Introduction
Hepatocellular carcinoma (HCC) is one of the most
threatening types of cancer, with high malignancy, poor prognosis
and high morbidity (1). It is ranked
as the fifth most common type of cancer and the third cause of
cancer-associated mortality worldwide (1). HCC is derived from chronic liver injury
with persistent inflammation leading to a progressive disease in
which the liver undergoes pathological changes, spanning hepatitis,
hepatic fibrosis, cirrhosis and finally HCC (2). However, at present there is no
effective treatment for HCC, as current treatment regimes are
accompanied by high recurrence rates and serious adverse reactions
(3). It would therefore be
beneficial to identify an effective, safe herbal medicine, whose
mechanism of action is well-characterized, to be used as an adjunct
therapy for HCC.
Pre-clinical and clinical studies have reported that
treatment with Astragalus membranaceus or Salvia
miltiorhiza effectively improves liver function and suppresses
hepatic fibrosis and cirrhosis (4–6). Based
on these findings and traditional Chinese medical theory, a formula
termed Compound Astragalus and Salvia miltiorrhiza
extract (CASE) was developed, comprising astragalosides, astragalus
polysaccharide and salvianolic acids extracted from Astragalus
membranaceus and Salvia miltiorhiza (7). Previous studies have revealed that CASE
has an anti-fibrotic effect in rats with carbon
tetrachloride-induced fibrosis and that the underlying mechanisms
are associated with modulation of the transforming growth factor-β
(TGF-β)/Smad signaling pathway (7,8). CASE
inhibits HepG2 cell proliferation and invasion by regulating the
TGF-β/Smad/plasminogen activator inhibitor 1 (PAI-1) signaling
pathway (9). Furthermore, CASE has
been demonstrated to have anti-cancer effects in rats with HCC
induced by diethylinitrosamine (DEN), which are achieved by
inhibiting fibrosis as well as modulating Smad protein expression
and PAI-1 transcription (6,10). However, it remains to be elucidated
how CASE modulates the expression of TGF-β1, specific
membrane receptors [TGF-β receptor type-I (TβRI) and TβRII] and
karyopherins [Importin (Imp)7 and Imp8] in the TGF-β/Smad signaling
pathway. The aim of the present study was to investigate the
effects of CASE on the expression of TGF-β1, TβRI, TβRII
and Imp7/8 during the development of HCC using DEN-induced
hepatocarcinogenesis in rats, rat myofibroblasts (MFBs, key
fibrogenic cells implicated in liver fibrosis) and the human
hepatoblastoma cell line HepG2.
Materials and methods
Preparation of CASE
The herbs of Astragalus membranaceus Bunge
(Leguminosae) and Salvia miltiorhiza Bunge (Lamiaceae) were
purchased from Bozhou Huqiao Pharmaceutical Co., Ltd. (Bozhou,
China) and authenticated by Professor Xiaoxiang Zhang (Department
of Pharmaceutical Engineering, Hefei University of Technology,
Hefei, China), a specialist in traditional Chinese herbal medicine.
Voucher specimens were deposited in the specimen room of
traditional Chinese medicine (Anhui University of Chinese
Traditional Medicine, Hefei, China). The processes of extracting
and preparing the three CASE components were performed as
previously described (7). Briefly,
astragalosides, astragalus polysaccharide and salvianolic acids
were made into powders, weighed and dissolved in 0.5% sodium
carboxymethylcellulose (CMC-Na) with distilled water according to a
standard ratio of 70:1:1.85.
DEN-induced hepatocarcinogenesis in
rats
A total of 150 male Sprague-Dawley rats (age, 6–7
weeks) weighing 180–200 g were purchased from Shanghai Xipuer-Bikai
Laboratory Animal Ltd., Co. (Shanghai, China) and housed in
conventional cages at 20–22°C with a 12-h light-dark cycle and a
40–70% relative humidity. Rats were supplied with laboratory chow
and water ad libitum. The rats were kept under these
conditions for ≥1 week prior to the experiment. The current study
was performed in accordance with the guidelines for the humane
treatment of animals set by the Association of Laboratory Animal
Sciences and the Center for Laboratory Animal Sciences at Anhui
Medical University based on Health Guide for the Care and Use of
Laboratory Animals from National and International Institutions
(11) and approved by the
Experimental Animal Ethics Committee of Anhui Medical University
(Hefei, China).
Rats were randomly divided into five groups (each,
n=30): The control group, the DEN group and three CASE treatment
groups. In the morning, rats in the DEN group and three CASE
treatment groups received 0.2% DEN dissolved in 0.5% CMC-Na and
distilled water at a dose of 10 mg/kg by gavage 5 times per week
for 14 weeks to induce hepatocarcinogenesis, synchronously, the
rats in control group administrated with equivalent 0.5% CMC-Na as
control. In the afternoon, rats in the three CASE groups were
concomitantly administered CASE at the doses of 60, 120 or 240
mg/kg/day by gavage for 16 weeks. Rats in the other two groups were
treated with equivalent 0.5% CMC-Na as control. Rats were
sacrificed 12 or 16 weeks following the start of DEN
administration. One lobe from each rat liver was harvested and
fixed in 10% formalin at room temperature for 3 days, dehydrated in
a graded series of alcohol, embedded in paraffin and cut into
4-µm-thick sections for further histological analysis. Other tissue
sections were preserved in liquid nitrogen for protein
detection.
Cell models of liver fibrosis and
liver cancer
Hepatic stellate cells (HSCs) were isolated from the
normal rat liver using collagenase IV and pronase-E (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) digestion according to a method
previously described (12). HSCs
were cultured on plastic dishes and activated to give MFBs as
previously described (13). The
human hepatoblastoma HepG2 cell line was purchased from the Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China). MFBs and HepG2 cells were seeded at a density of
1×106 cells in 25 cm2 culture flasks and
grown as sub-confluent monolayer cultures in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum
(Zhejiang Tianhang Biological Technology Co., Ltd., Huzhou,
Zhejiang, China) in a humidified 5% CO2 incubator at
37°C. Experiments were performed with cells in the log growth
phase. Cells in the experimental groups were incubated in
serum-free medium and CASE (20, 40 or 80 µg/ml) for 24 h, and
subsequently treated with TGF-β1 (40 pmol/l; R&D
Systems, Inc., Minneapolis, MN, USA) for 1 h. Cells in the control
group were incubated in serum-free medium. Total protein was
extracted using Cell lysis buffer for Western and IP (cat. no.
P0013; Beyotime Institute of Biotechnology, Shanghai, China)
according to the manufacturer's protocol. Each experiment was
repeated three times.
Histopathological investigation
Pathological changes in the liver were assessed in
each group using hematoxylin and eosin (H&E) staining, as
previously described (14). In
brief, paraffin sections were deparaffinized in xylene, rehydrated
in a graded series of alcohol, and stained with hematoxylin for 8
min at room temperature. Sections were then washed in running water
until a blue color was observed, following which they were stained
using eosin for 30 sec at room temperature and mounted under cover
slips. The pathological features were observed using a light
microscope (Nikon 80i; Nikon Corporation, Tokyo, Japan;
magnification, ×100).
Immunohistochemical examination
Paraffin sections were deparaffinized in xylene and
rehydrated in a decreasing graded alcohol series and distilled
water. Non-enzymatic antigen retrieval was performed by heating the
sections to 121°C in 0.01 M sodium citrate buffer (pH 6.0) for 10
min. Sections were cooled, rinsed in Tris-buffered saline
containing 0.1% Tween-20 (TBST) and incubated in methanol with 3%
H2O2 for 30 min at 37°C to quench endogenous
peroxidase activity. After rinsing with TBST 3 times, sections were
incubated with 5% bovine serum albumin (Nanjing KeyGen Biotech Co.,
Ltd., Nanjing, China) dissolved in TBST for 30 min at 37°C to block
non-specific antibody binding. The sections were then rinsed with
TBST and incubated with primary antibodies (Abs) for 1 h at room
temperature in a humid chamber. Primary Abs used in the present
study included rabbit anti-TGF-β1 Ab (cat. no. sc-146;
1:100) and rabbit anti-TβRI Ab (cat. no. sc-398; 1:100; each Santa
Cruz Biotechnology, Inc., Dallas, TX, USA). Sections were rinsed in
TBST again and incubated with peroxidase-labeled polymers
conjugated to goat anti-rabbit immunoglobulin antibodies (cat. no.
E0432; 1:2,500; Dako; Agilent Technologies, Inc., Santa Clara, CA,
USA) for 1 h at room temperature. Finally, sections were developed
with 3,3′-diaminobenzidine, counterstained with hematoxylin for 3
min at room temperature and mounted under cover slips. Images from
each section were randomly collected using a light microscope
(Nikon 80i; Nikon; magnification, ×200). Results were evaluated
using a semi-quantitative technique, assigning a score of 0–1 based
on the percentage of positively stained cells (0, 0% stained cells;
0.1, <10% stained cells; 0.2, 10–20% stained cells; etc. and 1,
>80% stained cells).
Western blot analysis
Frozen liver tissue specimens were homogenized in
cell lysis buffer (cat. no. P0013; Beyotime Institute of
Biotechnology) at 4°C for the extraction of whole protein. Total
protein was also extracted from MFBs and HepG2 cells. The protein
concentration of samples were measured using a BCA Protein Assay
kit (cat. no. P0012S; Beyotime Institute of Biotechnology)
according to the manufacturer's protocol. Proteins from each sample
(50 µg/lane) were loaded on 10% polyacrylamide gels, separated by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS/PAGE) and transferred onto polyvinylidene difluoride membranes
(EMD Millipore, Billerica, MA, USA) by the wet transfer method.
Non-specific antibody binding was blocked with 5% skim milk powder
dissolved in TBST for 2 h at room temperature. The membranes were
then incubated with the primary antibodies overnight at 4°C, washed
3 times with TBST for 10 min each time and incubated with
corresponding secondary antibodies for 2 h at room temperature. The
membranes were washed 3 times with TBST for 10 min each time and
developed using an ECL chemiluminescence system (GE Healthcare Life
Sciences, Little Chalfont, UK). The primary antibodies utilized
were as follows: Rabbit anti-TGF-β1 Ab (cat. no. sc-146;
1:800; Santa Cruz Biotechnology, Inc.), rabbit anti-TβRI Ab (cat.
no. sc-398; 1:800; Santa Cruz Biotechnology, Inc.) and goat
anti-TβRII Ab (cat. no. sc-33929; 1:800; Santa Cruz Biotechnology,
Inc.), mouse anti-GAPDH Ab (cat. no. TA-08; dilution, 1:5,000;
Origene Technologies, Inc., Rockville, MD, USA), rabbit anti-GST-P1
Ab (cat. no. SAB3500265; 1:1,000; Sigma-Aldrich, Merck KGaA),
rabbit anti-Imp7 Ab (cat. no. ab99273; 1:5,000; Abcam, Cambridge,
UK) and rabbit anti-Imp8 Ab (cat. no. ab72109; 1:5,000; Abcam).
Peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG; H+L)
(cat. no. ZB-2305; dilution, 1:10,000; Origene Technologies, Inc.),
peroxidase-conjugated goat anti-rabbit lgG (H+L; cat. no. ZB-2301;
1:10,000; Origene Technologies, Inc.) and peroxidase-conjugated
rabbit anti-goat IgG (H+L; cat. no. ZB-2306; 1:10,000; Origene
Technologies, Inc.) were used as second Abs. GAPDH was used as the
internal control. Results were densitometrically analyzed by ImageJ
version 2 software (National Institutes of Health, Bethesda, MD,
USA). These experiments were repeated three times.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analyses were performed using SPSS 11.0 (SPSS, Inc.,
Chicago, IL, USA). Experimental and control groups were compared
using one-way analysis of variance followed by a post-hoc
Least-Significant-Difference test). P<0.05 was considered to
indicate a statistically significant difference.
Results
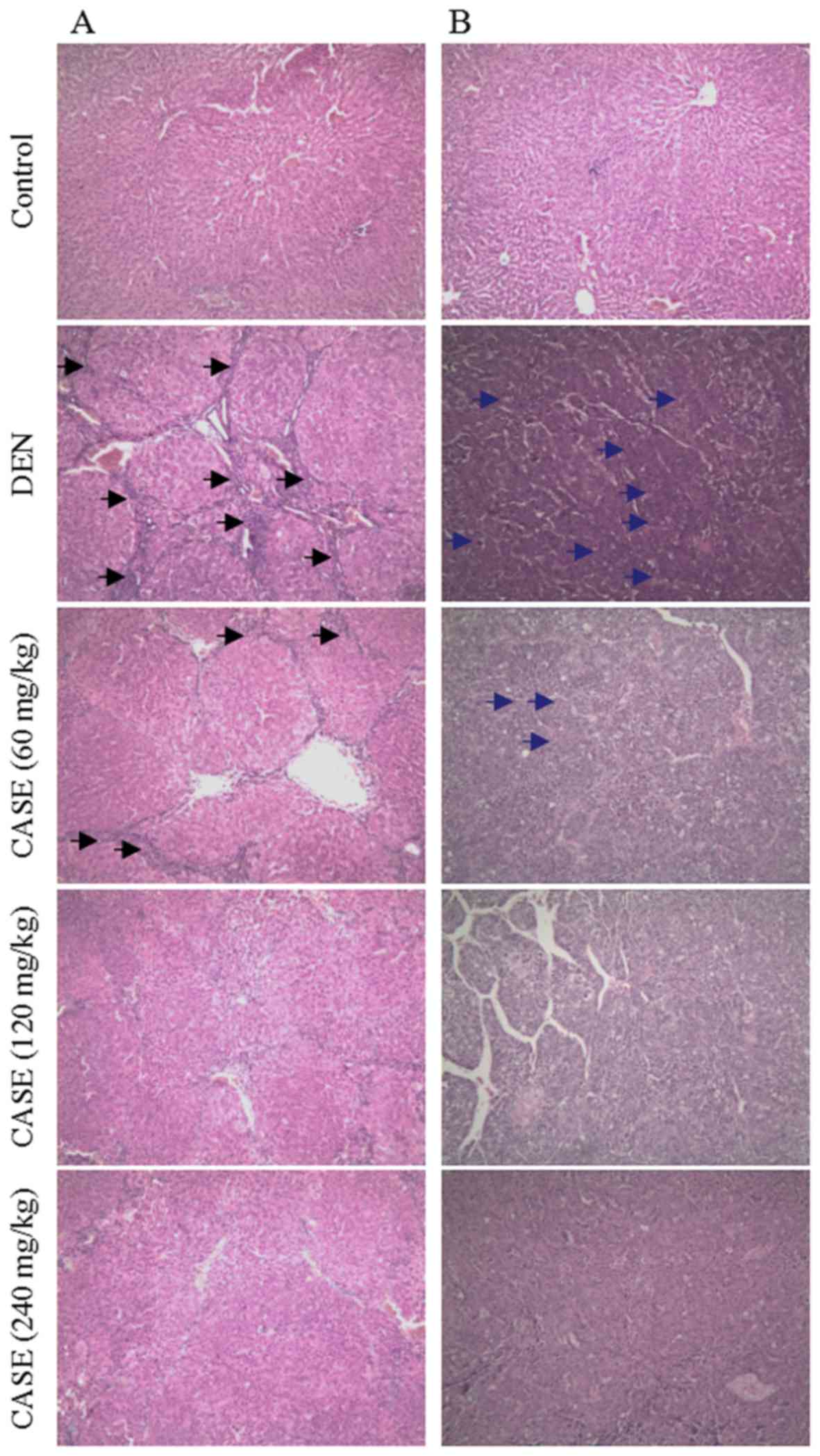
CASE decreases the degree of
histological changes in HCC
Representative hematoxylin and eosin-stained liver
sections are presented in Fig. 1. In
the DEN group, hepatic lobules were separated and/or encysted by
collagen bundles. Inflammatory cell infiltration and typical pseudo
lobule structures were observed in liver sections at week 12
(Fig. 1A). These pathological
changes were improved in the CASE treatment groups compared with
the DEN group. At week 16, HCC cells in the DEN group were poorly
differentiated with marked atypia and arranged in cord- or
crumby-like structures (Fig. 1B).
However, the degree of differentiation in the CASE treatment groups
increased in a dose-dependent manner, while the degree of HCC
malignancy decreased compared with the DEN group.
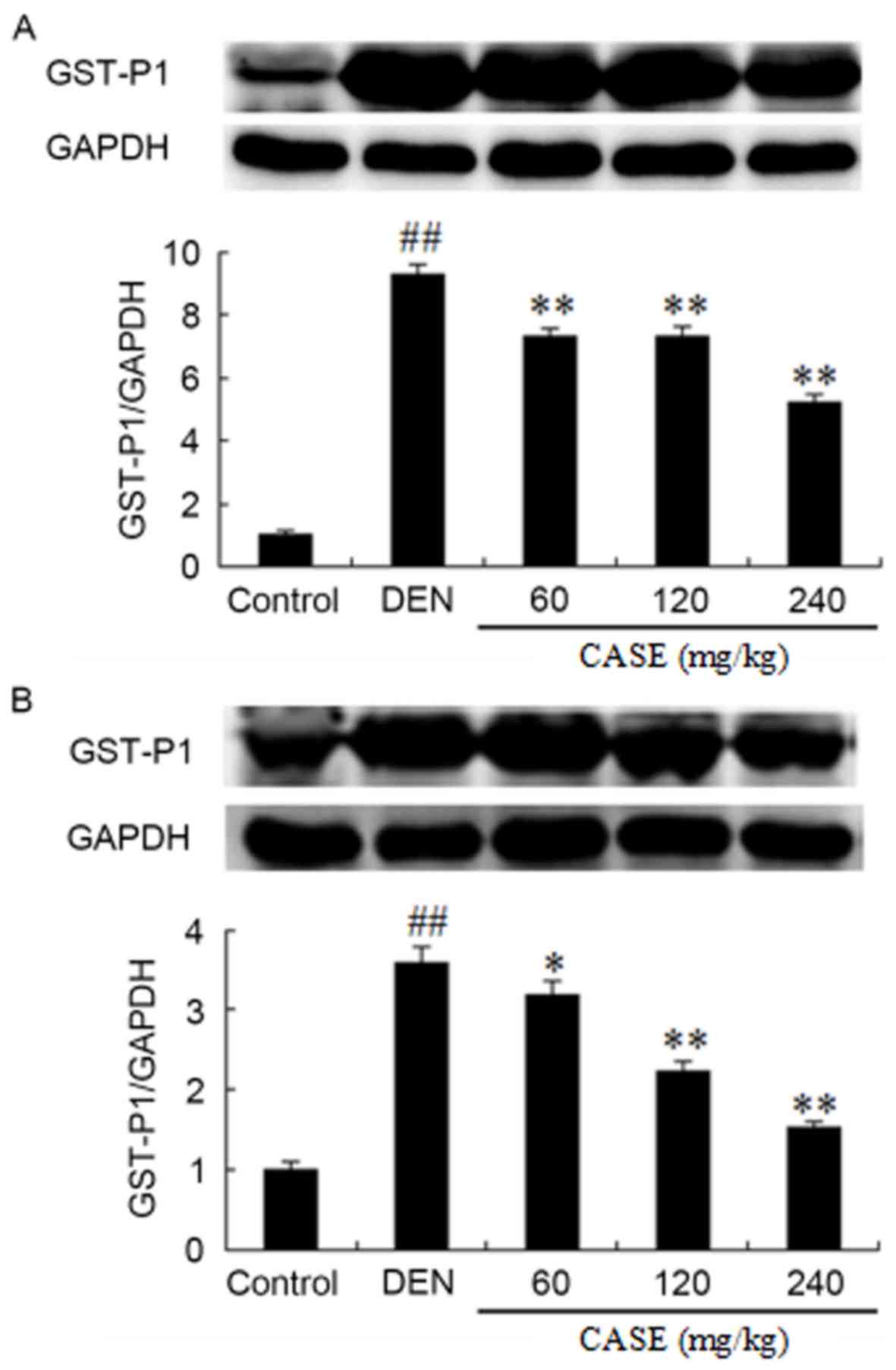
CASE downregulates GST-P1 protein
expression
DEN treatment significantly increased the expression
of GST-P1 protein in HCC tissues compared with the control groups
after week 12 (Fig. 2). CASE
treatment ameliorated DEN-induced GST-P1 upregulation in a
dose-dependent manner, especially CASE at the dose of 240 mg/kg,
which markedly decreased the level of GST-P1.
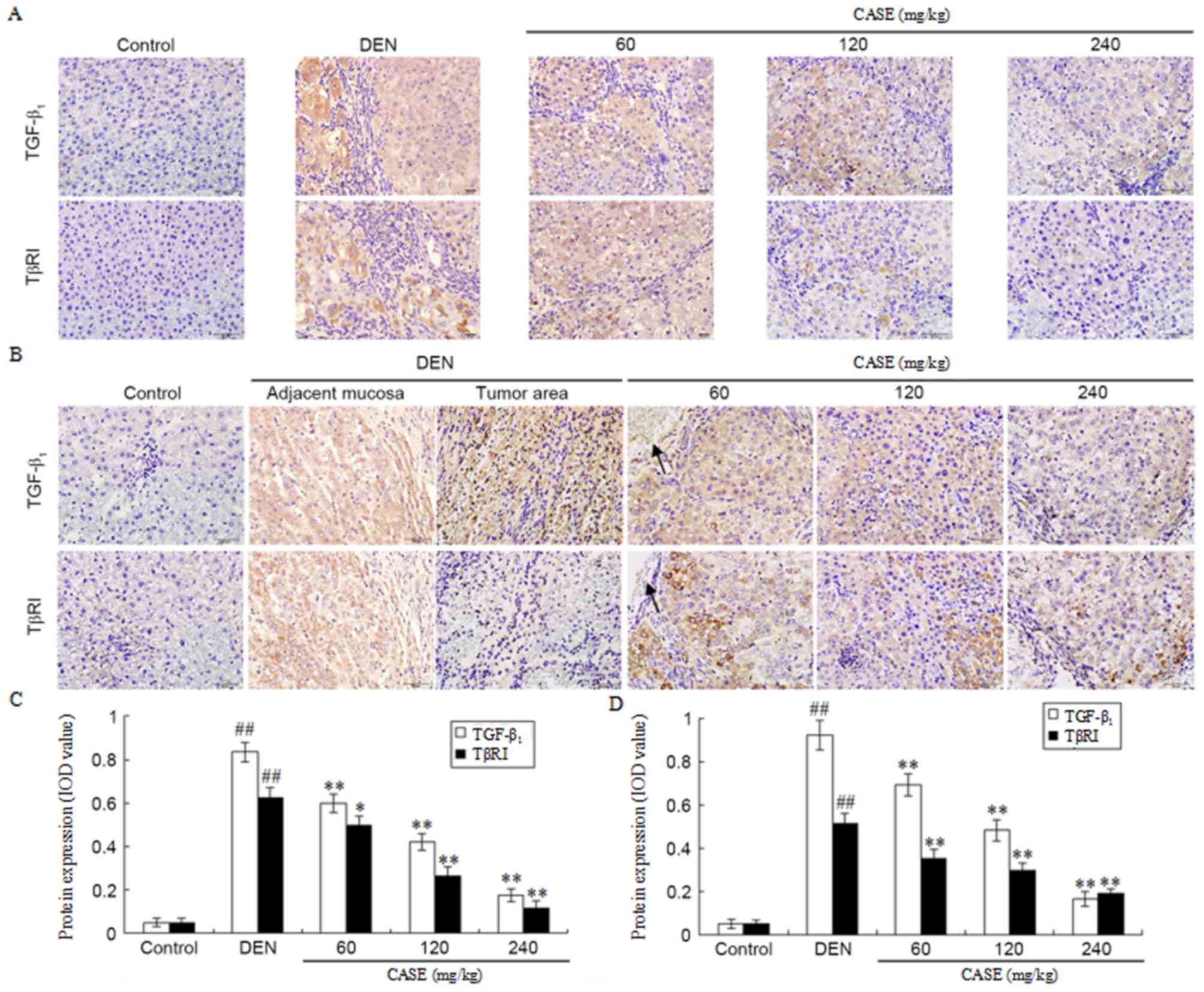
CASE decreases the protein expression
of TGF-β1, TβRI and TβRII
The number of TGF-β1- and TβRI-positive
immunoreactive cells were markedly increased in the DEN group
compared with the control group, but were decreased following CASE
treatment in a dose-dependent manner when compared with the DEN
group at week 12 (Fig. 3A). Positive
TGF-β1 staining was demonstrated in adjacent normal
liver tissues and hepatoma nodule areas, whilst positive TβRI
staining only occurred in adjacent normal liver tissues and was
markedly higher in DEN-treated rats compared with the control
group. Furthermore, the DEN-induced increase of TGF-β1-
and TβRI-positive cells was ameliorated by CASE treatment in a
dose-dependent manner when compared with the DEN group at week 16
(Fig. 3B). Additionally, The number
of TβRI-positive cells in the DEN group at week 16 was lower
compared with those at week 12. Semi-quantitative integral optical
density analysis revealed that there was significantly more
TGF-β1- and TβRI-positive staining in the DEN group
compared with the control group and the elevated number of
TGF-β1- and TβRI-positive cells was significantly
decreased following CASE treatment at week 12 (Fig. 3C) and week 16 (Fig. 3D).
The expression levels of TGF-β1, TβRI and
TβRII protein extracted from the liver tissues of rats at week 12
and 16 were measured. Compared with the control group, the
expression of TGF-β1 (Fig.
4A), TβRI (Fig. 4B) and TβRII
(Fig. 4C) increased significantly in
the DEN group, while these levels were downregulated following CASE
treatment in a dose-dependent manner when compared with the DEN
group at week 12. Additionally, CASE treatment significantly
decreased the DEN-induced increase of TGF-β1 (Fig. 4D), TβRI (Fig. 4E) and TβRII (Fig. 4F) expression compared with the DEN
group at week 16.
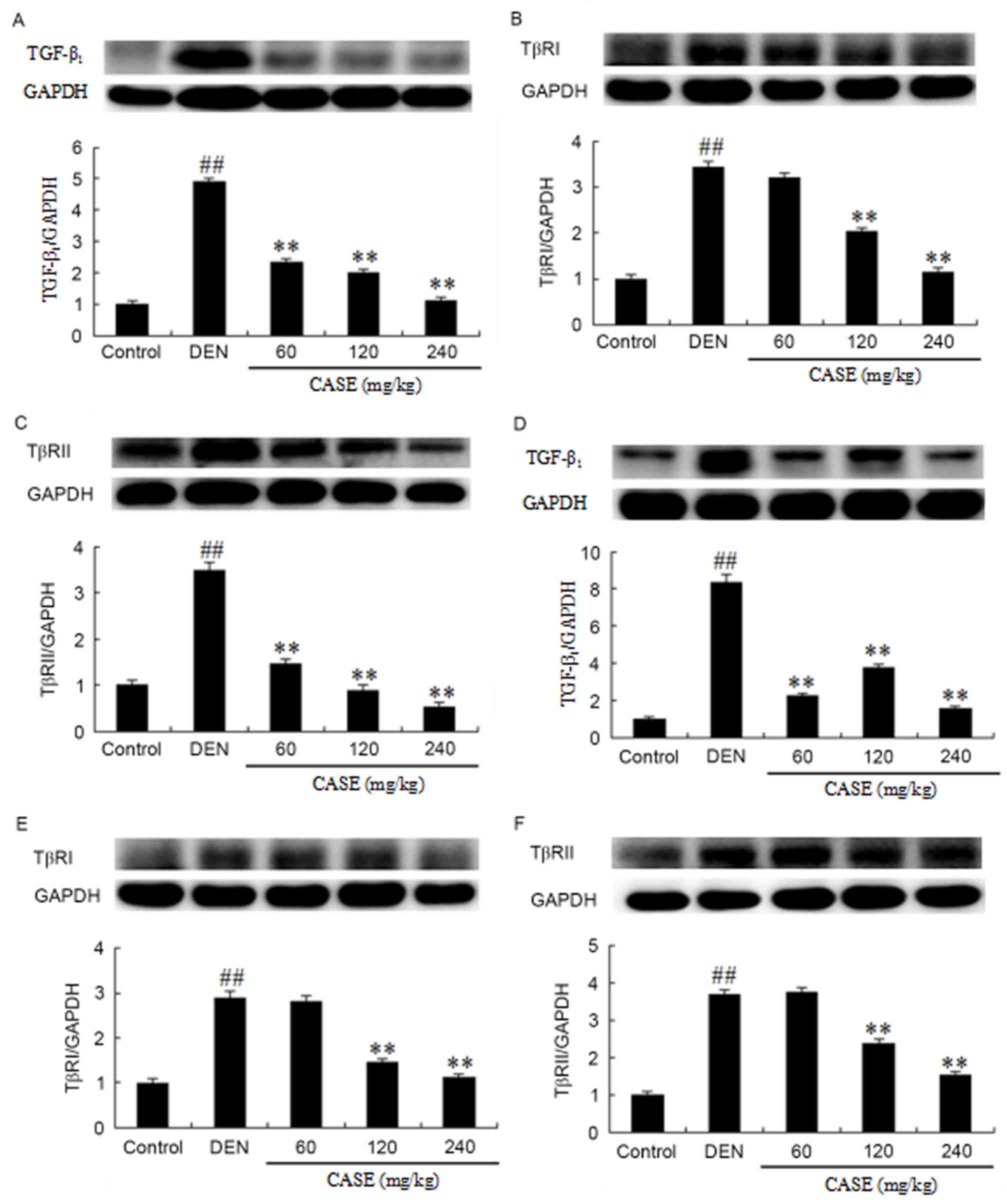 | Figure 4.CASE decreases the protein expression
of TGF-β1, TβRI and TβRII. The effects of CASE on
TGF-β1, TβRI and TβRII expression in rats treated with
DEN were assessed by western blotting (A-C) 12 and (D-F) 16 weeks
after the induction of hepatocellular carcinoma by DEN. The
proteins were extracted from frozen liver tissues.
TGF-β1, TβRI and TβRII proteins were analyzed using
anti-TGF-β1, -TβRI, -TβRII and -GAPDH antibodies.
Intensities of TGF-β1, TβRI and TβRII bands were
normalized to those of GAPDH in the corresponding treatment groups.
The ratios of the TGF-β1, TβRI or TβRII protein to GAPDH
in the normal groups were assigned a value of 1. Data are expressed
as mean ± standard deviation (n=3). ##P<0.01 vs. the
control group. **P<0.01 vs. the DEN group. DEN,
diethylinitrosamine; CASE, Compound Astragalus and Salvia
miltiorrhiza extract; TGF-β1, transforming growth factor
β-1; TβR, transforming growth factor-β receptor. |
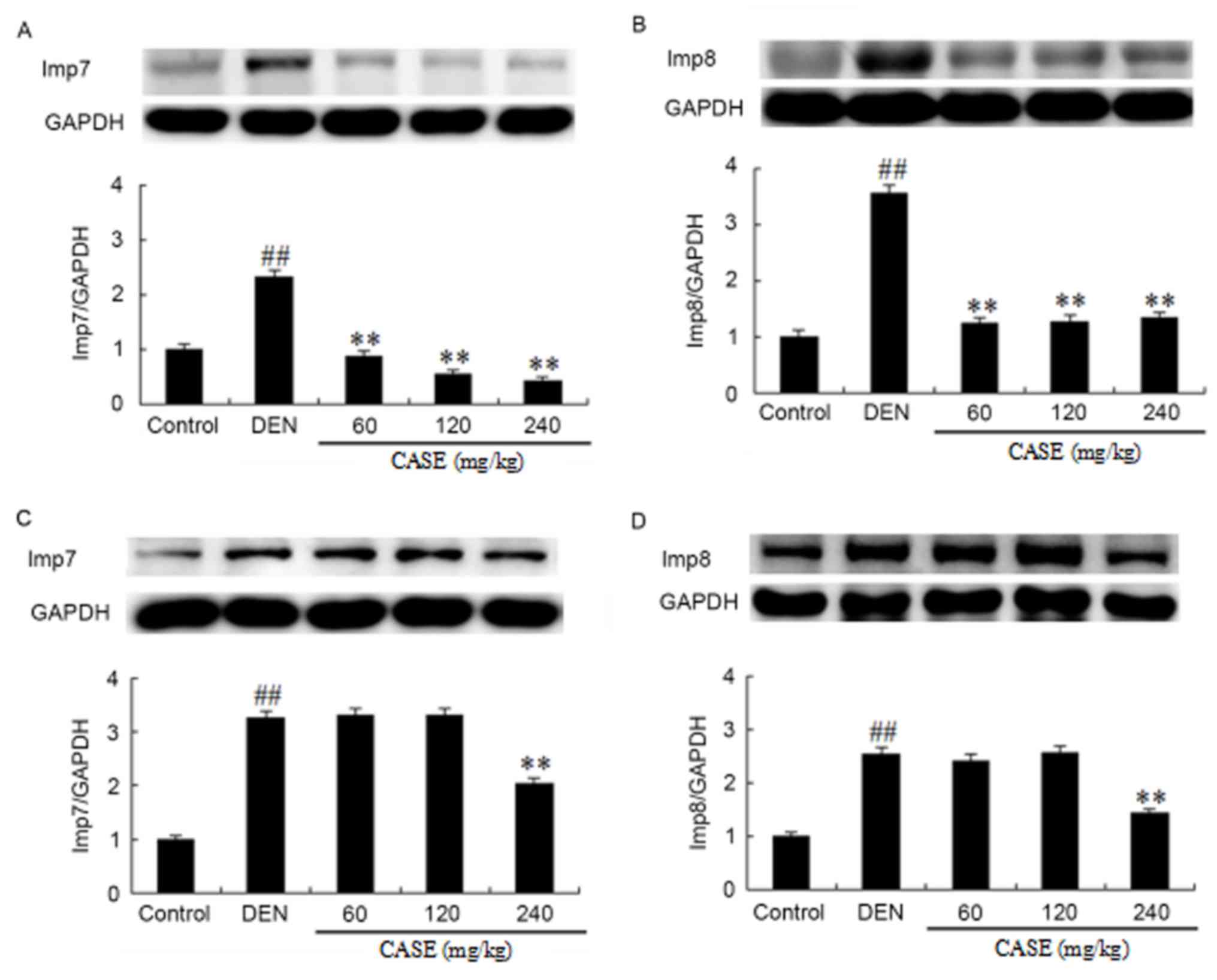
CASE decreases the expression of
Imp7/8
At week 12, DEN treatment induced a significant
increase in Imp7/8 protein expression compared with the control
group, while CASE treatment inhibited the DEN-induced
overexpression of Imp7/8 proteins during hepatocarcinogenesis
compared with the DEN group (Fig. 5A and
B). At week 16, DEN treatment significantly increased Imp7/8
protein expression compared with the control group. Simultaneously,
Imp7/8 expression was inhibited by CASE in the high dose (240
mg/kg) group, but not the low and middle dose (60 and 120 mg/kg,
respectively) groups compared with the DEN group (Fig. 5C and D).
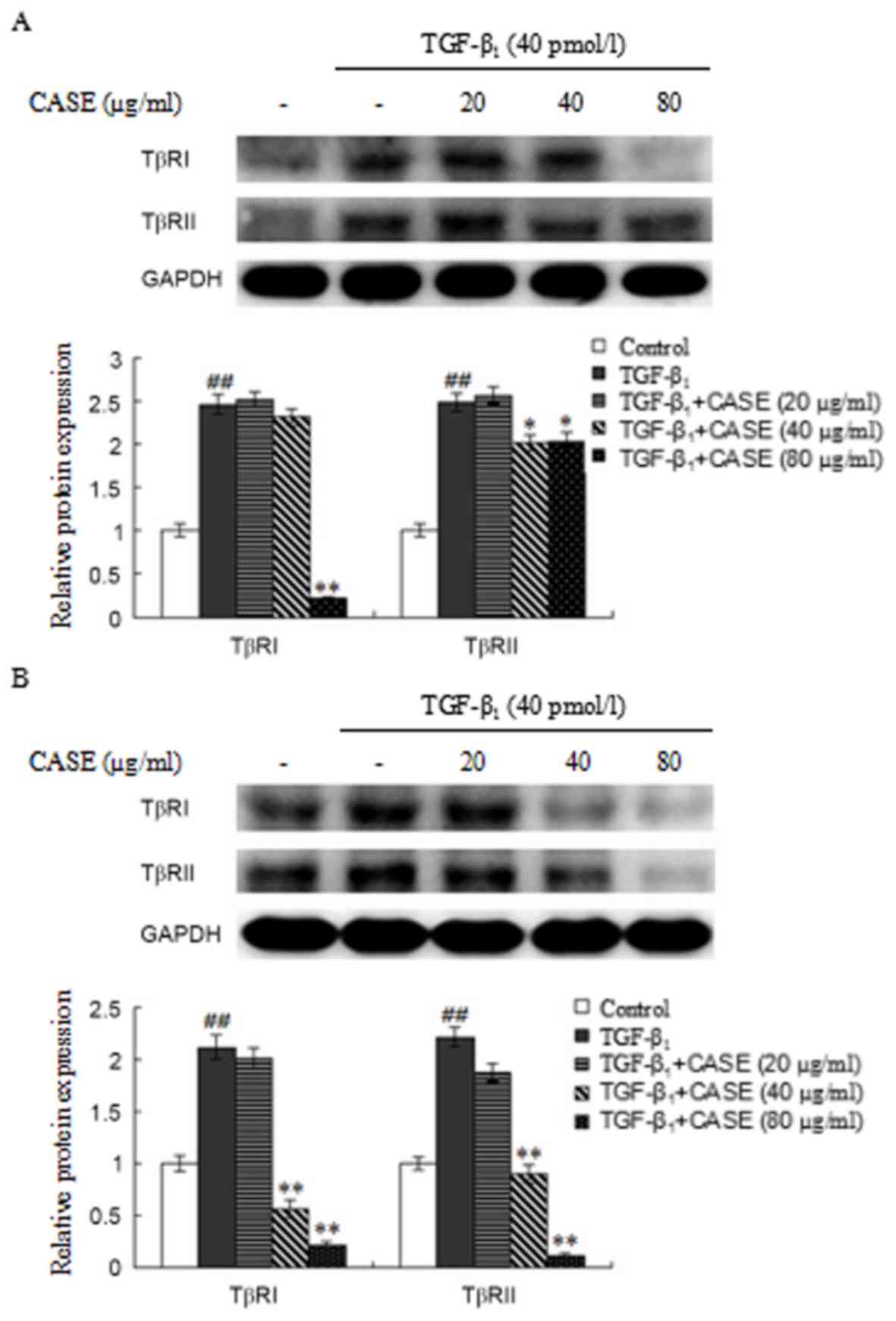
High and medium-dose CASE
downregulates TβRI and TβRII expression in MFBs and HepG2
cells
The expression of TβRI and TβRII in
TGF-β1-stimulated MFBs was assessed using western blot
analysis (Fig. 6A). Compared with
the control group, TβRI and TβRII expression was significantly
increased by TGF-β1-stimulation. CASE treatment
significantly decreased elevated TβRI protein levels at the highest
dose (80 µg/ml) and elevated TβRII protein levels at the medium and
high doses (40 and 80 µg/ml, respectively) compared with the
TGF-β1 group.
The expression of TβRI and TβRII protein in
TGF-β1-stimulated HepG2 cells was assessed using western
blot analysis (Fig. 6B). TβRI and
TβRII expression was significantly increased by
TGF-β1-stimulation compared with the control group. CASE
treatment (40 and 80 µg/ml) significantly decreased the
TGF-β1-induced elevation in TβRI and TβRII expression in
a dose-dependent manner compared with the TGF-β1
group.
Discussion
The results of the present study suggest that CASE
impedes DEN-induced hepatocarcinogenesis in rats via suppressing
GST-P1 expression and modulating upstream (TGF-β1, TβRI
and TβRII) and downstream (Imp7 and Imp8) mediators of the
TGF-β/Smad signaling pathway. Non-viral and non-alcohol-associated
hepatocarcinogenesis, including DEN-induced hepatocarcinogenesis,
typically progresses from chronic inflammation through fibrosis and
cirrhosis, finally becoming hepatocarcinogenesis (15). A number of studies have acknowledged
the link between chronic liver inflammation and
hepatocarcinogenesis, particularly HCC (16,17).
Similarly, other studies have indicated that the extent of fibrosis
and cirrhosis is directly associated with HCC progression (18,19).
Chronic inflammation, fibrosis and cirrhosis are therefore risk
factors for hepatocarcinogenesis. Pharmacological interventions
that are able to effectively disrupt or stop the collaboration
between chronic liver inflammation, fibrosis and cirrhosis have
become indispensable for reducing the risk factors of
hepatocarcinogenesis as well as limiting the progression and
severity of liver cancer.
Our research group previously demonstrated that CASE
attenuates DEN-induced hepatocarcinogenesis in rats via modulating
the TGF-β/Smad signaling pathway (6,10). CASE
inhibited the downstream mediators of the TGF-β/Smad signaling
pathway (Smad2 at the C-terminal and linker domains, Smad3
primarily at the linker phospho-domain and Smad2/3/4 complex) and a
TGF-β target gene (PAI-1), while it also upregulated inhibitory
Smad7 (6,10). However, it remained unclear whether
CASE could also modulate the upstream mediators of TGF-β/Smad
signaling (TGF-β1, TβRI and TβRII).
In the present study, a DEN-induced
hepatocarcinogenesis model was established in rats to imitate the
progression of liver cancer in humans and further investigate the
effects of CASE and its possible mechanisms. The preliminary
results revealed that CASE was able to significantly reduce the
incidence and multiplicity of HCC as well as levels of serum
biochemical indices, including alanine transaminase, aspartate
aminotransferase, albumin, alkaline phosphatase, total bilirubin,
direct bilirubin and gamma-glutamyltransferase (data not shown),
which are predictive of hepatic function. This confirmed the
results of an earlier study by our group (6). GST-P1 is a biomarker of neoplastic
cells (20) and has been used to
provide accurate assessments of HCC risk (21).
H&E staining and immunoblotting were used in the
present study to assess the expression of GST-P1. Liver tissues
from DEN-treated rats revealed increased GST-P1 protein expression,
which was associated with an increase in inflammatory cell
infiltration and the degree of fibrosis, as well as poor HCC cell
differentiation. However, CASE treatment significantly reversed the
effects of DEN treatment. Notably, CASE decreased GST-P1 protein
expression and the degree of fibrosis, as well as improving HCC
differentiation in a dose- and time-dependent manner. These
findings suggest that CASE exhibits a clear protective effect in
HCC and the mechanism involves inhibiting the expression of GST-P1
protein, the inflammatory reaction and fibrosis. Such inhibition is
achieved possibly via decreasing the synthesis and release of
pro-inflammatory and fibrogenic factors, including
TGF-β1.
The pathophysiological functions of
TGF-β1, particularly in hepatocarcinogenesis, are
directly associated with its dysregulated biosynthesis and over
secretion by cancerous cells (20).
Among the TGF-β family of cytokines, the TGF-β1 isoform
has been implicated as a crucial regulator of HCC progression
(22,23). TGF-β1 overexpression has
been reported in patients with cirrhosis (24) and HCC (25). However, TGF-β and its specific
serine/threonine kinase receptors (TβRI and TβRII) are crucial for
the TGF-β signaling cascade. Tan et al (26) demonstrated that TβRI and TβRII are
important molecules in TGF-β signaling. Accordingly, TβRI and TβRII
regulation was demonstrated to alter cellular responses to TGF-β
stimulation (27). The upstream
mediators of TGF-β serve roles in canonical TGF-β/Smad signaling to
regulate the transcription of target specific genes, which in turn
mediate the oncogenic roles of TGF-β (27). PAI-1 acts as the main inhibitor of
the urokinase-type plasminogen activator system; it also stimulates
cell migration and invasion by inhibiting cellular adhesion and
enhancing basement membrane degradation (28).
Canonical TGF-β signaling begins as a ligand
activation of constitutive transmembrane TβRII, which
transphosphorylates TβRI, leading to the TβRI-dependent
phosphorylation of Smad2 and Smad3 (23). Phosphorylated Smad2 and Smad3
oligomerize with Smad4 to form the Smad2/3/4 complex, which is
translocated to the nucleus via nuclear Imp7/8 proteins in order to
accurately target specific gene transcription (29,30).
Smad4 is crucial for the whole canonical signaling pathway,
particularly in terms spatio-temporal distribution (31). Smad4 is translocated into the nucleus
in of hetero-complex forms (typically the Smad3/4 and Smad2/3/4
complexes), where it modulates target gene expression by directly
binding with DNA or interacting with transcription factors,
co-activators and co-repressors (29). Yao et al (30) reported that nuclear import proteins,
including Imp7 and Imp8, were indispensable for the migration of
Smad4 from the cytoplasm to nucleus in TGF-β-stimulated Hela
cells.
The results of the present study suggest that CASE
significantly decreases the expression of TGF-β1 in the
livers of DEN-treated rats compared with those treated with DEN
alone and that this was associated with reduced inflammatory cell
infiltration and improved HCC differentiation. These results agree
in part with an earlier report by Liu et al (4), which revealed that Astragaloside IV,
the major active component of Astragalus membranaceus, could
decrease the level of TGF-β1. At the TGF-β-specific
receptor level, livers of DEN-treated rats exhibited increased TβRI
and TβRII staining, while CASE treatment significantly decreased
the expression of TβRI and TβRII proteins. Coincidentally, DEN
increased the protein expression of Imp7 and Imp8, while this
change was reversed by CASE treatment, particularly in the high
dose group (240 mg/kg), which is paralleled by the level of Smad4
protein in DEN-induced HCC reported in a previous study by our
group (10). The aforementioned
results demonstrate the ability of CASE to negatively modulate the
TGF-β/Smad signaling pathway in DEN-induced hepatocarcinogenesis at
multi-target levels and CASE's potential as an effective
hepatoprotective candidate drug.
To confirm the observed in vivo repression of
TβRI and TβRII by CASE, in vitro experiments were performed.
HSCs have been verified as a cell type vital to liver fibrogenesis
(32). The activation and
trans-differentiation of HSCs into MFBs by cytokines (primarily
TGF-β1) are considered to be central events in liver
fibrogenesis (33). MFBs directly
generate hepatocarcinogenesis by inducing autocrine TGF-β signaling
and nuclear β-catenin accumulation in neoplastic hepatocytes
(34). The HepG2 cell line is one of
the most common in vitro experimental models used in liver
cancer research and drug development (35). Therefore, in order to investigate the
effects of CASE on TβRI and TβRII, which are important targets of
the TGF-β signaling pathway, MFBs and HepG2 cells were used. The
results revealed that CASE decreased TβRI and TβRII expression in
MFBs and HepG2 cells. This coincides with the in vivo
results of the present study, implying that the receptors of TGF-β
may be likely direct targets in CASE's anti-HCC effects.
Collectively, the results of the current study indicate that CASE
suppresses DEN-induced hepatocarcinogenesis by downregulating
GST-P1 protein expression and negatively modulating the upstream
and downstream mediators of the TGF-β/Smad signaling pathway. These
results highlight that the multiple-target effects of CASE in the
prevention and treatment of HCC is a potential drug
candidature.
Acknowledgements
Not applicable.
Funding
The current study was supported by the National
Natural Science Foundation of China (grant nos. 81374012 and
81573652).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY designed and supervised the current study; CW,
HWK, MH, YFJ, JJW, JYW, XCY and AB conducted the experiments; CW,
HWK, MH and XL collected, analyzed and interpreted the data; CW, XL
and AB drafted the manuscript; and CW and YY were responsible for
the final revision of the manuscript.
Ethics approval and consent to
participate
The current study was approved by the Experimental
Animal Ethics Committee of Anhui Medical University (Hefei,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chiang JK and Kao YH: Predictors of high
healthcare costs in elderly patients with liver cancer in
end-of-life: A longitudinal population-based study. BMC Cancer.
17:5682017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kirstein MM and Vogel A: The pathogenesis
of hepatocellular carcinoma. Dig Dis. 32:545–553. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen KW, Ou TM, Hsu CW, Horng CT, Lee CC,
Tsai YY, Tsai CC, Liou YS, Yang CC, Hsueh CW and Kuo WH: Current
systemic treatment of hepatocellular carcinoma: A review of the
literature. World J Hepatol. 7:1412–1420. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu H, Wei W, Sun WY and Li X: Protective
effects of astragaloside iv on porcine-serum-induced hepatic
fibrosis in rats and in vitro effects on hepatic stellate cells. J
Ethnopharmacol. 122:502–508. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu C, Cao H, Zhou X, Dong C, Luo J, Zhang
C, Liu J and Ling Y: Meta-analysis of the clinical value of danshen
injection and huangqi injection in liver cirrhosis. Evid Based
Complement Alternat Med. 2013:8428242013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rui W, Xie L, Liu X, He S, Wu C, Zhang X,
Zhang L and Yang Y: Compound Astragalus and Salvia
miltiorrhiza extract suppresses hepatocellular carcinoma
progression by inhibiting fibrosis and PAI-1 mRNA transcription. J
Ethnopharmacol. 151:198–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang Y, Yang S, Chen M and Zhang X, Zou Y
and Zhang X: Compound Astragalus and Salvia
miltiorrhiza extract exerts anti-fibrosis by mediating
TGF-beta/smad signaling in myofibroblasts. J Ethnopharmacol.
118:264–270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang XX, Yang Y, Liu X, Wu C and Chen MZ:
Effects of compound traditional Astragalus and Salvia
miltiorrhiza extract on acute and chronic hepatic injury. TANG.
3:15.1–15.5. 2013.
|
|
9
|
Liu X, Yang Y, Zhang X, Xu S, He S, Huang
W and Roberts MS: Compound Astragalus and Salvia
miltiorrhiza extract inhibits cell invasion by modulating
transforming growth factor-beta/smad in HepG2 cell. J Gastroenterol
Hepatol. 25:420–426. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu X, Rui W, Wu C, He S, Jiang J, Zhang X
and Yang Y: Compound Astragalus and Salvia
miltiorrhiza extracts suppress hepatocarcinogenesis by
modulating transforming growth factor-β/smad signaling. J
Gastroenterol Hepatol. 29:1284–1291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McGrath JC, Drummond GB, McLachlan EM,
Kilkenny C and Wainwright CL: Guidelines for reporting experiments
involving animals: The ARRIVE guidelines. Br J Pharmacol.
160:1573–1576. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoshida K, Matsuzaki K, Mori S, Tahashi Y,
Yamagata H, Furukawa F, Seki T, Nishizawa M, Fujisawa J and Okazaki
K: Transforming growth factor-beta and platelet-derived growth
factor signal via c-jun n-terminal kinase-dependent smad2/3
phosphorylation in rat hepatic stellate cells after acute liver
injury. Am J Pathol. 166:1029–1039. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Furukawa F, Matsuzaki K, Mori S, Tahashi
Y, Yoshida K, Sugano Y, Yamagata H, Matsushita M, Seki T, Inagaki
Y, et al: p38 MAPK mediates fibrogenic signal through smad3
phosphorylation in rat myofibroblasts. Hepatology. 38:879–889.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu C, Jiang J, Boye A, Jiang Y and Yang Y:
Compound Astragalus and Salvia miltiorrhiza extract
suppresses rabbits' hypertrophic scar by modulating the TGF-β/Smad
signal. Dermatology. 229:363–368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen G, Dai ZK, Liang RG, Xiao SJ, He SQ,
Zhao HL and Xu Q: Characterization of diethylnitrosamine-induced
liver carcinogenesis in syrian golden hamsters. Exp Ther Med.
3:285–292. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsuzaki K: Modulation of TGF-beta
signaling during progression of chronic liver diseases. Front
Biosci (Landmark Ed). 14:2923–2934. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Q, Zhai YY, Dai JH, Li KY, Deng Q and
Hen ZG: SAMD9L inactivation promotes cell proliferation via
facilitating G1-S transition in hepatitis B virus-associated
hepatocellular carcinoma. Int J Biol Sci. 10:807–816. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Datta S, Ghosh A, Dasgupta D, Ghosh A,
Roychoudhury S, Roy G, Das S, Das K, Gupta S, Basu K, et al: Novel
point and combo-mutations in the genome of hepatitis B
virus-genotype D: Characterization and impact on liver disease
progression to hepatocellular carcinoma. PloS One. 9:e1100122014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ren W, Qi X, Yang Z, Han G and Fan D:
Prevalence and risk factors of hepatocellular carcinoma in
budd-chiari syndrome: A systematic review. Eur J Gastroenterol
Hepatol. 25:830–841. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ando N, Shimizu M, Okuno M,
Matsushima-Nishiwaki R, Tsurumi H, Tanaka T and Moriwaki H:
Expression of retinoid X receptor alpha is decreased in
3′-methyl-4-dimethylaminoazobenzene-induced hepatocellular
carcinoma in rats. Oncol Rep. 18:879–884. 2007.PubMed/NCBI
|
|
21
|
De Mattia E, Cecchin E, Polesel J,
Bignucolo A, Roncato R, Lupo F, Crovatto M, Buonadonna A, Tiribelli
C and Toffoli G: Genetic biomarkers for hepatocellular cancer risk
in a caucasian population. World J Gastroenterol. 23:6674–6684.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu W, Huang C, Wang Q, Huang T, Ding Y, Ma
C, Ma H and Chen W: MEF2 transcription factors promotes EMT and
invasiveness of hepatocellular carcinoma through TGF-β1
autoregulation circuitry. Tumour Biol. 35:10943–10951. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meindl-Beinker NM, Matsuzaki K and Dooley
S: TGF-β signaling in onset and progression of hepatocellular
carcinoma. Dig Dis. 30:514–523. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hidaka H, Nakazawa T, Shibuya A, Minamino
T, Takada J, Tanaka Y, Okuwaki Y, Watanabe M and Koizumi W: Effects
of 1-year administration of olmesartan on portal pressure and
TGF-beta1 in selected patients with cirrhosis: A randomized
controlled trial. J Gastroenterol. 46:1316–1323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee D, Chung YH, Kim JA and Lee YS, Lee D,
Jang MK, Kim KM, Lim YS, Lee HC and Lee YS: Transforming growth
factor beta 1 overexpression is closely related to invasiveness of
hepatocellular carcinoma. Oncology. 82:11–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tan Y, Xu Q, Li Y, Mao X and Zhang K:
Crosstalk between the p38 and TGF-β signaling pathways through
TβRI, TβRII and Smad3 expression in plancental choriocarcinoma
JEG-3 cells. Oncol Lett. 8:1307–1311. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu C, Xu P, Lamouille S, Xu J and Derynck
R: TACE-mediated ectodomain shedding of the type I TGF-beta
receptor downregulates TGF-beta signaling. Mol Cell. 35:26–36.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gramling MW and Church FC: Plasminogen
activator inhibitor-1 is an aggregate response factor with
pleiotropic effects on cell signaling in vascular disease and the
tumor microenvironment. Thromb Res. 125:377–381. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shi Y and Massague J: Mechanisms of
TGF-beta signaling from cell membrane to the nucleus. Cell.
113:685–700. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yao X, Chen X, Cottonham C and Xu L:
Preferential utilization of Imp7/8 in nuclear import of smads. J
Biol Chem. 283:22867–22874. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dong XM, Yin RH, Yang Y, Feng ZW, Ning HM,
Dong L, Zheng WW, Tang LJ, Wang J, Jia YX, et al: GATA-2 inhibits
transforming growth factor-β signaling pathway through interaction
with Smad4. Cell signal. 26:1089–1097. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Puche JE, Saiman Y and Friedman SL:
Hepatic stellate cells and liver fibrosis. Compr Physiol.
3:1473–1492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee UE and Friedman SL: Mechanisms of
hepatic fibrogenesis. Best Pract Res Clin Gastroenterol.
25:195–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mikula M, Proell V, Fischer AN and
Mikulits W: Activated hepatic stellate cells induce tumor
progression of neoplastic hepatocytes in a TGF-beta dependent
fashion. J Cell Physiol. 209:560–567. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qiu GH, Xie X, Xu F, Shi X, Wang Y and
Deng L: Distinctive pharmacological differences between liver
cancer cell lines HepG2 and Hep3B. Cytotechnology. 67:1–12. 2015.
View Article : Google Scholar : PubMed/NCBI
|