Introduction
Breast cancer is the most common type of cancer in
women (1) and its development is
associated with various factors, such as estrogen level, diet,
hereditary susceptibility and obesity (2). These factors contribute to gene
mutations, cell cycle abnormalities and loss of the control of
epigenetic modification, inducing alterations in a variety of
signaling pathways, such as phosphoinositide 3-kinase
(PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR),
RAS/RAF/mitogen-activated protein kinase (MAPK), estrogen receptor
(ER) and cyclin-dependent kinases (CDKs) (3,4).
Although the diagnosis and treatment of breast cancer have markedly
improved in recent years, the prognosis of patients with
advanced-stage disease remains poor. The incidence of breast cancer
is increasing in China, representing a major threat to the health
of women. Thus, it is crucial to identify potent new agents for the
treatment of breast cancer.
Natural products have attracted the attention of
several research scientists for the development of antitumor drugs,
due to their proven efficacy and safety. Natural products play an
important role in the discovery of lead compounds, and several
natural products have been developed and used in clinical practice
due to their potent antitumor properties, such as vincristine,
camptothecin and paclitaxel. Thus, natural products may represent
excellent sources of novel antitumor agents, and a number of them
must be further characterized. Curcumin is one of the most
important natural compounds, as it was found to possess multiple
antitumor properties, and may also sensitize tumor cells to
targeted therapy agents and reverse resistance to chemotherapeutic
drugs (5).
Several studies reported that curcumin may regulate
multiple signaling pathways, including PI3K/AKT, MAPK and nuclear
factor (NF)-κB (6). Curcumin exerts
synergistic effects when combined with other chemotherapeutic
agents. In breast cancer cell lines, curcumin and paclitaxel exert
complementary effects on the alteration of proteins involved in
apoptotic and inflammatory pathways (7). Curcumin was shown to induce endothelial
growth factor receptor degradation and potentiate the antitumor
activity of gefitinib in non-small-cell lung cancer cell lines and
xenograft mouse models; intriguingly, it also attenuated
gefitinib-induced gastrointestinal adverse effects via altering p38
activation (8). Curcumin was also
shown to increase the response of pancreatic cancer cells to
gemcitabine through attenuating EZH2 and lncRNA PVT1 expression
(9). In addition, curcumin was
reported to inhibit epithelial-to-mesenchymal transition (EMT) of
breast cancer cells (10,11). The focus of this study was the
antitumor effect of curcumin on breast cancer cell lines and its
underlying mechanism, in order to provide proof of the efficacy of
curcumin in the treatment of breast cancer.
Materials and methods
Cell lines
The breast cancer T47D, MCF7, MDA-MB-415, SK-BR-3,
MDA-MB-231, MDA-MB-468 and BT-20 cell lines were purchased from
American Type Culture Collection (Manassas, VA, USA). Cells were
routinely cultured in complete medium with 10% fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at
37°C in a 5% CO2 incubator.
Reagents
Curcumin was purchased from Sigma-Aldrich; Merck
KGaA (Darmstadt, Germany), diluted in dimethyl sulfoxide (DMSO) at
10 mM and stored at −20°C. The primary antibodies against p-Akt,
Akt, p-mTOR, mTOR, p-S6, B-cell lymphoma 2 (BCL2), BAX, cleaved
caspase 3 and β-actin were purchased from Cell Signaling
Technology, Inc., (Danvers, MA, USA). The antibodies against CDC25,
CDC2 and P21, and the secondary antibodies labelled with
horseradish peroxidase were purchased from Abcam (Cambridge, MA,
USA).
Cell proliferation determination
The breast cancer cells were seeded into 96-well
plates at a density of 3,000 cells per well in triplicates. After
overnight adherence, the cell lines were treated with various
concentrations of curcumin for 72 h. DMSO (0.1%) was added to the
control wells followed by incubation at 37°C for 4 h after addition
of 5 mg/ml
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
(MTT) to each well. Absorbance was measured on VersaMax (Molecular
Devices, LLC, Sunnyvale, CA, USA) at 570 nm after addition of 100
µl chromogenic triplex solution (10% SDS, 5% isobutyl alcohol and
0.01 M HCl). The IC50 was developed by an inhibition
curve and recorded as the mean ± standard deviation of three
independent experiments.
Cell cycle assessment
Cells in the logarithmic growth phase were seeded at
a density of 2×105 cells/well in a 6-well plate,
incubated at 37°C until adherent and treated with 10 or 30 µM
curcumin for 24 h, while the control cells were treated with 0.1%
DMSO. Cells were harvested into centrifuge tubes and washed with
ice-cold phosphate-buffered saline (PBS), then fixed with 700 µl
ethanol after being detached in PBS at 4°C overnight. The cells
were collected by centrifugation at 500 × g for 5 min, then
resuspended in 500 µl PBS containing 10 µg/ml RNase at 37°C for 15
min, followed by staining with 2 mg/ml propidium iodide (PI) at a
final concentration of 10 µg/ml and incubated for 15 min at 4°C in
the dark. Analysis was performed on a FACSCalibur analyzer (BD
Biosciences, Franklin Lakes, NJ, USA) and the data were analyzed
using CellQuest 3.3 software. The tests were performed in three
independent experiments.
Cell apoptosis measurement
To determine the effect of curcumin on cell
apoptosis, T47D and MCF7 cells in the logarithmic growth phase were
seeded at a density of 2×105 cells/well in a 6-well
plate and incubated at 37°C. Then, adherent cells were treated with
10 or 30 µM curcumin for 48 h; the control well was treated with
0.1% DMSO. Cells were detached and washed twice with cooled PBS.
Subsequently, the cells were collected and resuspended in cold 1×
binding buffer, and Annexin-V and PI (Beyotime Biotechnology,
Jiangsu, China) were added into the binding buffer and incubated
for 10 min at room temperature in the dark. Analysis was performed
on a FACSCalibur analyzer (BD Biosciences). The tests were
performed in three independent experiments.
Western blot analysis
Cells were treated with curcumin for 12 h at a
density of 2×105 cells/well in a 6-well plate.
Subsequently, the cells were detached and harvested in RIPA buffer,
then kept on ice for 30 min and centrifuged at 10,000 × g for 15
min. The total protein was quantitated with the BCA protein
quantitation reagent kit (Pierce; Thermo Fisher Scientific, Inc.),
followed by addition of equal volume of 2× SDS-PAGE loading buffer
containing 100 mM Tris-HCl (pH 6.8), 200 mM DTT, 4% SDS, 0.1
bromophenol blue and 20% glycerol, and boiling for 10 min for
denaturation. Equal amounts of total proteins were loaded on the
SDS-PAGE gel and electrophoresed in the Tris-glycine
electrophoresis buffer [25 mM Tris-HCl (pH 8.0), 250 mM glycine and
0.1% SDS] in 100 V for 1.5 h. The separated proteins were
transferred to Hybond-C nitrocellulose membranes in transferring
buffer (39 mM glycine, 48 mM Tris and 20% methanol) for 2 h. The
blots were blocked with 5% non-fat milk in TBST [20 mM Tris-HCl (pH
7.2–7.4), 150 mM NaCl and 0.1% (v/v) Tween-20] for 1 h at room
temperature, incubated with primary antibodies at 4°C overnight,
and then washed three times with TBST for 10 min, followed by
incubation with a horseradish peroxidase-conjugated secondary
antibody diluted in blocking buffer for 1 h and washing three times
with TBST for 10 min. The blots were developed with the ECL Plus
Western Blotting Detection system (Thermo Fisher Scientific, Inc.).
The tests were performed in three independent experiments.
Statistical analysis
Statistical analysis was performed using One-way
analysis of variance with the S-N-K method when comparing three
groups. P<0.05 was considered to indicate a statistically
significant difference. The statistical analysis was performed with
SPSS v.13.0 (SPSS, Inc., Chicago, IL, USA).
Results
Curcumin inhibited the proliferation
of breast cancer cells
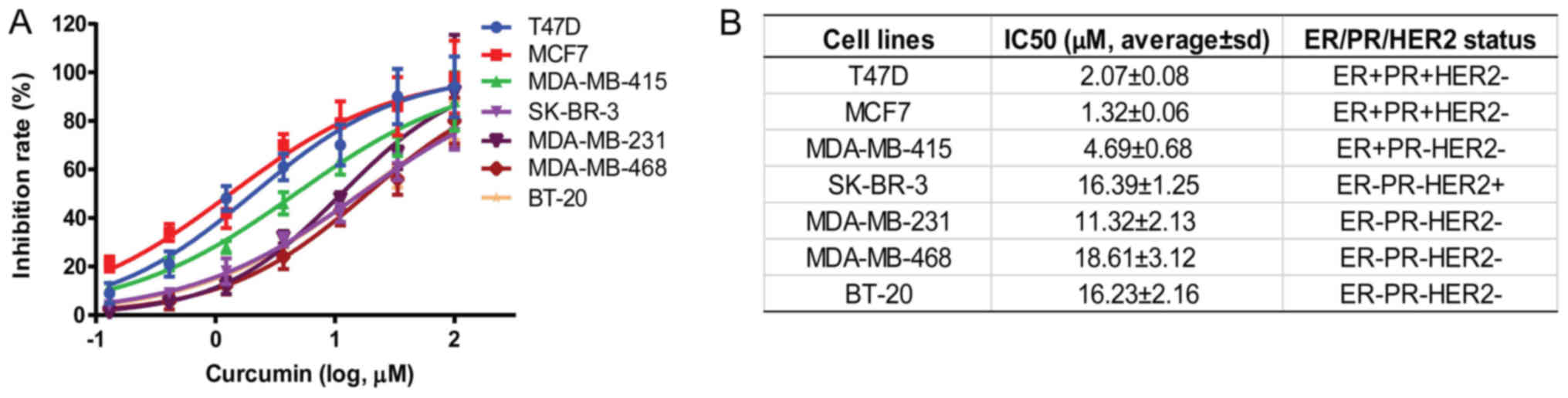
A variety of breast cancer cell lines, including
T47D, MCF7, MDA-MB-415, SK-BR-3, MDA-MB-231, MDA-MB-468 and BT-20,
with different ER, progesterone receptor (PR) and human epidermal
growth factor receptor-2 (HER2) statuses were selected, and the
inhibitory effect of curcumin on these cells was determined after
72 h of treatment. The results demonstrated that the proliferation
of breast cancer cells was dose-dependently inhibited by curcumin
(Fig. 1A). The IC50 of
curcumin in various cell lines was calculated, and the results
revealed different activity of breast cancer cells in response to
curcumin. Interestingly, curcumin was more active on ER+
breast cancer cells, such as T47D, MCF7 and MDA-MB-415, with an
IC50 of 2.07±0.08, 1.32±0.06 and 4.69±0.06 µM,
respectively (Fig. 1B). With regards
to the ER−PR−HER2− cells, such as
MDA-MB-231, MDA-MD-468 and BT-20 cells, the IC50 was
relatively weaker, namely 11.32±2.13 µM, 18.61±3.12 µM and
16.23±2.16 µM, respectively. These results demonstrated that
curcumin exerted a more potent effect on ER+ breast
cancer cells, such as T47D and MCF7; thus, these cells were
selected as models for further research on its mechanism of
action.
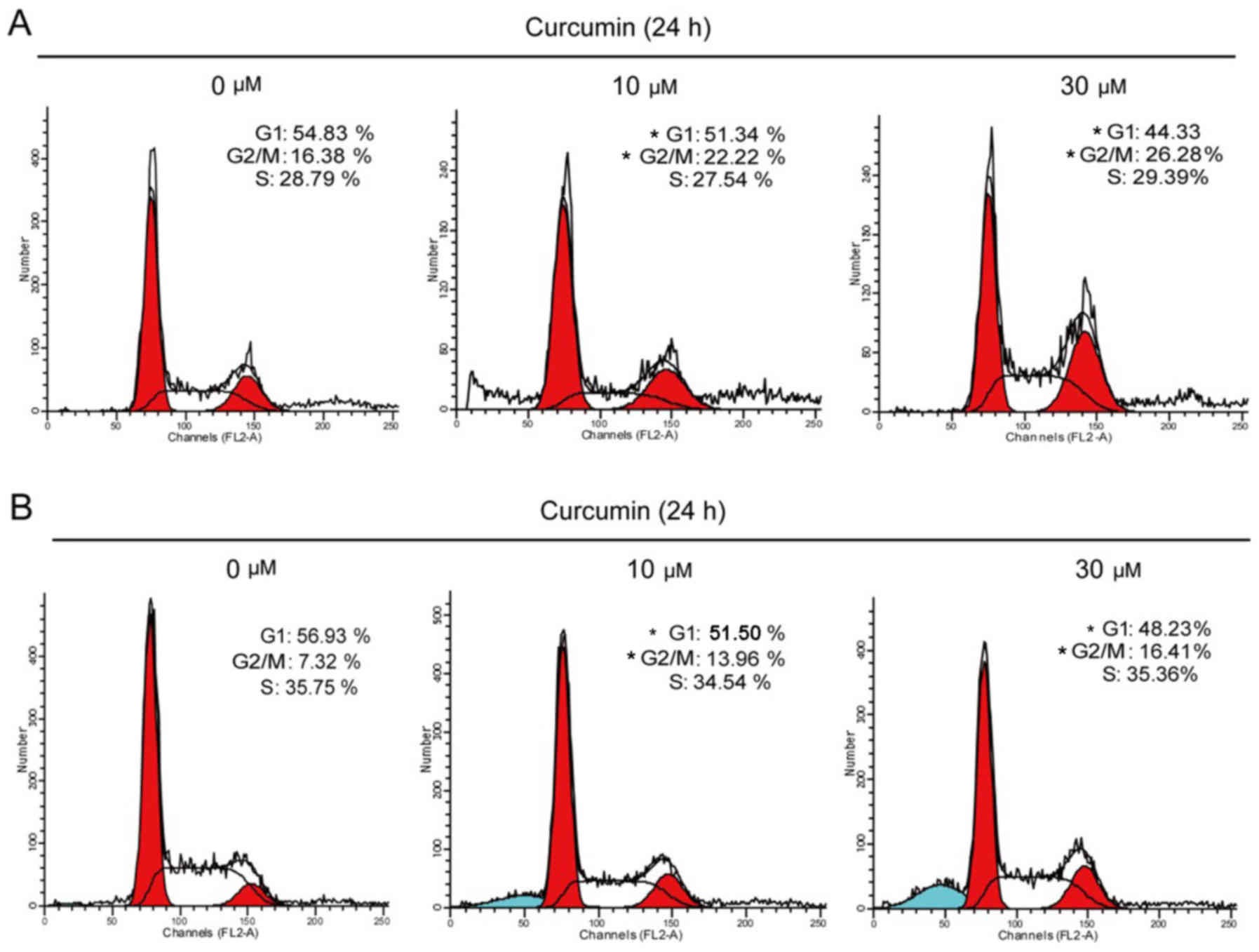
Curcumin induced G2/M cell cycle
arrest
To explore the mechanism underlying the
antiproliferative action of curcumin, alterations in the cell cycle
induced by curcumin were evaluated. T47D and MCF7 cells were
treated with 10 or 30 µM curcumin for 24 h, and the cell cycle was
evaluated by flow cytometry. Our results demonstrated that the
cells were arrested in the G2/M phase (Fig. 2). The percentage of T47D cells in the
G2/M phase increased from 16.38 to 22.22 and 26.28% after being
treated for 24 h with 10 and 30 µM curcumin, respectively. Similar
to T47D cells, the percentage of MCF7 cells in the G2/M phase
increased from 7.32 to 13.96 and 16.41% after being treated for 24
h with 10 and 30 µM curcumin, respectively (Fig. 2B).
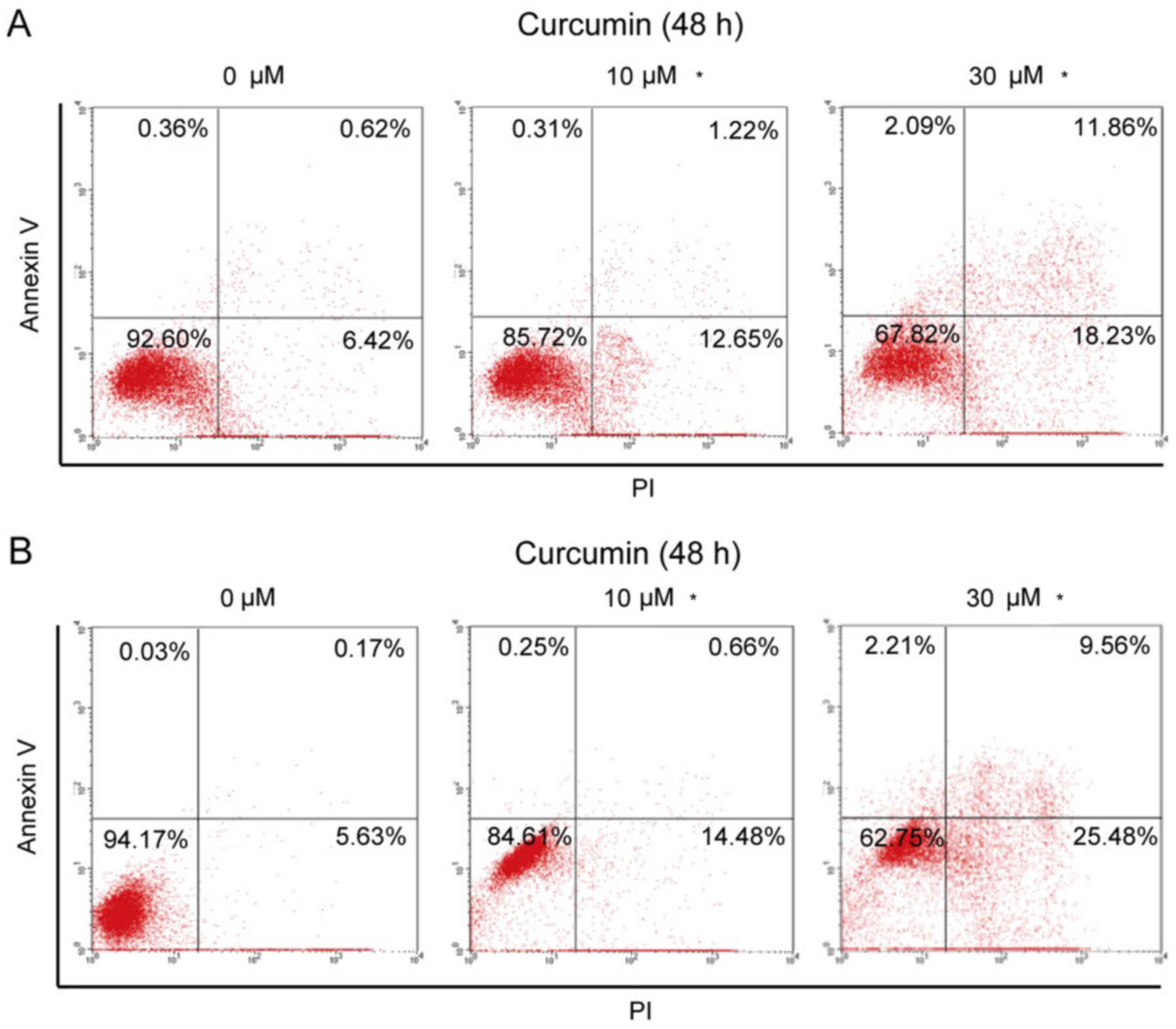
Curcumin promoted the apoptosis of
breast cancer cells
Next, the effect of curcumin on cell apoptosis was
investigated. T47D and MCF7 cells were treated with 10 or 30 µM
curcumin for 24 h. The results demonstrated that curcumin promoted
cell apoptosis. We calculated the early apoptosis (upper right) and
later apoptosis (lower right), and the total apoptotic ratio of
T47D cells increased from 7.04% at baseline to 13.87 and 30.09%
after treatment with 10 and 30 µM curcumin, respectively (Fig. 3A). Similarly, in MCT7 cells, the
total apoptosis rate increased from 5.8% at baseline to 15.14 and
35.04% after treatment with 10 and 30 µM curcumin, respectively. In
addition, curcumin at 30 µM induced some cell debris in T47D and
MCF7 cells, as the increased percentage of upper left area. These
results revealed that curcumin promoted the apoptosis of breast
cancer cells, and MCF7 cells appeared to be more sensitive to
curcumin-induced apoptosis compared with T47D cells.
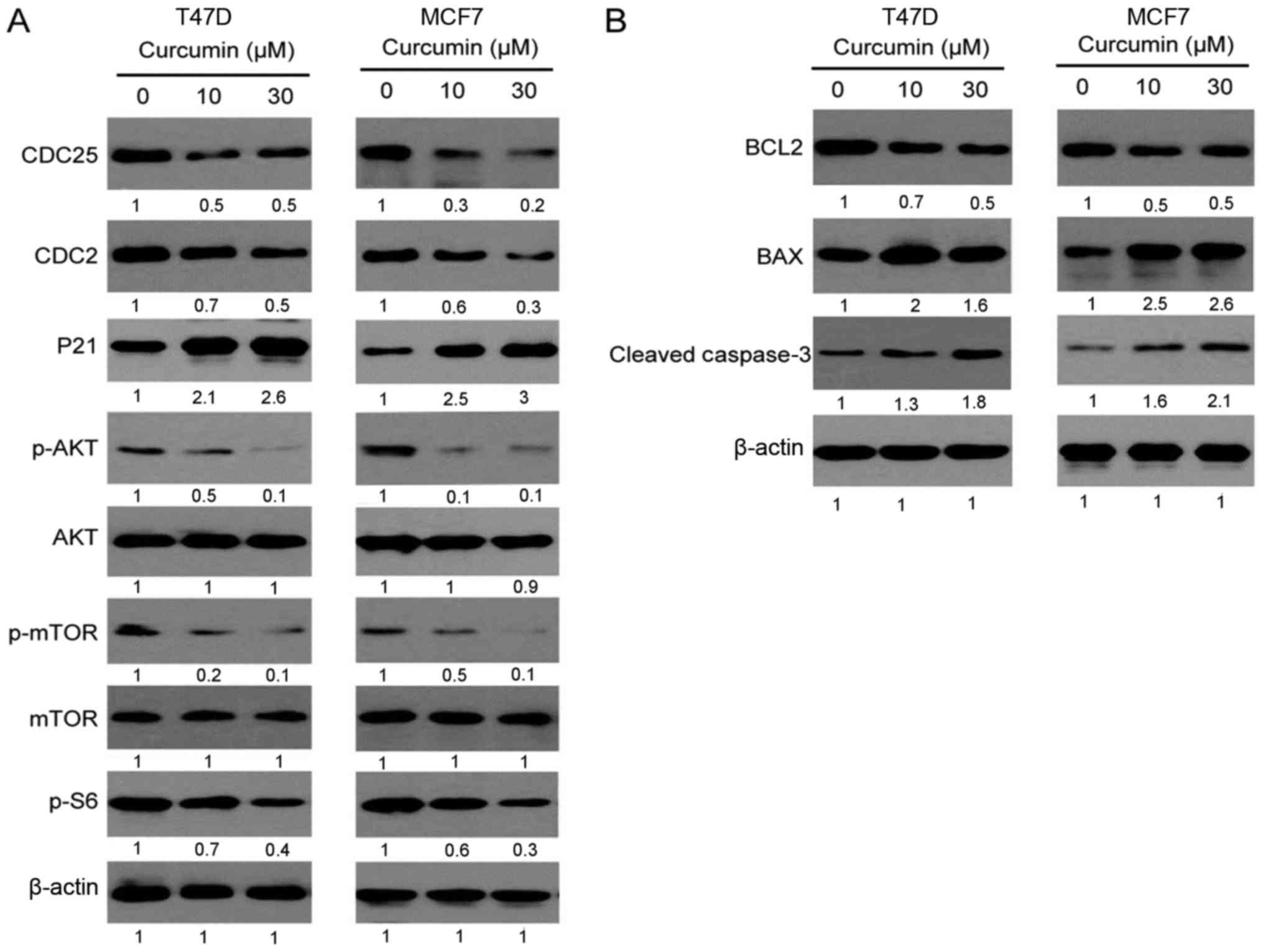
Curcumin regulates the signaling
pathways of cell cycle and apoptosis
To achieve a better understanding of how curcumin
inhibits the cell cycle and promotes apoptosis, the expression of
key signaling molecules involved in these processes was determined
by western blotting. The regulatory proteins related to the G2/M
phase of the cell cycle, such as CDC25, CDC2 and P21, were
detected. CDC25 and CDC2 are accelerators of the G2/M phase, while
P21 is an inhibitor. As seen in Fig.
4, the expression of CDC25 and CDC2 proteins was downregulated
and that of the P21 protein was markedly upregulated in both T47D
and MCF7 cell lines following treatment with curcumin for 12 h.
This result indicated that curcumin may affect the cell cycle by
regulating the expression of regulatory proteins in the G2/M cell
phase. In addition, curcumin was also found to inhibit the
phosphorylation of Akt, mTOR and their downstream proteins, which
are upstream of the cell cycle proteins, suggesting that curcumin
may induce cell cycle arrest through the inhibition of Akt/mTOR
signaling.
Curcumin was also found to promote apoptosis of
breast cancer cells; thus, the expression of cell apoptotic
proteins, such as BCL2, BAX and caspase 3, was also analyzed. BCL2
is an anti-apoptotic protein, while BAX is a pro-apoptotic protein.
As seen in Fig. 4B, the BCL2 level
was markedly decreased and the BAX level was increased in both T47D
and MCF7 cell lines following treatment with curcumin for 12 h and,
correspondingly, the expression of downstream cleaved caspase 3 was
increased, suggesting that curcumin may promote the mitochondrial
apoptotic pathway.
Discussion
Curcumin is a well-known natural compound, which has
been shown to have pleotropic pharmacological properties, such as
antifungal and antitumor properties (12–14). In
recent years, the antitumor activity of curcumin has been
extensively investigated, and compelling evidence demonstrated that
several proteins involved in cancer signaling pathways were
regulated by curcumin, such as tumor suppressors P53, P21 and P27,
inflammatory regulator NF-κB, and Akt/mTOR in pancreatic and colon
cancer (15–17). In the present study, we investigated
the antitumor activity of curcumin in breast cancer.
Curcumin was found to be a potent inhibitor on
breast cancer cells in vitro, with an IC50 at the
micromolar level. Moreover, it acted differently in breast cancer
cell lines with a different ER/PR/HER2 status, and favorably
inhibited ER+ cell lines, such as T47D and MCF7. To
further investigate the molecular mechanism underlying the
inhibitory effects of curcumin, T47D and MCF7 cells were selected,
as they were found to be more sensitive to its actions. The results
demonstrated that curcumin caused G2/M cell cycle arrest, which may
be one of the key mechanisms underlying the inhibition of cell
proliferation in breast cancer. This result is consistent with
previous reports of curcumin inducing G2/M arrest in bladder cancer
cells (18). The molecular mechanism
underlying this action of curcumin was further explored. CDC25 and
CDC2 are important positive regulators and P21 is a negative
regulator of the G2/M phase, and are closely associated with the
proliferation and response to chemotherapy of breast cancer cells.
We found that curcumin decreased the expression of CDC25 and CDC2
and increased the expression of P21. Taken together, our results
demonstrated that curcumin blocked the G2/M phase by decreasing the
CDC25 and CDC2 levels in addition to increasing the P21 level.
Breast cancer is a complex disease caused by a
variety of factors leading to activation of multiple signaling
pathways, including the PI3K/Akt/mTOR, RAF/MEK/ERK and ER pathways.
Compelling experimental evidence has demonstrated that targeting
the Akt/mTOR pathway is promising for the treatment of breast
cancer (19–21). In this study, we found that curcumin
exerted an inhibitory effect on Akt/mTOR phosphorylation. The mTOR
pathway, in addition to cancer, is also implicated in the
pathogenesis of autoimmune (22,23) and
infectious diseases (24–26). Thus, we hypothesized that curcumin
may also have therapeutic potential in autoimmune and infectious
diseases, such as HIV infection. In addition, curcumin may also
promote the mitochondrial apoptotic pathway in breast cancer cells,
further supporting the therapeutic value of curcumin in breast
cancer. Although the preclinical data of curcumin in antitumor
treatment are intriguing, several clinical studies with curcumin
have yielded disappointing results. Thus, several studies are
underway aiming to develop curcumin analogues of higher potency,
better bioavailability and longer half-life. For example, allylated
monocarbonyl analogues and enone analogues of curcumin were found
to promote mitotic arrest and apoptosis by reactive oxygen
species-mediated stress (27,28).
Novel curcumin analogues exhibited high potency in
castration-resistant prostate cancer (29) and nasopharyngeal carcinoma (30). Interestingly, novel curcumin
derivatives may exhibit high potency in triple-negative breast
cancer cells (31,32); curcumin exhibited lower activity in
these cancers cells in the present study, and suggested that
optimization of curcumin structure may expand its therapeutic
spectrum.
In conclusion, curcumin exerted a potent antitumor
effect on breast cancer by inducing cell cycle arrest at the G2/M
phase, likely mediated by the decreased expression of CDC25 and
CDC2, and the increased expression of P21. Curcumin inhibited the
phosphorylation of the Akt/mTOR signaling pathway, decreased the
expression of the anti-apoptotic protein BCL2, increased the
expression of the apoptotic protein BAX, and induced caspase 3
protein cleavage, leading to cell apoptosis. Thus, these results
may provide a basis for further study of curcumin in the treatment
of breast cancer.
Acknowledgements
The authors would like to thank the Science and
Technology Bureau of Shaoxing for awarding the grant.
Funding
The present study was supported by a grant from the
Science and Technology Bureau of Shaoxing (grant no.
2014B70079).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SH and HS were responsible for the conception and
design of the study. SH, LM and LH collaborated in the development
of methodology. SH, LM and HS acquired the data. SH, LM, YX and HS
wrote and revised the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
DeSantis CE, Ma J, Sauer Goding A, Newman
LA and Jemal A: Breast cancer statistics, 2017, racial disparity in
mortality by state. CA Cancer J Clin. 67:439–448. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Karimi Z, Jessri M, Houshiar-Rad A,
Mirzaei HR and Rashidkhani B: Dietary patterns and breast cancer
risk among women. Public Health Nutr. 17:1098–1106. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hart CD, Migliaccio I, Malorni L,
Guarducci C, Biganzoli L and Di Leo A: Challenges in the management
of advanced, ER-positive, HER2-negative breast cancer. Nat Rev Clin
Oncol. 12:541–552. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arnedos M, Vicier C, Loi S, Lefebvre C,
Michiels S, Bonnefoi H and Andre F: Precision medicine for
metastatic breast cancer-limitations and solutions. Nat Rev Clin
Oncol. 12:693–704. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou QM, Wang XF, Liu XJ, Zhang H, Lu YY,
Huang S and Su SB: Curcumin improves MMC-based chemotherapy by
simultaneously sensitising cancer cells to MMC and reducing
MMC-associated side-effects. Eur J Cancer. 47:2240–2247. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nagaraju GP, Aliya S, Zafar SF, Basha R,
Diaz R and El-Rayes BF: The impact of curcumin on breast cancer.
Integr Biol (Camb). 4:996–1007. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quispe-Soto ET and Calaf GM: Effect of
curcumin and paclitaxel on breast carcinogenesis. Int J Oncol.
49:2569–2577. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee JY, Lee YM, Chang GC, Yu SL, Hsieh WY,
Chen JJ, Chen HW and Yang PC: Curcumin induces EGFR degradation in
lung adenocarcinoma and modulates p38 activation in intestine: The
versatile adjuvant for gefitinib therapy. PLoS One. 6:e237562011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoshida K, Toden S, Ravindranathan P, Han
H and Goel A: Curcumin sensitizes pancreatic cancer cells to
gemcitabine by attenuating PRC2 subunit EZH2, and the lncRNA PVT1
expression. Carcinogenesis. 38:1036–1046. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gallardo M and Calaf GM: Curcumin inhibits
invasive capabilities through epithelial mesenchymal transition in
breast cancer cell lines. Int J Oncol. 49:1019–1027. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gallardo M and Calaf GM: Curcumin and
epithelial-mesenchymal transition in breast cancer cells
transformed by low doses of radiation and estrogen. Int J Oncol.
48:2534–2542. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khalil OAK, de Faria Oliveir OMM, Vellosa
JCR, Quadros AU, Dalposso LM, Karam TK, Mainardes RM and Khalil NM:
Curcumin antifungal and antioxidant activities are increased in the
presence of ascorbic acid. Food Chem. 133:1001–1005. 2012.
View Article : Google Scholar
|
|
13
|
Perrone D, Ardito F, Giannatempo G,
Dioguardi M, Troiano G, Lo Russo L, Lillo DE A, Laino L and Lo
Muzio L: Biological and therapeutic activities, and anticancer
properties of curcumin. Exp Ther Med. 10:1615–1623. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
El-Houseini ME, El-Agoza IA, Sakr MM and
El-Malky GM: NNovel protective role of curcumin and taurine
combination against experimental hepatocarcinogenesis. Exp Ther
Med. 13:29–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park MJ, Kim EH, Park IC, Lee HC, Woo SH,
Lee JY, Hong YJ, Rhee CH, Choi SH, Shim BS, et al: Curcumin
inhibits cell cycle progression of immortalized human umbilical
vein endothelial (ECV304) cells by up-regulating cyclin-dependent
kinase inhibitor, p21WAF1/CIP1, p27KIP1 and p53. Int J Oncol.
21:379–383. 2002.PubMed/NCBI
|
|
16
|
Kunnumakkara AB, Guha S, Krishnan S,
Diagaradjane P, Gelovani J and Aggarwal BB: Curcumin potentiates
antitumor activity of gemcitabine in an orthotopic model of
pancreatic cancer through suppression of proliferation,
angiogenesis, and inhibition of nuclear factor-kappaB-regulated
gene products. Cancer Res. 67:3853–3861. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hussain AR, Al-Rasheed M, Manogaran PS,
Al-Hussein KA, Platanias LC, Al Kuraya K and Uddin S: Curcumin
induces apoptosis via inhibition of PI3′-kinase/AKT pathway in
acute T cell leukemias. Apoptosis. 11:245–254. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park C, Kim GY, Kim GD, Choi BT, Park YM
and Choi YH: Induction of G2/M arrest and inhibition of
cyclooxygenase-2 activity by curcumin in human bladder cancer T24
cells. Oncol Rep. 15:1225–1231. 2006.PubMed/NCBI
|
|
19
|
Yang SX, Polley E and Lipkowitz S: New
insights on PI3K/AKT pathway alterations and clinical outcomes in
breast cancer. Cancer Treat Rev. 45:87–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abraham J: PI3K/AKT/mTOR pathway
inhibitors: The ideal combination partners for breast cancer
therapies? Expert Rev Anticancer Ther. 15:51–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Steelman LS, Martelli AM, Cocco L, Libra
M, Nicoletti F, Abrams SL and McCubrey JA: The therapeutic
potential of mTOR inhibitors in breast cancer. Br J Clin Pharmacol.
82:1189–1212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Donia M, Mangano K, Amoroso A, Mazzarino
MC, Imbesi R, Castrogiovanni P, Coco M, Meroni P and Nicoletti F:
Treatment with rapamycin ameliorates clinical and histological
signs of protracted relapsing experimental allergic
encephalomyelitis in Dark Agouti rats and induces expansion of
peripheral CD4+CD25+Foxp3+ regulatory T cells. J Autoimmun.
33:135–140. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oaks Z, Winans T, Huang N, Banki K and
Perl A: Activation of the mechanistic target of rapamycin in SLE:
Explosion of evidence in the last five years. Curr Rheumatol Rep.
18:732016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nicoletti F, Fagone P, Meroni P, McCubrey
J and Bendtzen K: mTOR as a multifunctional therapeutic target in
HIV infection. Drug Discov Today. 16:715–721. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Donia M, McCubrey JA, Bendtzen K and
Nicoletti F: Potential use of rapamycin in HIV infection. Br J Clin
Pharmacol. 70:784–793. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nicoletti F, Lapenta C, Donati S, Spada M,
Ranazzi A, Cacopardo B, Mangano K, Belardelli F, Perno C and Aquaro
S: Inhibition of human immunodeficiency virus (HIV-1) infection in
human peripheral blood leucocytes-SCID reconstituted mice by
rapamycin. Clin Exp Immunol. 155:28–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rajamanickam V, Zhu H, Feng C, Chen X,
Zheng H, Xu X, Zhang Q, Zou P, He G, Dai X, et al: Novel allylated
monocarbonyl analogs of curcumin induce mitotic arrest and
apoptosis by reactive oxygen species-mediated endoplasmic reticulum
stress and inhibition of STAT3. Oncotarget. 8:101112–101129. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Deck LM, Hunsaker LA, Vander Jagt TA,
Whalen LJ, Royer RE and Vander Jagt DL: Activation of anti-oxidant
Nrf2 signaling by enone analogues of curcumin. Eur J Med Chem.
143:854–865. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen S, Nimick M, Cridge AG, Hawkins BC
and Rosengren RJ: Anticancer potential of novel curcumin analogs
towards castrate-resistant prostate cancer. Int J Oncol.
52:579–588. 2018.PubMed/NCBI
|
|
30
|
Pan Y, Liu G, Xiao J, Su B, Zhou F and Wei
Y: A novel curcuminoid exhibits enhanced antitumor activity in
nasopharyngeal carcinoma. Int J Oncol. 48:2175–2183. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang LC, Hsieh MT, Yang JS, Lu CC, Tsai
FJ, Tsao JW, Chiu YJ, Kuo SC and Lee KH: Effect of
bis(hydroxymethyl) alkanoate curcuminoid derivative MTH-3 on cell
cycle arrest, apoptotic and autophagic pathway in triple-negative
breast adenocarcinoma MDA-MB-231 cells: An in vitro study. Int J
Oncol. 52:67–76. 2018.PubMed/NCBI
|
|
32
|
Taurin S, Nimick M, Larsen L and Rosengren
RJ: A novel curcumin derivative increases the cytotoxicity of
raloxifene in estrogen receptor-negative breast cancer cell lines.
Int J Oncol. 48:385–398. 2016. View Article : Google Scholar : PubMed/NCBI
|