Introduction
Age-related macular degeneration (AMD) is the
leading cause of irreversible vision loss in people older than 50 y
of age in the developed world (1).
Although the neovascular form accounts for only 20% of AMD cases,
it is the main cause of severe vision loss in almost 90% of
patients with AMD (2,3). Choroidal neovascularization (CNV) is
the primary pathology underlying wet AMD and is a pathological form
of neovascularization that can cause bleeding, hemorrhage,
fibrosis, and retinal pigment epithelium and neurosensory
functional damage, eventually resulting in vision loss (4).
At present, there are many treatments available for
AMD. The most common treatments are verteporfin photodynamic
therapy (PDT) and anti-vascular endothelial growth factor (VEGF)
drugs administered via intraocular injection (5). Bevacizumab is a humanized anti-VEGF
monoclonal IgG1 antibody that binds to all isoforms of VEGF and can
therefore prevent angiogenesis (6).
Off-label use of bevacizumab has been shown to be reasonably safe
and very effective in the treatment of AMD (7–9). Several
studies have shown that bevacizumab and ranibizumab have equivalent
efficacy and comparable safety (10–15).
Bevacizumab is still widely used for this off-label indication not
only because it has a mode of action similar to that of ranibizumab
but also because its cost is considerably lower than that of
ranibizumab (16,17). However, bevacizumab monotherapy
requires multiple reinjections (18)
and is associated with the risk of endophthalmitis, cataract
formation and uveitis (19–23), as well as an increased risk of
thromboembolic events (24).
PDT with verteporfin has historically been the
standard treatment for AMD and has been shown to benefit patients
affected by classic CNV in the setting of AMD (25–27).
Combining PDT with bevacizumab may reduce the number of required
treatments and preserve or even enhance visual acuity. Increasing
numbers of studies have reported that PDT combined with
intravitreal bevacizumab is an effective option for patients with
AMD, as this combination facilitates improvements in visual acuity,
decreases in central retinal thickness (CRT) and reductions in the
number of retreatments (28–31). Studies comparing combination therapy
with monotherapy have reported that combination therapy
significantly improves visual acuity compared to monotherapy
(32). However, the ideal
maintenance regimen for this combination remains an area of
scientific debate. Four clinical randomized controlled trials
(RCTs) showed that there were no significant differences in visual
gain between patients receiving the combination of PDT and
bevacizumab and patients receiving monotherapy, although these
studies found that the combination of the two agents reduced
reinjection rates (30,33–35).
Therefore, we performed a meta-analysis of RCTs to
compare the efficacy and safety of the combination of verteporfin
PDT and intravitreal bevacizumab therapy with those of bevacizumab
monotherapy in patients with AMD.
Materials and methods
Search strategy
A systematic English language search of PubMed,
EMBASE and the Cochrane Central Register of Controlled Trials for
human studies published up to October 2017 was conducted, with
language restrictions. Key terms included AMD, bevacizumab, avastin
and PDT. The search was restricted to RCTs. We manually searched
the reference lists of all original studies and review articles
identified by the electronic search to identify other potentially
eligible articles.
Inclusion criteria
We selected the following studies: i) studies
including patients with active CNV secondary to AMD; ii) studies
featuring a randomized controlled trial (RCT) design comparing the
combination of bevacizumab and PDT with bevacizumab monotherapy and
iii) studies measuring at least one outcome of interest.
Exclusion criteria
The following studies were excluded: i) studies that
were not RCTs; ii) studies of CNV not caused by AMD and iii)
unpublished conference abstracts.
Data extraction and quality
assessment
Titles and abstracts were reviewed by two reviewers
using the above selection criteria. Full-text versions of all
relevant studies were obtained for detailed evaluations. Data
extraction and quality assessments were conducted using the
modified Jadad assessment tool (36). Disagreements were resolved via
consensus after discussion. The following data were extracted from
each study: the name of the first author, the study design, and the
major inclusion and exclusion criteria, as well as information
regarding study population characteristics (age, sex, no. of eyes
in the study), intervention groups, follow-up durations and
outcomes (ocular and systemic adverse effects). Data regarding
changes in best-corrected visual acuity (BCVA), the numbers of
patients with gains of more than 15 letters, the average numbers of
bevacizumab retreatments, and changes in CRT were also
extracted.
Statistical analysis
The meta-analysis was conducted using RevMan v.5.3
software. Risk ratios (RRs) were measured using 95% confidence
intervals (CIs) for dichotomous data, while weighted mean
differences (WMDs) were measured using 95% CIs for continuous data.
Standard mean differences (SMDs) were used when all the trials
assessed the same outcomes in a variety of ways. The Q test or
I2 test was used to evaluate heterogeneity. An
I2 value of >50% accompanied with a P-value <0.05
for the Q test was determined to indicate the presence of
significant heterogeneity. Both fixed-effects and random-effects
models were used to obtain summary RRs, WMDs or SMDs. In the
absence of heterogeneity between groups, the fixed-effects model
and random-effects model yielded concordant results. When
heterogeneity was significant, the random effects model was
employed. Potential publication bias was estimated using the Egger
test and by visually evaluating a funnel plot.
Results
Literature search
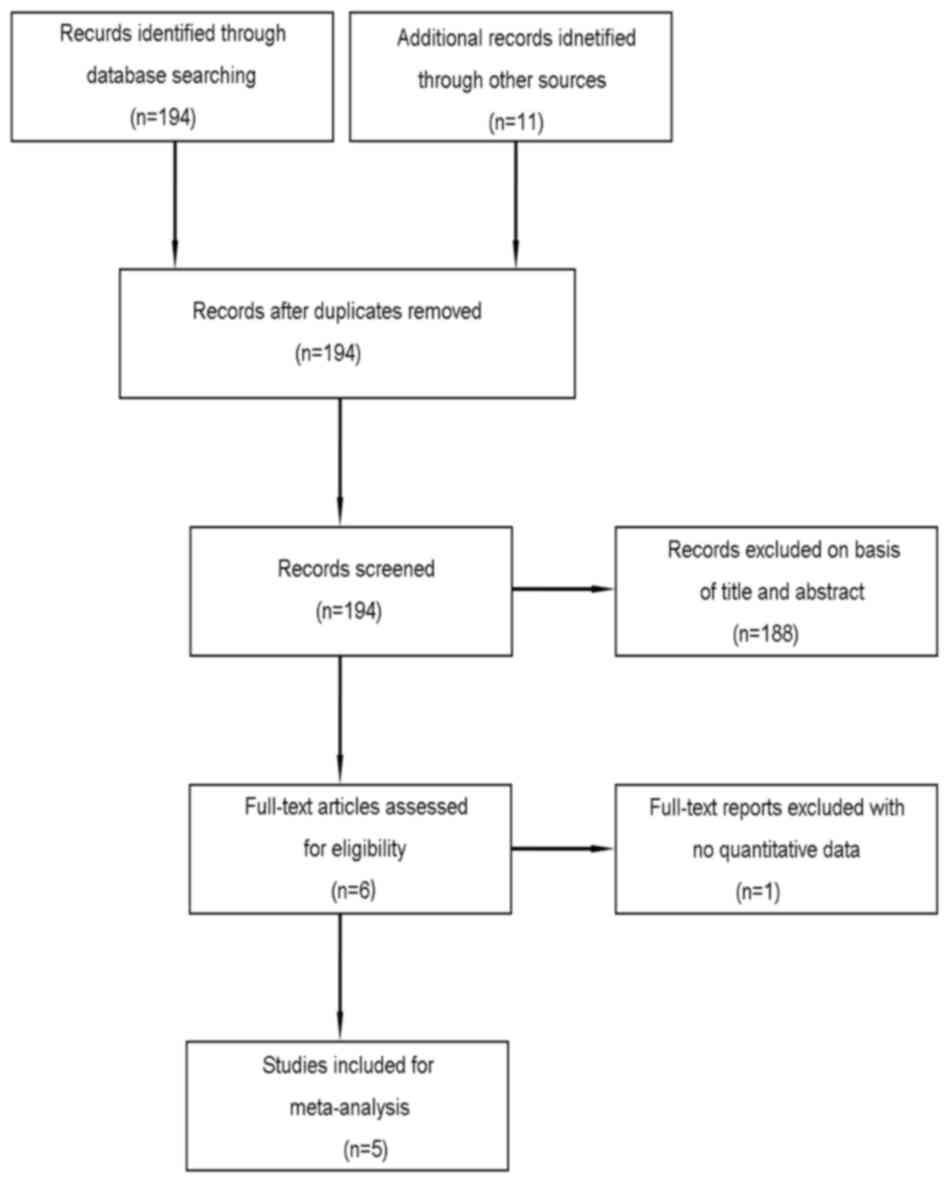
A flow chart of the selection process used to
identify eligible studies is shown in Fig. 1. A total of 205 articles were
initially identified. After duplicates were screened for
potentially relevant articles, 194 articles were deemed eligible
for further evaluation. We screened the titles and abstracts of
these articles and identified 6 eligible studies. We subsequently
read the text of each article and found 1 from the same study
group, which was excluded. Ultimately, five published (30,32–35)
articles were eligible for analysis.
Included studies
The basic characteristics of the five included
studies are shown in Table I. Sample
sizes ranged from 23 to 106 eyes. Mean patient ages ranged from
63.2 to 83.4 years. The dose of bevacizumab was 1.25 mg in the
bevacizumab monotherapy groups of the included studies. The doses
of verteporfin PDT and bevacizumab were 25 J/cm2
standard fluence (SF) and 1.25 mg, respectively, in the combination
therapy groups of the included studies, with the exception of the
Lazic and Gabric (32) study, in
which the dose of PDT was not mentioned. Moreover, the duration of
follow-up varied from 3 to 12 months among the studies. The five
studies were assessed regarding methodological quality, according
to the Jadad score and were determined to be of high quality.
 | Table I.Study characteristics of the included
four RCTs. |
Table I.
Study characteristics of the included
four RCTs.
| Author (Ref.) | Design | Inclusion
criteria | Sex (M/F) | Mean age,
years | No. of eyes | Intervention
groups | Follow-up
(months) | Jadad score |
|---|
| Costagliola 2010,
(30) | RCT | Naïve classic or
predominantly classic subfoveal CNV secondary to AMD | All: 38/47 | Group 1: 65.3 Group
2: 63.2 | Group 1: 45 Group
2: 40 | Group 1: IVB (1.25
mg) Group 2: IVB (1.25 mg) +PDT (25 J/cm2) | 12 | 3 |
| Datseris 2015,
(33) | RCT | Predominantly
classic and occult CNV due to AMD | All: 29/66 | Group 1: 74 Group
2: 73 | Group 1: 46 Group
2: 49 | Group 1: IVB (1.25
mg) Group 2: IVB (1.25 mg) +PDT (25 J/cm2) | 12 | 3 |
| Lazic and Gabric
2007, (32) | RCT | Minimally classic
or occult CNV due to AMD | Group 1: 17/37
Group 2: 18/34 Group 3: 15/35 | Group 1: 76.1 Group
2: 75.4 Group 3: 75.6 | Group 1: 54 Group
2: 52 Group 3: 50 | Group 1:IVB (1.25
mg) Group 2: IVB (1.25 mg) Group 3: PDT | 3 | 4 |
| Potter 2010,
(34) | RCT | New-onset CNV
secondary to AMD | Group 1: 3/8 | Group 1: 83.4 | Group 1: 11 | Group 1: IVB (1.25
mg) +PDT (25 J/cm2) | 6 | 5 |
|
|
|
| Group 2: 4/8 | Group 2: 78.3 | Group 2: 12 | Group 2: IVB (1.25
mg) +PDT (12 J/cm2) |
|
|
|
|
|
| Group 3: 4/8 | Group 3: 80.6 | Group 3: 12 | Group 3: IVB (1.25
mg) +sham PDT |
|
|
| Saviano 2016,
(35) | RCT | AMD-related
CNV | Group 1: 9/22 Group
2: 12/19 | Group 1: 77 Group
2: 31 | Group 1: 31 Group
2: 31 | Group 1: IVB (1.25
mg) Group 2: IVB (1.25 mg) +PDT (25 J/cm2) | 12 | 2 |
Estimation of outcomes
Changes in mean BCVA compared with
baseline
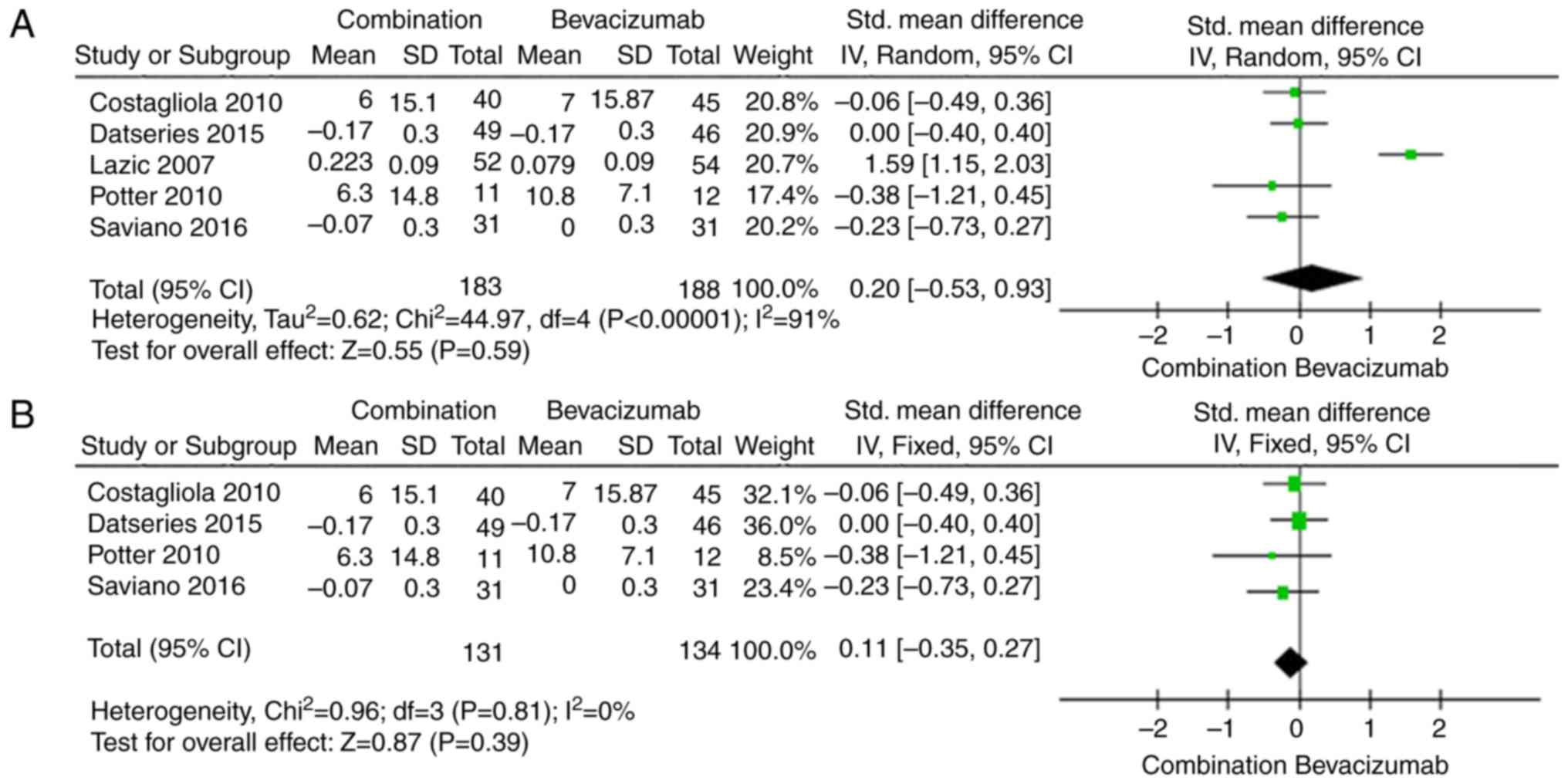
Visual acuity was the most important outcome measure
with respect to treatment efficacy. The results regarding changes
in mean BCVA are shown in Fig. 2A.
There were no significant differences in changes in BCVA between
the combination group and bevacizumab group (SMD 0.20; 95% CI
−0.53, 0.93, P=0.59). The random-effects model was used due to the
high heterogeneity of the effect size (I2=91%,
P<0.00001). The dose of PDT is not mentioned in Lazic and Gabric
(32) study and this may have
heterogeneous. So we removed the Lazic and Gabric (32) study to apply the sensitivity analysis
and found that the result of statistical analysis was still
insignificant (SMD, −0.11; 95% CI −0.35, 0.13, P=0.39; Fig. 2B).
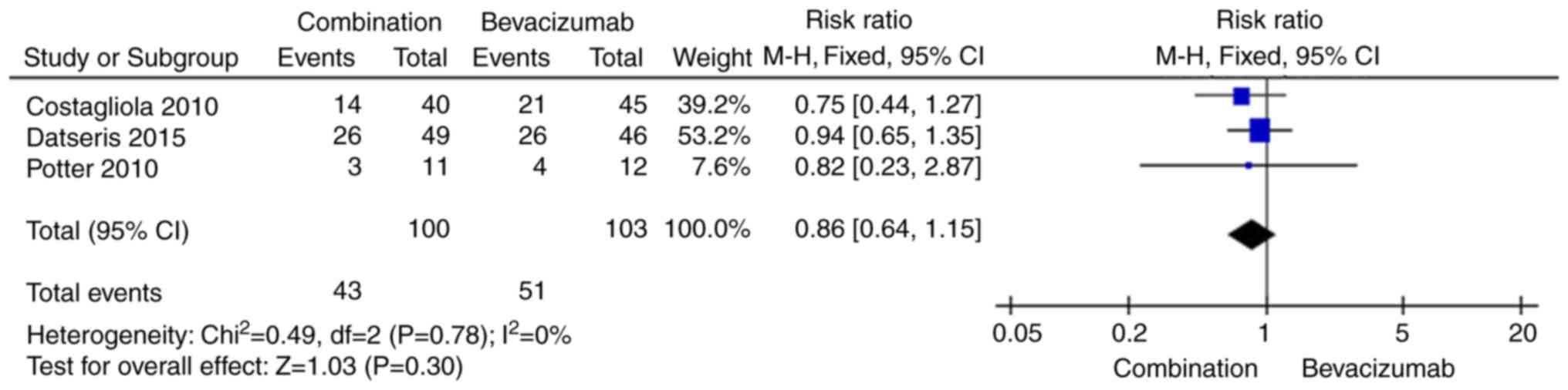
Number of patients who gained more
than 15 letters
We extracted the number of patients who gained more
than 15 letters. Because the data were not heterogeneous
(I2=0%, P=0.78), the fixed-effects model was used. The
pooled RR showed that there was no significant difference between
the two intervention groups regarding the number of patients who
gained more than 15 letters (RR 0.86, 95% CI 0.64, 1.15, P=0.30;
Fig. 3).
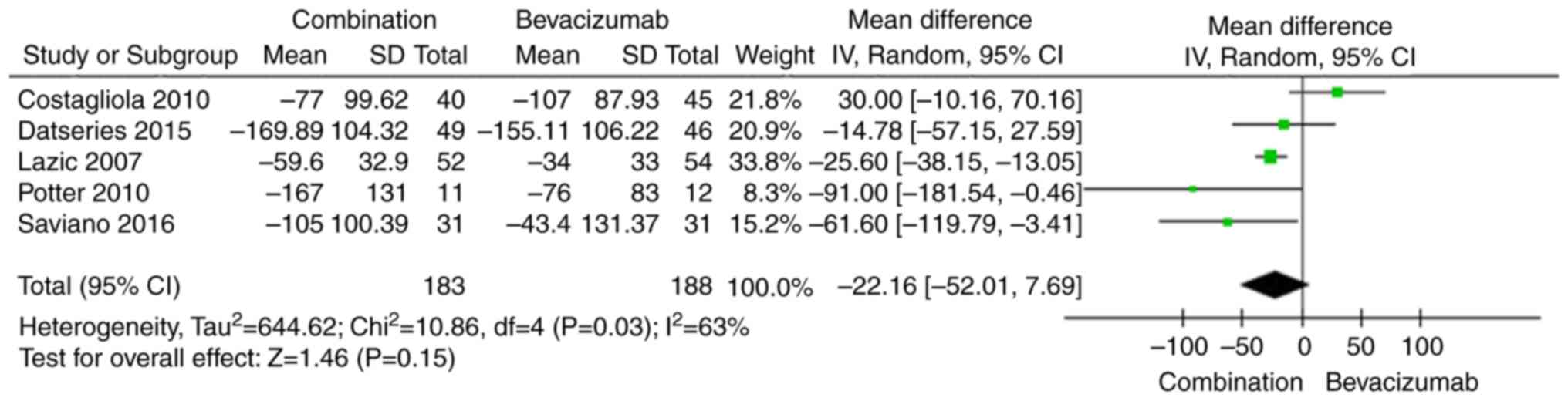
CRT
CRT is the most important anatomical change
associated with AMD treatment. The effects of the combination of
bevacizumab and PDT on CRT compared with those of bevacizumab
monotherapy are shown in Fig. 4. The
pooled results indicate that there was no significant difference
between the two groups regarding changes in CRT (WMD −22.16, 95% CI
−52.01 to 7.69, P=0.15).
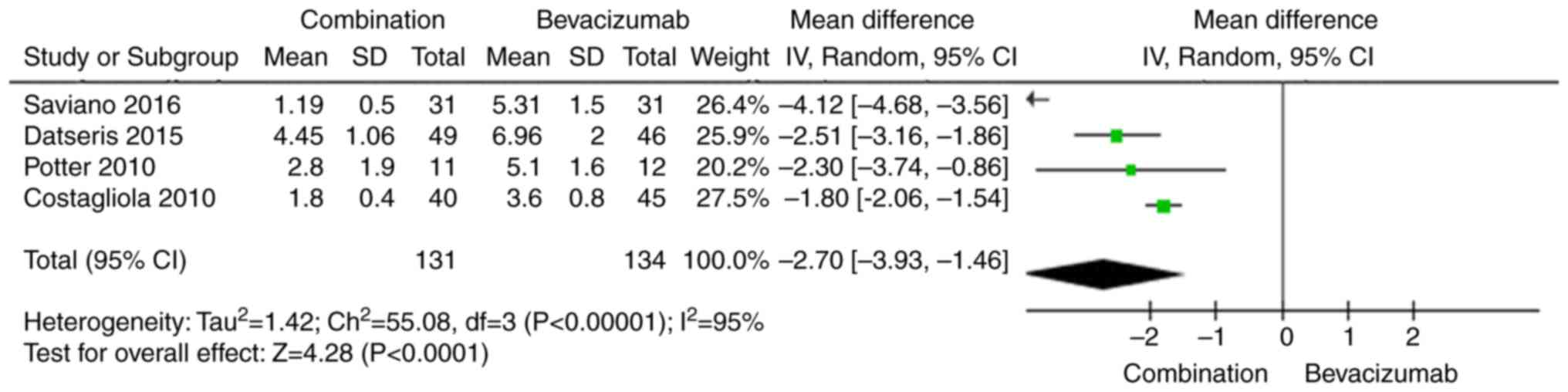
Average number of bevacizumab
retreatments
Three studies measured the average number of
bevacizumab retreatments. The pooled results indicate that the
average number of bevacizumab retreatments was significantly lower
in the combination therapy group than in the bevacizumab
monotherapy group (WMD −2.70, 95% CI −3.93 to −1.46, P<0.0001;
Fig. 5). The random-effects model
was used due to the high heterogeneity of the effect size
(I2=95%, P<0.00001).
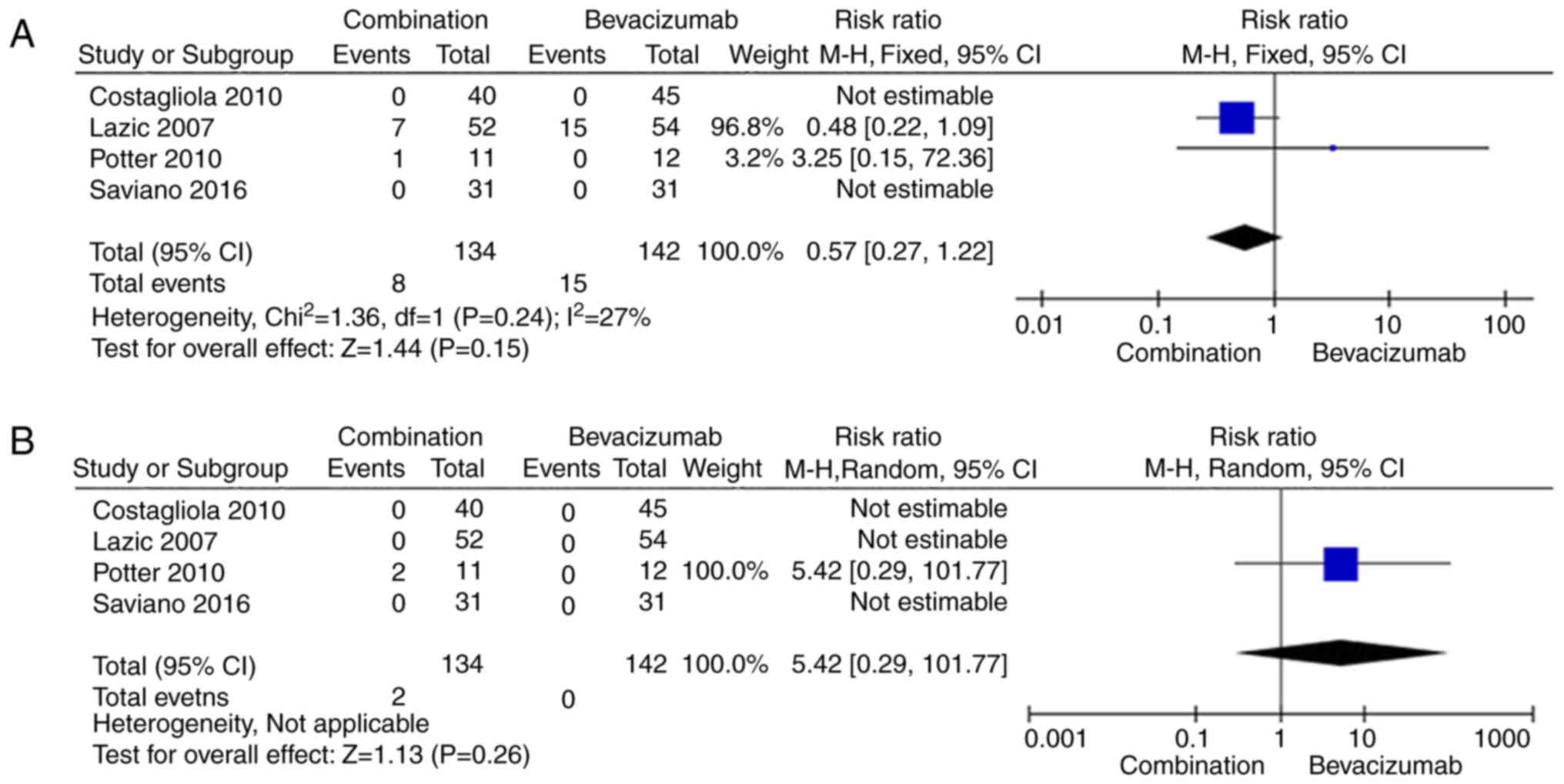
Adverse events
Four (30,32,34,35) of
five studies [excluding Datseris et al (33)] reported data regarding ocular adverse
events and systematic adverse events. We compared the numbers of
ocular adverse events and non-ocular adverse events in the
combination group with those in the bevacizumab monotherapy group
and noted that four were no significant differences between the two
intervention groups with respect to those parameters (Fig. 6). All adverse events reported in the
abovementioned three studies are shown in Table II.
 | Table II.Main ocular adverse events and
systemic adverse events reported in the three studies. |
Table II.
Main ocular adverse events and
systemic adverse events reported in the three studies.
|
| Combination | Bevacizumab |
|---|
|
|
|
|
|---|
| Side effects | Events | Total | Incidence (%) | Events | Total | Incidence (%) |
|---|
| Ocular adverse
events |
| Pigment
epithelium tears | 0 | 103 | 0 | 3 | 111 | 2.7 |
|
Posterior vitreous
detachments | 4 | 103 | 3.9 | 8 | 111 | 7.2 |
|
Cataract progressions | 3 | 103 | 2.9 | 4 | 111 | 3.6 |
| Vision
loss ≥20 letters | 1 | 103 | 1 | 0 | 111 | 0 |
|
Non-ocular adverse events | 1 | 103 | 1 | 0 | 111 | 0 |
|
Hypertension | 1 | 103 | 1 | 0 | 111 | 0 |
|
Myocardial infraction | 1 | 103 | 1 | 0 | 111 | 0 |
|
Mortalitya |
Heterogeneity, sensitivity analysis,
and publication bias
The dose of PDT is not mentioned in one study and
this may have heterogeneous. After excluding the study, the
analysis results not changed. A sensitivity analysis was conducted
to assess the stability of the results by sequential removal of
individual studies. When the analysis result is high heterogeneity,
we use random effects model. These sensitivity analyses indicated
that our conclusions were generally robust. Funnel plots and Egger
test were not used because there were less than ten studies for
each comparison.
Discussion
In this meta-analysis, we assessed four RCTs
including 371 patients (183 patients in the combination group and
188 patients in the bevacizumab group). Lazic and Gabric (32), noted significant improvements in BCVA
at 3 months after combination therapy with verteporfin PDT and
intravitreal bevacizumab. Some studies observed that there was no
significant differences in visual acuity improvement between the
bevacizumab monotherapy group and combination therapy group
(30,33–35). The
results of our meta-analysis indicated that the bevacizumab
monotherapy group experienced improvements in BCVA similar to those
of the combination therapy group, indicating that the efficacies of
the two therapy regimens were similar with respect to this
parameter. We assessed the numbers of patients who gained more than
15 letters and determined that there was no significant difference
between two groups with respect to this parameter. Regarding mean
changes in CRT, bevacizumab monotherapy demonstrated efficacy
equivalent to that of combination therapy. PDT does not have a
destructive impact on patient vision, as is the case with older
treatments, and stabilizes wet AMD progression (37). However, the combination of PDT and
bevacizumab can result in more rapid and permanent CNV occlusion
(8), resulting in increased ocular
VEGF levels (38). These findings
may explain the similar efficacies exhibited by the two treatment
regimens.
Reinjection rates were significantly lower in
patients treated with combination therapy than in patients treated
with bevacizumab monotherapy. In this meta-analysis, we noted that
the average number of bevacizumab reinjections in the combination
group was lower than that in the bevacizumab monotherapy group.
These findings support the hypothesis that combination treatment
exerts synergistic effects, resulting in a reduced need for
subsequent injections compared with monotherapy. Thus, combination
therapy may be a more cost-effective option than monotherapy for
the treatment of neovascular AMD.
The included RCTs indicated that both treatments
were safe. The majority of adverse events associated with
bevacizumab monotherapy and combination treatment were of moderate
severity. No serious adverse events, such as death or
endophthalmitis, were noted in any of the included RCTs. One
patient died of a stroke (34).
Ocular adverse events occurred more frequently in the bevacizumab
monotherapy group than in the combination therapy group, most
likely due to the use of intravitreal injections in the former
group. The most significant side effects associated with the two
treatments were posterior vitreous detachments and cataracts. Other
side effects, such as increased anterior chamber cell pigment
epithelium tears and vision loss of more than 20 letters, were
reported in three studies. However, there was no difference in the
incidence of ocular adverse events between the two groups. Systemic
adverse events, such as hypertension and myocardial infraction,
were reported only in the combination therapy group of one study
(34). We noted no significant
difference in the incidence of adverse events between the two
groups, findings consistent with those of related clinical trials.
However, as the number of studies included in our analysis was
small, additional RCTs comparing the efficacy and safety of
bevacizumab monotherapy and combination therapy among larger groups
of patients are necessary to confirm our findings.
This meta-analysis had some limitations. Verteporfin
PDT has been shown to be more effective at treating classic
choroidal neovascularization than mild or classic occult CNV, and
this analysis did not examine its efficacy with respect to the
different types of CNV. Thus, additional studies are required.
Furthermore, the differences in the durations of the included
trials (3 to 12 months) were a potential source of heterogeneity.
Some studies did not provide means and standard deviations,
electing to report only before- and after-treatment values or
medians and ranges, which may have resulted in data
conversion-related errors. Finally, the numbers of bevacizumab
treatments administered in the included trials were not uniform, as
the average numbers of bevacizumab injections differed among the
studies. Additionally, the dose of PDT was not mentioned in one of
the included studies, which have may have biased our results. And
the small number of studies included in this meta-analysis was a
limitation of the study, and that publication bias could therefore
not be assessed.
In conclusion, there were no significant differences
in mean BCVA changes, CRT increases, the proportions of patients
gaining more than 15 letters, or the incidences of ocular adverse
events and systemic adverse events between the two groups. However,
combination therapy may significantly reduce the average number of
bevacizumab retreatments compared with monotherapy. And this
systematic review and meta-analysis may provide a basis for
clinical treatment of wet AMD.
Acknowledgements
Not applicable.
Funding
The present study was financially supported by the
National Natural Science Foundation of China (grant no.
81470648).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JY and JW designed the study. QW, JL and QL screened
the literature. CR, WC and XL extracted the data from the
literature. QW, JL, JY and JW conducted the meta-analysis and wrote
the manuscript. JY and JW submitted the study.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bressler NM: Age-related macular
degeneration is the leading cause of blindness. JAMA.
291:1900–1901. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Position of the Retinological Society, the
German Ophthalmological Society and the Professional Association of
Ophthalmologists in Germany on the current therapeutic
possibilities for neovascular age-related macular degeneration.
Klin Monbl Augenheilkd. 224:559–566. 2007.PubMed/NCBI
|
|
3
|
Aiello LP, Northrup JM, Keyt BA, Takagi H
and Iwamoto MA: Hypoxic regulation of vascular endothelial growth
factor in retinal cells. Arch Ophthalmol. 113:1538–1544. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Buch H, Vinding T and Nielsen NV:
Prevalence and causes of visual impairment according to World
Health Organization and United States criteria in an aged, urban
Scandinavian population: The Copenhagen City Eye Study.
Ophthalmology. 108:2347–2357. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mitchell P, Annemans L, White R, Gallagher
M and Thomas S: Cost effectiveness of treatments for wet
age-related macular degeneration. Pharmacocconomics. 29:107–131.
2011. View Article : Google Scholar
|
|
6
|
Shao J, Choudhary MM and Schachat AP:
Neovascular age-related macular degeneration. Dev Ophthalmol.
55:125–136. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
EI-Mollayess GM, Noureddine BN and
Bashshur ZF: Bevacizumab and neovascular age related macular
degeneration: Pathogenesis and treatment. Semin Ophthalmol.
26:69–76. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leydolt C, Michels S, Prager F, Garhoefer
G, Georgopoulos M, Polak K and Schmidt-Erfurth U: Effect of
intravitreal bevacizumab (Avastin) in neovascular age-related
macular degeneration using a treatment regimen based on optical
coherence tomography: 6- and 12-month results. Acta Ophthalmol.
88:594–600. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takahashi M, Sato T and Kishi S:
Intravitreal bevacizumab for age-related macular degeneration with
good visual acuity. Jpn J Ophthalmol. 54:565–570. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Berg K, Hadzalic E, Gjertsen I, Forsaa V,
Berger LH, Kinge B, Henschien H, Fossen K, Markovic S, Pedersen TR,
et al: Ranibizumab or bevacizumab for neovascular age-related
macular degeneration according to the lucentis compared to avastin
study treat-and-extend protocol: Two-year results. Ophthalmology.
123:51–59. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Berg K, Pedersen TR, Sandvik L and
Bragadóttir R: Comparison of ranibizumab and bevacizumab for
neovascular age-related macular degeneration according to LUCAS
treat-and-extend protocol. Ophthalmology. 122:146–152. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moja L, Lucenteforte E, Kwag KH, Bertele
V, Campomori A, Chakravarthy U, D'Amico R, Dickersin K, Kodjikian
L, Lindsley K, et al: Systemic safety of bevacizumab versus
ranibizumab for neovascular age-related macular degeneration.
Cochrane Database Syst Rev. 15:CD011230. 2014. View Article : Google Scholar
|
|
13
|
Chen G, Li W, Tzekov R, Jiang F, Mao S and
Tong Y: Bevacizumab versus ranibizumab for neovascular age-related
macular degeneration: A meta-analysis of randomized controlled
trials. Retina. 35:187–193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kodjikian L, Decullier E, Souied EH,
Girmens JF, Durand EE, Chapuis FR and Huot L: Bevacizumab and
ranibizumab for neovascular age-related macular degeneration: An
updated meta-analysis of randomised clinical trials. Graefes Arch
Clin Exp Ophthalmol. 252:1529–1537. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang W and Zhang X: Systemic adverse
events after intravitreal bevacizumab versus ranibizumab for
age-related macular degeneration: A meta-analysis. PLoS One.
9:e1097442014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Solomon SD, Lindsley KB, Krzystolik MG,
Vedula SS and Hawkins BS: Intravitreal bevacizumab versus
ranibizumab for treatment of neovascular age-related macular
degeneration: Findings from a cochrane systematic review.
Ophthalmology. 123:70–77.e1. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stein JD, Newman-Casey PA, Mrinalini T,
Lee PP and Hutton DW: Cost-effectiveness of bevacizumab and
ranibizumab for newly diagnosed neovascular macular degeneration.
Ophthalmology. 121:936–945. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fong KC, Kirkpatrick N, Mohamed Q and
Johnston RL: Intravitreal bevacizumab (Avastin) for neovascular
age-related macular degeneration using a variable frequency regimen
in eyes with no previous treatment. Clin Experiment Ophthalmol.
36:748–755. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanchanaranya N, Rojdamrongratana D and
Piyasoonthorn P: Incidence of post-intravitreal anti-VEGF
endophthalmitis at Thammasat University Hospital. J Med Assoc Thai.
98:489–494. 2015.PubMed/NCBI
|
|
20
|
Haddock LJ, Ramsey DJ and Young LH:
Complications of subspecialty ophthalmic care: Endophthalmitis
after intravitreal injections of anti-vascular endothelial growth
factor medications. Semin Ophthalmol. 29:257–262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lyall DA, Tey A, Foot B, Roxburgh ST,
Virdi M, Robertson C and MacEwen CJ: Post-intravitreal anti-VEGF
endophthalmitis in the United Kingdom: Incidence, features, risk
factors, and outcomes. Eye (Lond). 26:1517–1526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stewart MW: Endophthalmitis after
injections of anti-vascular endothelial growth factor drugs.
Retina. 31:1981–1982. 2011.PubMed/NCBI
|
|
23
|
Inoue M, Kobayakawa S, Sotozono C, Komori
H, Tanaka K, Suda Y, Matsushima H, Kinoshita S, Senoo T, Tochikubo
T and Kadonosono K: Evaluation of the incidence of endophthalmitis
after intravitreal injection of anti-vascular endothelial growth
factor. Ophthalmologica. 226:145–150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carneiro AM, Barthelmes D, Falcão MS,
Mendonça LS, Fonseca SL, Gonçalves RM, Faria-Correia F and
Falcão-Reis FM: Arterial thromboembolic events in patients with
exudative age-related macular degeneration treated with
intravitreal bevacizumab or ranibizumab. Ophthalmologica.
225:211–221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schmidt-Erfurth UM and Pruente C:
Management of neovascular age-related macular degeneration. Prog
Retin Eye Res. 26:437–451. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Incorvaia C, Campa C, Parmeggiani F,
Menzione M, D'Angelo S, Della Corte M, Rinaldi M, Romano M,
Dell'omo R and Costagliola C: 12-month retrospective study and
review of photodynamic therapy with verteporfin for subfoveal
choroidal neovascularization in age-related macular degeneration.
Retina. 28:289–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yonekawa Y and Kim IK: Clinical
characteristics and current treatment of age-related macular
degeneration. Cold Spring Harb Perspect Med. 5:a0171782014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ladewig MS, Karl SE, Hamelmann V, Helb HM,
Scholl HP, Holz FG and Eter N: Combined intravitreal bevacizumab
and photodynamic therapy for neovascular age-related macular
degeneration. Graefes Arch Clin Exp Ophthalmol. 246:17–25. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim HW, Kim JL, Lee MH, Yoo HG, Chung IY
and Lee JE: Combined treatment of photodynamic therapy and
bevacizumab for choroidal neovascularization secondary to
age-related macular degeneration. Korean J Ophthalmol. 25:231–237.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Costagliola C, Romano MR, Rinaldi M,
dell'Omo R, Chiosi F, Menzione M and Semeraro F: Low fluence rate
photodynamic therapy combined with intravitreal bevacizumab for
neovascular age-related macular degeneration. Br J Ophthalmol.
94:180–184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kaiser PK; Registry of Visudyne AMD
Therapy Writing Committee. Boyer DS, Garcia R, Hao Y, Hughes MS,
Jabbour NM, Kaiser PK, Mieler W, Slakter JS, et al: Verteporfin
photodynamic therapy combined with intravitreal bevacizumab for
neovascular age-related macular degeneration. Ophthalmology.
116:747–755. 755.e12009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lazic R and Gabric N: Verteporfin therapy
and intravitreal bevacizumab combined and alone in choroidal
neovascularization due to age-related macular degeneration.
Ophthalmology. 114:1179–1185. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Datseris I, Kontadakis GA, Diamanti R,
Datseris I, Pallikaris IG, Theodossiadis P and Tsilimbaris MK:
Prospective comparison of low-fluence photodynamic therapy combined
with intravitreal bevacizumab versus bevacizumab monotherapy for
choroidal neovascularization in age-related macular degeneration.
Semin Ophthalmol. 30:112–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Potter MJ, Claudio CC and Szabo SM: A
randomised trial of bevacizumab and reduced light dose photodynamic
therapy in age-related macular degeneration: the VIA study. Br J
Ophthalmol. 94:174–179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Saviano S, Leon PE, Mangogna A and
Tognetto D: Combined therapy (intravitreal bevacizumab plus
verteporfin photodynamic therapy) versus intravitreal bevacizumab
monotherapy for choroidal neovascularization due to age-related
macular degeneration: A 1-year follow-up study. Digit J Ophthalmol.
22:46–53. 2016.PubMed/NCBI
|
|
36
|
Jadad AR, Moore RA, Carroll D, Jenkinson
C, Reynolds DJ, Gavaghan DJ and McQuay HJ: Assessing the quality of
reports of randomized clinical trials: Is blinding necessary?
Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nowak-Sliwinska P, Van den Bergh H,
Sickenberg M and Koh AH: Photodynamic therapy for polypoidal
choroidal vasculopathy. Prog Retin Eye Res. 37:182–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dabkeviciene D, Sasnauskiene A, Leman E,
Kvietkauskaite R, Daugelaviciene N, Stankevicius V, Jurgelevicius
V, Juodka B and Kirveliene V: mTHPC-mediated photodynamic treatment
up-regulates the cytokines VEGF and IL-1alpha. Photochem Photobiol.
88:432–439. 2012. View Article : Google Scholar : PubMed/NCBI
|