Introduction
Osteoporosis is a systemic disease, characterized by
reduced bone mass and a T-score [bone mineral density (BMD) score]
of ≤-2.5 (1). This disease is
frequently associated with an increased risk of fractures and is
considered a public health issue, threatening a large part of the
population aged >50 years (2,3).
Osteoclasts, which are essential in bone homeostasis, serve a key
role in the development of osteoporosis. Therefore, the study of
osteoclast function has become important in the investigation of
osteoporosis.
The regulation effect of matrix metalloproteinases
(MMPs) on the osteoclastic bone resorption has received increasing
attention (4). MMPs are a group of
proteolytic enzymes that dependent on Zn2+ hydrolysis.
To date, the MMP family has been found to only degrade fibrillar
collagen (5). As three members of
the MMP family, MMP-2, MMP-9 and MMP-13 serve an important role in
the activation of osteoclasts (6,7). MMP-2
has been reported to degrade type I collagen barrier bone cells
and, subsequently, active osteoclasts (8). MMP-9 is able to specifically degrade
non-mineralized cartilage and release extracellular matrix proteins
in combination with vascular endothelial growth factor (9). In addition, the serum MMP-9
concentration was observed to be negatively correlated with BMD and
is considered as a biochemical marker of bone resorption (10). Furthermore, MMP-13 enhances the
degradation of collagen fragments, leading to an increase of
specific integrins and collagen fragments in osteoclasts, and an
increase in bone remodeling and bone resorption (11). A previous study demonstrated that
decreased expression of MMP-13 reduced the osteoclastic bone
resorption and was negatively correlated with the percentage of
trabecular bone area (Tb.Ar) (12).
At present, although the contributions of MMP-2,
MMP-9 and MMP-13 in osteoporosis are increasingly investigated, the
dynamic alterations in in osteoporosis remain unclear. Therefore,
in the present study, it was hypothesized that the MMP-2, MMP-9 and
MMP-13 expression levels are continuously increased in
osteoporosis. To test this hypothesis, the study attempted to
demonstrate the dynamic expression of MMP-2, MMP-9 and MMP-13 in
ovariectomy (OVX)-induced osteoporosis model in rats.
Materials and methods
Animals
A total of 80 Sprague-Dawley specific-pathogen-free
female rats (3-month-old; weight, 284.72±20.32 g) obtained from the
Shanghai Animal Experiment Center [Shanghai, China; certification
no. SCXK(Hu)2012-0002] were used in the present study. The rats
were housed in the Experimental Animal Center of Fujian University
of Traditional Chinese Medicine (Fuzhou, China) under controlled
environmental conditions (12-h light/dark cycle; temperature,
~24°C; humidity, 40–70%) and were provided with standard food for
laboratory animals and water ad libitum. The present study
was approved by the Ethics Committee of Fujian University of
Traditional Chinese Medicine (project no. 20140120), and followed
the international guidelines for the Care and Use of Laboratory
Animals provided by the National Institutes of Health (Bethesda,
MD, USA).
OVX-induced osteoporosis rat
model
The rats were randomly divided into the OVX and sham
groups, with 40 rats in each group. Rats in the OVX group were
bilaterally ovariectomized following anesthesia with 2%
pentobarbital sodium. The ovaries were exposed and removed along
with the surrounding fat, oviduct and a small portion of the
uterus. Rats in the sham group underwent sham surgeries, during
which the ovary was exposed but left intact.
Sample collection
In total, 10 rats from each group were randomly
sacrificed at the 12, 16, 20 and 24 weeks after surgery. Following
anesthesia, the left tibia was removed, fixed in 10% neutral
buffered formalin and stored at room temperature for histological
examination. The right femur was also collected and stored in −80°C
prior to BMD analysis by dual-energy X-ray absorptiometry. The
first and second lumbar vertebrae were collected and stored in a
−80°C refrigerator for subsequent reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blotting, respectively.
Histological analysis
The left tibia were removed and immediately fixed in
10% neutral buffered formalin. Next, the sample was cleaned of soft
tissue, placed in decalcifying solution (10% EDTA/PBS; pH 7.4) for
~21 days at room temperature, dehydrated in 95% (v/v) ethanol and
then embedded in paraffin. Subsequently, three 5-mm
paraffin-embedded horizontal bone sections were cut from the upper
part of the left tibia, stained with hematoxylin-eosin and examined
by light microscopy. The quality of the bone and trabecular density
was assessed, according to the scores shown in Table I. The percentage of the Tb.Ar was
calculated as follows: Tb.Ar (%) = Tb.Ar/T.Ar (bone tissue area)
×100%.
 | Table I.Percentage of Tb.Ar of the two group
(mean ± standard deviation). |
Table I.
Percentage of Tb.Ar of the two group
(mean ± standard deviation).
|
| 12 weeks | 16 weeks | 20 weeks | 24 weeks |
|---|
|
|
|
|
|
|
|---|
| Group | n | Tb.Ar (%) | n | Tb.Ar (%) | n | Tb.Ar (%) | n | Tb.Ar (%) |
|---|
| Sham | 10 | 0.527±0.026 | 10 | 0.501±0.009 | 10 | 0.476±0.183 | 10 | 0.446±0.161 |
| OVX | 10 |
0.368±0.006a | 10 | 0.318±0.067a | 10 |
0.253±0.333a | 10 | 0.236±0.211a |
BMD assay
The BMD of the right femur was measured using
dual-energy X-ray absorptiometry (Lunar-DPX-IQ; GE Healthcare,
Chicago, IL, USA) equipped with Hologic APEX software (Hologic,
Inc., Marlborough, MA, USA), which assessed bone density in small
laboratory animals. A measured value of ±1.5% was considered as an
acceptable measurement. Whenever two points obtained in succession
were outside the limits of the quality control curve, the procedure
was repeated. Furthermore, the accuracy of the final BMD
measurements was determined by duplicate scans of the femur.
RT-qPCR analysis
Total RNA was extracted from the first lumbar
vertebra using TRIzol reagent according to the manufacturer's
protocol (R0016; Beyotime Institute of Biotechnology, Haimen,
China). The RNA concentration was measured by
NanoDrop™ 2000 (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). RNA was reverse transcribed into cDNA in each
sample using PrimeScript™ RT reagent kit (RR047A; Takara
Bio, Inc., Otsu, Japan), which was then used in PCR amplification.
The qPCR primers were as follows: MMP-2 (143 bp),
5′-TCCCGAGATCTGCAAGCAAG-3′ (forward) and 5′-AGAATGTGGCCACCAGCAAG-3′
(reverse); MMP-9 (173 bp), 5′-AGCCGGGAACGTATCTGGA-3′ (forward) and
5′-TGGAAACTCACACGCCAGAAG-3′ (reverse); MMP-13 (131 bp),
5′-CCCAGATGATGACGTTCAAGGA-3′ (forward) and
5′-CTCGGAGACTAGTAATGGCATCAAG-3′ (reverse); and β-actin (131 bp)
5′-GGAGATTACTGCCCTGGCTCCTA-3′ (forward) and
5′-GACTCATCGTACTCCTGCTTGCTG-3′ (reverse). β-actin was used as the
internal control. The conditions of qPCR conducted on a 7,500 Fast
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) were the following: One cycle at 95°C for 30 sec, 40 cycles
at 95°C for 5 sec and 60°C for 34 sec. The amplification curve and
Ct values were produced (13). To
obtain the dissolution curve, one cycle at 95°C for 30 sec, 40
cycles at 95°C for 3 sec and 60°C for 30 sec were conducted. The
expression levels of MMP-2, MMP-9 and MMP-13 were quantified and
normalized to β-actin expression.
Western blotting
Protein was extracted from the second lumbar
vertebral samples in each group, and the protein concentrations
were determined by the BCA assay (P0012S; Beyotime Institute of
Biotechnology). Next, 50 µg samples were loaded and separated by
10% SDS-PAGE. Following electrophoresis, the proteins were
transferred to polyvinylidene difluoride membranes in a
Tris-glycine transfer buffer (containing 20% v/v methanol, 3.03 g
Tris and 14.4 g glycine) using a wet transfer system. Subsequently,
the target proteins in the samples were detected with primary
antibodies against MMP-2 (1:1,000; cat. no. sc-10736), MMP-9
(1:1,000; cat. no. sc-10737) and MMP-13 (1:1,000; cat. no.
sc-30073; all from Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), as well as an anti-β-actin antibody (1:1,000; cat. no. 4970;
Cell Signaling Technology, Inc., Danvers, MA, USA) overnight at
4°C. The samples were then incubated with ImmunoPure®
goat anti-rabbit horseradish peroxidase-conjugated secondary
antibodies for 2 h at room temperature (1:3,000; cat. no. LP31460;
Xiamen Lulong Biotech Development Co., Ltd.). Proteins were
developed with BeyoECL Plus (P0018; Beyotime Institute of
Biotechnology). The optical density value of each band was read and
recorded using Image Laboratory software (version 5.0; Bio-Rad
Laboratories, Inc., Hercules, CA, USA), and normalized to the band
intensity of β-actin.
Statistical analysis
All experiments were repeated at least three times,
and representative experiment results are shown. Correlation
coefficient data were determined by Spearman's rank correlation
analysis. Other data are reported as the mean ± standard deviation.
Significance of differences between the mean of each group were
determined by one-way analysis of variance and Student-Newman-Keuls
test. P-values of <0.05 were considered to indicate differences
that were statistically significant.
Results
Morphology of the rat tibia
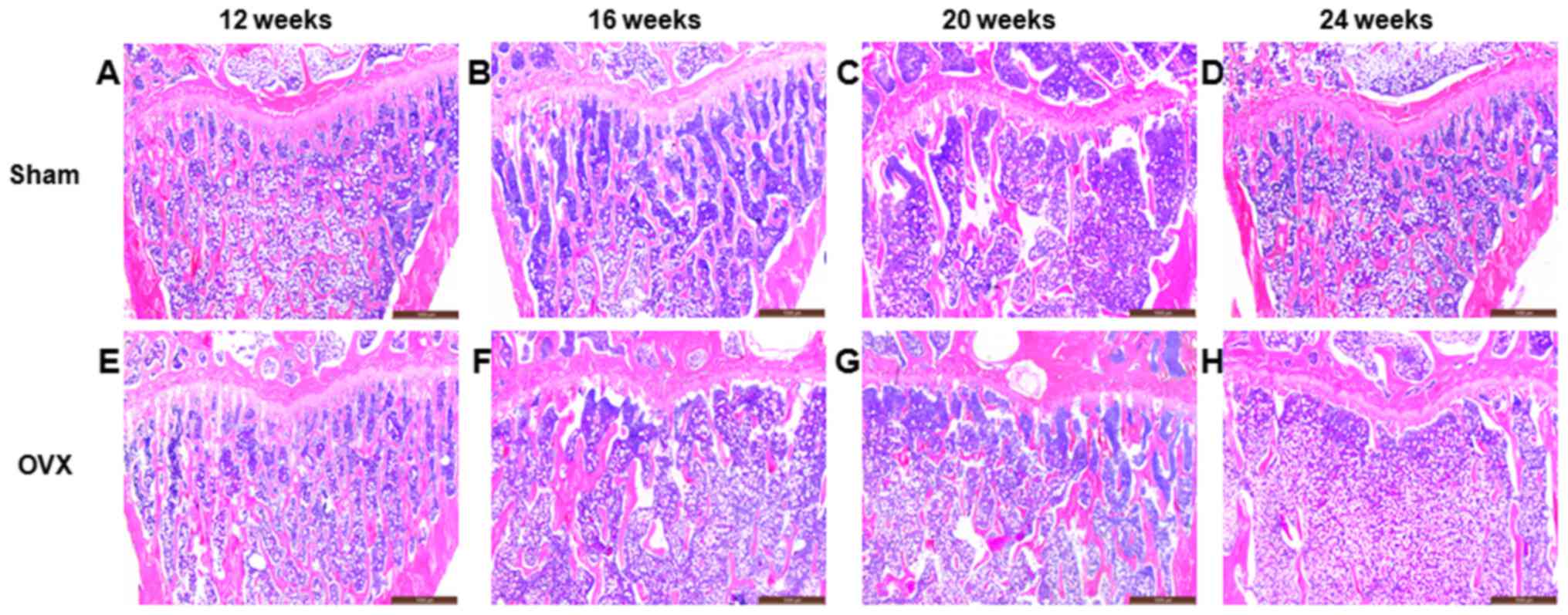
In the sham group, the surface of the trabecular
bone was smooth, and the trabecular plate remained intact (Fig. 1A-D). However, the structure of the
trabecular plate in the OVX group was absorbed and became
progressively thinner due to the increased osteoclast function. The
surface of the trabecular bone was rough, while the trabecular
plate underwent severe deterioration and was even completely
removed (Fig. 1E-H). Compared with
the sham group, the percentage of Tb.Ar in the OVX group at 12, 16,
20 and 24 weeks after surgery was significant lower (P<0.01;
Table I).
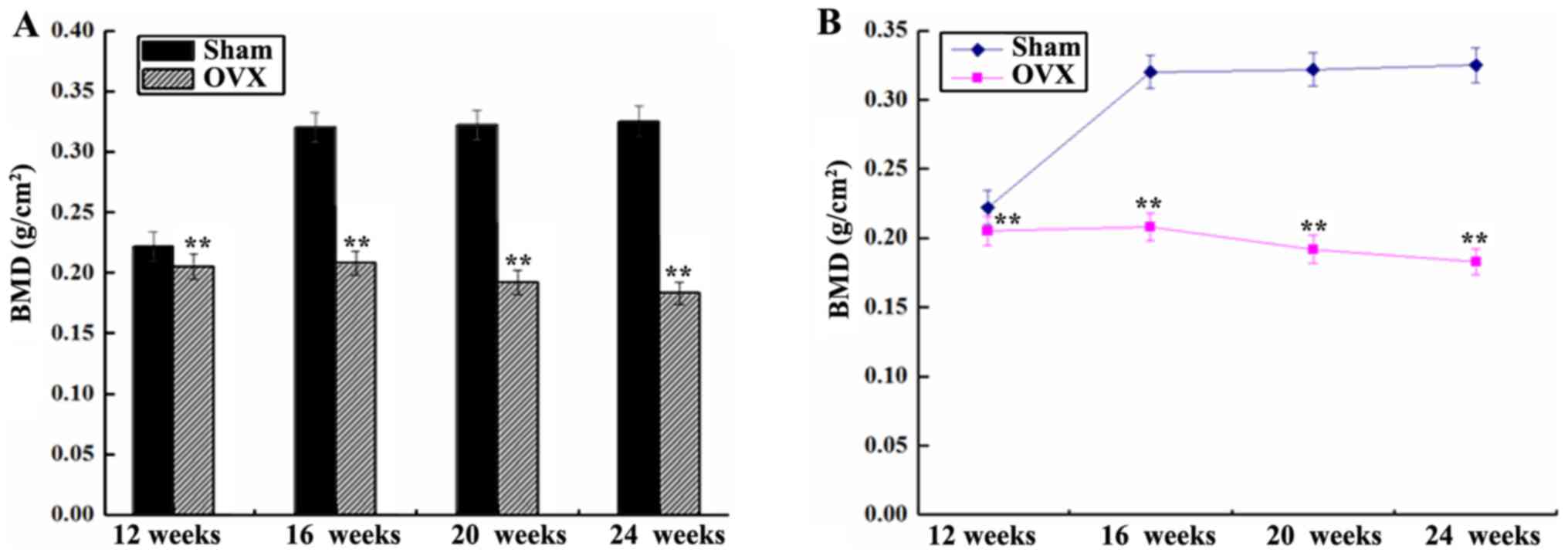
BMD of the right femur
The BMD of the right femur in the sham group was
significantly higher in comparison with that in the OVX group,
except at 12 weeks (P<0.01; Fig.
2). The BMD in the sham group was initially increased abruptly
between 12 and 16 weeks, and then remained stable between 16 and 24
weeks after surgery. However, the BMD in the OVX group demonstrated
a gradual and continuous downward trend (Fig. 2).
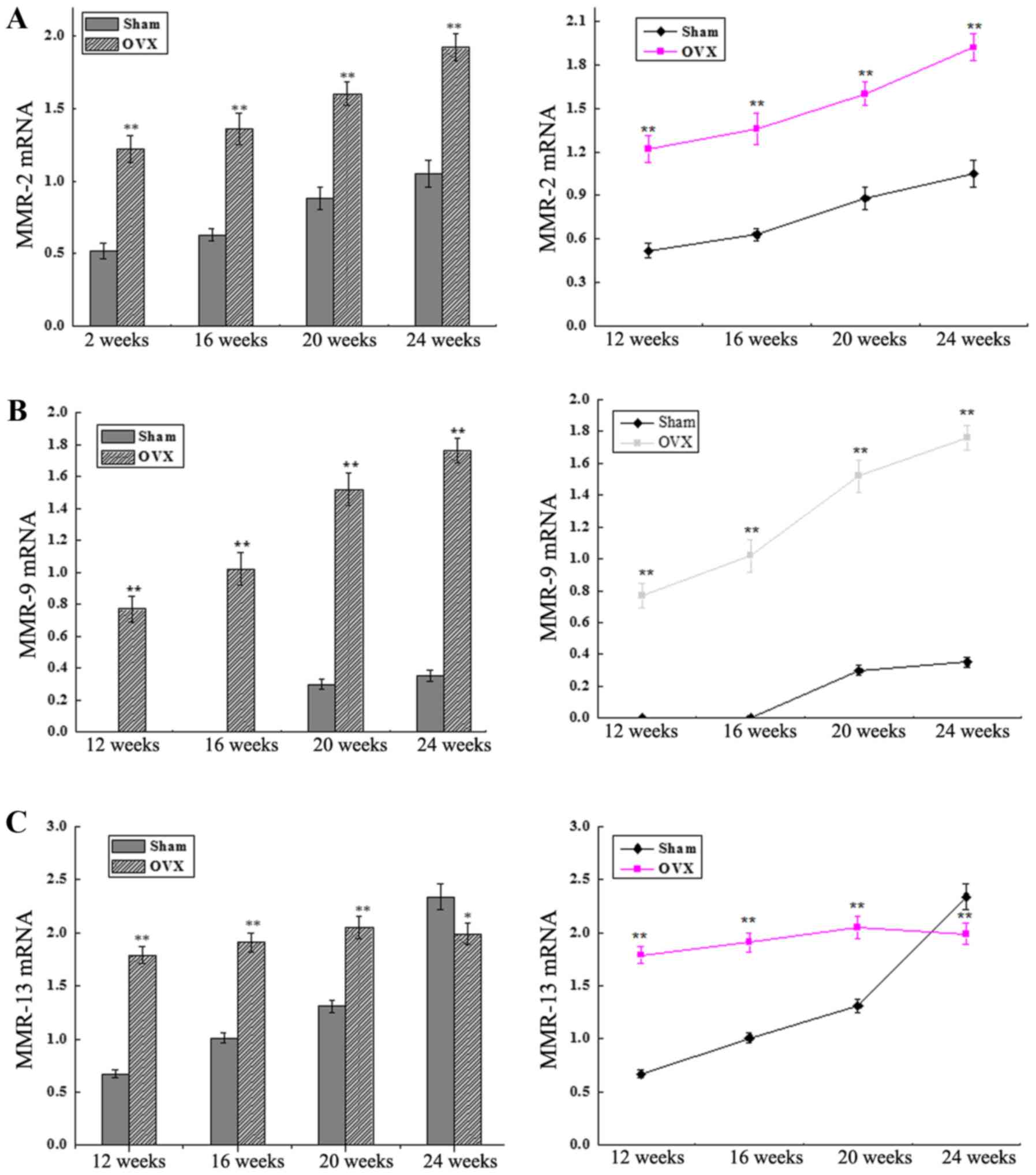
MMP-2, MMP-9 and MMP-13 mRNA
expression levels
The mRNA expression levels of MMP-2, MMP-9 and
MMP-13 were detected by RT-qPCR. As shown in Fig. 3A and B, the levels of MMP-2 and MMP-9
expression were significantly higher in the OVX group as compared
with those in the sham group at 12, 16, 20 and 24 weeks after
surgery (P<0.01). The MMP-9 mRNA expression in the OVX group
increased abruptly between 12 and 24 weeks after surgery. By
contrast, MMP-9 expression was extremely low in the sham group
between 12 and 16 weeks, while it increased steadily between 16 and
24 weeks after surgery. In addition, the MMP-13 expression level in
the OVX group was increased until 20 weeks and then decreased,
whereas a continuous increase was detected in the sham group
between 12 and 24 weeks after surgery, with a significant
difference observed between the two groups at all time-points (all
P<0.01 except at 24 weeks, P<0.05; Fig. 3C).
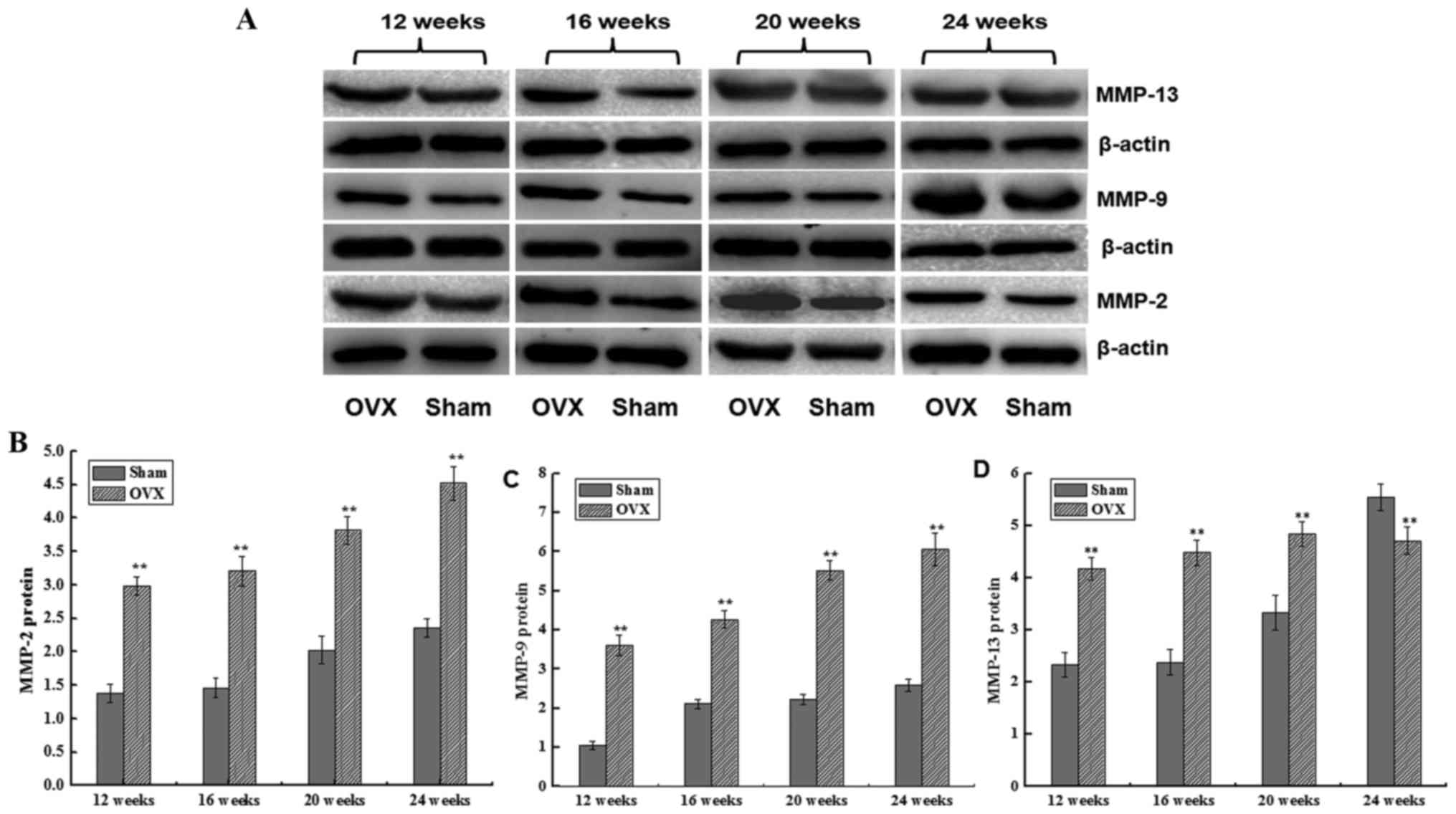
MMP-2, MMP-9 and MMP-13 protein
expression levels
The protein expression levels of MMP-2, MMP-9 and
MMP-13 were detected by western blotting. The MMP-2 protein
expression in the two groups increased between 12 and 24 weeks
after surgery. However, MMP-2 protein expression increased more
sharply in the OVX group as compared with that in the sham group,
with a significant difference observed (P<0.01; Fig. 4). A similar trend was also detected
for MMP-9 protein expression, which was significantly higher in
comparison with the sham group (P<0.01; Fig. 4). In addition, the MMP-13 expression
level initially increased and then slightly decreased in the OVX
group, while it was continuously increased in the sham group
between 12 and 24 weeks after surgery. A significant difference was
detected in MMP-13 expression between the two groups at all time
points (P<0.01; Fig. 4).
Furthermore, as shown in Table II,
a significantly negative correlation was identified between BMD and
the expression levels of MMP-2 and MMP-9, while MMP-13 expression
was positively correlated with the BMD.
 | Table II.Correlation coefficient (r) value
indicating the correlation of BMD with the protein expression
levels of MMP-2, MMP-9 and MMP-13 in the OVX group. |
Table II.
Correlation coefficient (r) value
indicating the correlation of BMD with the protein expression
levels of MMP-2, MMP-9 and MMP-13 in the OVX group.
| Parameter | 12 weeks | 16 weeks | 20 weeks | 24 weeks |
|---|
| MMP-2 | −0.645a | −0.795a | −0.733a | −0.753a |
| MMP-9 | −0.738a | −0.687a | −0.618a | −0.684a |
| MMP-13 | −0.602a | −0.679a | −0.588a | −0.745a |
Discussion
Osteoporosis is a bone disease mainly linked to
postmenopausal complications, which is characterized by low BMD and
microdamage of the bone structure. The use of ovariectomized rat or
mice models is a well-established and reproducible method of
simulating the postmenopausal conditions, and has been observed to
effectively cause postmenopausal cancellous bone loss over a
relatively short period of time (14). In the present study, the ovaries of
rats in the OVX group were removed, and this resulted in thinner
trabecular bone in the OVX group as compared with that in the sham
group (Fig. 1). In addition, the
Tb.Ar in the OVX group was significant lower in comparison with
that in the sham group (P<0.01; Table
I). The BMD of the OVX group was significantly lower compared
with that of the sham group between 12 and 24 weeks after the
surgery (P<0.01; Fig. 2A), and it
was decreased with time (Fig. 2B). A
low BMD and destructive trabecular morphology indicated that the
osteoporosis model was successfully established in the OVX
group.
MMPs are enzymes responsible for the degradation of
collagen fibrils. Accumulated evidence indicated that MMPs serve a
critical role in osteoclastic bone resorption and facilitate the
migration of osteoclasts to bone surfaces via the extracellular
matrix (15). To date, 18 different
mammalian MMPs have been identified, including MMP-2, MMP-9 and
MMP-13. Decrease of MMP-2, MMP-9 and MMP-13 expression levels is
known to reduce the bone resorption (4). However, little is known regarding the
dynamic expression of MMP-2, MMP-9 and MMP-13 in the bone during
the development of osteoporosis.
MMP-2 is mainly expressed in osteoblasts and
osteoclasts, which serve an important role in bone absorption
(7). The MMP-2 protein activates
bone absorption through enhancing the osteoclast MMP-2 activity and
promoting the degradation of the bone matrix (16), or through the degradation of type I
collagen barrier under the osteoblast bone cell and activation of
osteoclast functions (17). MMP-2
deficiency and the extracellular matrix breakdown defect create an
imbalance between bone synthesis and resorption (18). It was thus hypothesized that MMP-2
expression will continue increasing in the OVX group, whereas it
will remain low in the sham group. However, the present study
results clearly demonstrated that the expression of MMP-2 mRNA and
protein increased with time in the two groups. An interesting
finding in the present study was that the expression levels of
MMP-2 mRNA and protein in the OVX group increased more rapidly as
compared with those in the sham group between 20 and 24 weeks after
surgery, while they increased at the same rate between 12 and 20
weeks. The possible reason underlying this effect may be that the
estrogen level decreased rapidly in osteoporosis, weakening its
inhibition effect on MMP-2 activity, and consequently accelerating
BMD decrease. As for the sham group, the natural decline of
estrogen expression may have led to slow rise of the MMP-2 level
with the increase in the age of the rats. With the bone turnover
acceleration at the late stage of osteoporosis, the expression of
MMP-2 increased sharply between 20 and 24 weeks in the present
study, and was observed to be significantly negatively correlated
with BMD. Therefore, it is suggested that MMP-2 may serve an
important role at the late stage of osteoporosis.
MMP-9 is one of two known gelatinases in the MMP
family, along with MMP-2, and shares extensive structural
similarities with the MMP-2 gene (19). MMP-9 mainly degrades collagen,
integrin protein denaturation and protein polysaccharide. A high
expression of MMP-9 in osteoclasts has previously been detected
(7). The present study demonstrated
that, the levels of MMP-9 mRNA and protein expression in the OVX
group were significantly higher in comparison with those in the
sham group, which is consistent with the conclusions of a previous
study (20). Similar trends were
observed for MMP-9 expression as those detected for MMP-2
expression in the OVX group between 12 and 24 weeks after surgery.
Notably, an evidently low level of MMP-9 mRNA expression and a
relatively high level of MMP-9 protein expression were observed in
the sham group between 12 and 16 weeks, which indicated that the
expression levels of MMP-9 mRNA and protein were not exactly
associated. It has been reported that an adequate expression of
MMP-9 is necessary for osteoblast differentiation in the initial
phase of osteogenesis (21). In the
present study between 16 and 24 weeks after surgery, the level of
MMP-9 mRNA expression in the OVX group increased abruptly, while it
remained steady in the sham group. The high level of MMP-9 gene
expression in the OVX group and very low level in the sham group
between 12 and 16 weeks after surgery indicated that MMP-9 may
serve as an important marker for the early diagnosis of
osteoporosis. In addition, it was observed that MMP-9 expression
was negatively correlated with the BMD.
Another notable finding in the current study is that
the expression levels of MMP-13 gene and protein in the OVX group
were initially increased and then decreased. MMP-13 is one of the
most significantly expressed genes in patients with postmenopausal
osteoporosis (22). MMP-13 promotes
osteoclast precursor cells to differentiate into osteoclasts, and
indirectly promotes bone resorption (23). It is known that estrogen is able to
regulate the expression of MMP-13; thus, in theory, the decrease of
estrogen levels will lead to the significant increase of the
expression levels of MMP-13 mRNA and protein with the occurrence of
postmenopausal osteoporosis (24).
However, according to the findings of the present study, the
expression levels of MMP-13 gene and protein continued to increase
between 12 and 20 weeks after surgery, but gradually decreased at
24 weeks. By contrast, in the sham group, the expression levels of
MMP-13 gene and protein increased sharply, particularly at 24
weeks. These observations suggest that the levels of estrogen
decreased, while MMP-13 expression gradually increased at the late
phase of postmenopausal osteoporosis. Therefore, it is considered
that MMP-13 may serve an important role in the primary bone loss at
the early stage of postmenopausal osteoporosis. Due to the
reduction of regulatory factors, such as estrogen, during the late
stage, MMP-13 and BMD are positively correlated. Notably, the
present study demonstrated that MMP-13 expression decreased in the
OVX group and was significant lower in comparison with that in the
sham group at 24 weeks after surgery (P<0.05). However, the
mechanisms responsible for this trend in MMP-13 expression remain
unclear. It is hypothesized that high or low levels of estrogen may
exert a two-way regulation on MMP-13 expression, or other types of
hormones and cytokines may be associated with the negative
regulation on MMP-13. The mechanisms underlying this effect need to
be elucidated through long-term, multi-sample and multi-center
studies in the future.
In conclusion, MMP-2, MMP-9 and MMP-13 were
demonstrated to regulate the occurrence and development of
osteoporosis, and may serve as potent markers in the development of
novel prediction and diagnostic methods for postmenopausal
osteoporosis. Furthermore MMP-9 may be used as an important marker
for the early diagnosis of osteoporosis.
Acknowledgements
Not applicable.
Funding
The current study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81574003
and 81674054), the 2015 Strategic Emerging Industries Project of
Fujian Province (grant no. 2015Y0069), the Fujian Fuzhou Science
and Technology Project (grant no. 2016-S-125-3) and the Science and
Technology Project in Fujian Province Department of Education
(grant no. JK2015021).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XZ participated in the design of the study, all
experiment procedures and manuscript editing. YZ mainly developed
the postmenopausal osteoporosis model and performed the hematoxylin
and eosin staining. SG performed the bone mineral density of lumbar
vertebra analysis. WZ and JW performed the western blot analysis.
YL conceived the study, analyzed the data and prepared the
manuscript. All authors read, discussed and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Fujian University of Traditional Chinese Medicine
(project no. 20140120), and followed the international guidelines
for the Care and Use of Laboratory Animals provided by the National
Institutes of Health (Bethesda, MD, USA).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
OVX
|
ovariectomy
|
|
BMD
|
bone mineral density
|
|
MMP
|
matrix metalloproteinase
|
References
|
1
|
Kanis JA: Diagnosis of osteoporosis and
assessment of fracture risk. Lancet. 359:1929–1936. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hohenhaus MH, McGarry KA and Col NF:
Hormone therapy for the prevention of bone loss in menopausal women
with osteopenia: Is it a viable option? Drugs. 67:2311–2321. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Levine JP: Effective strategies to
identify postmenopausal women at risk for osteoporosis. Geriatrics.
62:22–30. 2007.PubMed/NCBI
|
|
4
|
Krane SM and Inada M: Matrix
metalloproteinases and bone. Bone. 43:7–18. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mott JD and Werb Z: Regulation of matrix
biology by matrix metalloproteinases. Curr Opin Cell Biol.
16:558–564. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Andersen TL, del Carmen Ovejero M,
Kirkegaard T, Lenhard T, Foged NT and Delaissé JM: A scrutiny of
matrix metalloproteinases in osteoclasts: Evidence for
heterogeneity and for the presence of MMPs synthesized by other
cell. Bone. 35:1107–1119. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tezuka K, Nemoto K, Tezuka Y, Sato T,
Ikeda Y, Kobori M, Kawashima H, Eguchi H, Hakeda Y and Kumegawa M:
Identification of matrix metalloproteinase-9 in rabbit osteoclasts.
J Biol Chem. 269:15006–15009. 1994.PubMed/NCBI
|
|
8
|
Inoue K, Mikuni-Takagaki Y, Oikawa K, Itoh
T, Inada M, Noguchi T, Park JS, Onodera T, Krane SM, Noda M and
Itohara S: A crucial role for matrix metalloproteinase 2 in
osteocytic canalicular formation and bone metabolism. J Biol Chem.
281:33814–33824. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Engsig MT, Chen QJ, Vu TH, Pedersen AC,
Therkidsen B, Lund LR, Henriksen K, Lenhard T, Foged NT, Werb Z and
Delaissé JM: Matrix metalloproteinase 9 and vascular endothelial
growth factor are essential for osteoclast recruitment into
developing long bones. J Cell Biol. 151:879–889. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Varghese S and Canalis E: Alendronate
stimulates collagenase 3 expression in osteoblasts by
posttranscriptional mechanisms. J Bone Miner Res. 15:2345–235l.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun B, Sun J, Han X, Liu H, Li J, Du J,
Feng W, Liu B, Cui J, Guo J, et al: Immunolocalization of MMP 2, 9
and 13 in prednisolone induced osteoporosis in mice. Histol
Histopathol. 31:647–656. 2016.PubMed/NCBI
|
|
12
|
Tang SY, Herber RP, Ho SP and Alliston T:
Matrix metalloproteinase-13 is required for osteocytic perilacunar
remodeling and maintains bone fracture resistance. J Bone Miner
Res. 27:1936–1950. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Egermann M, Goldhahn J and Schneider E:
Animal models for fracture treatment inosteoporosis. Osteoporosis
Int. 16 Suppl 2:S129–S138. 2005. View Article : Google Scholar
|
|
15
|
Samanna V, Ma T, Mak TW, Rogers M and
Chellaiah MA: Actin polymerization modulates CD44 surface
expression, MMP-9 activation, and osteoclast function. J Cell
Physiol. 213:710–720. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang P and Zhong M: Effects of
17beta-estradiol and progesterone on the expression of matrix
metalloproteinases and tissue inhibitors of metalloproteinases in
rat osteoblasts. Di Yi Jun Yi Da Xue Xue Bao. 21:929–931.
2001.PubMed/NCBI
|
|
17
|
Bachmeier BE, Iancu CM, Jochum M and
Nerlich AG: Matrix metalloproteinases in cancer: Comparison of
known and novel aspects of their inhibition as a therapeutic
approach. Expe Rev Anticancer Ther. 5:149–163. 2005. View Article : Google Scholar
|
|
18
|
Martignetti JA, Aqeel AA, Sewairi WA,
Boumah CE, Kambouris M, Mayouf SA, Sheth KV, Eid WA, Dowling O,
Harris J, et al: Mutation of the matrix metalloproteinase 2 gene
(MMP2) causes a multicentric osteolysis and arthritis syndrome. Nat
Genet. 28:261–265. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nyman JS, Lynch CC, Perrien DS, Thiolloy
S, O'Quinn EC, Patil CA, Bi X, Pharr GM, Mahadevan-Jansen A and
Mundy GR: Differential effects between the loss of MMP-2 and MMP-9
on structural and tissue-level properties of bone. J Bone Miner
Res. 26:1252–1260. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao H, Cai G, Du J, Xia Z, Wang L and Zhu
T: Expression of matrix metalloproteinase-9 mRNA in osteoporotic
bone tissues. J Tongji Med Univ. 17:28–31. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Filanti C, Dickson GR, Di Martino D, Ulivi
V, Sanguineti C, Romano P, Palermo C and Manduca P: The expression
of metalloproteinase-2, −9, and −14 and of tissue inhibitors-1 and
−2 is developmentally modulated during osteogenesis in vitro, the
mature osteoblastic phenotype expressing metalloproteinase-14. J
Bone Miner Res. 15:2154–2168. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jemtlan R, Holden M, Reppe S, Olstad OK,
Reinholt FP, Gautvik VT, Refvem H, Frigessi A, Houston B and
Gautvik KM: Molecular disease map of bone characterizing the
postmenopausal osteoporosis phenotype. J Bone Miner Res.
26:1793–1801. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pivetta E, Scapolan M, Pecolo M,
Wassermann B, Abu-Rumeileh I, Balestreri L, Borsatti E, Tripodo C,
Colombatti A and Spessotto P: MMP-13 stimulates osteoclast
differentiation and activation in tumour breast bone metastases.
Breast Cancer Res. 13:R1052011. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li J, Liao EY, Dai RC, Wei QY and Luo XH:
Effects of 17 beta-estradiol on the expression of interstitial
collagenases-8 and −13 (MMP-8 and MMP-13) and tissue inhibitor of
metalloproteinase-1 (TIMP-1) in ovariectomized rat osteoblastic
cells. J Mol Histol. 35:723–731. 2004. View Article : Google Scholar : PubMed/NCBI
|