Introduction
Asthma is a severe inflammatory disorder of the
airways that is characterized by hyperresponsiveness, obstruction
and inflammation of the airways (1).
It is a serious global health problem and individuals of all ages
may be affected by this chronic airway disorder (2). The prevalence of asthma is rising
globally (from 183 million in 1990 to ~242 million in 2013),
particularly among children in industrialized countries (3,4).
However, there is no cure for asthma at present, although symptoms
may be improved with certain medications (5). Therefore, an improved understanding of
the molecular mechanisms of this disease is required to discover
novel therapeutic targets for patients with asthma.
Asthma is believed to be caused by the interaction
of environmental and genetic factors (1). It has been suggested that environmental
exposure, such as smoking, and various home allergens, including
mold, dust mites and pollen, are associated with asthma (6). Estimates of heritability indicate that
35–80% of the variation in risk of developing asthma is due to
genetic variation (7). For example,
glutathione S-transferase mu 1, interleukin 10 (IL-10), cytotoxic
T-lymphocyte-associated protein 4, leukotriene C4 synthase, IL-4
receptor and ADAM metallopeptidase domain 33 have been implicated
in asthma (8). T-box 21 has been
suggested to have a vital role in asthma pathobiology via
regulation of T-helper 1 lineage commitment and interferon-γ
generation (9). Furthermore,
intracellular signaling component-associated pathways have been
implicated in asthma pathogenesis (10). Although some potential biomarkers and
functional pathways associated with asthma have been studied,
current knowledge is not adequate enough for the effective
diagnosis and treatment of this disease.
Bioinformatics has a vital role in identifying the
underlying genetic basis of human disease (11). A study by Laprise et al
(10) compared the expression of
genes and transcripts between healthy controls and allergic asthma
subjects in 2004 and deposited the gene expression profile,
GSE15823, in the Gene Expression Omnibus (ncbi.nlm.nih.gov/geo/). A study by Vaillancourt et
al (12) verified several
differentially expressed genes (DEGs) identified by Laprise et
al (10) as well as by
Chamberland et al (13).
However, Gene Ontology (GO), pathway functional enrichment and
protein-protein interaction (PPI) networks based on the DEGs were
not analyzed.
In the present study, bioinformatics methods were
employed to identify DEGs in lung tissue samples from patients with
asthma based on the gene expression profile of GSE15823. In
addition, GO functions, pathways and PPI networks were analyzed to
explore the potential functions, pathways and key genes involved in
asthma. Furthermore, module mining and functional analysis from PPI
networks were conducted. Notably, data validation based on another
gene expression profile dataset was performed in order to validate
the findings of the present study.
Materials and methods
Analysis of Affymetrix microarray
data
The gene expression profile of GSE15823, based on
the GPL8300 platform of Affymetrix Human Genome U95 Version 2
Array, was downloaded from the National Center of Biotechnology
Information GEO database (ncbi.nlm.nih.gov/geo/). According to the criteria of
the American Thoracic Society for the diagnosis of asthma (14), A total of 4 healthy subjects without
a history of allergy or asthma and 4 patients with asthma meeting
the criteria were recruited to the study by Laprise et al
(10). Lung tissue samples were
obtained from bronchial biopsies of the 8 subjects. In the present
study, gene expression data of the 8 lung tissue samples were used
for further analysis.
Data preprocessing and identification
of DEGs
Raw Affymetrix data (in the form of CEL files) were
downloaded and converted into expression values, followed by
background correction. Subsequently, gene expression data were
normalized using the robust multiarray average algorithm (15) of the Affy package (16) in Bioconductor software (version 3.4;
http://www.bioconductor.org/packages/release/bioc/),
followed by probe summarization.
Student's t-tests (17) were used to identify DEGs between
asthma and healthy control samples. Adjusted P-values were obtained
after the t-tests were performed and the fold-change (FC) was also
calculated. An adjusted P-value of P<0.1 and
|log2FC|>0.5 were selected as the criteria for DEG
screening.
GO and pathway functional enrichment
analyses of DEGs
Database for Annotation, Visualization and
Integrated Discovery (DAVID; david.ncifcrf.gov) (18) is a tool that provides an extensive
set of functional annotation. GO database (geneontology.org) (19)
is a tool used for functional unification of large-scale genomics
and includes three categories: Biological process (BP), molecular
function and cellular component. Kyoto Encyclopedia of Genes and
Genomes (KEGG) pathway database (20) covers various biochemical pathways. In
the present study, GO BP functional and KEGG pathway enrichment
analysis for upregulated and downregulated DEGs were performed with
DAVID. The modified Fisher's exact P-value of P<0.1 and count
>2 were used as thresholds.
PPI network construction and module
functional analysis
The Search Tool for the Retrieval of Interacting
Genes (STRING) (21) database
provides experimental and predicted interaction information. In the
present study, the STRING online tool was used to analyze the PPI
pairs of DEGs with a combined score of >0.4. The PPI network was
then visualized by Cytoscape (version 3.2.0) (22) and the proteins (genes) with the
highest connectivity degrees in the PPI network were regarded as
hub proteins (genes).
Modules may be defined in a data-driven fashion by
identifying subgroups of genes sharing similar expression patterns
across multiple conditions (23).
From the PPI network, the significant modules with the top three
Molecular Complex Detection (MCODE) scores were extracted for
analysis using MCODE of Cytoscape (24) with default thresholds. These
thresholds included: Degree cutoff, 2; node score cutoff, 0.2;
k-core, 2; and maximum depth, 100. Subsequently, the DEGs in
modules underwent GO and pathway enrichment analyses.
Data validation
In order to validate the reliability of the results
of the present study, expression profile data of GSE41649 (13) were downloaded from the GEO database,
based on the platform of GPL96 (HG-U133A) Affymetrix Human Genome
U133A (Affymetrix Inc., Santa Clara, CA, USA). Additionally, 8
samples, including 4 healthy controls without a history of allergy
or asthma and 4 patients with allergic asthma, were included in the
dataset. DEGs between allergic asthma and control samples were
identified and compared with the key DEGs identified in GSE15823.
The pathways and GO functions enriched by these DEGs were analyzed
using DAVID. P<0.05 and a count of >2 were considered to
indicate a statistically significant result.
Results
Identification of DEGs
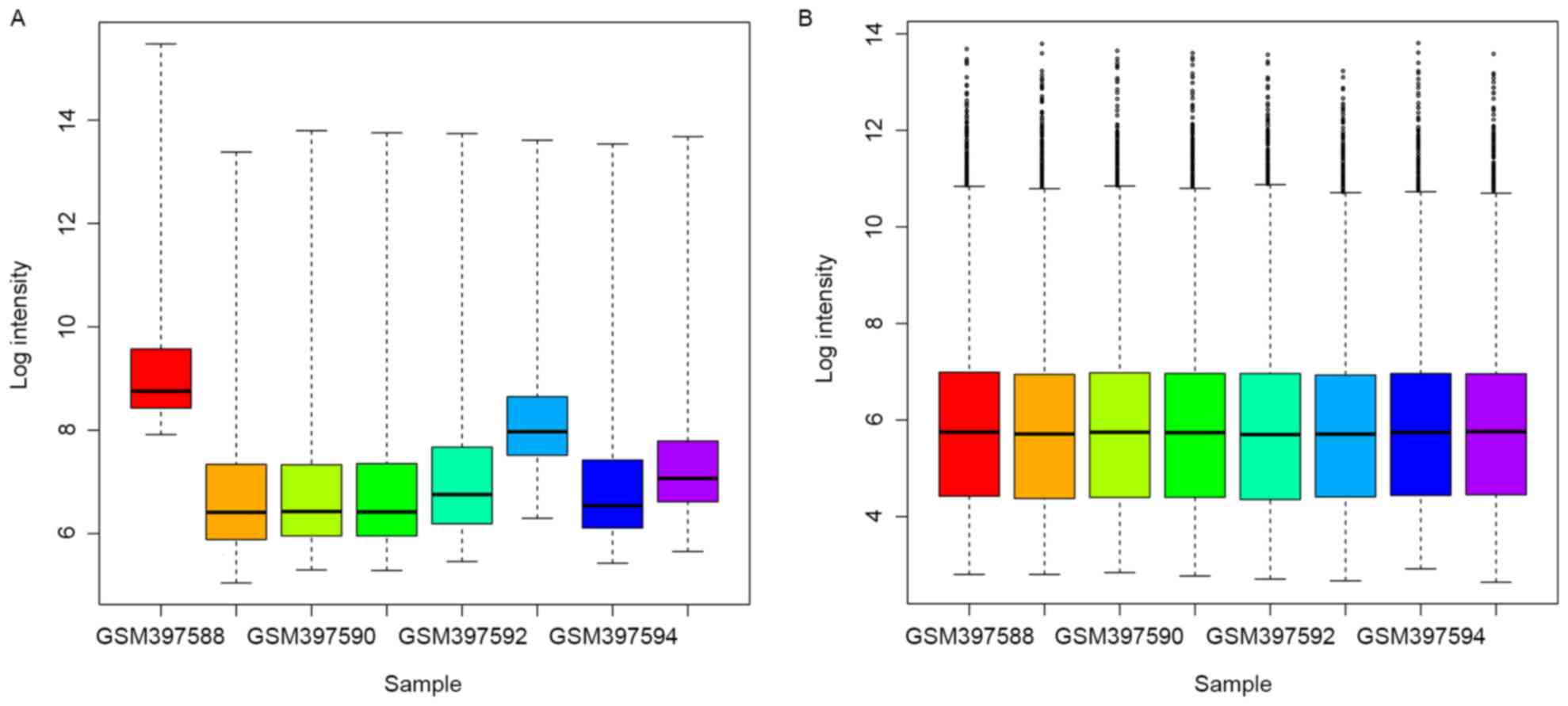
As demonstrated in Fig.
1, the raw expression data were well-normalized after
preprocessing. A total of 318 DEGs were obtained between asthma
samples and healthy subjects after microarray analysis, including
43 upregulated and 275 downregulated DEGs.
GO and KEGG pathway enrichment
analysis
The enriched functions of upregulated and
downregulated DEGs are demonstrated in Table I. The upregulated DEGs, such as
nitric oxide synthase 2 inducible (NOS2), were enriched in the BPs
associated with oxidation reduction and nitric oxide metabolism.
The downregulated DEGs, such as chemokine (C-C motif) ligand 21
(CCL21) and Cys-X-Cys ligand (CXCL9), were predominantly enriched
in the BPs associated with the immune response.
 | Table I.GO functional enrichment of
upregulated and downregulated DEGs. |
Table I.
GO functional enrichment of
upregulated and downregulated DEGs.
| Category | Description | Count | P-value |
|---|
| Upregulated
DEGs |
|
|
|
| BP | GO: 0055114:
Oxidation reduction | 6 | 1.62E-02 |
| BP | GO: 0043523:
Regulation of neuron apoptosis | 3 | 1.91E-02 |
| BP | GO: 0006809: Nitric
oxide biosynthetic process | 2 | 2.80E-02 |
| BP | GO: 0046209: Nitric
oxide metabolic process | 2 | 3.03E-02 |
| BP | GO: 0042981:
Regulation of apoptosis | 6 | 3.88E-02 |
| BP | GO: 0043067:
Regulation of programmed cell death | 6 | 4.03E-02 |
| BP | GO: 0010941:
Regulation of cell death | 6 | 4.08E-02 |
| BP | GO: 0031214
Biomineral formation | 2 | 8.40E-02 |
| Downregulated
DEGs |
|
|
|
| BP | GO: 0006955: Immune
response | 44 | 1.89E-14 |
| BP | GO: 0002449:
Lymphocyte mediated immunity | 11 | 1.86E-07 |
| BP | GO: 0016064:
Immunoglobulin mediated immune response | 10 | 1.98E-07 |
| BP | GO: 0019724: B cell
mediated immunity | 10 | 2.74E-07 |
| BP | GO: 0002252: Immune
effector process | 14 | 2.87E-07 |
| BP | GO: 0002460:
Adaptive immune response based on somatic recombination of immune
receptors built from immunoglobulin superfamily domains | 11 | 4.66E-07 |
| BP | GO: 0002250:
Adaptive immune response | 11 | 4.66E-07 |
| BP | GO: 0006952:
Defense response | 29 | 8.73E-07 |
| BP | GO: 0002443:
Leukocyte mediated immunity | 11 | 1.32E-06 |
| BP | GO: 0002684:
Positive regulation of immune system process | 17 | 1.85E-06 |
The KEGG pathways of upregulated and downregulated
DEGs are demonstrated in Table II.
Upregulated DEGs were enriched in caffeine metabolism.
Downregulated DEGs were enriched in immune-associated pathways,
such as systemic lupus erythematosus, allograft rejection, and
complement and coagulation cascades.
 | Table II.KEGG pathway enrichment of
upregulated and downregulated DEGs. |
Table II.
KEGG pathway enrichment of
upregulated and downregulated DEGs.
| Category | Description | Count | P-value |
|---|
| Upregulated
DEGs |
|
|
|
|
KEGG | hsa00232: Caffeine
metabolism | 2 | 1.91E-02 |
| Downregulated
DEGs |
|
|
|
|
KEGG | hsa05322: Systemic
lupus erythematosus | 12 | 7.52E-06 |
|
KEGG | hsa04672:
Intestinal immune network for IgA production | 8 | 7.43E-05 |
|
KEGG | hsa05330: Allograft
rejection | 7 | 1.03E-04 |
|
KEGG | hsa05416: Viral
myocarditis | 9 | 1.27E-04 |
|
KEGG | hsa05332:
Graft-versus-host disease | 7 | 1.63E-04 |
|
KEGG | hsa04940: Type I
diabetes mellitus | 7 | 2.49E-04 |
|
KEGG | hsa05310:
Asthma | 6 | 3.39E-04 |
|
KEGG | hsa04610:
Complement and coagulation cascades | 8 | 6.54E-04 |
|
KEGG | hsa05320:
Autoimmune thyroid disease | 7 | 7.29E-04 |
|
KEGG | hsa04612: Antigen
processing and presentation | 8 | 1.96E-03 |
|
KEGG | hsa04514: Cell
adhesion molecules | 10 | 2.06E-03 |
|
KEGG | hsa05340: Primary
immunodeficiency | 5 | 6.48E-03 |
|
KEGG | hsa00071: Fatty
acid metabolism | 5 | 1.04E-02 |
|
KEGG | hsa04060:
Cytokine-cytokine receptor interaction | 13 | 1.05E-02 |
|
KEGG | hsa04062: Chemokine
signaling pathway | 10 | 1.92E-02 |
|
KEGG | hsa04640:
Hematopoietic cell lineage | 6 | 3.77E-02 |
|
KEGG | hsa05020: Prion
diseases | 4 | 3.91E-02 |
PPI network construction
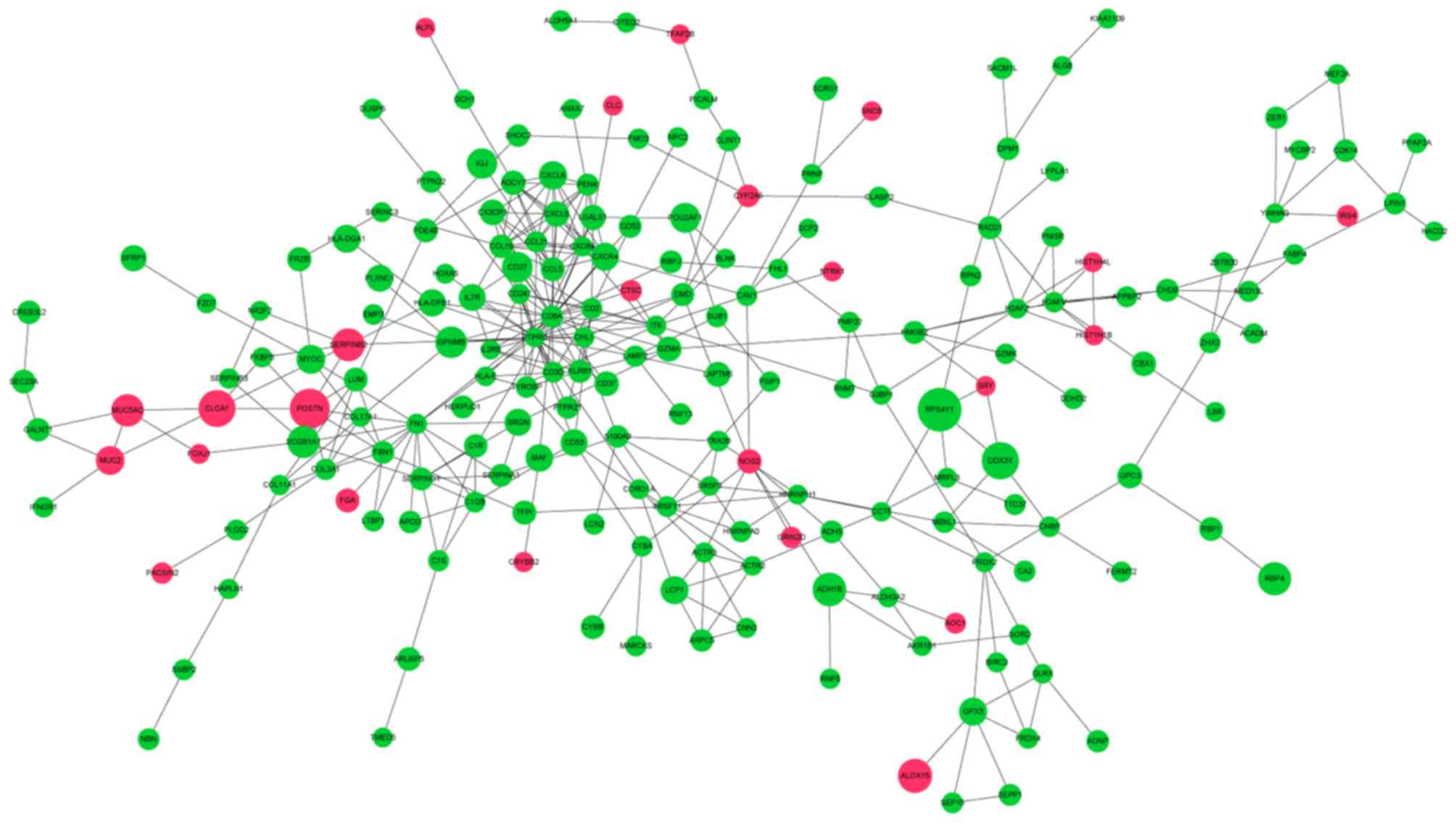
In total, 220 DEGs with 381 PPI interaction pairs
were identified in the PPI network (Fig.
2). The top 20 nodes with the highest degrees in the PPI
networks are demonstrated in Table
III and were defined as hub proteins (genes). These hub
proteins (genes) included cluster of differentiation 8a molecule
(CD8A; degree, 30), protein tyrosine phosphatase receptor type C
(PTPRC; degree, 26), fibronectin 1 (FN1; degree, 14), CCL21
(degree, 13) and CXCL9 (degree, 10).
 | Table III.Top 20 genes with the highest
connectivity degrees in the protein-protein interaction
network. |
Table III.
Top 20 genes with the highest
connectivity degrees in the protein-protein interaction
network.
| Gene name | Degree | Adjusted
P-value |
Log2FC |
|---|
| CD8A | 30 | 0.23 | −0.66 |
| PTPRC | 26 | 0.22 | −0.50 |
| FN1 | 14 | 0.24 | −0.56 |
| CCL19 | 13 | 0.26 | −0.76 |
| CCL5 | 12 | 0.24 | −0.78 |
| CCL21 | 11 | 0.20 | −0.60 |
| CXCR4 | 11 | 0.20 | −1.04 |
| CXCL9 | 10 | 0.32 | −0.86 |
| ADCY7 | 10 | 0.17 | −0.80 |
| CXCR6 | 10 | 0.25 | −0.52 |
| CD2 | 10 | 0.20 | −0.60 |
| CXCL6 | 9 | 0.20 | −1.07 |
| ITK | 8 | 0.23 | −0.53 |
| CD247 | 8 | 0.23 | −0.55 |
| PENK | 8 | 0.22 | −0.65 |
| H2AFV | 8 | 0.21 | −0.56 |
| POSTN | 7 | 0.21 | 1.81 |
| SERPING1 | 7 | 0.21 | −0.72 |
| KLRB1 | 7 | 0.23 | −0.74 |
| COL3A1 | 7 | 0.29 | −0.61 |
Module mining and functional
analysis
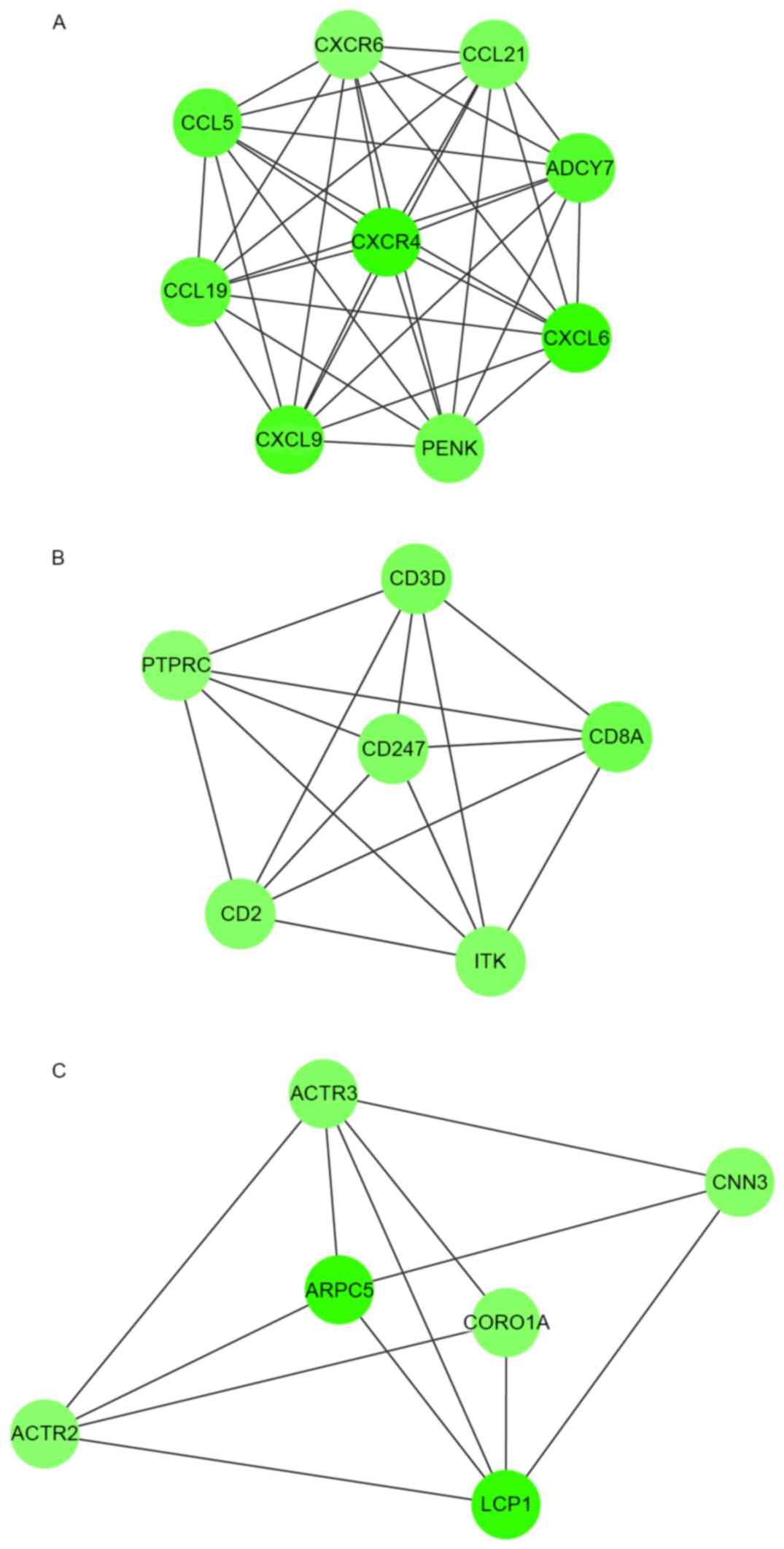
Module analysis demonstrated that there were 9, 6
and 6 nodes in the 3 modules, respectively, and the DEGs involved
in these modules were all downregulated (Fig. 3). Their MCODE scores were 9, 6 and 8,
respectively.
As demonstrated in Table
IV, DEGs in module 1 were linked with the G-protein coupled
receptor protein signaling pathway, immune response and defense
response. DEGs in module 2 were enriched in BPs related to T cell
activation and differentiation, and lymphocyte activation and
differentiation. DEGs in module 3 were enriched in actin
cytoskeleton-related BPs.
 | Table IV.GO and KEGG pathway functional
enrichment of DEGs in modules. |
Table IV.
GO and KEGG pathway functional
enrichment of DEGs in modules.
| Category | Term | Description | Count | P-value |
|
|---|
| Module 1 |
|
|
|
|
|
| BP | GO: 0007186 | G-protein coupled
receptor protein signaling pathway | 9 | 2.20E-09 |
| BP | GO: 0006935 | Chemotaxis | 6 | 1.18E-08 |
| BP | GO: 0042330 | Taxis | 6 | 1.18E-08 |
| BP | GO: 0007610 | Behavior | 7 | 4.44E-08 |
| BP | GO: 0007166 | Cell surface
receptor linked signal transduction | 9 | 1.24E-07 |
| BP | GO: 0007626 | Locomotory
behavior | 6 | 1.75E-07 |
| BP | GO: 0006952 | Defense
response | 7 | 2.23E-07 |
| BP | GO: 0006954 | Inflammatory
response | 6 | 4.10E-07 |
| BP | GO: 0009611 | Response to
wounding | 6 | 4.60E-06 |
| BP | GO: 0006955 | Immune
response | 6 | 1.68E-05 |
|
KEGG | hsa04062 | Chemokine signaling
pathway | 8 | 8.15E-11 |
|
KEGG | hsa04060 | Cytokine-cytokine
receptor interaction | 7 | 1.19E-07 |
| Module 2 |
|
|
|
|
| BP | GO: 0042110 | T cell
activation | 4 | 7.78E-06 |
| BP | GO: 0046649 | Lymphocyte
activation | 4 | 3.07E-05 |
| BP | GO: 0045321 | Leukocyte
activation | 4 | 5.51E-05 |
| BP | GO: 0001775 | Cell
activation | 4 | 9.16E-05 |
| BP | GO: 0030217 | T cell
differentiation | 3 | 2.25E-04 |
| BP | GO: 0030098 | Lymphocyte
differentiation | 3 | 5.66E-04 |
| BP | GO: 0002521 | Leukocyte
differentiation | 3 | 9.13E-04 |
| BP | GO: 0007166 | Cell surface
receptor linked signal transduction | 5 | 1.57E-03 |
| BP | GO: 0045059 | Positive thymic T
cell selection | 2 | 2.58E-03 |
| BP | GO: 0030097 | Hemopoiesis | 3 | 2.93E-03 |
|
KEGG | hsa04660 | T cell receptor
signaling pathway | 5 | 9.47E-07 |
|
KEGG | hsa05340 | Primary
immunodeficiency | 3 | 4.54E-04 |
|
KEGG | hsa04640 | Hematopoietic cell
lineage | 3 | 2.74E-03 |
|
KEGG | hsa04514 | Cell adhesion
molecules | 3 | 6.35E-03 |
| Module 3 |
|
|
|
|
| BP | GO: 0030036 | Actin cytoskeleton
organization | 4 | 4.49E-05 |
| BP | GO: 0030029 | Actin
filament-based process | 4 | 5.44E-05 |
| BP | GO: 0030833 | Regulation of actin
filament polymerization | 3 | 1.55E-04 |
| BP | GO: 0008064 | Regulation of actin
polymerization or depolymerization | 3 | 1.98E-04 |
| BP | GO: 0030832 | Regulation of actin
filament length | 3 | 2.12E-04 |
| BP | GO: 0032271 | Regulation of
protein polymerization | 3 | 2.47E-04 |
| BP | GO: 0007010 | Cytoskeleton
organization | 4 | 3.17E-04 |
| BP | GO: 0006928 | Cell motion | 4 | 4.08E-04 |
| BP | GO: 0032956 | Regulation of actin
cytoskeleton organization | 3 | 4.23E-04 |
| BP | GO: 0043254 | Regulation of
protein complex assembly | 3 | 4.32E-04 |
Additionally, KEGG pathway analysis for DEGs
(Table IV) in module 1 demonstrated
that these DEGs predominantly participated in the pathways of
chemokine signaling and cytokine-cytokine receptor interaction.
DEGs in module 2 participated in T cell receptor signaling
pathways, primary immunodeficiency, hematopoietic cell lineage and
cell adhesion molecules. However, no pathway was observed to be
enriched by the DEGs in module 3.
Data validation
Data validation demonstrated that the hub genes
obtained in GSE15823, such as PTPRC, FN1 and CXCL9, were
downregulated. The downregulation of these genes was also observed
in GSE41649. Additionally, the expression of CCL21 (enriched in BPs
associated with immune response) and NOS2 (enriched in BPs related
to oxidation reduction and nitric oxide metabolism) demonstrated
the same variation directions in the two datasets.
In addition, following functional enrichment
analysis, the DEGs identified in GSE41649 were also demonstrated to
be significantly enriched in pathways associated with immune
responses (P<0.05), such as systemic lupus erythematosus,
complement and coagulation cascades, cytokine-cytokine receptor
interaction and chemokine signaling pathways. Furthermore, they
were significantly enriched in BPs associated with immune responses
and oxidoreductase activity (P<0.05; data not shown).
Discussion
In the present study, the GSE15823 gene expression
profile was analyzed and the underlying molecular mechanisms of
asthma were explored using bioinformatics methods. A total of 43
upregulated and 275 downregulated DEGs were identified in asthma
samples compared with healthy subjects. Upregulated DEGs, such as
NOS2, were enriched in BPs related to oxidation, reduction
and nitric oxide metabolism. Downregulated DEGs, such as CCL21,
CXCL9 and PTPRC, were enriched in immune
response-associated BPs, and immune and inflammation-associated
pathways. Additionally, 20 DEGs were considered as hub genes in the
PPI network, such as PTPRC, CCL21 and CXCL9. The DEGs
in module 1 were significantly involved in chemokine signaling
pathways. Notably, the 4 DEGs (NOS2, PTPRC, CCL21 and
CXCL9), pathways and BP terms were confirmed in
GSE41649.
In asthmatic patients, impaired immune response to
viral infections is a proposed mechanism for susceptibility to
infection (25); however, the
relationships between viral infections, innate immunity and asthma
are complex and remain to be fully elucidated. In the present
study, the majority of the downregulated DEGs were enriched in
immune response-associated BPs and pathways, such as CCL21 and
CXCL9. Additionally, PTPRC was enriched in BPs
related to immune effector processes.
PTPRC was identified as a hub gene with the
second highest degree in the PPI network. PTPRC encodes CD45
leukocyte common antigen, which is essential for normal lymphocyte
function (26). A study by
Trowbridge et al (27)
suggested that CD45 has an important role in intracellular signal
transduction in the immune system. As such, it has also been
demonstrated that the absence of CD45 may result in severe combined
immunodeficiency (28). CD45 may be
expressed by all leucocytes, including eosinophils (29), which have been implicated as effector
cells in asthma and other allergic diseases (30). In asthma, eosinophils and their
secreted mediators are believed to be a major contributor to the
inflammation underlying the pathophysiological changes in the
airways (31). Therefore, we
speculated that CD45 (PTPRC) may have an important role in
the progression of asthma.
CCL21 and CXCL9 were also identified as hub
genes in the PPI network. These genes were also involved in module
1, which was associated with the chemokine signaling pathway.
Chemokines are significant regulators of immune cell trafficking,
and malfunction of the chemokine signaling pathway has a key role
in allergic asthma (32). In
allergic asthma, mature dendritic cells migrate to lymph nodes
through the interaction of chemokine (C-C motif) receptor 7 and CC
chemokine ligands of CCL21, where they effectively present antigens
to naive T cells (33). It has
previously been demonstrated that the administration of
antigen-pulsed dendritic cells induces allergic inflammation
(34). CCL21 is understood to be
crucial for lymphoid cell trafficking and for the structural
organization of lymphoid tissues (35). A study by Xu et al (36) suggested that a lack of lymphoid
chemokine ligand, CCL21, enhances allergic airway inflammation by
modulating the recruitment of CD4+ T cells into the
lungs. CXCL9 may be produced by eosinophils following stimulation
with interferon-γ, indicating that CXCL9 may be involved in the
downregulation of allergic inflammation (37,38).
Taken together these findings suggest that CCL21 and
CXCL9 may be involved in immune response-related BPs and
have important roles in the progression of asthma.
In addition to immune responses, oxidants may also
have a critical role in the pathogenesis of asthma (39). The lungs are highly susceptible to
oxidative injury, which has been implicated in various lung
diseases, including asthma (40).
Increased levels of reactive oxygen species may induce apoptosis
and result in increased airway reactivity and secretions, which may
augment existing inflammation in asthma (41). In the present study, BPs related to
oxidation reduction and nitric oxide metabolism were enriched by
several upregulated DEGs, including NOS2. NOS2 is
able to endogenously produce NO, which has been suggested to have
an important role in the physiological regulation of airway
functions (42,43). Notably, a previous study demonstrated
that NOS2 was significantly associated with the fraction of
exhaled NO in asthmatic children (44). Additionally, a study by Dweik
(45) reported an increase in NOS2
expression during inflammation, which was in accordance with the
results of the present study. Taken together these findings suggest
that NOS2 may be a candidate molecular marker associated
with asthma progression through oxidation reduction.
In the present study, there were 4 lung tissue
samples obtained from bronchial biopsies of healthy controls and 4
from subjects with allergic asthma. All 4 of the asthmatic patients
were women, whereas the 4 healthy subjects included 2 women and 2
men. Notably, Laprise et al (10) stated that gene expression
measurements were similar in control subjects, which indicated that
there was no gender difference in our data. Similarly, a study by
Prescott et al (46)
demonstrated that there was no gender difference in the prevalence
of asthma.
The dataset of GSE41649 confirmed the key genes,
pathways and GO functions identified in the present study; however,
some limitations existed. In the process of data analysis, only 8
samples were used. Furthermore, the investigation was implemented
by means of bioinformatics methods and the screened genes and
pathways have not been validated by experiments, although the
dataset of GSE41649 was used for verification.
In conclusion, the results of the present study
demonstrate that the immune response, oxidants and nitric oxide
metabolism may have important roles in the progression of asthma.
Additionally, DEGs, such as PTPRC, CXCL9, CCL21 and
NOS2, may have the potential to be used as targets for
asthma diagnosis and treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SQ, HC and GL conceived of and designed this study,
and drafted the manuscript. NH acquired the data. XD analyzed and
interpreted the data. WL performed the statistical analysis. HC
revised the manuscript for important intellectual content and
agreed to be accountable for all aspects of the work; ensuring that
questions related to the accuracy or integrity of any part of the
work are appropriately investigated and resolve. All authors have
read and approved the manuscript, and ensure that the information
is correct.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maddox L and Schwartz DA: The
pathophysiology of asthma. Annu Rev Med. 53:477–498. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bateman ED, Hurd SS, Barnes PJ, Bousquet
J, Drazen JM, FitzGerald JM, Gibson P, Ohta K, O'Byrne P, Pedersen
SE, et al: Global strategy for asthma management and prevention:
GINA executive summary. Eur Respir J. 31:143–178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koehoorn M, Tamburic L, McLeod CB, Demers
P, Lynd L and Kennedy SM: Population-based surveillance of asthma
among workers in British Columbia, Canada. Chronic Dis Inj Can.
33:88–94. 2013.PubMed/NCBI
|
|
4
|
Lai C, Beasley R, Crane J, Foliaki S, Shah
J and Weiland S: International Study of Asthma and Allergies in
Childhood Phase Three Study Group: Global variation in the
prevalence and severity of asthma symptoms: Phase three of the
international study of asthma and allergies in childhood (ISAAC).
Thorax. 64:476–483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leutholtz BC and Ripoll I: Exercise and
disease management. 2nd edition. CRC Press; Boca Raton, FL:
2011
|
|
6
|
Reponen T, Lockey J, Bernstein DI, Vesper
SJ, Levin L, Hershey Khurana GK, Zheng S, Ryan P, Grinshpun SA,
Villareal M and Lemasters G: Infant origins of childhood asthma
associated with specific molds. J Allergy Clin Immunol.
130:639–644.e5. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Torgerson DG, Ampleford EJ, Chiu GY,
Gauderman WJ, Gignoux CR, Graves PE, Himes BE, Levin AM, Mathias
RA, Hancock DB, et al: Meta-analysis of genome-wide association
studies of asthma in ethnically diverse North American populations.
Nat Genet. 43:887–892. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ober C and Hoffjan S: Asthma genetics
2006: The long and winding road to gene discovery. Genes Immun.
7:95–100. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang G, Liu CT, Wang ZL, Jiang LL, Yan CL
and Luo FM: Antisense oligonucleotides-induced local blockade of
T-bet expression leads to airway inflammation in rats1. Acta
Pharmacol Sin. 27:561–567. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Laprise C, Sladek R, Ponton A, Bernier MC,
Hudson TJ and Laviolette M: Functional classes of bronchial mucosa
genes that are differentially expressed in asthma. BMC genomics.
5:212004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moore JH, Asselbergs FW and Williams SM:
Bioinformatics challenges for genome-wide association studies.
Bioinformatics. 26:445–455. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vaillancourt VT, Bordeleau M, Laviolette M
and Laprise C: From expression pattern to genetic association in
asthma and asthma-related phenotypes. BMC Res Notes. 5:6302012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chamberland A, Madore AM, Tremblay K,
Laviolette M and Laprise C: A comparison of two sets of microarray
experiments to define allergic asthma expression pattern. Exp Lung
Res. 35:399–410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Standards for the diagnosis and care of
patients with chronic obstructive pulmonary disease (COPD) and
asthma. This official statement of the American Thoracic Society
was adopted by the ATS Board of Directors, November 1986. Am Rev
Respir Dis. 136:225–244. 1987.PubMed/NCBI
|
|
15
|
Irizarry RA, Hobbs B, Collin F,
Beazer-Barclay YD, Antonellis KJ, Scherf U and Speed TP:
Exploration, normalization, and summaries of high density
oligonucleotide array probe level data. Biostatistics. 4:249–264.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gautier L, Cope L, Bolstad BM and Irizarry
RA: affy-analysis of Affymetrix GeneChip data at the probe level.
Bioinformatics. 20:307–315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dubitzky W, Wolkenhauer O, Yokota H and
Cho KH: Student'st-TestEncyclopedia of Systems Biology. Springer;
New York, NY: pp. 2023–2025. 2013, View Article : Google Scholar
|
|
18
|
da Huang W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The Gene
Ontology Consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Franceschini A, Szklarczyk D, Frankild S,
Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C
and Jensen LJ: STRING v9.1: Protein-protein interaction networks,
with increased coverage and integration. Nucleic Acids Res.
41:(Database Issue). D808–D815. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stuart JM, Segal E, Koller D and Kim SK: A
gene-coexpression network for global discovery of conserved genetic
modules. science. 302:249–255. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bader GD and Hogue CW: An automated method
for finding molecular complexes in large protein interaction
networks. BMC Bioinformatics. 4:22003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wark PA, Johnston SL, Bucchieri F, Powell
R, Puddicombe S, Laza-Stanca V, Holgate ST and Davies DE: Asthmatic
bronchial epithelial cells have a deficient innate immune response
to infection with rhinovirus. J Exp Med. 201:937–947. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Differentiation CO: Protein tyrosine
phosphatase receptor type C. Springer; New York, NY: 2012
|
|
27
|
Trowbridge IS, Ostergaard HL and Johnson
P: CD45: A leukocyte-specific member of the protein tyrosine
phosphatase family. Biochim Biophys Acta. 1095:46–56. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hermiston ML, Xu Z and Weiss A: CD45: A
critical regulator of signaling thresholds in immune cells. Annu
Rev Immunol. 21:107–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Matsumoto K, Bochner BS, Wakiguchi H and
Kurashige T: Altered expression of CD11b and CD62L after
cross-linking of CD45 isoforms on human eosinophils. Int Arch
Allergy Immunol. 117 Suppl 1:S34–S39. 1998. View Article : Google Scholar
|
|
30
|
Blaylock MG, Lipworth BJ, Dempsey OJ,
Duncan CJ, Lee DK, Lawrie A, Douglas JG and Walsh GM: Eosinophils
from patients with asthma express higher levels of the
pan-leucocyte receptor CD45 and the isoform CD45RO. Clin Exp
Allergy. 33:936–941. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Walsh GM: Advances in the immunobiology of
eosinophils and their role in disease. Crit Rev Clin Lab Sci.
36:453–496. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nguyen KD, Vanichsarn C, Fohner A and
Nadeau KC: Selective deregulation in chemokine signaling pathways
of CD4+CD25(hi)CD127(lo)/(−) regulatory T cells in human allergic
asthma. J Allergy Clin Immunol. 123:933–939.e10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamashita N, Tashimo H, Matsuo Y, Ishida
H, Yoshiura K, Sato K, Yamashita N, Kakiuchi T and Ohta K: Role of
CCL21 and CCL19 in allergic inflammation in the ovalbumin-specific
murine asthmatic model. J Allergy Clin Immunol. 117:1040–1046.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lambrecht BN, Peleman RA, Bullock GR and
Pauwels RA: Sensitization to inhaled antigen by intratracheal
instillation of dendritic cells. Clin Exp Allergy. 30:214–224.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Takamura K, Fukuyama S, Nagatake T, Kim
DY, Kawamura A, Kawauchi H and Kiyono H: Regulatory role of
lymphoid chemokine CCL19 and CCL21 in the control of allergic
rhinitis. J Immunol. 179:5897–5906. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu B, Aoyama K, Kusumoto M, Matsuzawa A,
Butcher EC, Michie SA, Matsuyama T and Takeuchi T: Lack of lymphoid
chemokines CCL19 and CCL21 enhances allergic airway inflammation in
mice. Int Immunol. 19:775–784. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Meller S, Lauerma AI, Kopp FM, Winterberg
F, Anthoni M, Müller A, Gombert M, Haahtela A, Alenius H, Rieker J,
et al: Chemokine responses distinguish chemical-induced allergic
from irritant skin inflammation: Memory T cells make the
difference. J Allergy Immuno. 119:1470–1480. 2007. View Article : Google Scholar
|
|
38
|
Tworek D, Kuna P, Młynarski W, Górski P,
Pietras T and Antczak A: MIG (CXCL9), IP-10 (CXCL10) and I-TAC
(CXCL11) concentrations after nasal allergen challenge in patients
with allergic rhinitis. Arch Med Sci. 9:849–853. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sackesen C, Ercan H, Dizdar E, Soyer O,
Gumus P, Tosun BN, Büyüktuncer Z, Karabulut E, Besler T and Kalayci
O: A comprehensive evaluation of the enzymatic and nonenzymatic
antioxidant systems in childhood asthma. J Allergy Clin Immunol.
122:78–85. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Andreadis AA, Hazen SL, Comhair SA and
Erzurum SC: Oxidative and nitrosative events in asthma. Free Radic
Biol Med. 35:213–225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nadeem A, Raj HG and Chhabra SK: Increased
oxidative stress and altered levels of antioxidants in chronic
obstructive pulmonary disease. Inflammation. 29:23–32. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ghosh S and Erzurum SC: Nitric oxide
metabolism in asthma pathophysiology. Biochim Biophys Acta.
1810:1008–1016. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Alderton WK, Cooper CE and Knowles RG:
Nitric oxide synthases: Structure, function and inhibition. Biochem
J. 357:593–615. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Salam MT, Bastain TM, Rappaport EB, Islam
T, Berhane K, Gauderman WJ and Gilliland FD: Genetic variations in
nitric oxide synthase and arginase influence exhaled nitric oxide
levels in children. Allergy. 66:412–419. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dweik RA: Nitric oxide, hypoxia, and
superoxide: The good, the bad, and the ugly! Thorax. 60:265–267.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Prescott E, Lange P and Vestbo J: Effect
of gender on hospital admissions for asthma and prevalence of
self-reported asthma: A prospective study based on a sample of the
general population. Copenhagen City Heart Study Group. Thorax.
52:287–289. 1997. View Article : Google Scholar : PubMed/NCBI
|