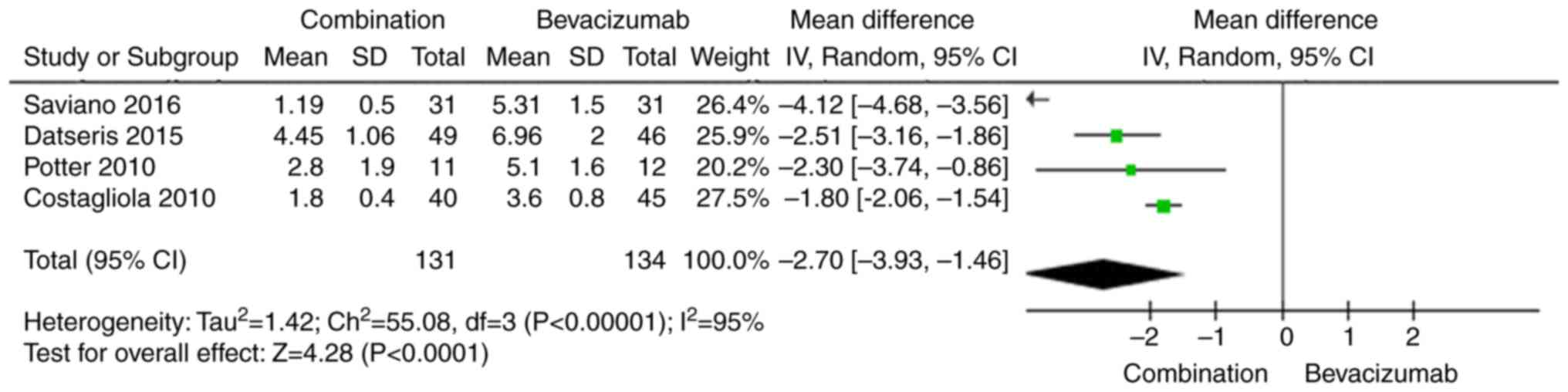
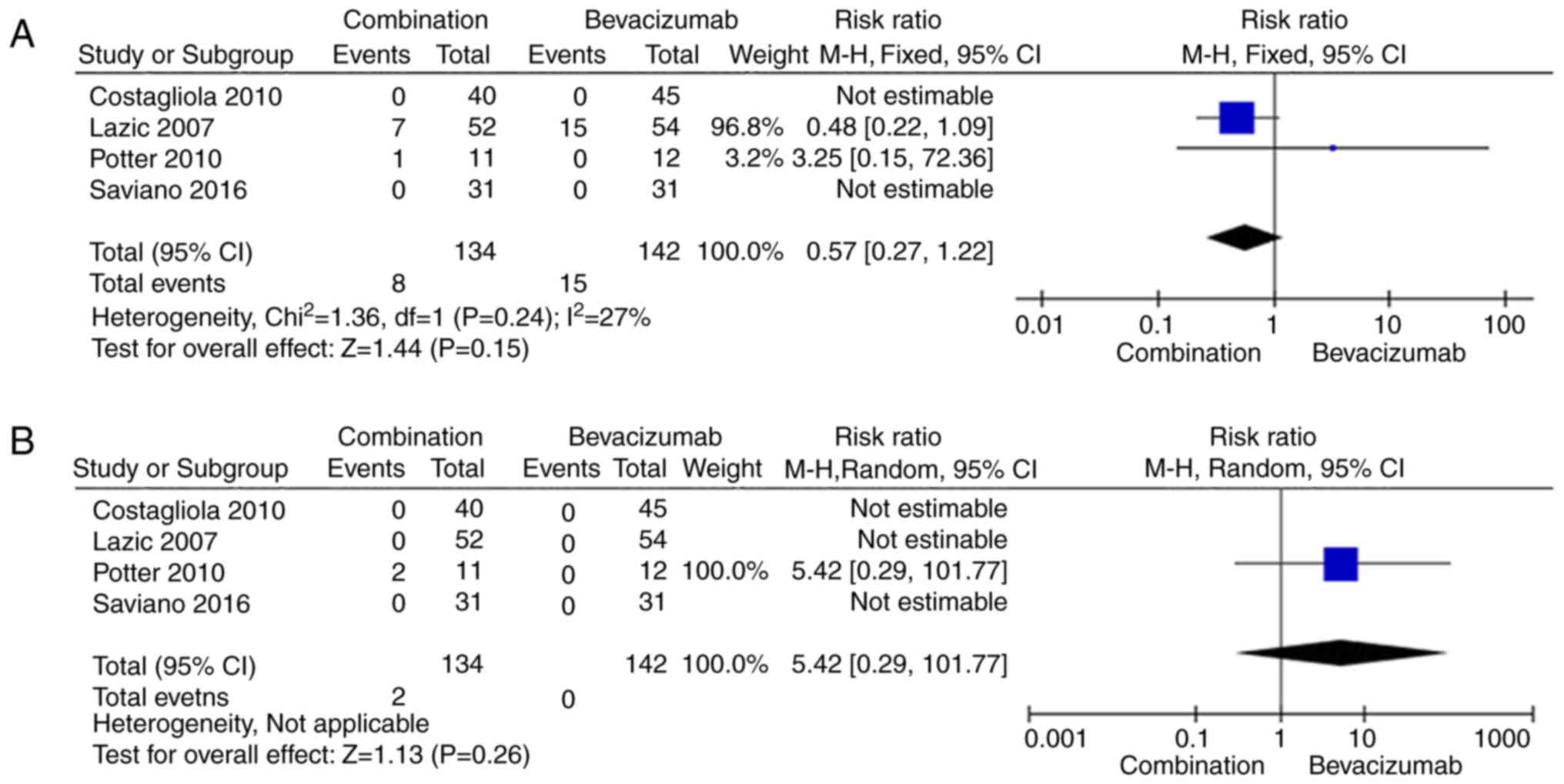
|
1
|
Bressler NM: Age-related macular
degeneration is the leading cause of blindness. JAMA.
291:1900–1901. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Position of the Retinological Society, the
German Ophthalmological Society and the Professional Association of
Ophthalmologists in Germany on the current therapeutic
possibilities for neovascular age-related macular degeneration.
Klin Monbl Augenheilkd. 224:559–566. 2007.PubMed/NCBI
|
|
3
|
Aiello LP, Northrup JM, Keyt BA, Takagi H
and Iwamoto MA: Hypoxic regulation of vascular endothelial growth
factor in retinal cells. Arch Ophthalmol. 113:1538–1544. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Buch H, Vinding T and Nielsen NV:
Prevalence and causes of visual impairment according to World
Health Organization and United States criteria in an aged, urban
Scandinavian population: The Copenhagen City Eye Study.
Ophthalmology. 108:2347–2357. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mitchell P, Annemans L, White R, Gallagher
M and Thomas S: Cost effectiveness of treatments for wet
age-related macular degeneration. Pharmacocconomics. 29:107–131.
2011. View Article : Google Scholar
|
|
6
|
Shao J, Choudhary MM and Schachat AP:
Neovascular age-related macular degeneration. Dev Ophthalmol.
55:125–136. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
EI-Mollayess GM, Noureddine BN and
Bashshur ZF: Bevacizumab and neovascular age related macular
degeneration: Pathogenesis and treatment. Semin Ophthalmol.
26:69–76. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leydolt C, Michels S, Prager F, Garhoefer
G, Georgopoulos M, Polak K and Schmidt-Erfurth U: Effect of
intravitreal bevacizumab (Avastin) in neovascular age-related
macular degeneration using a treatment regimen based on optical
coherence tomography: 6- and 12-month results. Acta Ophthalmol.
88:594–600. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takahashi M, Sato T and Kishi S:
Intravitreal bevacizumab for age-related macular degeneration with
good visual acuity. Jpn J Ophthalmol. 54:565–570. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Berg K, Hadzalic E, Gjertsen I, Forsaa V,
Berger LH, Kinge B, Henschien H, Fossen K, Markovic S, Pedersen TR,
et al: Ranibizumab or bevacizumab for neovascular age-related
macular degeneration according to the lucentis compared to avastin
study treat-and-extend protocol: Two-year results. Ophthalmology.
123:51–59. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Berg K, Pedersen TR, Sandvik L and
Bragadóttir R: Comparison of ranibizumab and bevacizumab for
neovascular age-related macular degeneration according to LUCAS
treat-and-extend protocol. Ophthalmology. 122:146–152. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moja L, Lucenteforte E, Kwag KH, Bertele
V, Campomori A, Chakravarthy U, D'Amico R, Dickersin K, Kodjikian
L, Lindsley K, et al: Systemic safety of bevacizumab versus
ranibizumab for neovascular age-related macular degeneration.
Cochrane Database Syst Rev. 15:CD011230. 2014. View Article : Google Scholar
|
|
13
|
Chen G, Li W, Tzekov R, Jiang F, Mao S and
Tong Y: Bevacizumab versus ranibizumab for neovascular age-related
macular degeneration: A meta-analysis of randomized controlled
trials. Retina. 35:187–193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kodjikian L, Decullier E, Souied EH,
Girmens JF, Durand EE, Chapuis FR and Huot L: Bevacizumab and
ranibizumab for neovascular age-related macular degeneration: An
updated meta-analysis of randomised clinical trials. Graefes Arch
Clin Exp Ophthalmol. 252:1529–1537. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang W and Zhang X: Systemic adverse
events after intravitreal bevacizumab versus ranibizumab for
age-related macular degeneration: A meta-analysis. PLoS One.
9:e1097442014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Solomon SD, Lindsley KB, Krzystolik MG,
Vedula SS and Hawkins BS: Intravitreal bevacizumab versus
ranibizumab for treatment of neovascular age-related macular
degeneration: Findings from a cochrane systematic review.
Ophthalmology. 123:70–77.e1. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stein JD, Newman-Casey PA, Mrinalini T,
Lee PP and Hutton DW: Cost-effectiveness of bevacizumab and
ranibizumab for newly diagnosed neovascular macular degeneration.
Ophthalmology. 121:936–945. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fong KC, Kirkpatrick N, Mohamed Q and
Johnston RL: Intravitreal bevacizumab (Avastin) for neovascular
age-related macular degeneration using a variable frequency regimen
in eyes with no previous treatment. Clin Experiment Ophthalmol.
36:748–755. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanchanaranya N, Rojdamrongratana D and
Piyasoonthorn P: Incidence of post-intravitreal anti-VEGF
endophthalmitis at Thammasat University Hospital. J Med Assoc Thai.
98:489–494. 2015.PubMed/NCBI
|
|
20
|
Haddock LJ, Ramsey DJ and Young LH:
Complications of subspecialty ophthalmic care: Endophthalmitis
after intravitreal injections of anti-vascular endothelial growth
factor medications. Semin Ophthalmol. 29:257–262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lyall DA, Tey A, Foot B, Roxburgh ST,
Virdi M, Robertson C and MacEwen CJ: Post-intravitreal anti-VEGF
endophthalmitis in the United Kingdom: Incidence, features, risk
factors, and outcomes. Eye (Lond). 26:1517–1526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stewart MW: Endophthalmitis after
injections of anti-vascular endothelial growth factor drugs.
Retina. 31:1981–1982. 2011.PubMed/NCBI
|
|
23
|
Inoue M, Kobayakawa S, Sotozono C, Komori
H, Tanaka K, Suda Y, Matsushima H, Kinoshita S, Senoo T, Tochikubo
T and Kadonosono K: Evaluation of the incidence of endophthalmitis
after intravitreal injection of anti-vascular endothelial growth
factor. Ophthalmologica. 226:145–150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carneiro AM, Barthelmes D, Falcão MS,
Mendonça LS, Fonseca SL, Gonçalves RM, Faria-Correia F and
Falcão-Reis FM: Arterial thromboembolic events in patients with
exudative age-related macular degeneration treated with
intravitreal bevacizumab or ranibizumab. Ophthalmologica.
225:211–221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schmidt-Erfurth UM and Pruente C:
Management of neovascular age-related macular degeneration. Prog
Retin Eye Res. 26:437–451. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Incorvaia C, Campa C, Parmeggiani F,
Menzione M, D'Angelo S, Della Corte M, Rinaldi M, Romano M,
Dell'omo R and Costagliola C: 12-month retrospective study and
review of photodynamic therapy with verteporfin for subfoveal
choroidal neovascularization in age-related macular degeneration.
Retina. 28:289–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yonekawa Y and Kim IK: Clinical
characteristics and current treatment of age-related macular
degeneration. Cold Spring Harb Perspect Med. 5:a0171782014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ladewig MS, Karl SE, Hamelmann V, Helb HM,
Scholl HP, Holz FG and Eter N: Combined intravitreal bevacizumab
and photodynamic therapy for neovascular age-related macular
degeneration. Graefes Arch Clin Exp Ophthalmol. 246:17–25. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim HW, Kim JL, Lee MH, Yoo HG, Chung IY
and Lee JE: Combined treatment of photodynamic therapy and
bevacizumab for choroidal neovascularization secondary to
age-related macular degeneration. Korean J Ophthalmol. 25:231–237.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Costagliola C, Romano MR, Rinaldi M,
dell'Omo R, Chiosi F, Menzione M and Semeraro F: Low fluence rate
photodynamic therapy combined with intravitreal bevacizumab for
neovascular age-related macular degeneration. Br J Ophthalmol.
94:180–184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kaiser PK; Registry of Visudyne AMD
Therapy Writing Committee. Boyer DS, Garcia R, Hao Y, Hughes MS,
Jabbour NM, Kaiser PK, Mieler W, Slakter JS, et al: Verteporfin
photodynamic therapy combined with intravitreal bevacizumab for
neovascular age-related macular degeneration. Ophthalmology.
116:747–755. 755.e12009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lazic R and Gabric N: Verteporfin therapy
and intravitreal bevacizumab combined and alone in choroidal
neovascularization due to age-related macular degeneration.
Ophthalmology. 114:1179–1185. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Datseris I, Kontadakis GA, Diamanti R,
Datseris I, Pallikaris IG, Theodossiadis P and Tsilimbaris MK:
Prospective comparison of low-fluence photodynamic therapy combined
with intravitreal bevacizumab versus bevacizumab monotherapy for
choroidal neovascularization in age-related macular degeneration.
Semin Ophthalmol. 30:112–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Potter MJ, Claudio CC and Szabo SM: A
randomised trial of bevacizumab and reduced light dose photodynamic
therapy in age-related macular degeneration: the VIA study. Br J
Ophthalmol. 94:174–179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Saviano S, Leon PE, Mangogna A and
Tognetto D: Combined therapy (intravitreal bevacizumab plus
verteporfin photodynamic therapy) versus intravitreal bevacizumab
monotherapy for choroidal neovascularization due to age-related
macular degeneration: A 1-year follow-up study. Digit J Ophthalmol.
22:46–53. 2016.PubMed/NCBI
|
|
36
|
Jadad AR, Moore RA, Carroll D, Jenkinson
C, Reynolds DJ, Gavaghan DJ and McQuay HJ: Assessing the quality of
reports of randomized clinical trials: Is blinding necessary?
Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nowak-Sliwinska P, Van den Bergh H,
Sickenberg M and Koh AH: Photodynamic therapy for polypoidal
choroidal vasculopathy. Prog Retin Eye Res. 37:182–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dabkeviciene D, Sasnauskiene A, Leman E,
Kvietkauskaite R, Daugelaviciene N, Stankevicius V, Jurgelevicius
V, Juodka B and Kirveliene V: mTHPC-mediated photodynamic treatment
up-regulates the cytokines VEGF and IL-1alpha. Photochem Photobiol.
88:432–439. 2012. View Article : Google Scholar : PubMed/NCBI
|