullayev J, Sabashnikov A,
Zeriouh M, Schmack B, Farag M, Popov AF, Dohmen PM, Choi YH,
Wahlers T and Weymann A: Cardiac hypertrophy: An introduction to
molecular and cellular basis. Med Sci Monit Basic Res. 22:75–79.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gerdts E, Okin PM, Boman K, Wachtell K,
Nieminen MS, Dahlöf B and Devereux RB: Association of heart failure
hospitalizations with combined electrocardiography and
echocardiography criteria for left ventricular hypertrophy. Am J
Hypertens. 25:678–683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aronow WS, Ahn C, Kronzon I and
Koenigsberg M: Congestive heart failure, coronary events and
atherothrombotic brain infarction in elderly blacks and whites with
systemic hypertension and with and without echocardiographic and
electrocardiographic evidence of left ventricular hypertrophy. Am J
Cardiol. 67:295–299. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu YC, Zhu YZ, Gohlke P, Stauss HM and
Unger T: Effects of angiotensin-converting enzyme inhibition and
angiotensin II AT1 receptor antagonism on cardiac parameters in
left ventricular hypertrophy. Am J Cardiol. 80:110A–117A. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang N, Yang Z, Yuan Y, Li F, Liu Y, Ma
Z, Liao H, Bian Z, Zhang Y, Zhou H, et al: Naringenin attenuates
pressure overload-induced cardiac hypertrophy. Exp Ther Med.
10:2206–2212. 2015. View Article : Google Scholar : PubMed/NCBI
|