Introduction
Systemic lupus erythematosus (SLE) is a chronic
inflammatory autoimmune disease that can affect various organ
systems. In China, SLE occurs at an incidence of ~31-70 per 100,000
individuals (1). SLE is
characterized by high heterogeneity, a complex pathophysiology and
various clinical manifestations; thus, no test alone is
sufficiently sensitive or specific for diagnosis (2).
Currently, the SLE disease activity index 2000
(SLEDAI-2K) score is widely utilized to assess disease activity in
SLE patients (3,4). However, it could be too complex to use
in routine clinical practice. Conventional methods, including
erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP)
are not in complete accordance with the activity of SLE (5,6).
Therefore, there is significant interest in the identification of
biomarkers that can predict SLE and quantify disease activity,
although a single biomarker is unlikely to replace clinical
evaluation due to the heterogeneity of this disease (7).
The neutrophil-to-lymphocyte ratio (NLR) and
hemoglobin are parts of a routine complete blood count. NLR can be
easily calculated using peripheral blood, and it has been
identified as a new index of inflammatory biomarkers in a number of
diseases (8–10). Hu et al (11) reported that increased NLR was
positively correlated with primary Sjögren's syndrome (pSS).
Similarly, Sen et al (12)
reported that NLR may reflect systemic inflammation in psoriasis
patients. Although Qin et al (13) found that NLR may reflect inflammatory
responses and disease activity in SLE patients, the predictive
value of NLR in connection with hemoglobin levels in SLE has not
been determined. The hemoglobin level may be used as an indicator
of active inflammatory disease (14,15). Low
hemoglobin levels have been found to be significantly associated
with disease activity in rheumatoid arthritis (16). However, to the best of our knowledge,
the predictive value of the hemoglobin level in SLE remains
unclear.
In this study, we analyzed the medical records of
212 SLE patients and 201 healthy individuals to determine the
possible association of NLR and hemoglobin level with disease
activity and inflammatory responses in SLE patients, in order to
evaluate the predictive value of NLR and hemoglobin level in
SLE.
Patients and methods
Participants
A total of 212 adult patients with SLE were involved
in this retrospective study, according to the diagnostic criteria
of SLE developed by the American College of Rheumatology in 1997.
All of the patients were originally recruited to the First Hospital
of Lanzhou University (Lanzhou, China) between July 2013 and
January 2017; their medical records were obtained for retrospective
use in the present study. The SLE patients with hematological
diseases, blood transfusion, malignancies, other autoimmune
diseases, lymphoproliferative disorders, infections or
hepatosplenic diseases were excluded. In addition, 201 age- and
sex-matched healthy subjects who underwent routine physical
examinations in the same hospital during the same period were used
as the healthy control group. The study protocol was approved by
the Research Ethics Committee of the First Hospital of Lanzhou
University (no. LDYYLL201731).
Laboratory values and clinical
assessment
Age and sex were extracted from the electronic
medical records system database. Blood tests, such as red blood
cell count, white blood cell count, neutrophil count, neutrophil
ratio in the white blood cell count, lymphocyte count, lymphocyte
ratio in the white blood cell count, and hemoglobin, were performed
using a hematology analyzer (Sysmex XE-5000; Sysmex Corporation,
Kobe, Japan) with its supporting reagents. The NLR was calculated
as the absolute neutrophil count divided by the absolute lymphocyte
count. Furthermore, CRP, complement component 3 (C3) and C4 were
analyzed using an automatic protein analyzer (Siemens Healthcare
Diagnostics, Eschborn, Germany). ESR was measured with an automatic
ESR analyzer (ORON-200; HORRON XLH Medical Electronics, Shenzhen,
China). In addition, SLEDAI-2K, a global index based on symptoms
and laboratory findings, was used to assess SLE disease activity
based on the symptoms and laboratory test results (17).
Statistical analysis
The descriptive statistics are presented as mean ±
standard deviation for continuous variables and percentages of the
number for categorical variables. The unpaired t-test or
Mann-Whitney U-test was used to compare the two independent groups
according to distribution state. Pearson's approach was used to
quantify the correlation between variables. A receiver operating
characteristic (ROC) curve was constructed to determine the
predictive value of NLR and hemoglobin in the patient group. A
P-value of <0.05 was considered to indicate statistically
significant differences. All statistical studies were conducted
with the SPSS program v.16.0 (SPSS, Inc., Chicago, IL, USA).
Results
Characteristics of the
participants
The demographic characteristics, clinical
characteristics and laboratory findings of the 212 SLE patients and
201 healthy controls are listed in Table
I. There was no significant difference in age, sex or
neutrophil count between the SLE and healthy control groups. The
neutrophil ratio in the white blood cell count was markedly
increased, and the red blood cell count, white blood cell count,
lymphocyte count and lymphocyte ratio in the white blood cell count
were significantly decreased in SLE patients (P<0.001; Table I).
 | Table I.Characteristics of the
participants. |
Table I.
Characteristics of the
participants.
|
| SLE patients
(n=212) | Healthy controls
(n=201) |
|
|---|
|
|
|
|
|
|---|
| Characteristic | n | Results | n | Results | P-value |
|---|
| Age (years) | 212 | 40.19±15.24 | 201 | 41.45±12.08 | 0.351 |
| Sex
(male/female) | 212 | 23/189 | 201 | 20/181 | 0.766 |
| RBC
(×1012/l) | 174 | 3.93±0.89 | 201 | 4.66±0.36 |
<0.0001c |
| WBC
(×109/l) | 212 | 5.36±2.94 | 201 | 6.04±1.28 | 0.002b |
| Lym
(×109/l) | 212 | 1.19±0.70 | 201 | 0.96±0.46 |
<0.0001c |
| Lymphocyte (%) | 212 | 24.40±11.09 | 201 | 32.76±5.18 |
<0.0001c |
| Neu
(×109/l) | 212 | 3.93±2.48 | 201 | 3.65±0.93 | 0.048a |
| Neutrophil (%) | 212 | 66.60±12.70 | 201 | 60.07±5.44 |
<0.0001c |
| CRP (mg/l) | 189 | 14.59±2.37 | 30 | 2.90±1.04 |
<0.0001c |
| C3 (g/l) | 201 | 0.70±0.33 | 30 | 1.20±0.27 |
<0.0001c |
| C4 (g/l) | 194 | 0.14±0.11 | 30 | 0.34±0.08 |
<0.0001c |
| ESR (mm/h) | 192 | 42.08±3.36 | – | – | – |
| SLEDAI-2K | 141 | 10.67±6.63 | – | – |
|
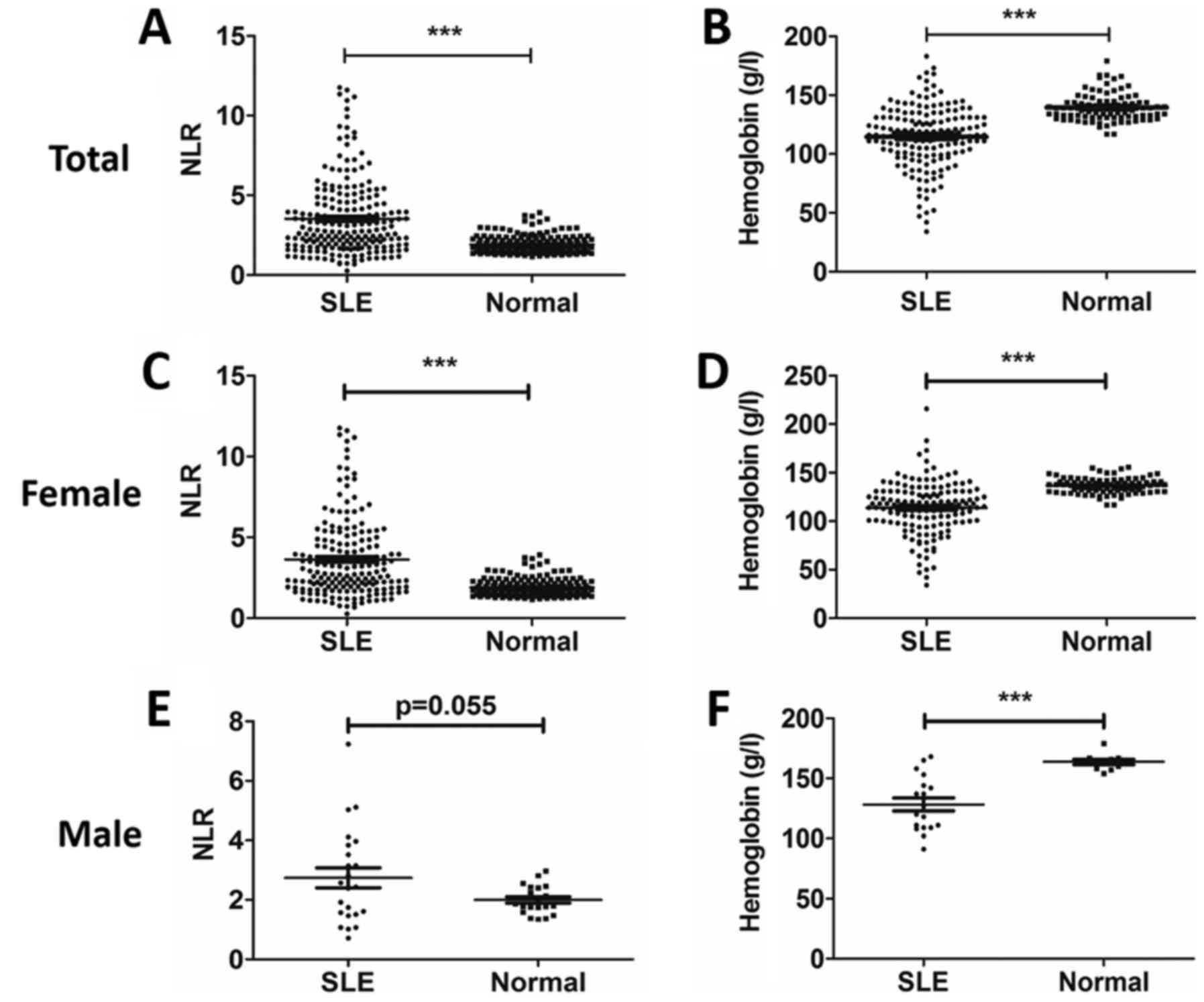
NLR was increased and hemoglobin was
decreased in SLE patients compared with healthy controls
As shown in Fig. 1,
NLR was significantly elevated (P<0.001; Fig. 1A) and the hemoglobin level was
markedly decreased (P<0.001; Fig.
1B) in total SLE patients compared with healthy controls. In
order to investigate the association of NLR and hemoglobin levels
with SLE patient sex, the cohort was divided into females and
males. NLR was significantly elevated in female SLE patients
compared with healthy female controls (P<0.001), whereas there
was no statistically significant difference between male SLE
patients compared with healthy male controls (Fig. 1C and D). The hemoglobin level was
markedly decreased in both female and male patients with SLE
(Fig. 1E and F).
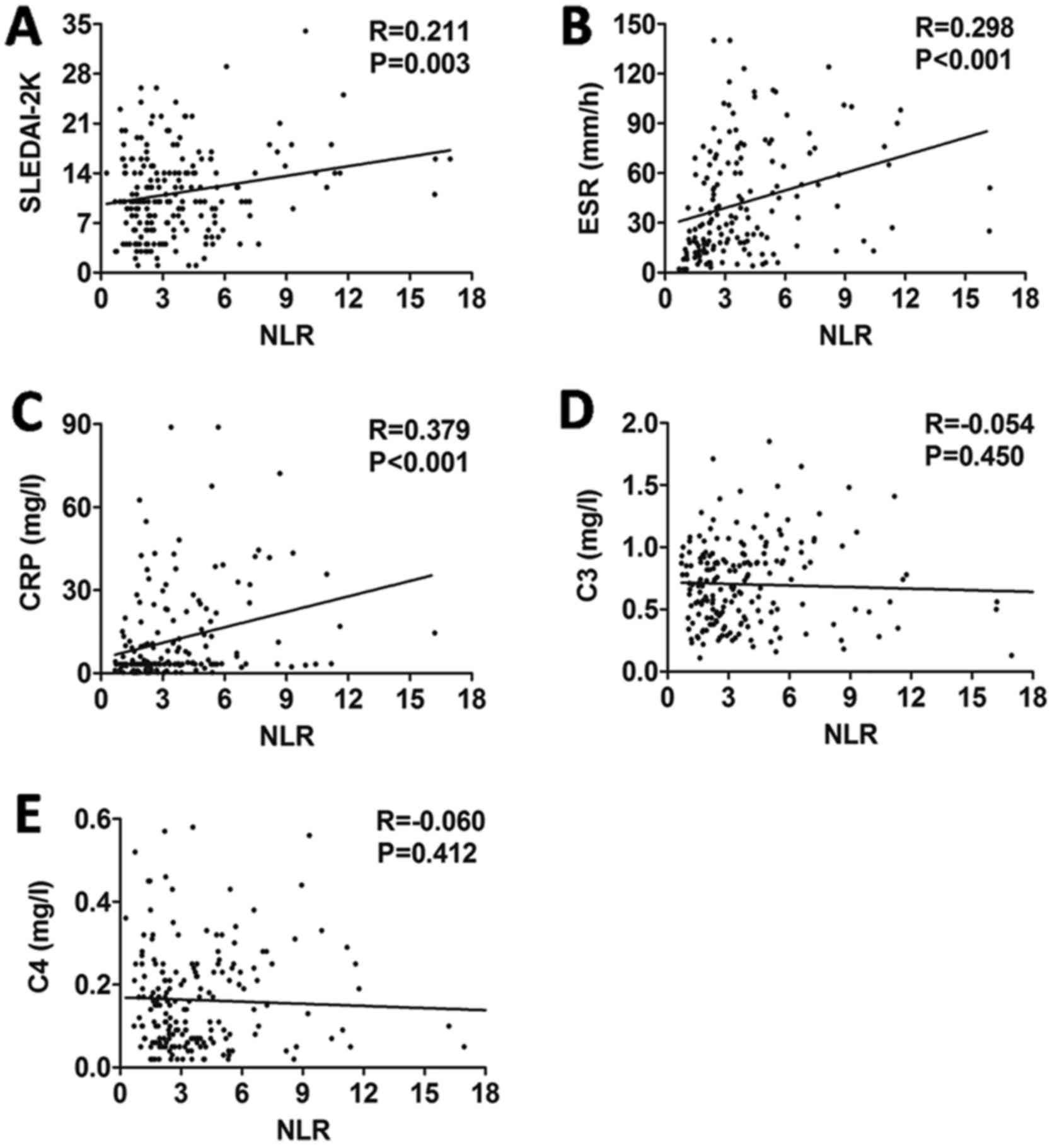
Correlations of NLR and hemoglobin
with disease activity in SLE
The study demonstrated that NLR was positively
correlated with SLEDAI-2K (r=0.211, P=0.003; Fig. 2A), ESR (r=0.298, P<0.001; Fig. 2B) and CRP (r=0.379, P<0.001;
Fig. 2C), but not with C3 (r=−0.054,
P=0.450; Fig. 2D) or C4 (r=−0.060,
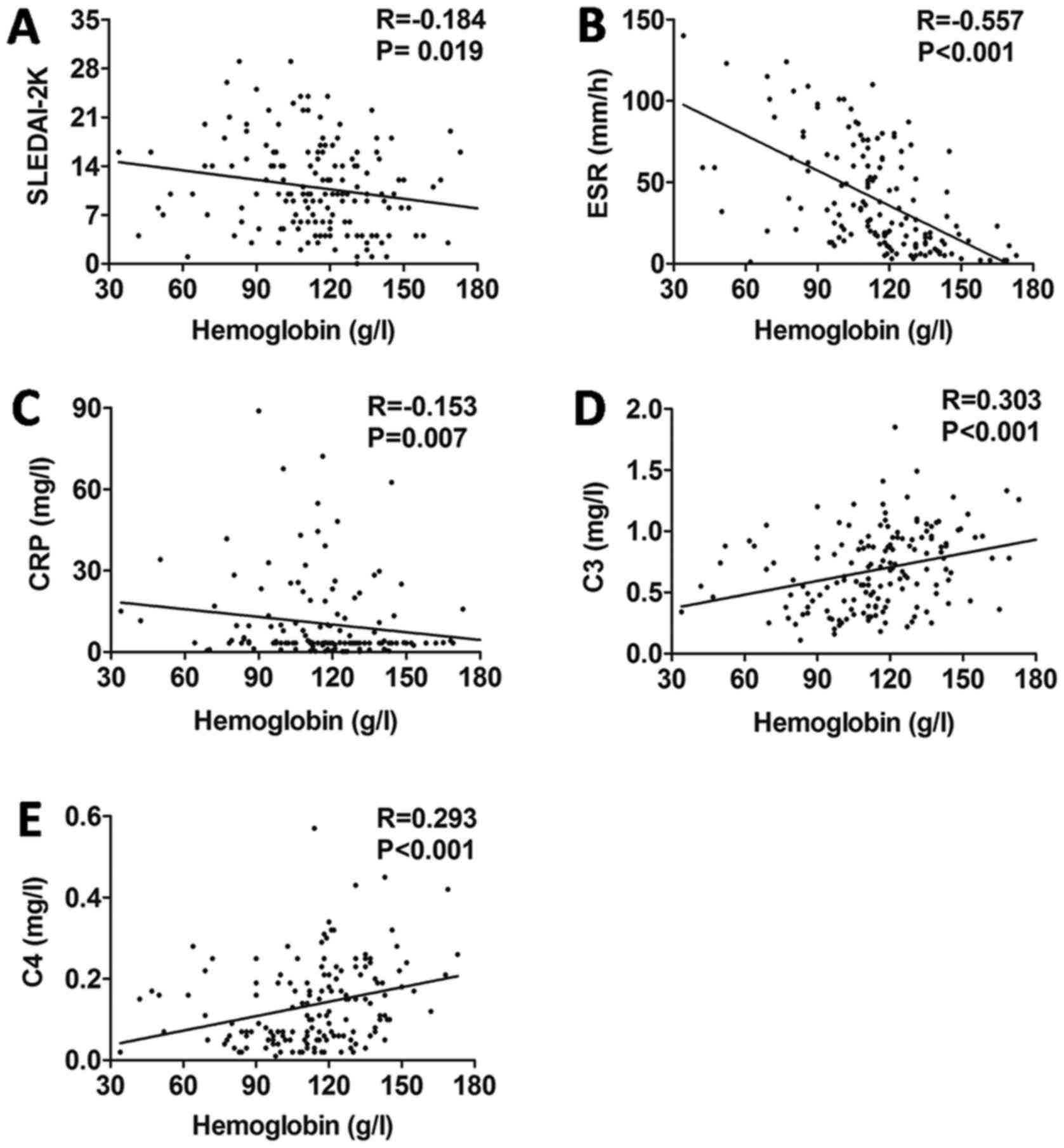
P=0.412; Fig. 2E). Furthermore, the
hemoglobin level was negatively correlated with SLEDAI-2K
(r=−0.184, P=0.019; Fig. 3A), ESR
(r=−0.557, P<0.001; Fig. 3B) and
CRP (r=−0.157, P<0.007; Fig. 3C),
whereas it was positively correlated with C3 (r=0.303, P<0.001;
Fig. 3D) and C4 (r=0.293, P=0.003;
Fig. 3E).
NLR and hemoglobin may predict the
development of SLE
The risk factors were entered into a logistic
regression analysis. The results demonstrated that NLR
[EXP(B)=1.986; 95% CI, 1.432-2.753; P=0.001], hemoglobin
[EXP(B)=0.947; 95% CI, 0.929-0.965; P=0.001], C3 (P=0.001) and C4
(P=0.070) were independent predictive factors in SLE patients
(Table II).
 | Table II.Logistic regression analysis of
factors independently associated with SLE. |
Table II.
Logistic regression analysis of
factors independently associated with SLE.
| Variable | P-value | EXP(B) | 95% CI of
EXP(B) |
|---|
| NLR | 0.001 | 1.986 | 1.432-2.753 |
| Hemoglobin | 0.001 | 0.947 | 0.929-0.965 |
| C3 | 0.001 | 0.004 | 0.000-0.123 |
| C4 | 0.007 | 0.049 | 0.005-0.430 |
| CRP | 0.080 | 1.393 | 0.961-2.017 |
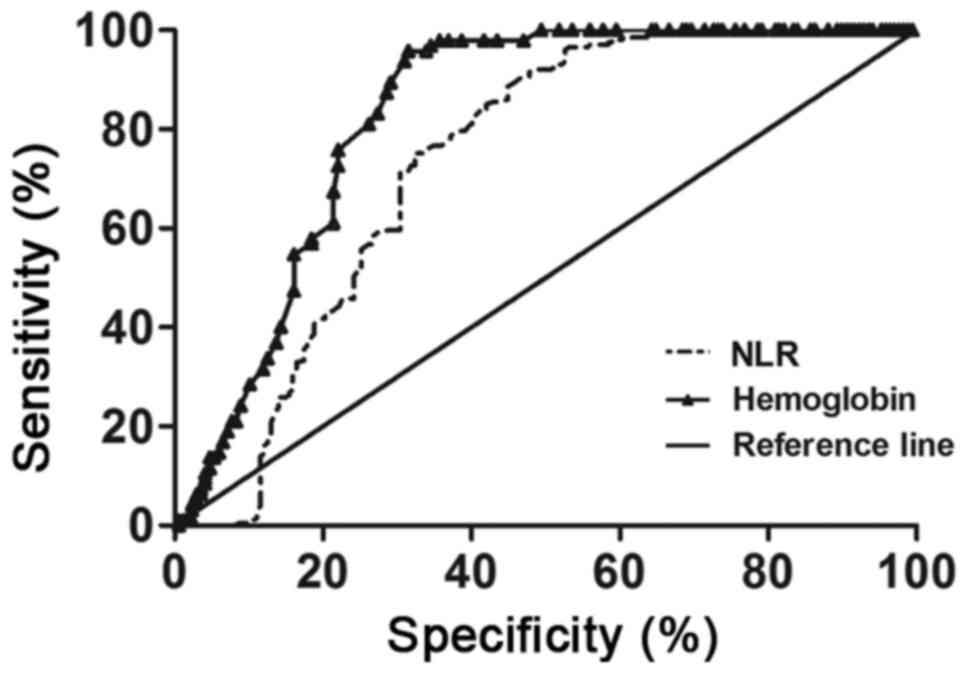
ROC curve analysis was performed to determine the
cut-off value of NLR and hemoglobin to predict SLE, and it revealed
that the optimal clinical cut-off level of NLR was 2.075, with a
sensitivity of 71.14% and a specificity of 69.57%. The optimal
cut-off level of hemoglobin was 131.5 g/l, with a sensitivity of
75.79% and a specificity of 77.98%. There was no statistically
significant difference in the area under the ROC curve between NLR
and hemoglobin (P>0.05; Fig. 4
and Table III).
 | Table III.Analysis of the area under the ROC
curve for NLR and hemoglobin. |
Table III.
Analysis of the area under the ROC
curve for NLR and hemoglobin.
|
|
|
|
| Asymptotic 95%
confidence interval |
|---|
|
|
|
|
|
|
|---|
| Test result
variable | Area | Standard
errora | Asymptotic
significanceb | Lower bound | Upper bound |
|---|
| NLR | 0.735 | 0.026 | 0.000 | 0.684 | 0.786 |
| Hemoglobin
levels | 0.825 | 0.025 | 0.000 | 0.775 | 0.874 |
Predictive value of NLR and hemoglobin
in SLE
Although all positive likelihood ratios of high NLR
and low hemoglobin levels were <10 in predicting SLE,
simultaneously high NLR and low hemoglobin levels, and high NLR or
low hemoglobin levels, had higher positive predictive values (86.05
and 66.95, respectively) and positive likelihood ratios (3.42 and
1.14, respectively) compared with a high NLR level alone or a low
hemoglobin level alone (Table IV).
Moreover, high NLR with low hemoglobin levels and high NLR or low
hemoglobin exhibited higher sensitivity (64.91 and 92.40,
respectively) and specificity (64.91 and 18.95, respectively),
compared with a high NLR level alone or a low hemoglobin level
alone (Table IV).
 | Table IV.Statistical evaluation of NLR and
hemoglobin levels in predicting SLE. |
Table IV.
Statistical evaluation of NLR and
hemoglobin levels in predicting SLE.
| Characteristic | Sensitivity
(%) | Specificity
(%) | Accuracy (%) | Positive predicted
value (%) | Negative predicted
value (%) | Positive likelihood
ratio | Negative likelihood
ratio |
|---|
| NLR up alone | 6.43 | 78.95 | 32.33 | 35.48 | 31.91 | 0.31 | 1.19 |
| Hemoglobin down
alone | 22.81 | 61.05 | 36.47 | 51.32 | 30.53 | 0.59 | 1.26 |
| NLR up and
hemoglobin down | 64.91 | 81.05 | 70.68 | 86.05 | 56.2 | 3.42 | 0.43 |
| NLR up or
hemoglobin down | 92.40 | 18.95 | 66.17 | 66.95 | 58.06 | 1.14 | 0.4 |
Discussion
This study demonstrated that NLR was increased and
hemoglobin was decreased in SLE patients, no matter in female or
male. Another key finding was that NLR was positively correlated
with SLEDAI-2K, ESR and CRP, while hemoglobin level was negatively
correlated with SLEDAI-2K, ESR and CRP, and positively correlated
with C3 and C4. Furthermore, NLR and hemoglobin were independent
predictive factors for SLE. These findings indicate that NLR and
hemoglobin, which are easily detectable and applicable laboratory
parameters, maybe predictive factors for SLE and reflect
inflammatory response and disease activity in SLE patients.
It is well known that the blood composition
undergoes relative changes under conditions of systemic
inflammation, typically represented by neutrophilia, lymphopenia
and anemia (18). In recent years,
neutrophil count, lymphocyte count and hemoglobin levels have been
identified as biomarkers of inflammation in several diseases. NLR
has been used in combination with other inflammatory markers to
quantify systemic inflammation in both auto- and non-autoimmune
diseases (13). Accumulating
evidence has revealed that NLR is a reliable indicator of
inflammation (11,19,20). Qin
et al (13) reported that NLR
was increased in SLE patients in a study of a cohort of 154 SLE
patients and 151 healthy controls. In accordance with the results
of previous studies, our results further confirmed that NLR was
positively correlated with SLEDAI-2K, a widely utilized indicator
to assess disease activity in SLE patients, and ESR and CRP, which
are inflammatory indices also used as markers of SLE disease
activity. Interestingly, our research also demonstrated that there
is no correlation of NLR with C3 and C4, which are biomarkers of
SLE disease activity. This inconsistency may be the reason why
medical intervention in SLE patients markedly affects C3 and C4
(21), or an indication that the
abovementioned parameters are involved in separate biological
processes in SLE.
Anemia in SLE usually develops in the context of
chronic systemic inflammation, and low hemoglobin levels have been
found to be an indicator of subclinical inflammatory disease
(14,15). However, hemoglobin is not considered
to be a readily available acute phase response biomarker, unlike
ESR and CRP, which are routinely used to estimate the activity of
systemic inflammation. Previous studies reported that the
hemoglobin level was increased after starting targeted rheumatoid
arthritis treatment (22,23), but failed to report longitudinal
changes in composite disease activity indices (23). To the best of our knowledge, there
are no studies on the direct association between hemoglobin and
SLE. The present study demonstrated that the level of hemoglobin
was significantly lower in SLE patients compared with that in
healthy controls. In order to further elucidate the association
between hemoglobin and SLE, we evaluated the correlations between
hemoglobin level and SLEDAI-2K, ESR, CRP, C3 and C4 in patients
with SLE, and found that hemoglobin is negatively correlated with
SLE disease activity. All these findings suggest that NLR and
hemoglobin may be used to reflect the inflammatory response and
disease activity in SLE patients.
In this study, logistic regression analysis revealed
that SLE was independently associated with NRL, hemoglobin, C3 and
C4, while NLR exhibited a moderate association [Exp(B)=1.986],
hemoglobin a moderate association [Exp(B)=0.947], and C3 and C4 a
less significant association [Exp(B)=0.004, 0.049, respectively].
Moreover, the area under the ROC curve of NLR and hemoglobin was
0.735 (with a sensitivity of 71.14% and a specificity of 69.57%)
and 0.825 (with a sensitivity of 75.79% and a specificity of
77.98%), respectively, in differentiating between SLE patients and
normal subjects. Therefore, the two indices NLR and hemoglobin have
predictive power for SLE, and the increased NLR and decreased
hemoglobin levels observed at the onset of inflammation are
significantly associated with the presence of SLE. In addition, the
combined results in the present study demonstrated that
simultaneously high NLR and low hemoglobin levels, and high NLR or
low hemoglobin levels, had higher positive predictive values (86.05
and 66.95, respectively), with higher sensitivity (64.91 and 92.40,
respectively) and specificity (84.05 and 18.95, respectively) in
diagnosing SLE compared with high NLR alone or low hemoglobin
alone, although all positive likelihood ratios of high NLR and low
hemoglobin levels were <10. This suggests that NLR and
hemoglobin should be measured simultaneously for a more reliable
clinical diagnosis in SLE. NLR and hemoglobin measurement is easy,
cost-effective and readily available compared with tests for other
inflammatory cytokines. Therefore, they are valuable clinical tools
that may serve as biomarkers for inflammatory response and for
predicting SLE. Due to the complexity of NLR and hemoglobin, they
are not included in major management recommendations for SLE at
present, as further clinical decision-making is required.
There were certain limitations to the present study.
First, this was a single-center retrospective study, and additional
larger, multicenter studies are needed to verify our results.
Furthermore, the exact mechanism underlying the involvement of NLR
and hemoglobin in SLE was not investigated. Finally, the
participants were only Chinese, and the inclusion of other ethnic
groups is required in further investigations.
In conclusion, the present study has demonstrated
that increased NLR and decreased hemoglobin levels were found in
SLE patients, and that they were correlated with inflammatory
response and disease activity. These findings suggest that NLR and
hemoglobin may be useful markers in predicting SLE and assessing
disease activity. However, further studies are required to
elucidate the exact role of NLR and hemoglobin in SLE.
Acknowledgements
Not applicable.
Funding
This study was supported by the Health Industry
Research Program Management of Gansu Province (grant no. GWGL
2014-48) and the First Hospital of Lanzhou University Foundation
(grant no. ldyyyn2017-19).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HY conceived and designed the present study,
performed analysis and acquired funding. LY and XH collected the
data. RL, CG and NS performed the experiments. HY drafted the
manuscript. LJ designed and revised the manuscript. HY, LJ, LY, CG,
XH, RL and NS gave final approval of the version to be
published.
Ethics approval and consent to
participate
The study protocol was approved by the Research
Ethics Committee of the First Hospital of Lanzhou University (no.
LDYYLL201731).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zeng QY, Chen R, Darmawan J, Xiao ZY, Chen
SB, Wigley R, Le Chen S and Zhang NZ: Rheumatic diseases in China.
Arthritis Res Ther. 10:R172008. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu CC and Ahearn JM: The search for lupus
biomarkers. Best Pract Res Clin Rheumatol. 23:507–523. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yuan J, Li LI, Wang Z, Song W and Zhang Z:
Dyslipidemia in patients with systemic lupus erythematosus:
Association with disease activity and B-type natriuretic peptide
levels. Biomed Rep. 4:68–72. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Urowitz MB, Isenberg DA and Wallace DJ:
Safety and efficacy of hCDR1 (Edratide) in patients with active
systemic lupus erythematosus: Results of phase II study. Lupus Sci
Med. 2:e0001042015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Osei-Bimpong A, Meek JH and Lewis SM: ESR
or CRP? A comparison of their clinical utility. Hematology.
12:353–357. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gaitonde S, Samols D and Kushner I:
C-reactive protein and systemic lupus erythematosus. Arthritis
Rheum. 59:1814–1820. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Petri M: Disease activity assessment in
SLE: Do we have the right instruments? Ann Rheum Dis. 66 Suppl
3:iii61–iii64. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Afari ME and Bhat T: Neutrophil to
lymphocyte ratio (NLR) and cardiovascular diseases: An update.
Expert Rev Cardiovasc Ther. 14:573–577. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Balta S, Celik T, Mikhailidis DP, Ozturk
C, Demirkol S, Aparci M and Iyisoy A: The relation between
atherosclerosis and the neutrophil-lymphocyte ratio. Clin Appl
Thromb Hemost. 22:405–411. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim DS, Shin D, Lee MS, Kim HJ, Kim DY,
Kim SM and Lee MG: Assessments of neutrophil to lymphocyte ratio
and platelet to lymphocyte ratio in Korean patients with psoriasis
vulgaris and psoriatic arthritis. J Dermatol. 43:305–310. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu ZD, Sun Y, Guo J, Huang YL, Qin BD, Gao
Q, Qin Q, Deng AM and Zhong RQ: Red blood cell distribution width
and neutrophil/lymphocyte ratio are positively correlated with
disease activity in primary Sjögren's syndrome. Clin Biochem.
47:287–290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sen BB, Rifaioglu EN, Ekiz O, Inan MU, Sen
T and Sen N: Neutrophil to lymphocyte ratio as a measure of
systemic inflammation in psoriasis. Cutan Ocul Toxicol. 33:223–227.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qin B, Ma N, Tang Q, Wei T, Yang M, Fu H,
Hu Z, Liang Y, Yang Z and Zhong R: Neutrophil to lymphocyte ratio
(NLR) and platelet to lymphocyte ratio (PLR) were useful markers in
assessment of inflammatory response and disease activity in SLE
patients. Mod Rheumatol. 26:372–376. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moller B, Scherer A, Förger F, Villiger PM
and Finckh A: Swiss Clinical Quality Management Program for
Rheumatic Diseases, Anaemia may add information to standardised
disease activity assessment to predict radiographic damage in
rheumatoid arthritis: A prospective cohort study. Ann Rheum Dis.
73:691–696. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van Steenbergen HW, van Nies JA and van
der Helm-van Mil AH: Anaemia to predict radiographic progression in
rheumatoid arthritis. Ann Rheum Dis. 72:e162013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smyrnova G: The relationship between
hemoglobin level and disease activity in patients with rheumatoid
arthritis. Rev Bras Reumatol. 54:437–440. 2014.(In Portuguese).
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gladman DD, Ibañez D and Urowitz MB:
Systemic lupus erythematosus disease activity index 2000. J
Rheumatol. 29:288–291. 2002.PubMed/NCBI
|
|
18
|
Zahorec R: Ratio of neutrophil to
lymphocyte count-rapid and simple parameter of systemic
inflammation in critically ill. Bratisl Lek Listy. 102:5–14.
2001.(In English, Slovak). PubMed/NCBI
|
|
19
|
Ahsen A, Ulu MS, Yuksel S, Demir K, Uysal
M, Erdogan M and Acarturk G: As a new inflammatory marker for
familial Mediterranean fever: Neutrophil-to-lymphocyte ratio.
Inflammation. 36:1357–1362. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu Y, Chen Y, Yang X, Chen L and Yang Y:
Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte
ratio (PLR) were associated with disease activity in patients with
systemic lupus erythematosus. Int Immunopharmacol. 36:94–99. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stohl W, Hiepe F, Latinis KM, Thomas M,
Scheinberg MA, Clarke A, Aranow C, Wellborne FR, Abud-Mendoza C,
Hough DR, et al: Belimumab reduces autoantibodies, normalizes low
complement levels, and reduces select B cell populations in
patients with systemic lupus erythematosus. Arthritis Rheum.
64:2328–2337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hashimoto M, Fujii T, Hamaguchi M, Furu M,
Ito H, Terao C, Yamamoto K, Yamamoto W, Matsuo T, Mori M, et al:
Increase of hemoglobin levels by anti-IL-6 receptor antibody
(tocilizumab) in rheumatoid arthritis. PLoS One. 9:e982022014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Isaacs JD, Harari O, Kobold U, Lee JS and
Bernasconi C: Effect of tocilizumab on haematological markers
implicates interleukin-6 signalling in the anaemia of rheumatoid
arthritis. Arthritis Res Ther. 15:R2042013. View Article : Google Scholar : PubMed/NCBI
|