Introduction
Mitochondria are dynamic organelles, which are
continuously remodeled through the dynamic processes, including
fusion, fission and mitophagy (1).
Fusion and fission are opposing actions that are crucial for
maintaining the number, size, shape and function of mitochondria
(2,3). Mitochondrial fusion is regulated by 3
GTPases, namely mitofusin 1 (Mfn1), mitofusin 2 (Mfn2) and optic
atrophy 1 (OPA1), while dynamin-related protein 1 (Drp1) and
fission 1 (Fis1) have roles in mitochondrial fragmentation
(4,5). Mitochondrial fusion contributes to the
formation of mitochondrial networks and facilitates the exchange of
proteins, lipids and nucleic acids among defective mitochondria
(6,7). Conversely, mitochondrial fission
divides the tubular mitochondrial network to ensure that a
sufficient number of normally functional mitochondria is present
(8). Therefore, balanced
mitochondrial dynamics are not only crucial for maintaining proper
mitochondrial morphology, but also regulate its functions inside
the eukaryotic cells (9).
Heme oxygenase (HO)-1, a low-molecular weight heat
shock protein, is thought to protect against pro-oxidant heme
release caused by numerous agents, including lipopolysaccharides
(LPS), cytokines and reactive oxygen species (ROS) (10,11).
In vitro and in vivo, the beneficial effects of HO-1
through regulating apoptosis and inflammation, protection against
oxidative injury as well as contribution to angiogenesis have been
demonstrated (12–14). As one of the cytoprotective enzymes,
HO-1 eliminates heme and produces carbon monoxide (CO), biliverdin
and free iron. In this decade, intensive investigations have
demonstrated that endogenous CO also conveys a protective effect
through the modulation of antioxidative, anti-apoptotic,
anti-proliferative and anti-inflammatory processes (15–17).
Beyond these, CO increases mitochondrial ROS leakage by binding to
cytochrome oxidase, which promotes the expression of genes
associated with mitochondrial biogenesis (10,18).
Of note, previous studies by our group have
confirmed that the induction of HO-1/CO by LPS protects organs from
septic shock in rats (18–20). Furthermore, in vivo and in
vitro experiments by our group have also demonstrated that the
HO-1/CO system contributes to the attenuation of LPS-induced acute
lung injury by increasing the expression of mitochondrial fusion
proteins and decreasing the levels of mitochondrial fission
proteins (20,21). Therefore, it has been known that
mitochondrial dynamics may be regulated by the HO-1/CO system
during septic shock, but the precise mechanisms have remained to be
systematically demonstrated.
The protein kinase C (PKC) family comprises a group
of multi-functional serine/threonine kinases that have a key role
in signal transduction (22). The
PKC family may be classified into three major groups (conventional,
novel and atypical PKCs) according to their modes of activation and
primary structures (23,24). PKC-α belongs to the conventional
classical PKCs (cPKCs; types α, βI, βII and γ), which depend on
calcium and diacylglycerol/phorbol esters for activation (23). It has been demonstrated that PKC-α is
an important modulator in the upregulation of HO-1 stimulated by
LPS in human monocytic cells (25).
However, whether the PKC-α/HO-1 signaling pathway is involved in
the adjustment of mitochondrial dynamics in LPS-activated
macrophages has remained to be elucidated.
Therefore, in the present study, it was hypothesized
that HO-1 may protect cells from oxidative stress and improve
mitochondrial dynamics in rat macrophages stimulated by LPS via the
PKC-α/HO-1 signaling pathway. To the best of our knowledge, the
results of the present study are the first to support the notion
that the PKC-α/HO-1 signaling pathway regulates mitochondrial
dynamics by altering the expression of fusion and fission
proteins.
Materials and methods
Reagents
The murine NR8383 macrophage cell line was obtained
from the American Type Culture Collection (Manassas, VA, USA). LPS
(Escherichia coli serotype O111:B4), Go6976 and
phorbol-12-myristate-13-acetate (PMA) were purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Antibodies specific
for PKC-α (cat. no. ab32376), HO-1 (cat. no. ab13248), β-actin
(cat. no. ab8227) were obtained from Abcam (Cambridge, UK).
Antibodies specific for Mfn1 (cat. no. sc-50330), Mfn2 (cat. no.
sc-50331), OPA1 (cat. no. sc-367890), Drp1 (cat. no. sc-32898),
Fis1 (cat. no. sc-376447) were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The superoxide dismutase
(SOD; cat. no. A001-1) and malondialdehyde (MDA; cat. no. A003-1)
kits were supplied by Nanjing Jiancheng Bioengineering Institute
(Nanjing, China).
Cell culture
Alveolar macrophages (AMs) were incubated in Ham's
F-12K medium containing 15% heat-inactivated fetal bovine serum
(both Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), 100 U/ml penicillin and 100 pg/ml streptomycin at 37°C in a
humidified atmosphere containing 5% CO2. Cells were
seeded in 6-well plates at a density of 1×106 cells/ml.
The medium was replaced every 2–3 days. Cell viability was assessed
by trypan blue exclusion.
Experimental grouping
The wells containing AMs were randomly divided into
7 groups (n=5 each): The control (group C), LPS (group L),
LPS+Go6976 (group LG), LPS+PMA (group LP), LPS+DMSO (group LD),
Go6976 (group G) and PMA (group P) groups. Group L was stimulated
with 10 µg/ml LPS (L2630; Sigma-Aldrich; Merck KGaA) to establish
the experimental endotoxemia model. The cells in group C received
an equal amount of normal saline. To block the PKC-α/HO-1 signaling
pathway, group LG was pre-treated with 5 µM Go6976 (an inhibitor of
PKC-α) for 30 min prior to stimulation with LPS. Conversely, PMA, a
direct PKC activator, was used to active PKC-α; 100 nM PMA was
added for 30 min prior to the incubation of LPS in group LP. Group
LD was pre-treated with an equivalent amount of drug vehicle
dimethylsulfoxide (DMSO) instead. Group G and group P were
pre-treated with Go6976 and PMA respectively. Go6976 and PMA were
dissolved in 0.1% DMSO in normal saline.
Measurement of intracellular ROS
production
Intracellular ROS levels were detected by using the
dichloro-dihydro-fluorescein diacetate (DCFH-DA) probe (Beyotime
Institute of Biotechnology, Nanjing, China). In brief, after
treatment with LPS for 24 h, macrophages were incubated with 10 µM
DCFH-DA at 37°C for 20 min and then washed twice in PBS (26). DCF fluorescence was monitored with
excitation and emission wavelengths of 485 and 535 nm. The results
were recorded as the differences in fluorescence relative to the
initial one.
Assessment of SOD and MDA
The activities of SOD and contents of MDA were
measured using commercial reagent kits (Nanjing Jiancheng
Bioengineering Institute). All of the measurements and calculations
were performed according to the manufacturer's protocols. The
content of MDA and the activity of SOD were expressed as millimoles
per milligram and U per milligram of protein respectively.
Mitochondrial respiratory control
ratio
According to the methods described by Carlson et
al (27), mitochondria were
isolated and stored at 0°C in a buffer containing 250 mM sucrose,
10 mM Tris-hydrochloric acid (HCl), 0.5 mM EDTA, and 0.5 g/l fatty
acid-free bovine serum albumin (BSA; Invitrogen; Thermo Fisher
Scientific, Inc.) (pH 7.2) (21).
The respiratory control ratio (RCR) was measured by a Clark-type
oxygen electrode (Hansatech, King's Lynn, United Kingdom) at 37°C
(28). The respiration medium
contained 110 mM sucrose, 60 mM K-lactobionate, 0.5 mM EGTA, 1 g/l
BSA essentially fatty acid-free, 3 mM MgCl2, 20 mM
taurine, 10 mM KH2PO4, 20 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.1) and 280
IU/ml catalase (Nanjing Jiancheng Bioengineering Institute)
(28). A 60-µl mitochondrial
suspension was incubated in the chamber for 10 min at 25°C.
Subsequently, 0.1 or 0.2 mM K-ADP (Nanjing Jiancheng Bioengineering
Institute) was added for the determination of the State 3
respiration (27). Next, 2 µM
carboxyatractyloside was added to induce a State 4 respiration.
Finally, the RCR was calculated as RCR=State 3 respiration/State 4
respiration.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells using TRIzol
(Thermo Fisher Scientific, Inc.). Complementary DNA was synthesized
using the Reverse Transcription Kit (Takara, Otsu, Japan) and
Real-time PCR was performed using a SYBR Premix Ex Taq Kit (cat.
no. DRR041; Takara) according to the manufacturer's protocols. The
expression of PKC-α, HO-1, Mfn1, Mfn2, OPA1, Drp1 and Fis1 was
assessed at the same time. The primers were as follows: β-actin,
forward: 5′-TGTGTCCGTCGTGGATCTGA-3′ and reverse:
5′-TTGCTGTTGAAGTCGCAGGAG-3′ (149 bp); PKCα, forward:
5′-TGGCAAGGTCATGCTCTCAG-3′ and reverse: 5-GGAAGCAGGAATGGAGCTGA-3
(133 bp); HO-1, forward: 5′-GAATCGAGCAGAACCAGCCT-3′ and reverse:
5′-CTCAGCATTCTCGGCTTGGA-3′ (135 bp); Mfn1, forward:
5′-ACTGTAGGAGGAAGCGGACT-3′ and reverse: 5′-CACAATCTCCGCAAGGCATC-3′
(102 bp); Mfn2, forward: 5′-ACTTCTCCTCTGTTCCAGTTGT-3 and reverse:
5′-GTGCTTGAGAGGGGAAGCAT-3′ (181 bp); OPA1, forward:
5′-ACCTTGCCAGTTTAGCTCCC-3′ and reverse: 5′-ACCTAACAAGAGAAGGGCCTC-3′
(131 bp); Drp1, forward: 5′-GCCTCAGATCGTCGTAGTGG-3′ and reverse:
5′-TGCTTCAACTCCATTTTCTTCTCC-3′ (187 bp); and Fis1, forward:
5′-TACCCCGAGGCTGTCCTAAG-3′ and reverse: 5′-CAGGACATTAGGCCCAGAGC-3′
(147 bp). PCR was performed in a final volume of 20 µl. The PCR
products were amplified using the following thermocycling
conditions: 95°C for 30 sec, followed by 40 cycles of 95°C for 5
sec and 60°C for 34 sec. An ABI-7500 Sequence Detection System
(Applied Biosystems; Thermo Fisher Scientific, Inc.) was used for
product detection. The ΔΔCq method was used for determining the
mRNA levels. Finally, based on the equation F=2−∆∆Cq,
the quantity of mRNA was calculated (29).
Western blot analysis
The proteins of cell lysates were extracted using a
total and nuclear protein isolation kit (Thermo Fischer Scientific,
Inc.). Furthermore, the protein concentration was determined using
a bicichoninic acid assay kit (Thermo Fischer Scientific, Inc.).
Equal amounts of extracted protein (50 µg) were fractionated by 12%
SDS-PAGE and then transferred to a polyvinylidene difluoride
membrane (EMD Millipore, Billerica, MA, USA). The membranes were
blocked with blocking buffer (PBS with 5% skimmed milk and 0.05%
Tween 20) and incubated overnight at 4°C with primary antibodies
against PKC-α (1:800 dilution), HO-1 (1:800 dilution), Mfn1 (1:800
dilution), Mfn2 (1:800 dilution), OPA1 (1:500 dilution), Drp1
(1:800 dilution) and Fis1 (1:500 dilution). Next, the blots were
incubated at 37°C for 1 h with the horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G (1:3,000
dilution; cat. no. CW0156S; Beijing ComWin Biotech Co., Ltd.,
Beijing, China) after three washes for 10 min each with
Tris-buffered saline containing 0.05% Tween-20. The blots were
visualized by enhanced chemiluminescence (30) and quantified by densitometry
(Molecular Analyst image analysis software; version 3.0; both
Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Values are expressed as the mean ± standard
deviation. Parametric data were analyzed by one-way analysis of
variance, followed by Dunnett's post-hoc test to determine
significant differences between groups. The analyses were performed
using GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of PKC-α activity on HO-1 in
LPS-activated NR8383 cells
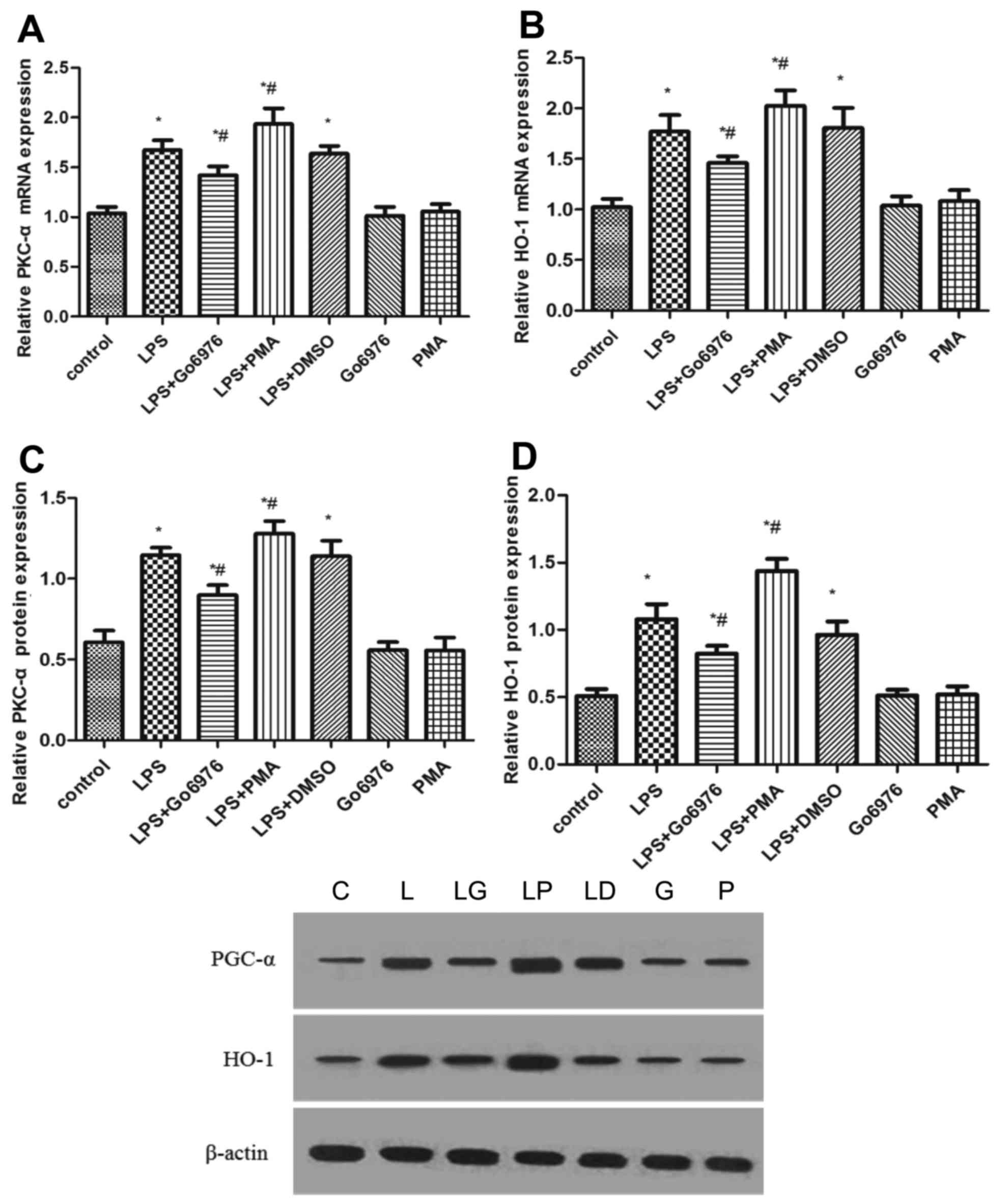
To evaluate the effect of PKC-α activity on the
expression of HO-1, NR8383 cells were pre-treated with 5 µM Go6976
and 100 nM PMA for 30 min prior to incubation with 10 µg/ml LPS for
24 h. As presented in Fig. 1,
compared with those in the control group, the gene and protein
expression of PKC-α and HO-1 were increased by treatment with LPS.
Furthermore, the protein and mRNA expression of PKC-α and HO-1
exhibited a reduction when cells were pre-treated with Go6976
(Fig. 1). Conversely, the expression
of PKC-α and HO-1 was increased when the LPS-activated NR8383
macrophages were pre-treated with PMA (Fig. 1). Of note, Go6976 or PMA alone had no
effect on the expression of PKC-α and HO-1 (Fig. 1).
Levels of ROS and MDA, as well as the
RCR and the SOD activity in LPS-induced NR8383 cells
To investigate the effect of PKC-α/HO-1 signaling on
oxidative stress, the levels of ROS and MDA, the RCR and the
activity of SOD were analyzed (Fig.
2). Compared with group C, groups L, LG, LP and LD exhibited an
apparent increase in the ROS (Fig.
2B) and MDA (Fig. 2A) content
and a decline of SOD activity (Fig.
2C) and the RCR (Fig. 2D) after
administration of 10 µg/ml LPS to NR8383 cells. Pre-treatment with
100 nM PMA, a direct PKC-α activator, increased the RCR (Fig. 2D), improved the SOD activity
(Fig. 2C) and decreased the
generation of MDA (Fig. 2A) and ROS
(Fig. 2B) in the LP group compared
with that in the L group. In comparison, pre-treatment with 5 µM
Go6976 in the LG group had the opposite effect, while the effect on
the activity of SOD was not significant.
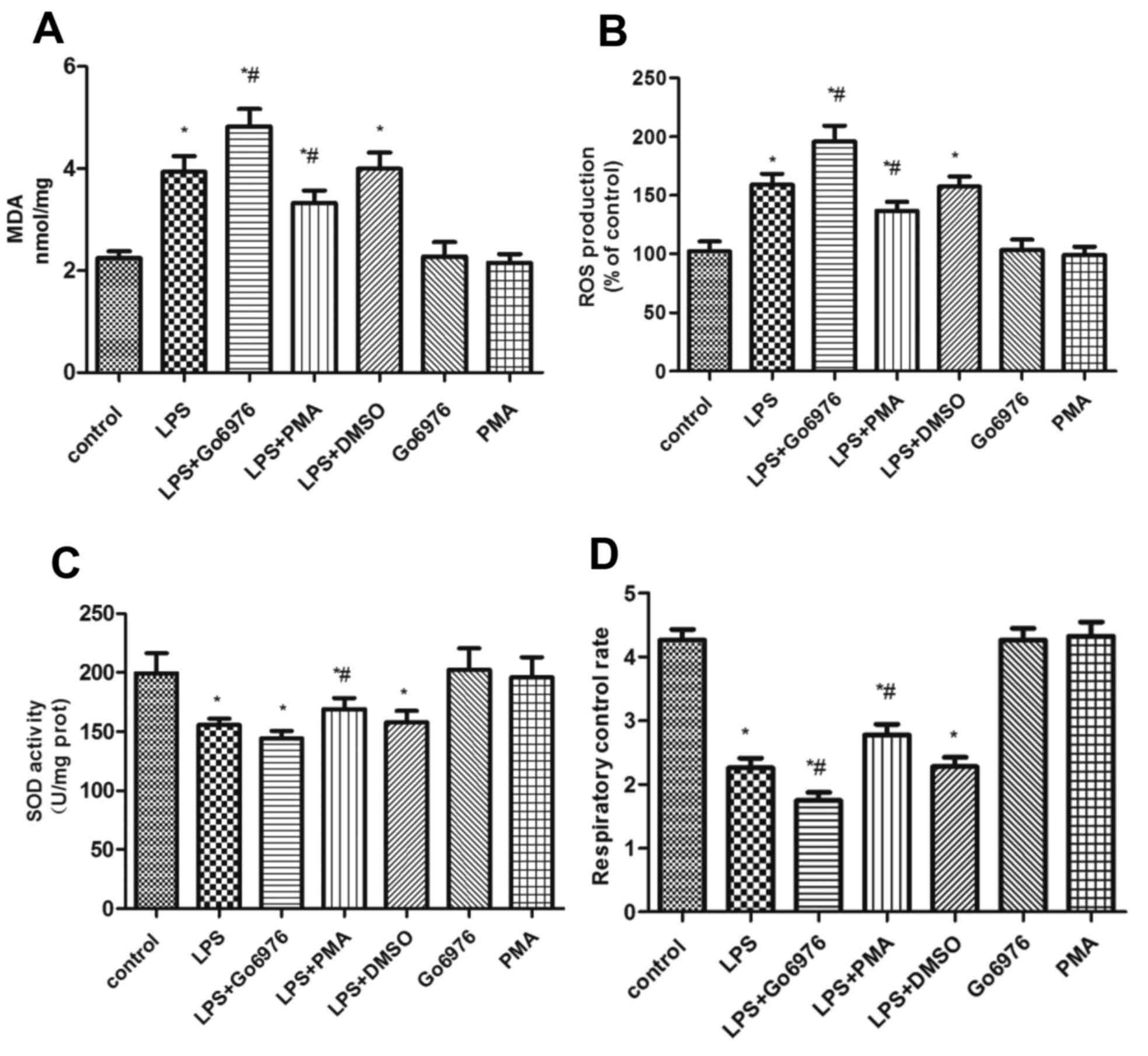 | Figure 2.Induction of oxidative stress in
NR8383 cells. (A) Levels of MDA, (B) ROS content, (C) SOD activity
and (D) RCR levels in the NR8383 cell line following stimulation
with LPS. *P<0.05 vs. control, #P<0.05 vs. LPS
group; analysis of variance followed by Dunnett's post-hoc test
(n=5). MDA, malondialdehyde; ROS, reactive oxygen species; SOD,
superoxide dismutase; RCR, respiratory control rate; PMA,
phorbol-12-myristate-13-acetate; DMSO, dimethylsulfoxide; LPS,
lipopolysaccharide. |
The activation of PKC-α/HO-1 signaling
pathway improves mitochondrial dynamics in LPS-activated NR8383
cells
To further address the effect of the PKC-α/HO-1
signaling pathway on mitochondrial dynamics, western blot and
RT-qPCR were used to measure the mRNA and protein contents of Mfn1,
Mfn2, OPA1, Drp1 and Fis1 in all groups. Compared with those in the
control group, LPS significantly induced the mRNA and protein
expression of Drp1 and Fis1, while concurrently decreasing the
expression of Mfn1, Mfn2 and OPA1 (Figs.
3 and 4). Therefore, the
mitochondrial dynamic equilibrium was disturbed by endotoxin in
macrophages of rats. To examine whether the activation of the
PKC-α/HO-1 signaling pathway has any effect on mitochondrial
dynamics in NR8383 cells, cells were pre-treated with 5 µM Go6976
or 100 nM PMA for 30 min, followed by incubation with 10 µg/ml LPS
for 24 h. The results indicated that Go6976 blocked the mRNA and
protein expression of Mfn1, Mfn2 and OPA1, while increasing Drp1
and Fis1 in the LG group compared with those in the L group
(Figs. 3 and 4). However, pre-treatment with PMA caused
an elevation of the mRNA and protein levels of Mfn1, Mfn2 and OPA1,
and a downregulation of Drp1 and Fis1 in the LP group vs. those in
the L group (Figs. 3 and 4).
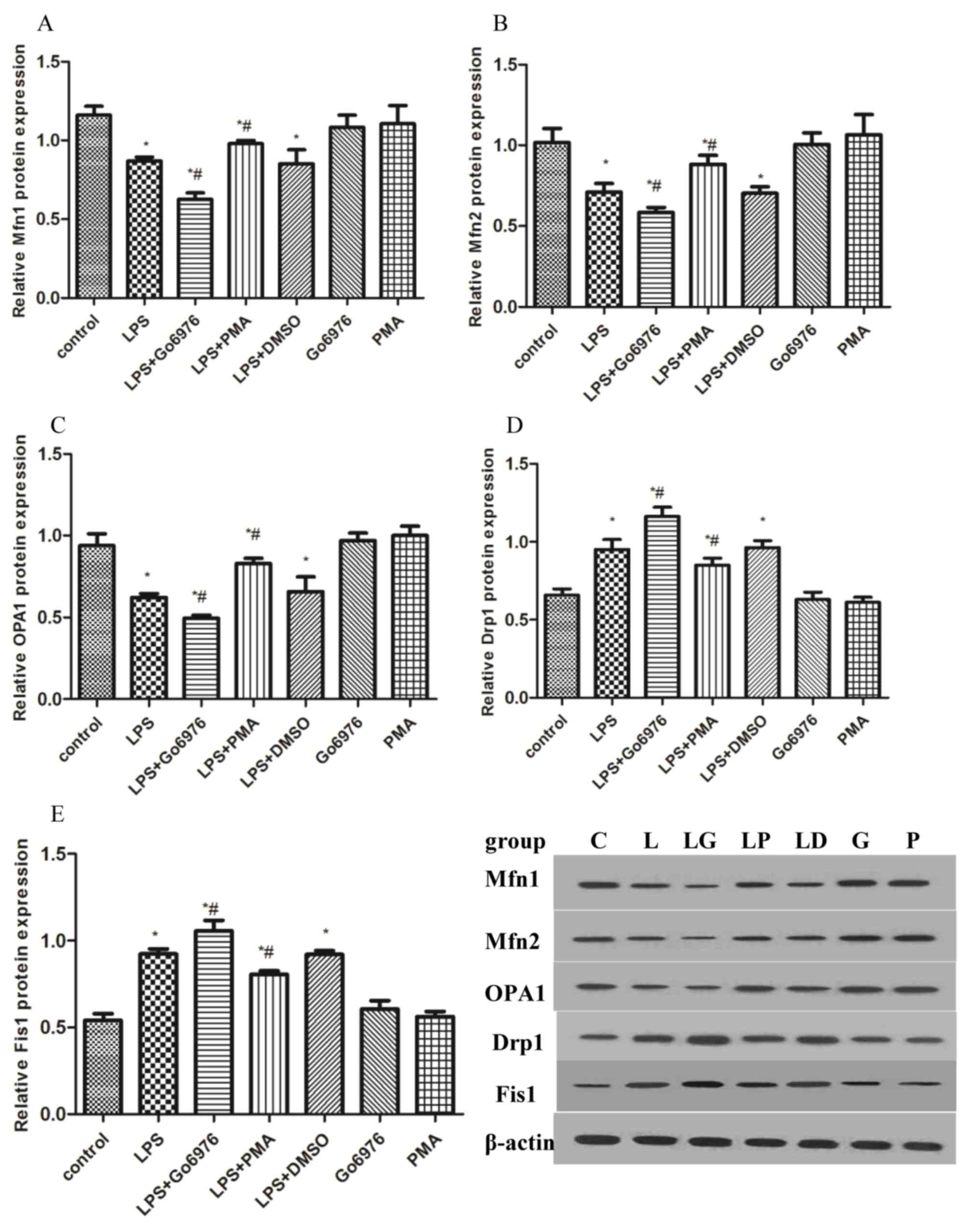 | Figure 3.Effects of PKC-α/HO-1 signaling
pathway on mitochondrial fusion/fission proteins in response to LPS
in NR8383 cells. To evaluate the implication of the PKC-α/HO-1
signaling pathway in the effects of LPS on mitochondrial dynamic
markers, NR8383 cells were pre-treated with 5 µM Go6976 and 100 nM
PMA for 30 min prior to incubation with 10 µg/ml LPS for 24 h. The
protein levels of the mitochondrial dynamic markers (A) Mfn1, (B)
Mfn2, (C) OPA1, (D) Drp1 and (E) Fis1 were assessed by western blot
analysis. *P<0.05 vs. control, #P<0.05 vs. LPS
group; analysis of variance followed by Dunnett's post-hoc test
(n=5). PMA, phorbol-12-myristate-13-acetate; DMSO,
dimethylsulfoxide; LPS, lipopolysaccharide; Drp1, dynamin-related
protein 1; Fis1, fission 1; Mfn, mitofusin; OPA1, optic atrophy
1. |
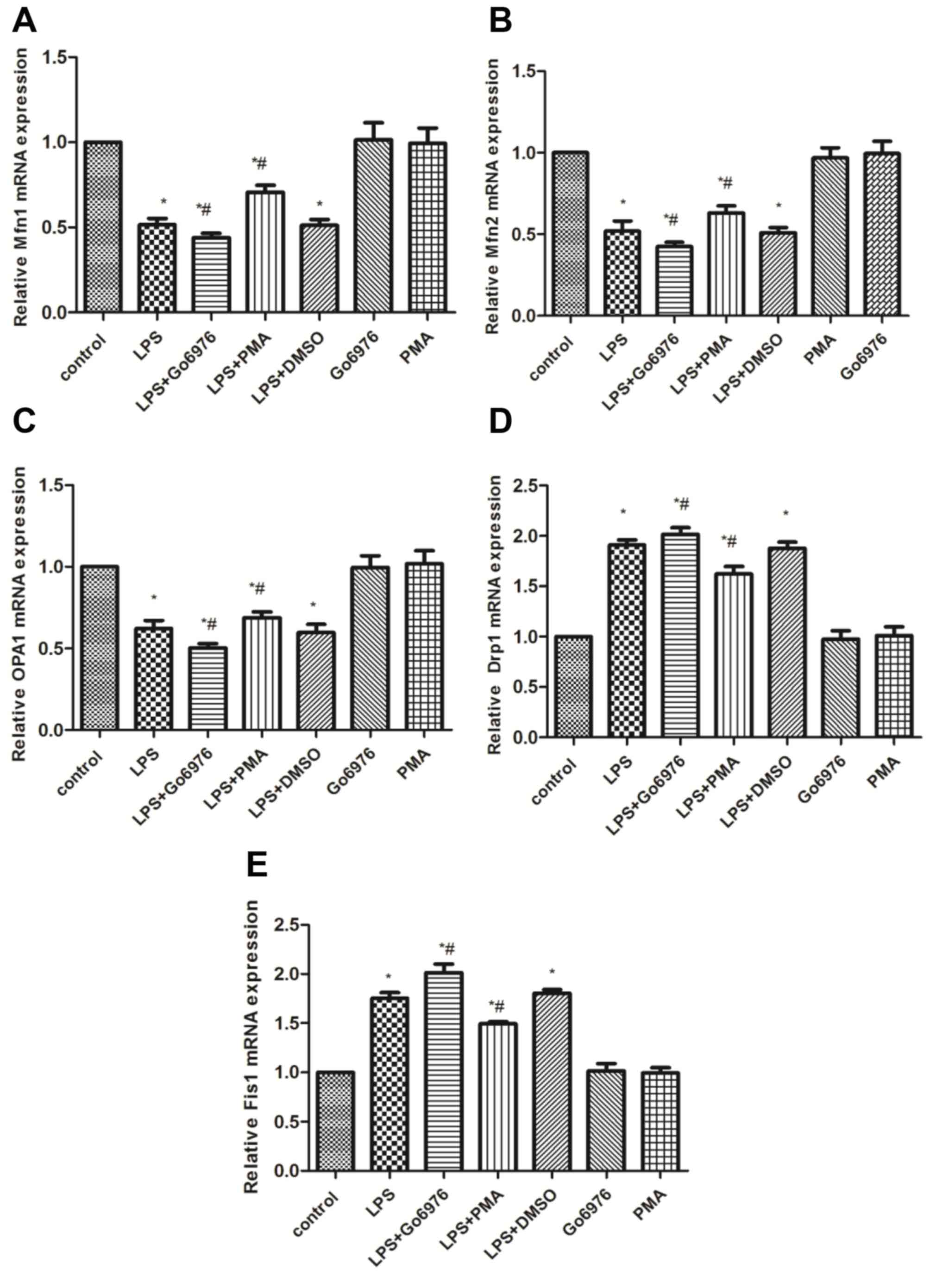 | Fig 4.Effects of p PKC-α/HO-1 signaling
pathway on the mRNA levels of mitochondrial fusion/fission markers
in NR8383 cells induced with LPS. To evaluate the effect of
PKC-α/HO-1 signaling pathway on mitochondrial dynamic markers,
NR8383 cells were pretreated with 5 µM Go6976 and 100 nM
phorbol-12-myristate-13-acetate for 30 min prior to the incubation
of 10 µg/ml LPS for 24 h. The mRNA levels of the mitochondrial
dynamic markers (A) Mfn1, (B) Mfn2, (C) OPA1, (D) Drp1 and (E) Fis1
were assessed by reverse transcription-quantitative polymerase
chain reaction analysis. *P<0.05 vs. control,
#P<0.05 vs. LPS group; analysis of variance followed
by Dunnett's post-hoc test (n=5). LPS, lipopolysaccharide; Drp1,
dynamin-related protein 1; Fis1, fission 1; Mfn, mitofusin; OPA1,
optic atrophy 1. |
Discussion
In the present study, it was demonstrated that
activation of the PKC-α/HO-1 signaling pathway in LPS-activated
NR8383 cells markedly increased the expression of Mfn1, Mfn2 and
OPA1, while decreasing the levels of Drp1 and Fis1, in parallel
with an increased expression of HO-1 and PKC-α proteins.
Furthermore, activation of the PKC-α/HO-1 signaling pathway
significantly increased the SOD activity and the RCR, and
diminished the MDA and ROS content in LPS-activated NR8383 cells.
However, blockade of the PKC-α/HO-1 signaling pathway by
pre-treatment with Go6976, a specific inhibitor of PKC-α, had the
opposite effect to that of the activator PMA on LPS-activated
macrophages.
The RCR is a crucial and versatile indicator of
mitochondrial health due to its close association with oxidative
phosphorylation (OXPHOS). State 3 respiration reflects the amount
of oxygen utilized for ATP production, which is measured following
the addition of ADP and phosphoric acid groups. State 4 respiration
is obtained after ADP is completely phosphorylated to ATP during
OXPHOS. Therefore, the mitochondrial RCR expresses the ratio
between the oxygen consumption rate in State 3 vs. the oxygen
consumption rate in State 4 (31). A
high RCR (>2.5) may be regarded as an indicator of good-quality
mitochondrial respiration (31).
Consistent with a previous study by our group (21), the present results indicated that
NR8383 cells induced by LPS had a lower RCR compared with that of a
vehicle-treated control. However, the induction of the PKC-α/HO-1
signaling pathway by PMA effectively attenuated the LPS-induced
depression of mitochondrial function via restoring the RCR. By
contrast, exposure to Go6976 significantly reduced the RCR, thereby
aggravating the effect of LPS.
HO-1 is an important antioxidant enzyme and exerts a
crucial cytoprotective effect in various disease states and organ
systems (32–34). In addition, the protective effect of
HO-1 is closely associated with the normal function of
mitochondria. A recent study has also indicated that HO-1 partially
mediates cardiac protection by regulating mitochondrial quality
control, comprising mitochondrial dynamics, biogenesis and
mitophagy (33). Importantly,
overexpression of HO-1 abrogated increases in the expression of
Fis1 and elevated the expression of Mfn1 and Mfn2 in mice with
doxorubicin-induced dilated cardiomyopathy (33). In line with these results, preceding
studies by our group indicated that the HO-1/CO system exerted
anti-oxidant effects via increasing the expression of mitochondrial
fusion proteins and decreasing the levels of mitochondrial fission
proteins in an endotoxin-induced acute lung injury (ALI) model and
in LPS-activated RAW 264.7 cells (21). According to previous studies, the
experimental model of LPS-activated NR8383 cells was applied in the
present study. HO-1 expression was induced by stimulation of NR8383
cells with LPS. Furthermore, after pre-treatment with Go6976, low
HO-1 expression coincided with low expression of Mfn1, Mfn2 and
OPA1, as well as high levels of Drp1 and Fis1, which supports the
protective effect of HO-1 in improving mitochondrial dynamics.
ALI associated with sepsis is the leading cause of
mortality in intensive care units. During the course of ALI,
alveolar macrophages generate and release a variety of mediators
once activated by bacteria and/or viruses, which in turn promotes
excessive recruitment of leukocytes (35). Furthermore, accompanied with an
uncontrolled inflammatory response, numerous kinases and signaling
pathways may be stimulated in LPS-activated monocytes (36,37). The
activation of PKC, particularly in the PKC-α/HO-1 signaling
pathway, has been reported to be involved in the protection against
oxidative stress, inflammation and apoptosis (38–40).
However, whether HO-1 has any effect on mitochondrial dynamics
through PKC-α pathways has remained to be elucidated. In the
present study, Go6976, a specific PKC-α inhibitor, decreased the
mRNA and protein expression of PKC-α and HO-1 in LPS-induced NR8383
cells, along with the depression of Mfn1, Mfn2 and OPA1, and the
increase of Drp1 and Fis1. Furthermore, suppression of PKC-α and
HO-1 led to increases in the levels of ROS and MDA, and a reduction
of the RCR. Therefore, the present study hypothesized and
experimentally verified that the PKC-α/HO-1 signaling pathway is a
candidate mechanism via which cells may be protected from oxidative
stress injury and mitochondrial function may be regulated by
improving mitochondrial dynamics in LPS-activated macrophages.
In summary, the present study indicated that the
activation of PKC-α by LPS stimulated the expression of HO-1
protein, which in turn ameliorated mitochondrial injury by
increasing the expression of Mfn2 and OPA1, and decreasing the
levels of Drp1 and Fis1 in NR8383 cells. Furthermore, PKC-α/HO-1
signaling pathway was identified to be implicated in the
anti-oxidant and cytoprotective effects against LPS-induced
activation in macrophages. Based on the present results, it may be
concluded that the PKC-α/HO-1 signaling pathway constitutes at
least one critical signal transduction pathway for modulating
mitochondrial dynamics in LPS-activated macrophages. Therefore, the
present results provide an approach for further investigation into
the function of HO-1 in improving the quality of mitochondrial
dynamics as well as the underlying mechanisms, which may be a
potential and efficient strategy to protect cells against
sepsis.
Acknowledgements
The authors would like to thank Dr Donghua Li
(Department of Pharmacology, Institute of Integrated Traditional
Chinese and Western medicine for Acute Abdominal Disease, Tianjin,
China) for the technical assistance.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant number 81772106;
Beijing, China).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
XL and YZ designed the study and drafted the
manuscript. XL, RM, LW, JS and DL conducted the experiments. JY, LG
and YZ conceived and supervised the study, and revised the
manuscript. All of the authors read and approved the final
manuscript, and each author believes that the manuscript represents
honest work.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ALI
|
acute lung injury
|
|
CO
|
carbon monoxide
|
|
Drp1
|
dynamin-related protein 1
|
|
Fis1
|
fission 1
|
|
HO-1
|
heme oxygenase-1
|
|
LPS
|
lipopolysaccharide
|
|
MDA
|
malondialdehyde
|
|
Mfn1
|
mitofusin 1
|
|
OPA1
|
optic atrophy 1
|
|
PKC-α
|
protein kinase C-α
|
|
RCR
|
pespiratory control ratio
|
|
ROS
|
reactive oxygen species
|
|
SOD
|
superoxide dismutase
|
References
|
1
|
DeBalsi KL, Hoff KE and Copeland WC: Role
of the mitochondrial DNA replication machinery in mitochondrial DNA
mutagenesis, aging and age-related diseases. Ageing Res Rev.
33:89–104. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sheridan C and Martin SJ: Mitochondrial
fission/fusion dynamics and apoptosis. Mitochondrion. 10:640–648.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kieper N, Holmström KM, Ciceri D, Fiesel
FC, Wolburg H, Ziviani E, Whitworth AJ, Martins LM, Kahle PJ and
Krüger R: Modulation of mitochondrial function and morphology by
interaction of Omi/HtrA2 with the mitochondrial fusion factor OPA1.
Exp Cell Res. 316:1213–1224. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu J, Wu J, Xie P, Maimaitili Y, Wang J,
Xia Z, Gao F, Zhang X and Zheng H: Sevoflurane postconditioning
attenuates cardiomyocyte hypoxia/reoxygenation injury via restoring
mitochondrial morphology. PeerJ. 4:e26592016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhuang X, Maimaitijiang A, Li Y, Shi H and
Jiang X: Salidroside inhibits high-glucose induced proliferation of
vascular smooth muscle cells via inhibiting mitochondrial fission
and oxidative stress. Exp Ther Med. 14:515–524. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Q, Tamura Y, Roy M, Adachi Y, Iijima
M and Sesaki H: Biosynthesis and roles of phospholipids in
mitochondrial fusion, division and mitophagy. Cell Mol Life Sci.
71:3767–3778. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin L, Zhang M, Yan R, Shan H, Diao J and
Wei J: Inhibition of Drp1 attenuates mitochondrial damage and
myocardial injury in Coxsackievirus B3 induced myocarditis. Biochem
Biophys Res Commun. 484:550–556. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han XJ, Tomizawa K, Fujimura A, Ohmori I,
Nishiki T, Matsushita M and Matsui H: Regulation of mitochondrial
dynamics and neurodegenerative diseases. Acta medica Okayama.
65:1–10. 2011.PubMed/NCBI
|
|
9
|
Hu C, Huang Y and Li L: Drp1-dependent
mitochondrial fission plays critical roles in physiological and
pathological progresses in mammals. Int J Mol Sci. 18:pii: E144.
2017. View Article : Google Scholar
|
|
10
|
Rayamajhi N, Kim SK, Go H, Joe Y, Callaway
Z, Kang JG, Ryter SW and Chung HT: Quercetin induces mitochondrial
biogenesis through activation of HO-1 in HepG2 cells. Oxid Med Cell
Longev. 2013:1542792013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu JB, Zhou F, Yao SL, Tang ZH, Wang M and
Chen HR: Effect of heme oxygenase-1 on the kidney during septic
shock in rats. Transl Res. 153:283–287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Constantin M, Choi AJ, Cloonan SM and
Ryter SW: Therapeutic potential of heme oxygenase-1/carbon monoxide
in lung disease. Int J Hypertens. 2012:8592352012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Loboda A, Damulewicz M, Pyza E, Jozkowicz
A and Dulak J: Role of Nrf2/HO-1 system in development, oxidative
stress response and diseases: an evolutionarily conserved
mechanism. Cell Mol Life Sci. 73:3221–3247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee SE, Yang H, Son GW, Park HR, Park CS,
Jin YH and Park YS: Eriodictyol protects endothelial cells against
oxidative stress-induced cell death through modulating
ERK/Nrf2/ARE-Dependent heme oxygenase-1 expression. Int J Mol Sci.
16:14526–14539. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin YT, Chen YH, Yang YH, Jao HC, Abiko Y,
Yokoyama K and Hsu C: Heme oxygenase-1 suppresses the infiltration
of neutrophils in rat liver during sepsis through inactivation of
p38 MAPK. Shock. 34:615–621. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fredenburgh LE, Perrella MA and Mitsialis
SA: The role of heme oxygenase-1 in pulmonary disease. The role of
heme oxygenase-1 in pulmonary disease. 36:158–165. 2007.
|
|
17
|
Liu XM, Peyton KJ and Durante W: Ammonia
promotes endothelial cell survival via the heme
oxygenase-1-mediated release of carbon monoxide. Free Radic Biol
Med. 102:37–46. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
MacGarvey NC, Suliman HB, Bartz RR, Fu P,
Withers CM, Welty-Wolf KE and Piantadosi CA: Activation of
mitochondrial biogenesis by heme oxygenase-1-mediated NF-E2-related
factor-2 induction rescues mice from lethal Staphylococcus aureus
sepsis. Am J Respir Crit Care Med. 185:851–861. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu JB and Yao SL: Effect of heme
oxygenase-endogenous carbon monoxide on mortality during septic
shock in rats. Ir J Med Sci. 178:491–496. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu J, Wang Y, Li Z, Dong S, Wang D, Gong
L, Shi J, Zhang Y, Liu D and Mu R: Effect of heme oxygenase-1 on
mitofusin-1 protein in LPS-induced ALI/ARDS in rats. Sci Rep.
6:365302016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu J, Shi J, Wang D, Dong S, Zhang Y, Wang
M, Gong L, Fu Q and Liu D: Heme oxygenase-1/carbon
monoxide-regulated mitochondrial dynamic equilibrium contributes to
the attenuation of endotoxin-induced acute lung injury in rats and
in lipopolysaccharide-activated macrophages. Anesthesiology.
125:1190–1201. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang G, Chen Z, Zhang F, Jing H, Xu W,
Ning S, Li Z, Liu K, Yao J and Tian X: Blockade of PKCβ protects
against remote organ injury induced by intestinal ischemia and
reperfusion via a p66shc-mediated mitochondrial apoptotic pathway.
Apoptosis. 19:1342–1353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hug H and Sarre TF: Protein kinase C
isoenzymes: Divergence in signal transduction? Biochem J.
291:329–343. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou H, Wang Y, Zhou Q, Wu B, Wang A,
Jiang W and Wang L: Down-regulation of protein kinase C-ε by
prolonged incubation with PMA inhibits the proliferation of
vascular smooth muscle cells. Cell Physiol Biochem. 40:379–390.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rushworth SA, Chen XL, Mackman N, Ogborne
RM and O'Connell MA: Lipopolysaccharide-induced heme oxygenase-1
expression in human monocytic cells is mediated via Nrf2 and
protein kinase C. J Immunol. 175:4408–4415. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yuan ZH, Liang ZE, Wu J, Yi JE, Chen XJ
and Sun ZL: A potential mechanism for the anti-apoptotic property
of koumine involving mitochondrial pathway in LPS-mediated RAW
264.7 macrophages. Molecules. 21:pii: E1317. 2016. View Article : Google Scholar
|
|
27
|
Carlson DE, Pumplin DW, Ghavam S, Fiedler
SM, Chiu WC and Scalea TM: ATP accelerates respiration of
mitochondria from rat lung and suppresses their release of hydrogen
peroxide. J Bioenerg Biomembr. 37:327–338. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Labieniec-Watala M, Siewiera K and Jozwiak
Z: Resorcylidene aminoguanidine (RAG) improves cardiac
mitochondrial bioenergetics impaired by hyperglycaemia in a model
of experimental diabetes. Int J Mol Sci. 12:8013–8026. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Balogun E, Hoque M, Gong P, Killeen E,
Green CJ, Foresti R, Alam J and Motterlini R: Curcumin activates
the haem oxygenase-1 gene via regulation of Nrf2 and the
antioxidant-responsive element. Biochem J. 371:887–895. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jacoby RP, Millar AH and Taylor NL:
Assessment of respiration in isolated plant mitochondria using
Clark-type electrodes. Methods Mol Biol. 1305:165–185. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chi X, Guo N, Yao W, Jin Y, Gao W, Cai J
and Hei Z: Induction of heme oxygenase-1 by hemin protects lung
against orthotopic autologous liver transplantation-induced acute
lung injury in rats. J Transl Med. 14:352016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hull TD, Boddu R, Guo L, Tisher CC,
Traylor AM, Patel B, Joseph R, Prabhu SD, Suliman HB, Piantadosi
CA, et al: Heme oxygenase-1 regulates mitochondrial quality control
in the heart. JCI insight. 1:e858172016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhong ZY and Tang Y: Upregulation of
periostin prevents high glucose-induced mitochondrial apoptosis in
human umbilical vein endothelial cells via activation of Nrf2/HO-1
signaling. Cell Physiol Biochem. 39:71–80. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ding X, Jin S, Tong Y, Jiang X, Chen Z,
Mei S, Zhang L, Billiar TR and Li Q: TLR4 signaling induces TLR3
up-regulation in alveolar macrophages during acute lung injury. Sci
Rep. 7:342782017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guha M and Mackman N: LPS induction of
gene expression in human monocytes. Cell Signal. 13:85–94. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee TY, Lee KC, Chen SY and Chang HH:
6-Gingerol inhibits ROS and iNOS through the suppression of
PKC-alpha and NF-kappaB pathways in lipopolysaccharide-stimulated
mouse macrophages. Biochem Biophys Res Commun. 382:134–139. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang Z, Cui W, Li G, Yuan S, Xu D, Hoi
MP, Lin Z, Dou J, Han Y and Lee SM: Baicalein protects against
6-OHDA-induced neurotoxicity through activation of Keap1/Nrf2/HO-1
and involving PKCα and PI3K/AKT signaling pathways. J Agric Food
Chem. 60:8171–8182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin M, Zhai X, Wang G, Tian X, Gao D, Shi
L, Wu H, Fan Q, Peng J4, Liu K and Yao J: Salvianolic acid B
protects against acetaminophen hepatotoxicity by inducing Nrf2 and
phase II detoxification gene expression via activation of the PI3K
and PKC signaling pathways. J Pharmacol Sci. 127:203–210. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee H, Park YH, Jeon YT, Hwang JW, Lim YJ,
Kim E, Park SY and Park HP: Sevoflurane post-conditioning increases
nuclear factor erythroid 2-related factor and haemoxygenase-1
expression via protein kinase C pathway in a rat model of transient
global cerebral ischaemia. Br J Anaesth. 114:307–318. 2015.
View Article : Google Scholar : PubMed/NCBI
|