Introduction
Central nervous system (CNS) infections can occur at
any age and may be caused by a variety of pathogens, of which
bacteria and fungi are the most prevalent (1,2). With
rising antibiotic resistance and various surgical operations,
pathogens are adapting accordingly (2–4).
Previous studies (5–7) have demonstrated that 8-10% of the
regional population (including Egypt, Iran and China) has
experienced bacterial and fungal infections of CNS, which expose
patients to a higher risk of disability and mortality. Therefore,
an accurate, more rapid etiologic identification is key to
diagnosis and treatment. However, previous studies (1,2,5,1) have
reported that the majority of intracranial infections were not
clearly diagnosed early enough during hospitalization, which may
partially be attributed to limits of traditional detection methods.
The conventional methods of cerebrospinal fluid (CSF) examination,
including culture and microscopy, have a limited role in severe
infections due to its low positive rate (8-20% in the majority of
studies) and time-consuming process (2-7 days) (6,7,9–11). A
number of novel etiological methods have been proposed as a
supplement or substitute for the conventional technique, such as
polymerase chain reaction (PCR), quantitative PCR (5,11),
multiplex PCR techniques (3,12) and DNA sequencing based on 16S
ribosomal DNA (16S rDNA) in bacteria or the internal transcribed
spacer (ITS) in fungi (6,13–16). To
the best of our knowledge, PCR and multiplex PCR may only be
effective for detecting common clinically suspected organisms
(3,15). Furthermore, using more primers will
decrease the sensitivity and specificity (15). Finally, DNA sequencing of CSF in
clinical application has been limited because of the high cost and
low specificity associated with second-generation sequencing
(15,17).
Thus, a more rapid, broad-spectrum, accurate, and
relatively inexpensive screening method that identifies pathogens
from CSF is required. Previous studies (18,19) have
demonstrated the diagnostic value and importance of DNA microarray
in the identification of viral CNS infections. This technology has
been proposed to have the potential to simultaneously detect a
large number of species with high specificity and has already
resulted in its widespread adoption in clinical practice for the
diagnosis of viral infections (20–22).
However, previous studies have not examined if this technique can
simultaneously detect bacterial and fungal CNS infections. The
present study established two gene chips, containing 18 probes
selected based on specific DNA sequence, to de bacterial and fungal
CNS infections and to assess their clinical application value.
Materials and methods
Clinical CSF samples and strains
A total of 88 CSF samples were collected from
patients with suspected CNS infections and analyzed with DNA
microarray from January 2014 to July 2016 at Xuanwu Hospital,
Capital Medical University (Beijing, China). The present study was
approved by the Ethics Committee of Xuanwu Hospital, Capital
Medical University. All patients provided written informed consent.
The clinical diagnosis of CNS infection was based on the Harrison
standard (23), combined with
certain risk factors (CSF leak and surgery), clinical
manifestations (fever and headache), and CSF examination results
(white blood cell count, glucose and protein). The reference
strains purchased from American Type Culture Collection (ATCC;
Manassas, VA, USA) were as follows: Streptococcus pneumoniae strain
ATCC 49619, S. aureus strain ATCC 29213, E. coli strain ATCC 25922,
Listeria monocytogenes strain ATCC 7644, Enterococcus faecalis
strain ATCC 29212, K. pneumonia strain ATCC 700603, P. aeruginosa
strain ATCC 27853, Enterobacter cloacae strain ATCC 700323,
Haemophilus influenzae strain ATCC 49766, Cryptococcus neoformans
strain ATCC 32269, Candida albicans strain ATCC 10231, Candida
tropicalis strain ATCC 14058 and Candida glabrata strain ATCC
15126. The clinical isolates (Enterococcus faecium, A. baumannii,
Stenotrophomonas maltophilia, coagulase negative staphylococcus,
Neisseria meningitides, Burkholderia cepacia, Candida parapsilosis,
Candida krusei, Cryptococcus gattii) used in the study were
collected and identified by Vitek 2 Compact (bioMerieux SA,
Marcy-l'Étoile, France), Matrix Assisted Laser Desorption
Ionization Time of Flight Mass Spectrometry (MALDI-TOF MS; Bruker
Daltonics; Bruker Corporation, Billerica, MA, USA) and DNA
sequencing (24).
DNA extraction from strains and
CSF
DNA of bacterial and fungal strains were extracted
using Bacterial Genomic DNAiso kit (DP302; Tiangen Biotech Co.,
Ltd., Beijing, China) and Yeast DNAiso kit (DP307; Tiangen Biotech
Co., Ltd., Beijing, China) according to the manufacturer's
protocol. Each CSF sample (1-2 ml) was centrifuged at 12,000 × g
for 5 min at room temperature (15-25°C). The supernatant was
discarded and the sediment was pretreated using QIAamp UCP Pathogen
Mini kit (Qiagen GmbH, Hilden, Germany), and DNeasy®
Blood & Tissue kit (Qiagen GmbH) was used to extract the sample
DNA. The DNA was stored at −20°C.
Primer design and PCR
amplification
Based on a previous study (15), the bacterial and fungal universal
primers, 16S rRNA and ITS, were designed to be capable of producing
PCR products from standard strains and common organisms causing CNS
infection as follows: 27F (forward, 5′-AGAGTTTGATCCTGGCTCAG-3′) and
1492R (reverse, 5′-GGYTACCTTGTTACGACTT-3′), ITS1 (forward,
5′-GCCGTAGGTGAACCTGCGG-3′) and ITS4 (reverse,
5′-TCCTCCGCTTATTGATATGC-3′) were used for bacteria and fungi
amplification, respectively. Other pairs of primers adding biotin
were developed for amplification and hybridization as follows: B1
(forward, 5′-CCTACGGGAGGCAGCAG-3′) and B2 (reverse,
5′biotin-TACGGYTACCTTGTTACGACTT-3′; where Y is C or T), P1
(forward, 5′-CAATAAGCGGAGGAAAAGAAAC-3′) and P2 (reverse, 5′
biotin-ACTCCTTGGTCCGTGTTTCA-3′) were used for bacteria and fungi
amplification, respectively. PCR was performed according to the
protocol of a previous study (15)
with a slight modification of annealing temperature of 56°C for 25
sec and extension of 72°C for 25 sec. The PCR products were
analyzed using 1% agarose gel electrophoresis with ultraviolet gel
imaging system (Syngene, Frederick, MD, USA), and the amplification
bands of fungi and bacteria were observed.
Synthesis of probes
The specific DNA sequences were screened for 14
types of bacteria and 4 fungi from GenBank (http://www.ncbi.nlm.nih.gov/genbank/), using the
software Primer Premier 5.0 (Premier Biosoft International, Palo
Alto, CA, USA) to design probes. The 16S universal probes as a
positive reference for all bacteria were designed according to the
sequences of conserved domain of 16S rDNA, and specific primers or
probes were designed based on the sequence of variable domain of
16S rDNA. With the exceptions of E. coli and K. pneumonia, the
genus probe was designed for other enterobacteria due to high
homology. Each probe comprised a modified 19-33 amino acid sequence
followed by a spacer of (Poly-dt)16 or
(Poly-da)16 and a stretch of specific sequence (Table I). All primers and probes were
synthesized by Shanghai Invitrogen Biotechnology; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). The probe sequence was
verified through the comparison of the probe sequence with the PCR
product sequence in standard strains and clinical isolates.
 | Table I.Sequences of microarray hybridization
probes for bacteria and fungi. |
Table I.
Sequences of microarray hybridization
probes for bacteria and fungi.
| No. of spot | Name | Probe sequence
(5′-3′) |
|---|
| Bacteria |
|
|
| 1 | Klebsiella
pneumonia |
NH2-ttttttttttttttttAGCTAATACCGCATAATGTCGCAAGACCAAAGT |
| 2 | Acinetobacter
baumannii |
NH2-ttttttttttttttttCGGTCGCAAGACTAAAACTCAA |
| 3 | Pseudomonas
aeruginosa |
NH2-ttttttttttttttttCCAAAAGCTACTGAGCTAGAGTACGGTA |
| 4 | Escherichia
coli |
NH2-ttttttttttttttttCGGTTTGTTAAGTCAGATGTG |
| 5 | Enterobacter
spp. |
NH2-ttttttttttttttttCGGGGAGGAAGGTGTTGTGGTTAAT |
| 6 | Haemophilus
influenzae |
NH2-ttttttttttttttttGATGTGTTAATAGCACATCAAATTGACGTT |
| 7 | Stenotrophomonas
maltophilia |
NH2-ttttttttttttttttCGCTAATACCGCATACGACCTACGGGTGAAAGC |
| 8 | Neisseria
meningitidis |
NH2-ttttttttttttttttCAACCTGATTGCTTGGTAGCGTAG |
| 9 | Enterococcus
faecalis |
NH2-ttttttttttttttttCGTTAGTAACTGAACGTCCCCTG |
| 10 | Enterococcus
faecium |
NH2-ttttttttttttttttATGCAAGTCGAACGCTTCTTTTTCCACCGG |
| 11 | Listeria
monocytogenes |
NH2-ttttttttttttttttAGAACAAGGATAAGAGTAACTGC |
| 12 | Staphylococcus.
aureus |
NH2-ttttttttttttttttAGAACATATGTGTAAGTAACTGTGCACATC |
| 13 | Streptococcus.
pneumoniae |
NH2-ttttttttttttttttAGAAGAACGAGTGTGAGAGTGGAAAGTTCAC |
| 14 | Coagulase negative
staphylococcus |
NH2-ttttttttttttttttGATGAAGGTCTTCGGATCGTAAAACTCTGTTAT |
| 15 | Blank control | – |
| 16 | Gram-negative |
NH2-ttttttttttttttttCTGATGCAGCCGCGTGTGTGAAG |
| 17 | Gram-positive |
NH2-ttttttttttttttttGATGACGTCAAATCATCATGCCCCTTATG |
| 18 | Bacterial Universal
probe |
NH2-ttttttttttttttttAACAGGATTAGATACCCTGGTAGTCCA |
| 19 | Negative
control |
NH2-ttttttttttttttttATTTGTCTTTGTAGATCTTCCCT |
| Fungi |
|
|
| 1 | Candida
albicans |
NH2-ttttttttttttttttGCATGCTGCTCTCTCGGG |
| 2 | Candida
tropicalis |
NH2-ttttttttttttttttACTGGCTCTTTCAGAGTCCGA |
| 3 | Candida
glabrata |
NH2-ttttttttttttttttACTGGTACCTTTGGTGCCCGA |
| 4 | Cryptococcus
neoformans |
NH2-ttttttttttttttttTGACACGATCACCAGTGCTC |
| 5 | Fungal universal
probe |
NH2-aaaaaaaaaaaaaaaaTGGGTGGTAAATTCCATCTAAAGC |
| 6 | Blank control | – |
Microarray preparation and
hybridization
Each array contained repeated triplicate identical
sets of probes and arranged in a bacteria-specific matrix of 7×12
or fungi-specific matrix of 3×9 (Fig.
1). Each matrix had ‘landing-light’ spots (spots of an internal
control probe or a coordinate probe; Shanghai BaiO Technology Co.,
Ltd., Shanghai, China) made with cyanine 3 (Cy3)- or Cy5-labeled
nucleotides to mark the array orientation. Following labeling the
product, both control and sample DNAs were processed with a Hybrid
chromogenic reagent kit (BST03021; Shanghai BaiO Technology Co.,
Ltd.) and hybridized using a BaiO® e-Hyb Automatic
hybrid instrument (BSE03011; Shanghai BaiO Technology Co., Ltd.)
according to the manufacturers' protocol. The hybridization
temperatures of bacterial and fungal chips were 42 and 43°C,
respectively, with each occurring for 30 min. Hybridization and
color-substrate reaction procedures are listed in Table II.
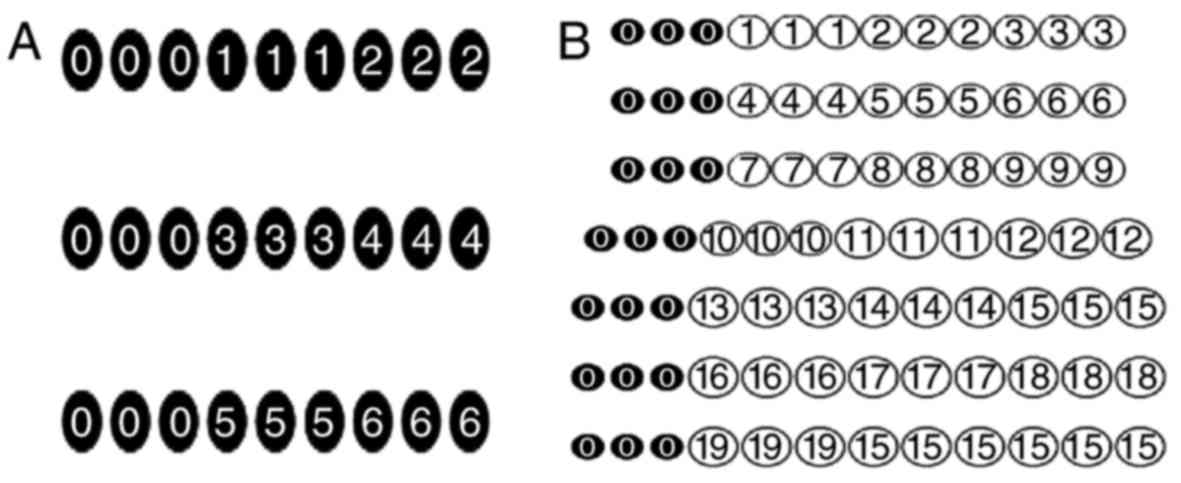 | Figure 1.The probe site arrangement of the
bacteria and fungi gene-detecting chips. (A) Fungi: 1, Candida
albicans; 2, Candida tropicalis; 3, Candida
glabrata; 4, Cryptococcus neoformans; 5, ITS; and 6,
blank control. (B) Bacteria: 1, Klebsiella pneumoniae; 2,
Acinetobacter baumannii; 3, Pseudomonas aeruginosa;
4, Escherichia coli; 5, enterobacter; 6, Haemophilus
influenzae; 7, Stenotrophomonas maltophilia; 8,
Neisseria meningitidis; 9, Enterococcus faecalis; 10,
Enterococcus faecium; 11, Listeria monocytogenes; 12,
Staphylococcus aureus; 13, Streptococcus pneumoniae;
14, Coagulase negative staphylococci; 15, blank control; 16,
Gram-negative bacteria; 17, Gram-negative bacteria; 18, 16S; 19,
experimental water; 0, internal control probes. |
 | Table II.Protocols of bacterial and fungal
chip hybridization. |
Table II.
Protocols of bacterial and fungal
chip hybridization.
| Procedure | Reagent | Volume (µl) | Time (min) | Sampling times | Temperature
(°C) |
|---|
| 1 | Prehybridization
solution | 1,200 | 5 | 1 | 42,43 |
| 2 | Hybridization
solution |
200 | 30 | 1 | 42,43 |
| 3 | Wash solution1 |
800 | 6 | 2 | 42,43 |
| 4 | Wash solution2 | 1,600 | 5 | 2 | 28 |
| 5 | Antibody
solution |
200 | 20 | 1 | 28 |
| 6 | Wash solution2 | / | 5 | 2 | 28 |
| 7 | Wash solution3 |
400 | 3 | 1 | 28 |
| 8 | Color-substrate
solution |
200 | 20 | 1 | 42,43 |
| 9 | Prehybridization
solution | / | 2 | 2 | 28 |
The hybridized chip was scanned in BaiO®
BE-2.0 Biochip reading meter (BSE01011; Shanghai BaiO Technology
Co., Ltd.). Image analysis, fluorescence signal intensity detection
and quantification of each probe set was performed with
BaiO® Gene chip image analysis software V2.4 (Shanghai
BaiO Technology Co., Ltd.). The identification of the infected
specimens was determined, with ≥2 of the bacterial and fungal
probes on the array displaying positive hybridization signals.
Analytical sensitivity, specificity
and reproducibility of microarray
The analytical sensitivity and specificity of the
bacterial and fungal assay was calculated by analysis and
comparison with the identified results of culture and sequencing
method. A total of 18 clinical isolates (Stenotrophomonas
maltophilia, Burkholderia cepacia and Candida parapsilosis; 6
isolates each) with no probes located in the chips, were used to
analyze specificity. In addition, the following closely related
type and reference strains were analyzed the analytical specificity
of the assay: K. pneumoniae and E. coli, P. aeruginosa and
Stenotrophomonas maltophilia, etc. Furthermore, genomic DNA of 2
types of pathogens with the sequenced isolates or reference strains
were mixed and used as templates for testing the cross-reaction.
The detection limit was determined with a 10-fold dilution series,
ranging from 1 to 100,000,000 copies of the study strains. To test
the reproducibility of the assay, all reference strains and
sequenced clinical strains (S. aureus, E. coli and Cryptococcus
neoformans) were analyzed and evaluated. DNA extraction, PCR
amplification and gene chip hybridization experiment were repeated
6 times, in the same batch and different batches of microarrays.
The clinical sensitivity, specificity, false-positive rate and
false-negative rates of microarray technique were evaluated with
clinical strains from 88 suspected infection CSF samples and 20
negative CSF samples.
Statistical analysis
All data were analyzed using SPSS 19.0 software (IBM
Corp., Armonk, NY, USA), and the comparison of ratios expressed via
the χ2 test or Fisher's exact test. P≤ was considered to
indicate a statistically significant difference.
Results
PCR amplification and probe
verification
The universal primers of bacteria and fungi were
assessed by PCR and sequencing with standard strains and clinical
isolates. The PCR products were confirmed to show clear bands of
the appropriate size for fungi and bacteria (~800 and 1,500 bp,
respectively) on agarose gel electrophoresis (Fig. 2). No bands were detected in the
negative control and blank control (data not shown). The PCR
products of the universal primers and DNA template from standard
strains or clinical isolates were sequenced. The sequencing results
are consistent with the sequences of bacteria or fungi from
GenBank, thus confirming the efficiency and accuracy of designed
primers. Furthermore, each probe matched that of the PCR products
in all standard strains and clinical isolates.
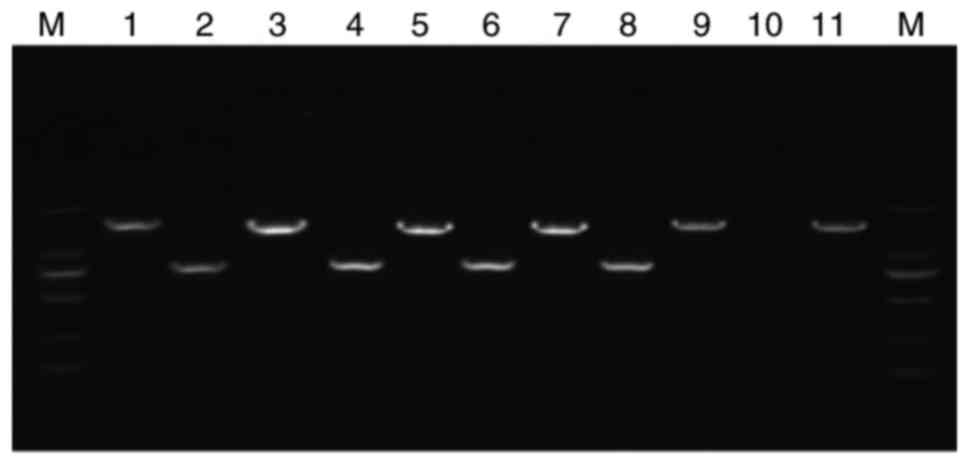 | Figure 2.Detection of bacterial and fungal
strains using electrophoresis of the polymerase chain reaction
products. Lane 1, Escherichia coli; lane 2, Candida
albicans; lane 3, Staphylococcus aureus; lane 4,
Candida tropicalis; lane 5, Enterococcus faecalis;
lane 6, Candida glabrata; lane 7, Klebsiella
pneumoniae; lane 8, Cryptococcus neoformans; lane 9,
Pseudomonas aeruginosa; lane 10, negative control; lane 11,
Enterobacter cloacae; M, DNA marker. |
Microarray hybridization
A total of 13 standard strains and 15 clinical
isolates were used to assess the chips. Coded DNA samples from
species were randomly selected and hybridized to gene chips, with
the typical and specific yellow fluorescent signals produced in
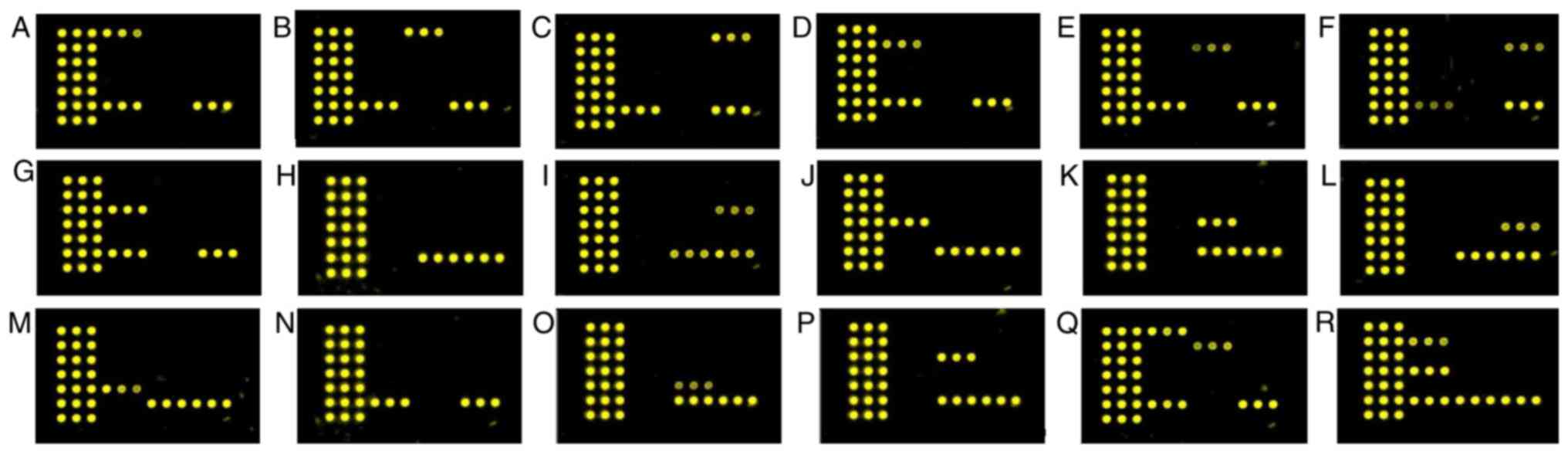
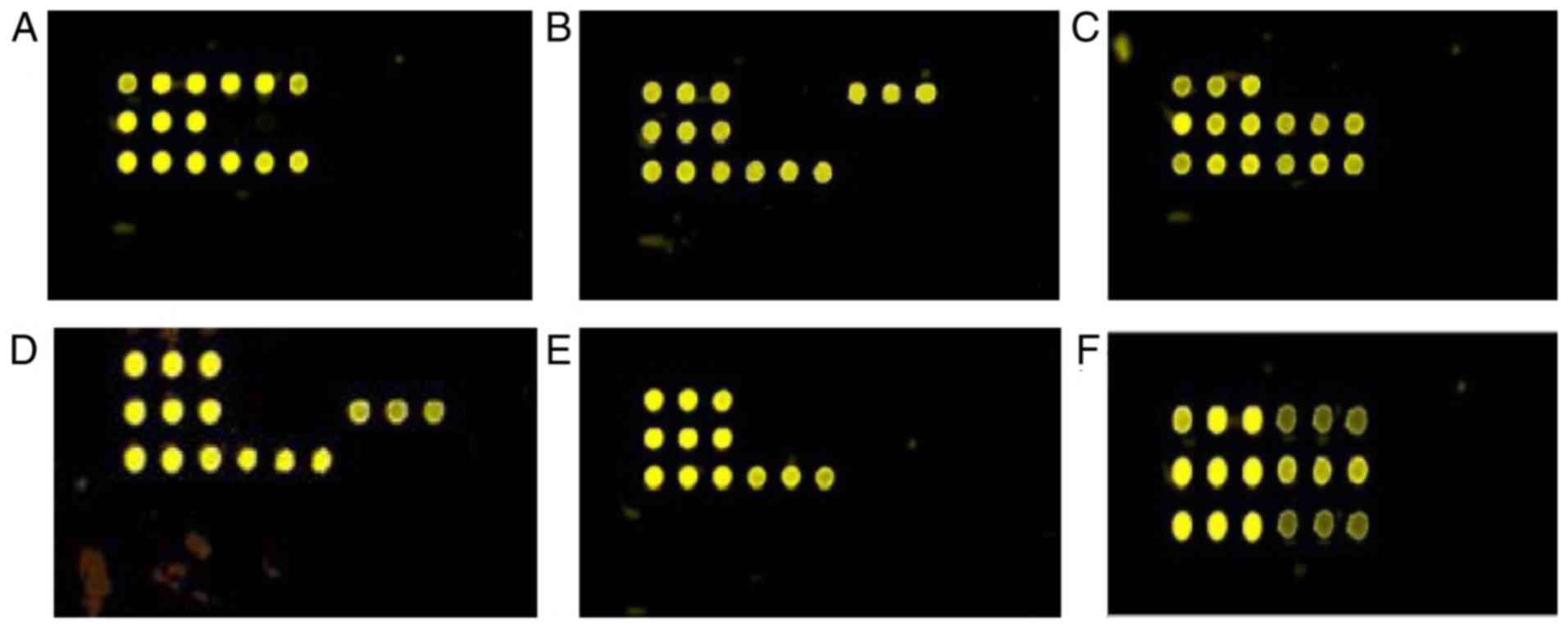
corresponding microarray spots (Figs.
3 and 4). The results matched
98% of the results achieved by conventional detection methods
except for some subspecies of coagulase negative Staphylococcus.
The negative control displayed no signal.
Assessment of microarray
hybridization
The results of mixed hybridization revealed that the
designed probes are able to detect a mix of 2 types of bacteria or
2 fungal isolates simultaneously (Figs.
3Q and R, and 4F). Using
bacterial products in the fungal microarray, no fluorescent signals
were detected, and vice versa. No cross-reactions with bacterial
species (K. pneumoniae and E. coli) tested and fungal species
(Candida albicans and Candida tropicalis) tested by chips were
detected. In the fixed condition of PCR, the minimum concentration
of detection was 10 cfu ml−1 for Gram-negative bacteria,
and 100 cfu ml−1 for Gram-positive bacterial and fungal
species. The results also demonstrated that the fluorescent signals
were directly associated with the concentration of bacterial and
fungal suspension from 10-10,000 cfu ml−1. However, at
the concentration ≥100,000 cfu ml−1, all pathogens
exhibited the same strength of fluorescent signals. The result of
reliability of gene chips with strains demonstrated that all the
batch of gene chips reported the same identified results and the
coefficient of variation (CV) for within-run and between-run assays
were 5.0-8.0 and 5.0-7.0%, respectively, demonstrating both
reproducibility and accuracy.
Primary clinical application of the
microarray
A total of 88 CSF samples from patients with
suspected intracranial infections and 20 negative CSF samples were
selected for microarray analysis. Culture testing took 4-7 days to
complete the identification, whereas the microarray analysis took
only 1 day. Culture testing presented 28 positive results (23
bacteria and 5 fungi), whereas the microarray analysis presented 41
positive cases of bacteria and fungi (35 bacteria and 6 fungi). The
sensitivity of gene chip was higher than that of the culture method
(100 vs. 68.3%, χ2=4.03, P<0.05; Table III). The results demonstrated that
the specificity, false-positive rate and false-negative rate of
microarray technique and culture method were 97.1 vs. 100%, 2.9 vs.
0%, and 0 vs. 31.7%, respectively.
 | Table III.Positive rates of pathogen detected
with DNA microarray and culture method in cerebrospinal fluid. |
Table III.
Positive rates of pathogen detected
with DNA microarray and culture method in cerebrospinal fluid.
|
| DNA microarray | Culture |
|
|---|
|
|
|
|
|
|---|
|
| Positive | Negative | Positive | Negative |
|
|---|
| Specimen group | Bacteria | Fungi | Bacteria | Fungi | Bacteria | Fungi | Bacteria | Fungi | P-value |
|---|
| Study group
(n=88) | 35 | 6 | 33 | 14 | 23 | 5 | 45 | 15 | <0.05 |
| Control group
(n=20) | 0 | 0 | 10 | 10 | 0 | 0 | 10 | 10 | >0.05 |
| Total (n=108) | 35 | 6 | 43 | 24 | 23 | 5 | 55 | 25 |
|
A total of 13 extra pathogens that were determined
negative using the culture method, were detected using the gene
chip technique, including P. aeruginosa (n=2), E.
coli (n=2), Ochrobactrum anthropi (n=2),
Streptococcus pneumonia (n=1), A. baumannii (n=1),
Cryptococcus neoformans (n=1), 16S positive (n=4). The 2 16S
positive but unidentified strains were successfully sequenced and
confirmed to be Brucella spp., but sequencing of the 2 other
strains failed. Of the 13 cases, 2 patients were discharged from
hospital prior to etiological diagnosis, 8 patients were treated
accordingly based on DNA microarray analysis and recovered, but the
3 more serious cases infected with Ochrobactrum anthropi, P.
aeruginosa and A. baumannii succumbed to
encephalocele.
Discussion
The rapid identification of bacteria and fungi can
be achieved by the detection of characteristic bacterial genes and
fungal genes in CSF specimens, which is important for clinical
diagnosis and therapy. Certain non-culture methods have been
developed to detect pathogens from CSF samples and may be more
sensitive than conventional methods. Previous studies (6,13–16) have
usually chosen the 16S rRNA and ITS as a target for universal
primers to amplify bacteria and fungi, respectively. The 16S rRNA
is composed of conserved regions and variable regions, which are
widely used in classification and identification of bacteria
(14–16). The conserved region and variable
region provide the premise for the design of bacterial
species-specific primers and probes. In addition, detection of
variations of ITS within fungal rDNA spacer regions has been
demonstrated to be effective for the identification and
classification of fungi (19). The
microarray-based virus detection provides a diagnostic tool for
viral CNS infections (3). As such,
the two technologies of universal primers PCR and DNA microarray
were combined in the present study to detect 14 types of bacteria
and 4 fungi. The detection was performed in 1 day, facilitating the
rapid detection of pathogens causing CNS infection.
The diagnostic probes designed in the microarray
covered >95% of the bacterial and fungal pathogens responsible
for intracranial infection. The DNA microarray exhibited positive
fluorescence at the corresponding sites when validating the array
with standard strains, except for certain subspecies of coagulase
negative Staphylococcus, which exhibited negative signals, possibly
because a single probe of coagulase negative Staphylococcus cannot
completely detect all subspecies. In our future study, more probes
will be designed to detect these subspecies.
The minimum concentration of detection of chips,
serving a key role in positive rate, was analyzed in the present
study. The results demonstrated that the gene chips were able to
identify pathogens at a concentration of 10 cfu ml−1 (10
µl/well) for bacteria and 100 cfu ml−1 (10 µl/well) for
fungi. In contrast, PCR sequencing was only able to identify
pathogens accurately at >10,000 cfu ml−1 (10
µl/well). Hence, DNA microarray is more sensitive in the detection
of all bacteria and fungi, and even more sensitive for detection of
Gram-negative bacteria than PCR sequencing. A possible explanation
is that Gram-positive bacteria and fungi cell membranes are
notoriously difficult to break (13,19),
which may lead to poor DNA extraction. The results are notable as
the low detection limit will allow direct detection of the DNA from
patient specimens, thus improving the detection rate. Furthermore,
the results demonstrated that the hybridization signal was linearly
dependent on the concentration of targeted microbes. This
potentially makes it possible for quantification and automation in
the future. The results demonstrated that every probe was specific
for its corresponding pathogen, and there was no cross-reaction
between them, indicating high specificity in the microarray
technique. The results of reproducibility demonstrated that the
repeatability of detection using chips had a high CV (5.0-8.0%).
However, the reproducibility was tested with purified DNA and
simulated specimens instead of real clinical samples in the present
study, therefore the latter may have issues of incomplete DNA
isolation, inhibitors being present, etc. which may affect
sensitivity.
The rate of false-positive results of microarray
technique was 2.9%, which may be due to the contamination of
samples. Effective methods for the removal of these substances will
be developed and the present protocol will be modified with
rigorous use of controls, including the extraction protocol, PCR
amplification, hybridization steps and analysis of fluorescence
signals.
A total of 23 cases of bacterial and 5 cases of
fungal positive culture specimens were identified to have the same
results using the microarray assay. A further 12 bacterial and 1
fungal cases of culture-negative specimen were identified, 9 were
identified using gene chip hybridization technique, demonstrating
the presence of infection. This suggests that microarray technology
may be more sensitive than conventional CSF culture methods, which
is consistent with other findings (3,6). The 4
unidentified strains that were demonstrated to be positive only in
the universal probe sites (16S), which may be due to the pathogens
not belonging to any of the species included in the study design.
The 2 16S positive unidentified strains were successfully sequenced
and confirmed to be Brucella spp., and the infection was
controlled with accordingly antimicrobial agents and recovered. Of
the 13 cases, 10 patients were recovering and discharged,
indicating a higher clinical diagnostic value. The availability of
species identification by use of this rapid and sensitive method
will enable physicians to treat with appropriate antimicrobial
agents in the absence of positive-culture in the CSF in a timely
manner, and in turn reduce the inappropriate use of antibiotics and
improve patient management.
Based on these findings, the DNA microarray assay of
the present study is suitable for detecting and analyzing a large
number of pathogens simultaneously, which may facilitate detection
and identification of the pathogens responsible for serious
infection, especially in the initial screening of patients with
suspected CNS infections. Although DNA microarray is not currently
the widely accepted clinical diagnostic method, previous studies
(12,20,22) and
the present study have demonstrated the potential clinical
application value of DNA microarray due to its high sensitivity,
specificity and reproducibility, while also being less
time-consuming. The DNA microarray can also be proposed as a
valuable supplement or substitute for the traditional methods
(microscopy and culture) in clinical diagnosis of intracranial
infections. Furthermore, this method can simultaneously detect a
large number of pathogens, suggesting that this technique is
helpful in the initial screening of patients with suspected CNS
infections. However, the present study was limited by the
single-center design and the small number of patients. To improve
the clinical value and application of DNA microarray for CNS
infections, multi-center large-sample studies with standardized
diagnostic criteria and procedures will be conducted in the future.
Studies are currently under way to increase the range of species,
add ≥2 more probes for each species that can be identified and to
apply the small chip directly to CSF specimens and other clinical
specimens.
The findings of the present study suggest that
high-density probe of the assay can greatly enhance detection range
of species and reduce the cost of testing. Furthermore, it is vital
to conduct comprehensive analysis in the future to avoid
false-positive results caused by contamination and false-negative
results caused by improper DNA extraction methods.
Acknowledgements
The authors wish to thank their department and
research team for their help and dedication.
Funding
The present study was supported by Beijing Council
of Science and Technology (grant no. Z141107002514012).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JCa and PW designed the present study and drafted
the manuscript; JCa, SG, JCh and BZ performed the experiments and
collected the data; JCa and BZ performed the statistical analysis;
JCh collected important background information. RM collected
important information regarding CSF pathogens, analyzed and
interpreted the data, and made substantial contributions in the
design and preparation of the gene chips. The final version of the
manuscript has been read and approved by all authors, and each
author believes that the manuscript represents honest work.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Xuanwu Hospital, Capital Medical University (Beijing,
China). All patients provided written informed consent.
Patient consent for publication
All patients provided written informed consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Amin M, Ghaderpanah M and Navidifar T:
Detection of Haemophilus influenzae type b, Streptococcus
agalactiae, Streptococcus pneumoniae and Neisseria
meningitidis in CSF specimens of children suspicious of
Meningitis in Ahvaz, Iran. Kaohsiung J Med Sci. 32:501–506. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Taj A and Jamil N: Detection of
meningococcal meningitis in cerebrospinal fluid of patients with
neurological disorders in government hospitals of Karachi. J Pak
Med Assoc. 66:1418–1421. 2016.PubMed/NCBI
|
|
3
|
Bøving MK, Pedersen LN and Møller JK:
Eight-Plex PCR and liquid-array detection of bacterial and viral
pathogens in cerebrospinal fluid from patients with suspected
meningitis. J Clin Microbiol. 47:908–913. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gade L, Scheel CM, Pham CD, Lindsley MD,
Iqbal N, Cleveland AA, Whitney AM, Lockhart SR, Brandt ME and
Litvintseva AP: Detection of fungal DNA in human body fluids and
tissues during a multistate outbreak of fungal meningitis and other
infections. Eukaryot Cell. 12:677–683. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khater WS and Elabd SH: Identification of
common bacterial pathogens causing meningitis in culture-negative
cerebrospinal fluid samples using real-time polymerase chain
reaction. Int J Microbiol. 2016:41971872016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sarookhani MR, Ayazi P, Alizadeh S,
Foroughi F, Sahmani A and Adineh M: Comparison of 16S rDNA-PCR
amplification and culture of cerebrospinal fluid for diagnosis of
bacterial meningitis. Iran J Pediatr. 20:471–475. 2010.PubMed/NCBI
|
|
7
|
Xiao X, Zhang Y, Zhang L, Kang P and Ji N:
The diagnostic value of cerebrospinal fluid lactate for
post-neurosurgical bacterial meningitis: A meta-analysis. BMC
Infect Dis. 16:4832016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shen J, Guan Y, Zhang J, Tang J, Lu X and
Zhang C: Application of microarray technology for the detection of
intracranial bacterial infection. Exp Ther Med. 7:496–500. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin AL and Safdieh JE: The evaluation and
management of bacterial meningitis: Current practice and emerging
developments. Neurologist. 16:143–151. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rajesh NT, Dutta S, Prasad R and Narang A:
Effect of delay in analysis on neonatal cerebrospinal fluid
parameters. Arch Dis Child Fetal Neonatal Ed. 95:F25–F29. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Filippis I, de Andrade CF, Caldeira N,
de Azevedo AC and de Almeida AE: Comparison of PCR-based methods
for the simultaneous detection of Neisseria meningitidis,
Haemophilus influenzae and Streptococcus pneumoniae in
clinical samples. Braz J Infect Dis. 20:335–341. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leveque N, Van Haecke A, Renois F,
Boutolleau D, Talmud D and Andreoletti L: Rapid virological
diagnosis of central nervous system infections by use of a
multiplex reverse transcription-PCR DNA microarray. J Clin
Microbiol. 49:3874–3879. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Esparcia O, Montemayor M, Ginovart G,
Pomar V, Soriano G, Pericas R, Gurgui M, Sulleiro E, Prats G,
Navarro F and Coll P: Diagnostic accuracy of a 16S ribosomal DNA
gene-based molecular technique (RT-PCR, microarray and sequencing)
for bacterial meningitis, early-onset neonatal sepsis and
spontaneous bacterial peritonitis. Diagn Microbiol Infect Dis.
69:153–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Srinivasan L, Pisapia JM, Shah SS, Halpern
CH and Harris MC: Can broad-range 16S ribosomal ribonucleic acid
gene polymerase chain reactions improve the diagnosis of bacterial
meningitis? A systematic review and meta-analysis. Ann Emerg Med.
60:609–620.e2. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Klindworth A, Pruesse E, Schweer T,
Peplies J, Quast C, Horn M and Glöckner FO: Evaluation of general
16S ribosomal RNA gene PCR primers for classical and
next-generation sequencing-based diversity studies. Nucleic Acids
Res. 41:e12013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu A, Wang, C1 Liang Z, Zhou ZW, Wang L,
Ma Q, Wang G, Zhou SF and Wang Z: High-throughput sequencing of 16S
rDNA amplicons characterizes bacterial composition in cerebrospinal
fluid samples from patients with purulent meningitis. Drug Des
Devel Ther. 9:4417–4429. 2015.PubMed/NCBI
|
|
17
|
Zhong D, Koepfli C, Cui L and Yan G:
Molecular approaches to determine the multiplicity of
Plasmodium infections. Malar J. 17:1722018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bumgarner R: Overview of DNA microarrays:
Types, applications and their future. Curr Protoc Mol Biol.
101:22.1.1–22.1.11. 2013.
|
|
19
|
McCarthy MW and Walsh TJ: PCR methodology
and applications for the detection of human fungal pathogens.
Expert Rev Mol Diagn. 16:1025–1036. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rupp S: Microarray technologies in fungal
diagnostics. Methods Mol Biol. 1508:385–409. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ben RJ, Kung S, Chang FY, Lu JJ, Feng NH
and Hsieh YD: Rapid diagnosis of bacterial meningitis using a
microarray. J Formos Med Assoc. 107:448–453. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Patel A and Cheung SW: Application of DNA
microarray to clinical diagnostics. Methods Mol Biol. 1368:111–132.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Raich T and Powell S: Identification of
bacterial and fungal pathogens from positive blood culture bottles:
A microarray-based approach. Methods Mol Biol. 1237:73–90. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van Veen SQ, Claas EC and Kuijper EJ:
High-throughput identification of bacteria and yeast by
matrix-assisted laser desorption ionization-time of flight mass
spectrometry in conventional medical microbiology laboratories. J
Clin Microbiol. 48:900–907. 2010. View Article : Google Scholar : PubMed/NCBI
|