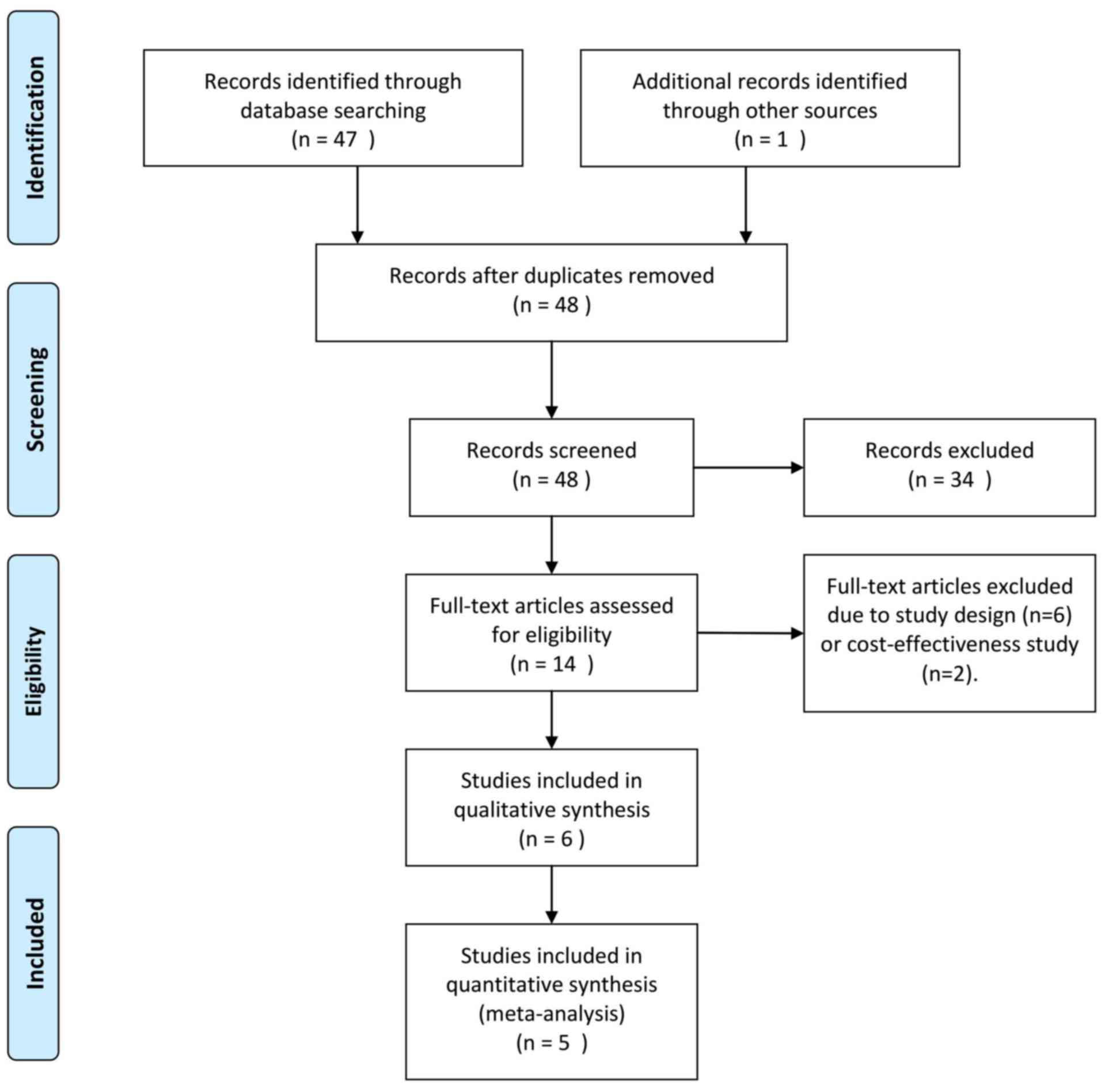
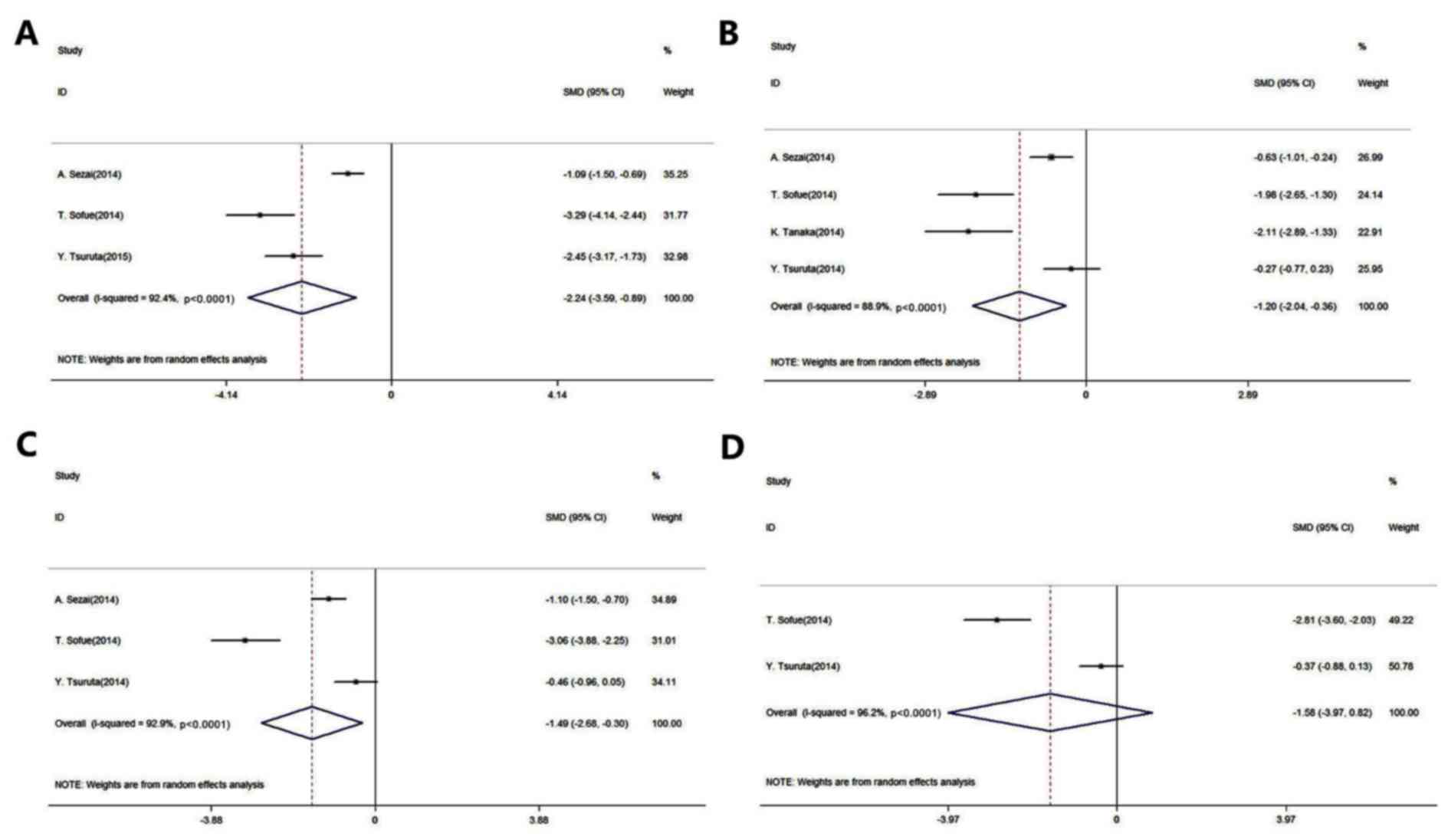
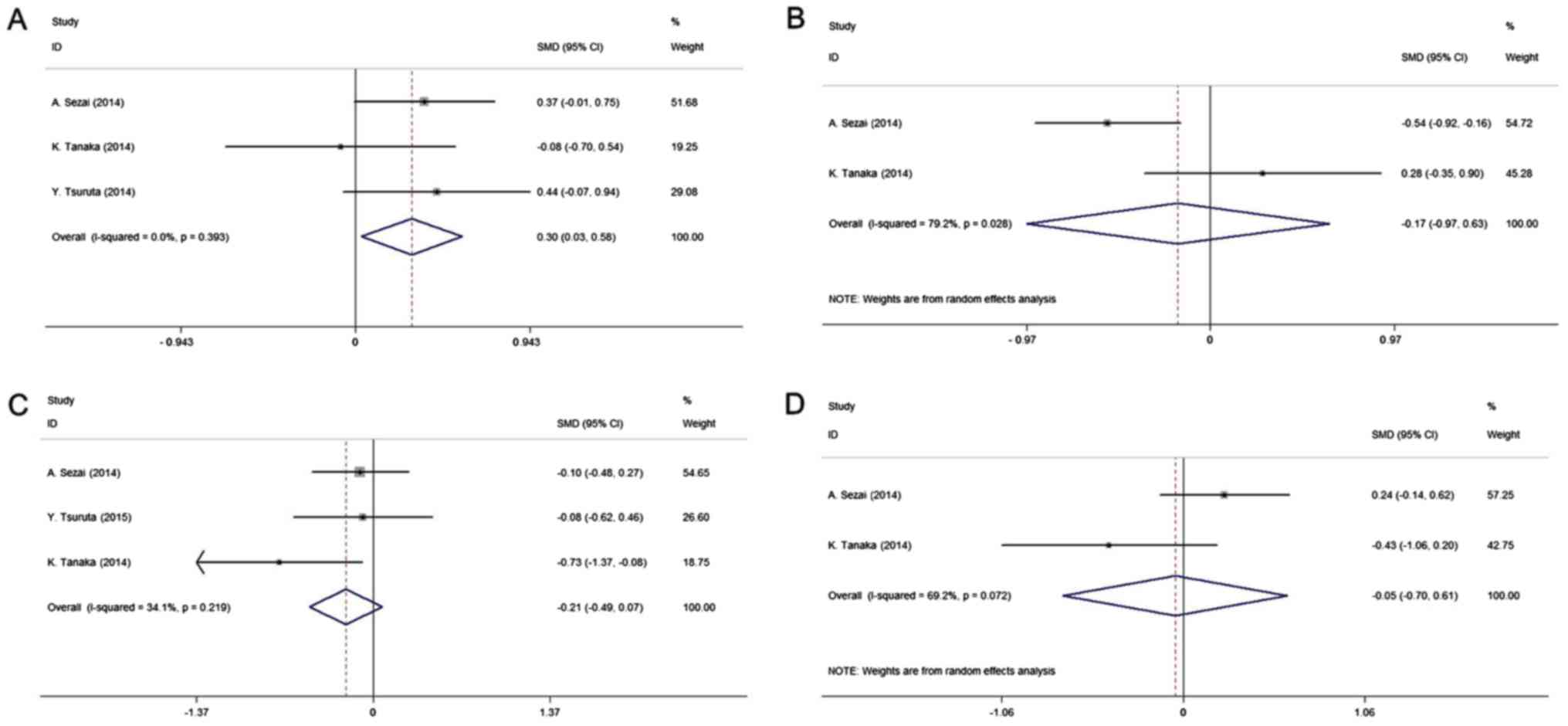
|
1
|
Woo KT, Choong HL, Wong KS, Tan HB and
Chan CM: The contribution of chronic kidney disease to the global
burden of major noncommunicable diseases. Kidney Int. 81:1044–1045.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hasegawa S, Jao TM and Inagi R: Dietary
metabolites and chronic kidney disease. Nutrients. 9:E3582017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Niizuma S, Iwanaga Y, Yahata T and
Miyazaki S: Renocardiovascular biomarkers: From the perspective of
managing chronic kidney disease and cardiovascular disease. Front
Cardiovasc Med. 4:102017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee SM, Lee AL, Winters TJ, Tam E, Jaleel
M, Stenvinkel P and Johnson RJ: Low serum uric acid level is a risk
factor for death in incident hemodialysis patients. Am J Nephrol.
29:79–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weiner DE, Tighiouart H, Elsayed EF,
Griffith JL, Salem DN and Levey AS: Uric acid and incident kidney
disease in the community. J Am Soc Nephrol. 19:1204–1211. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li L, Yang C, Zhao Y, Zeng X, Liu F and Fu
P: Is hyperuricemia an independent risk factor for new-onset
chronic kidney disease? A systematic review and meta-analysis based
on observational cohort studies. BMC Nephrol. 15:1222014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takano Y, Hase-Aoki K, Horiuchi H, Zhao L,
Kasahara Y, Kondo S and Becker MA: Selectivity of febuxostat, a
novel non-purine inhibitor of xanthine oxidase/xanthine
dehydrogenase. Life Sci. 76:1835–1847. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chinchilla SP, Urionaguena I and
Perez-Ruiz F: Febuxostat for the chronic management of
hyperuricemia in patients with gout. Expert Rev Clin Pharmacol.
9:665–673. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frampton JE: Febuxostat: A review of its
use in the treatment of hyperuricaemia in patients with gout.
Drugs. 75:427–438. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li S, Yang H, Guo Y, Wei F, Yang X, Li D,
Li M, Xu W, Li W, Sun L, et al: Comparative efficacy and safety of
urate-lowering therapy for the treatment of hyperuricemia: A
systematic review and network meta-analysis. Sci Rep. 6:330822016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun Y, Li L, Zhou TY and Lu W: A
model-based meta-analysis to compare urate-lowering response rate
of febuxostat and allopurinol in gout patient. Yao Xue Xue Bao.
49:1674–1683. 2014.(In Chinese). PubMed/NCBI
|
|
12
|
Faruque LI, Ehteshami-Afshar A, Wiebe N,
Tjosvold L, Homik J and Tonelli M: A systematic review and
meta-analysis on the safety and efficacy of febuxostat versus
allopurinol in chronic gout. Semin Arthritis Rheum. 43:367–375.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Omori H, Kawada N, Inoue K, Ueda Y,
Yamamoto R, Matsui I, Kaimori J, Takabatake Y, Moriyama T, Isaka Y
and Rakugi H: Use of xanthine oxidase inhibitor febuxostat inhibits
renal interstitial inflammation and fibrosis in unilateral ureteral
obstructive nephropathy. Clin Exp Nephrol. 16:549–556. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sezai A, Soma M, Nakata K, Osaka S, Ishii
Y, Yaoita H, Hata H and Shiono M: Comparison of febuxostat and
allopurinol for hyperuricemia in cardiac surgery patients with
chronic kidney disease (NU-FLASH trial for CKD). J Cardiol.
66:298–303. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shekelle PG, Newberry SJ, FitzGerald JD,
Motala A, O'Hanlon CE, Tariq A, Okunogbe A, Han D and Shanman R:
Management of gout: A systematic review in support of an american
college of physicians clinical practice guideline. Ann Intern Med.
166:37–51. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luís Â, Domingues F and Pereira L: Can
cranberries contribute to reduce the incidence of urinary tract
infections? A systematic review with meta-analysis and trial
sequential analysis of clinical trials. J Urol. 198:614–621. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Beddhu S, Filipowicz R, Wang B, Wei G,
Chen X, Roy AC, DuVall SL, Farrukh H, Habib AN, Bjordahl T, et al:
A Randomized controlled trial of the effects of Febuxostat therapy
on adipokines and markers of kidney fibrosis in asymptomatic
hyperuricemic patients with diabetic nephropathy. Can J Kidney
Health Dis. 3:20543581166753432016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sofue T, Inui M, Hara T, Nishijima Y,
Moriwaki K, Hayashida Y, Ueda N, Nishiyama A, Kakehi Y and Kohno M:
Efficacy and safety of febuxostat in the treatment of hyperuricemia
in stable kidney transplant recipients. Drug Des Devel Ther.
8:245–253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsuruta Y, Kikuchi K, Tsuruta Y, Sasaki Y,
Moriyama T, Itabashi M, Takei T, Uchida K, Akiba T, Tsuchiya K and
Nitta K: Febuxostat improves endothelial function in hemodialysis
patients with hyperuricemia: A randomized controlled study.
Hemodial Int. 19:514–520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tanaka K, Nakayama M, Kanno M, Kimura H,
Watanabe K, Tani Y, Hayashi Y, Asahi K, Terawaki H and Watanabe T:
Renoprotective effects of febuxostat in hyperuricemic patients with
chronic kidney disease: A parallel-group, randomized, controlled
trial. Clin Exp Nephrol. 19:1044–1053. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsuruta Y, Mochizuki T, Moriyama T,
Itabashi M, Takei T, Tsuchiya K and Nitta K: Switching from
allopurinol to febuxostat for the treatment of hyperuricemia and
renal function in patients with chronic kidney disease. Clin
Rheumatol. 33:1643–1648. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Viggiano D, Gigliotti G, Vallone G,
Giammarino A, Nigro M and Capasso G: Urate-lowering agents in
asymptomatic hyperuricemia: Role of urine sediment analysis and
musculoskeletal ultrasound. Kidney Blood Press Res. 43:606–615.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grassi D, Pontremoli R, Bocale R, Ferri C
and Desideri G: Therapeutic approaches to chronic hyperuricemia and
gout. High Blood Press Cardiovasc Prev. 21:243–250. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uloric (febuxostat) tablets (prescribing
information). https://www.ulorichcp.com/Takeda Pharaceuticals
North America; Deerfield, IL: 2009 June 1st–2018
|
|
26
|
Gray CL and Walters-Smith NE: Febuxostat
for treatment of chronic gout. Am J Health Syst Pharm. 68:389–398.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Borghi C and Perez-Ruiz F: Urate lowering
therapies in the treatment of gout: A systematic review and
meta-analysis. Eur Rev Med Pharmacol Sci. 20:983–992.
2016.PubMed/NCBI
|
|
28
|
Becker MA, Schumacher HR Jr, Wortmann RL,
MacDonald PA, Eustace D, Palo WA, Streit J and Joseph-Ridge N:
Febuxostat compared with allopurinol in patients with hyperuricemia
and gout. N Eng J Med. 353:2450–2461. 2005. View Article : Google Scholar
|
|
29
|
Schumacher HR Jr, Becker MA, Wortmann RL,
Macdonald PA, Hunt B, Streit J, Lademacher C and Joseph-Ridge N:
Effects of febuxostat versus allopurinol and placebo in reducing
serum urate in subjects with hyperuricemia and gout: A 28-week,
phase III, randomized, double-blind, parallel-group trial.
Arthritis Rheum. 59:1540–1548. 2008. View Article : Google Scholar : PubMed/NCBI
|