Introduction
Given its high morbidity and mortality rates,
chronic kidney disease (CKD) is recognized as a worldwide public
health problem (1). Due to the
decreased excretion of metabolites, patients with CKD develop
various complications, including cardiovascular events, digestive
system disorders and endocrine dysfunction (2,3). Of such
complications, hyperuricemia, defined as a serum concentration of
uric acid (UA) that exceeds the limit of solubility (7.0 mg/dl), is
a common complication that affects ~70% of hemodialysis patients
(4). Several studies on patients
with CKD have confirmed the association of hyperuricemia with the
development and progression of kidney disease, indicating the
requirement for treatment even in the absence of symptoms (5,6).
Febuxostat, a chemically engineered and selective
inhibitor of xanthine oxidase, has been approved by the US Food and
Drug Administration for the long-term management of hyperuricemia
in patients with gout (7). Xanthine
oxidase inhibitors, including febuxostat and allopurinol, reduce
serum UA levels by impeding the transformation of hypoxanthine to
xanthine and of xanthine to UA (8).
Structurally differing from allopurinol, which lacks a modified
purine ring, febuxostat inhibits the oxidized and reduced forms of
xanthine oxidase, whereas oxypurinol, the active metabolite of
allopurinol, only inhibits the reduced form of xanthine oxidase
(9). Recent evidence-based studies
have indicated that febuxostat had a better efficacy and safety
than other drugs for lowering UA levels, including allopurinol
(10,11). However, another study comparing the
safety and efficacy of febuxostat and allopurinol in the treatment
of chronic gout reported conflicting results (12). These results suggest a lack of
consistency in the efficacy and safety of febuxostat and other
drugs for treating hyperuricemia.
Experimental and clinical studies have indicated the
renoprotective effects of febuxostat against decreased kidney
function via inhibition of the formation of renal interstitial
fibrosis and macrophage infiltration (13,14).
Furthermore, febuxostat is excreted through the urinary and fecal
pathways, and may be well-tolerated in the short-term and long-term
by patients with CKD with mild to moderate renal dysfunction
without a dose reduction, indicating that febuxostat may be
superior to other agents used for UA-lowering therapy (15). However, the clinical efficacy and
safety of febuxostat in the treatment of hyperuricemia in patients
with CKD have remained to be fully determined.
In the present study, a comprehensive, systematic
review and meta-analysis was performed to assess the efficacy and
safety of febuxostat used for UA-lowering therapy in patients with
CKD and renal transplant recipients.
Materials and methods
Literature search
The present meta-analysis was performed based on the
criteria of the Preferred Reporting Items for Systematic Reviews
and Meta-Analysis protocol (16).
Relevant studies published until April 1, 2017 were identified by a
systematic search of the MEDLINE, EMBASE and the Cochrane Library
databases, using the following key words: (‘chronic kidney disease’
OR ‘CKD’ OR ‘end-stage renal disease’ OR ‘ESRD’) OR (‘kidney
transplantation’ OR ‘renal transplant’) AND (‘febuxostat’ OR ‘TEI
6720’ OR ‘TEI-6720’ OR ‘TEI6720’) in combination with ‘gout’ OR
‘hyperuricemia’. In addition, the reference lists of eligible
studies were scanned to identify potential relevant studies.
Study selection
Original studies evaluating the efficacy and safety
of febuxostat in patients with CKD with hyperuricemia were reviewed
and selected if they met the following inclusion criteria: i) The
study was a randomized or non-randomized controlled trial; ii)
patients in the intervention and the control group were diagnosed
with CKD or were renal transplant recipients; iii) hyperuricemia
was diagnosed unequivocally; and iv) the study included at least
one predefined outcome measure. The exclusion criteria were as
follows: i) Case reports; ii) reviews; iii) animal experiments,
chemical synthesis papers or in vitro studies; iv) studies
in a language other than English or Chinese. Two authors (XL and
SS) reviewed the articles independently for potential inclusion in
the present meta-analysis.
Data extraction
Two authors (XL and SS) extracted the data
independently; disputes were resolved by discussion or by
consultation of a third investigator (ZX). The following
information was extracted from each study: First author,
publication year, ethnicity of the subjects, study design, number
of cases, age, number of male/female participants, time-points for
measurement, inclusion criteria for patients, and treatment in the
intervention and control groups. Furthermore, laboratory parameters
prior to and after the administration of febuxostat were extracted:
UA levels, estimated glomerular filtration rate (eGFR), serum
creatinine (Scr), low-density lipoprotein (LDL) and high-density
lipoprotein (HDL). The corresponding items in the control group
were also extracted.
Quality assessment
The quality of all identified studies was assessed
based on the Newcastle-Ottawa quality assessment scale (NOS)
(17). The scale included three
aspects: Selection of the study population, comparability within
the study population and ascertainment of outcomes; the score of
each study ranged from 0 to 9 points. The quality of an eligible
study was considered high if the total score was >8 points and
moderate if the total score ranged from 5–7 points.
Statistical analysis
The pooled data were used to assess the efficacy and
safety of febuxostat in reducing UA levels in patients with CKD or
in renal transplant recipients based on the standard mean
difference (SMD) with 95% confidence intervals (95% CIs). P<0.05
was considered to indicate a statistically significant difference.
Heterogeneity among trials was determined by the I2
test, with I2=(Q-df)/Q ×100%, where Q was Cochran's
heterogeneity statistical parameter and df the degree of freedom.
In cases of low statistical inconsistency (I2<50%), a
fixed-effects model was used for meta-analysis; otherwise, in cases
of heterogeneity, a random-effects model was used, which is better
adapted to clinical and statistical variations. Sensitivity
analysis was performed using the leave-one-out method, which
comprises removal of one study at a time and repetition of the
analysis. In addition, publication bias was explored using Begg's
rank correlation and Egger's weighted regression tests. All
statistical analyses were performed using STATA software (release
12.0; StataCorp, College Station, TX, USA).
Results
Screening and inclusion of
studies
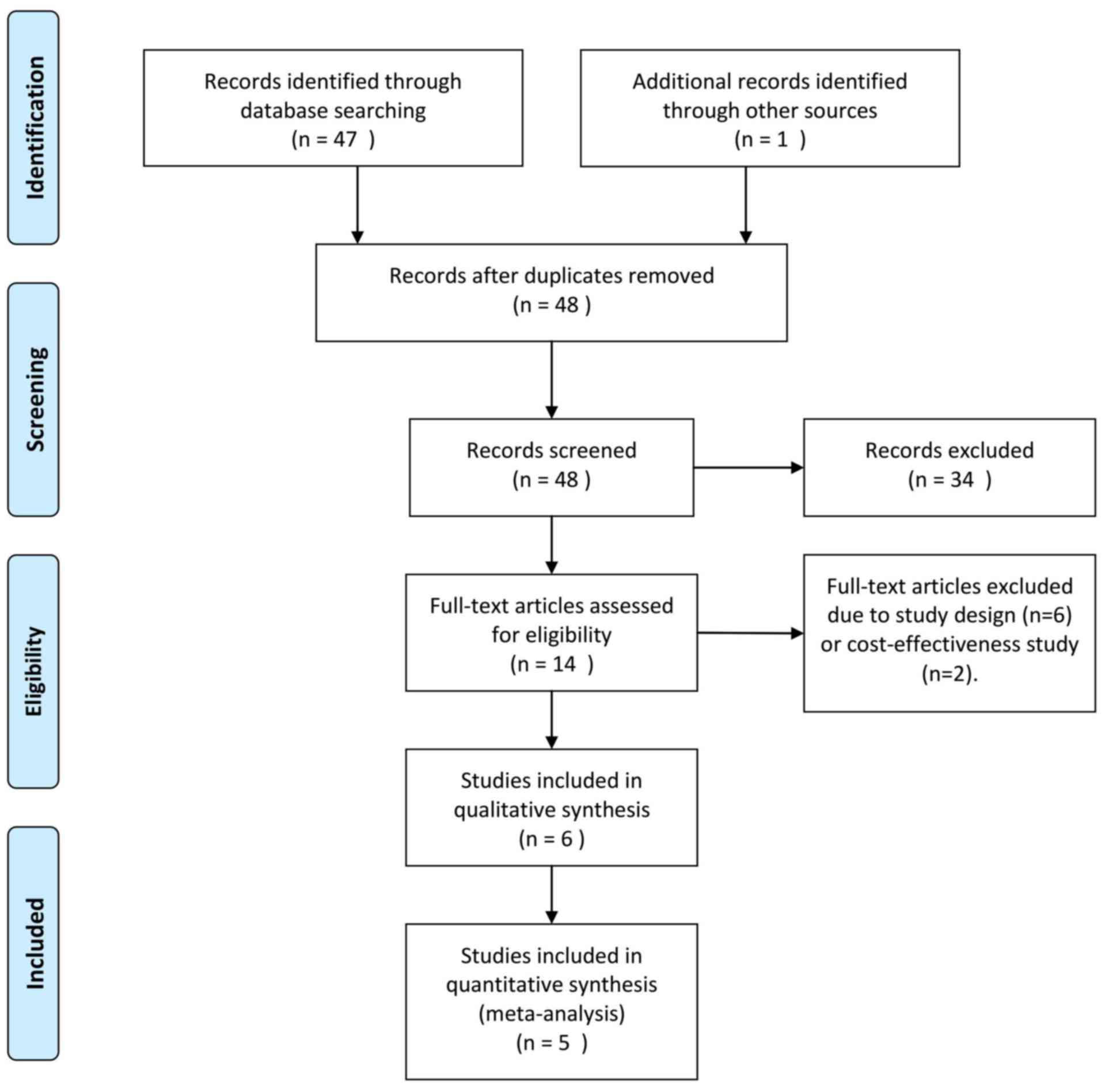
In the present meta-analysis, a total of 48 relevant
studies were identified, and their titles and abstracts were
reviewed. Subsequently, 34 papers were excluded, as they were case
reports, reviews or articles written in a language other than
English or Chinese. After full-text review of the remaining
studies, eight studies were excluded due to study design and the
focus of the study being inappropriate (drug cost-effectiveness).
Finally, six clinical trials comprising 402 patients were eligible
for systematic review and meta-analysis (Fig. 1) (14,18–22),
five of which were included for further data analysis (14,19–22).
Quantitative statistics were unable to be extracted from the study
by Beddhu et al (18) and
could only be systematically reviewed.
The clinical characteristics of all included
patients included in the present systematic review and
meta-analysis are presented in Table
I. Among the six studies included, participants in five trials
were of Asian ethnicity (14,19–22)
and of Caucasian ethnicity in one trial (18). The studies all compared the efficacy
of febuxostat with control agents (placebo or allopurinol), and
four were randomized controlled trials (14,18,20,21).
More importantly, one trial focused on the effect and safety of
febuxostat in renal transplant recipients (19).
 | Table I.Basic characteristics of eligible
studies included in the present systematic review and
meta-analysis. |
Table I.
Basic characteristics of eligible
studies included in the present systematic review and
meta-analysis.
| First author
(year) | Ethnicity | Study design | Cases
(FX/control) | Age (mean ± SD,
years) | M/F | Time-points of
measurement (months) | Inclusion
criteria | Intervention | NOS scale | (Refs.) |
|---|
| Sezai (2014) | Asian | Prospective,
randomized, controlled | 56/53 | FX: 69.4±10.0; Con:
69.1±9.2 | 85/24 | 6 | Patients with eGFR
<60 ml/min/ 1.73 m2 prior to treatment | A maximum of 60
mg/day for FX or 300 mg/day for allopurinol; in patients with an
eGFR <30 ml/min/1.73 m2, the maximum daily dose was 40 mg for FX
and 200 mg for allopurinol. | 7 | (14) |
| Sofue (2014) | Asian | Retrospective
observational study | 26/25 | FX: 48.6; Con:
54.1 | 43/8 | 1, 3, 6, 12 | UA >7.0 mg/dl or
requirement for treatment with conventional UA-lowering drugs | FX started at 10
mg/day and increased to 20 mg/day if serum UA levels remained >7
mg/dl. | 6 | (19) |
| Tanaka (2014) | Asian | Prospective,
randomized, controlled | 21/19 | FX: 70.1±9.5; Con:
66.1±7.0 | 35/5 | 1, 2, 3 | Adult subjects with
hyperuricemia (serum UA ≥7.0 mg/dl) who were known to have CKD
stage 3. | FX was allowed to
be increased to 40 mg/day. | 8 | (21) |
| Tsuruta (2014) | Asian | Retrospective
observational study | 51/22 | FX: 67.4±12.3; Con:
72.9±10.7 | 45/28 | 3, 6, 9, 12 | Presence of CKD as
manifested by an eGFR <45 ml/min; current UA-lowering therapy
with allopurinol; stable renal function; stable clinical
condition. | NA | 7 | (22) |
| Tsuruta (2015) | Asian | Prospective,
randomized, controlled | 27/26 | FX: 67.7±12.4; Con:
68.9±12.7 | 34/29 | 1 | Outpatients on
maintenance hemodialysis; age of >20 years; serum UA levels of
≥7.0 mg/dl; stable clinical condition | FX (10 mg/day) or
control group using the minimization method for age and UA
levels | 8 | (20) |
| Beddhu (2016) | Caucasian | Prospective,
randomized, controlled | 37/39 | 68±10 | 52/28 | 2 weeks, 1, 3,
6 | Serum UA levels
≥327 µmol/l in males and ≥274 µmol/l in females; adults with
diagnosis of type 2 diabetes and kidney disease defined as eGFR
30–59 ml/min/1.73 m2 (body surface area) or eGFR ≥60 ml/min/1.73 m2
with urine dipstick ≥1+ proteinuria or urine albumin/creatinine
≥3.4 mg/mmol | Oral FX 80 mg/day
or matching placebo for 24 weeks | 8 | (18) |
Table I also presents
the results of the quality assessment of the included studies.
Three studies each were deemed as being of high quality and another
three as being of moderate quality.
Evaluation of the efficacy of
febuxostat in reducing UA
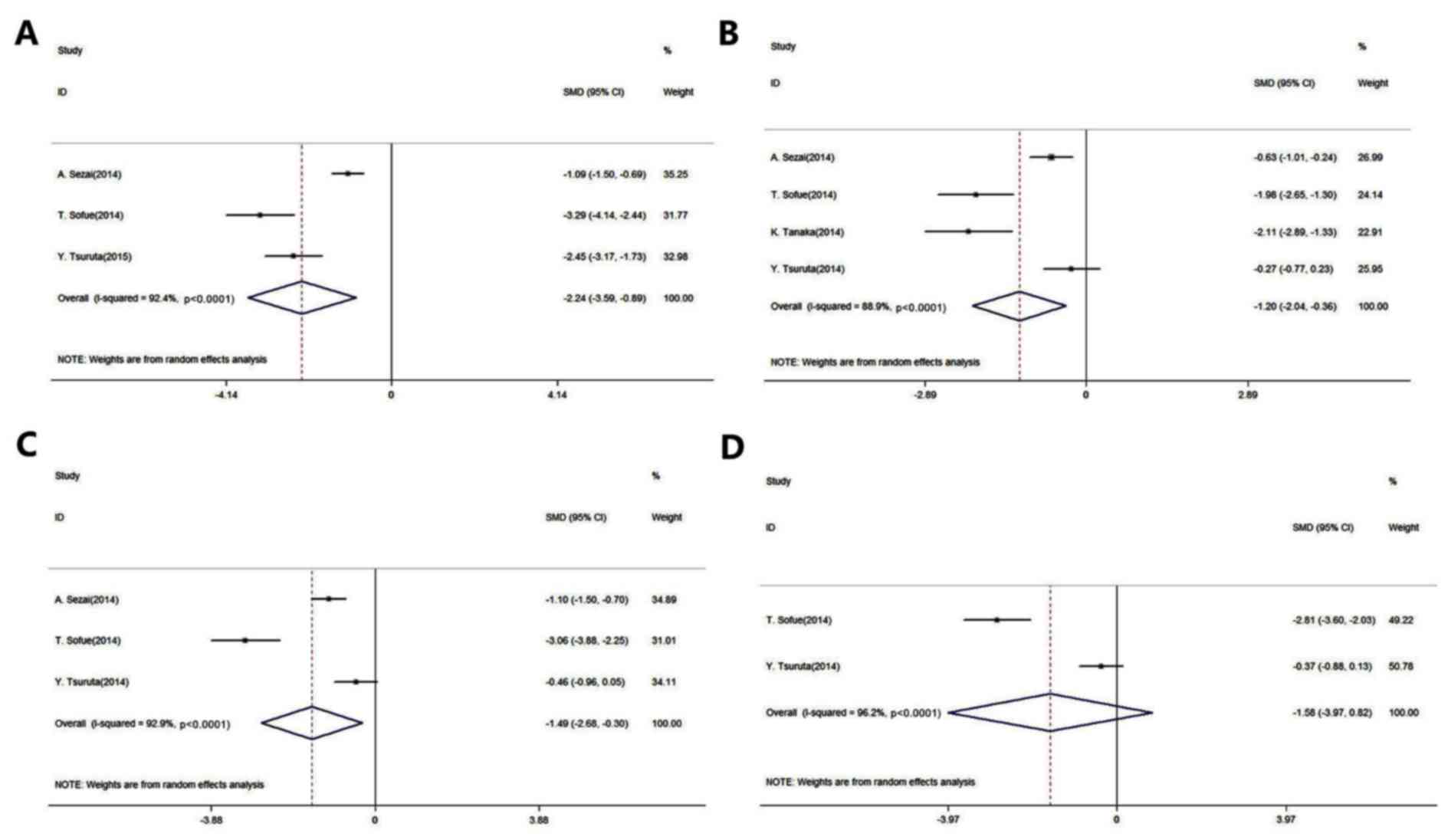
Fig. 2 presents the
forest plots for the meta-analysis of UA level reduction in
patients with CKD and in renal transplant recipients (The P-values
presented in Fig. 2 are
representative of heterogeneity). A total of five studies were
included in this evaluation (14,19–22). The
results of this meta-analysis indicated that febuxostat
significantly reduced the UA levels when compared to the control
agents (placebo or allopurinol) after 1, 3 and 6 months of
administration [1 month: SMD, −2.24; 95% CI, −3.59 to −0.89;
P-value of SMD=0.001; I2, 92.4% (Fig. 2A); 3 months: SMD, −1.20; 95% CI,
−2.04 to −0.36; P-value of SMD=0.005; I2, 88.9%
(Fig. 2B); 6 months: SMD, −1.49; 95%
CI, −2.68 to −0.30; P-value of SMD=0.014; I2, 92.9%
(Fig. 2C)]. However, there was no
significant difference in UA levels between the administration of
febuxostat and the control agents after 12 months (SMD, −1.58; 95%
CI, −3.97 to 0.82; P-value of SMD=0.196; I2, 96.2%;
Fig. 2D).
Evaluation of safety regarding renal
function and lipid metabolism
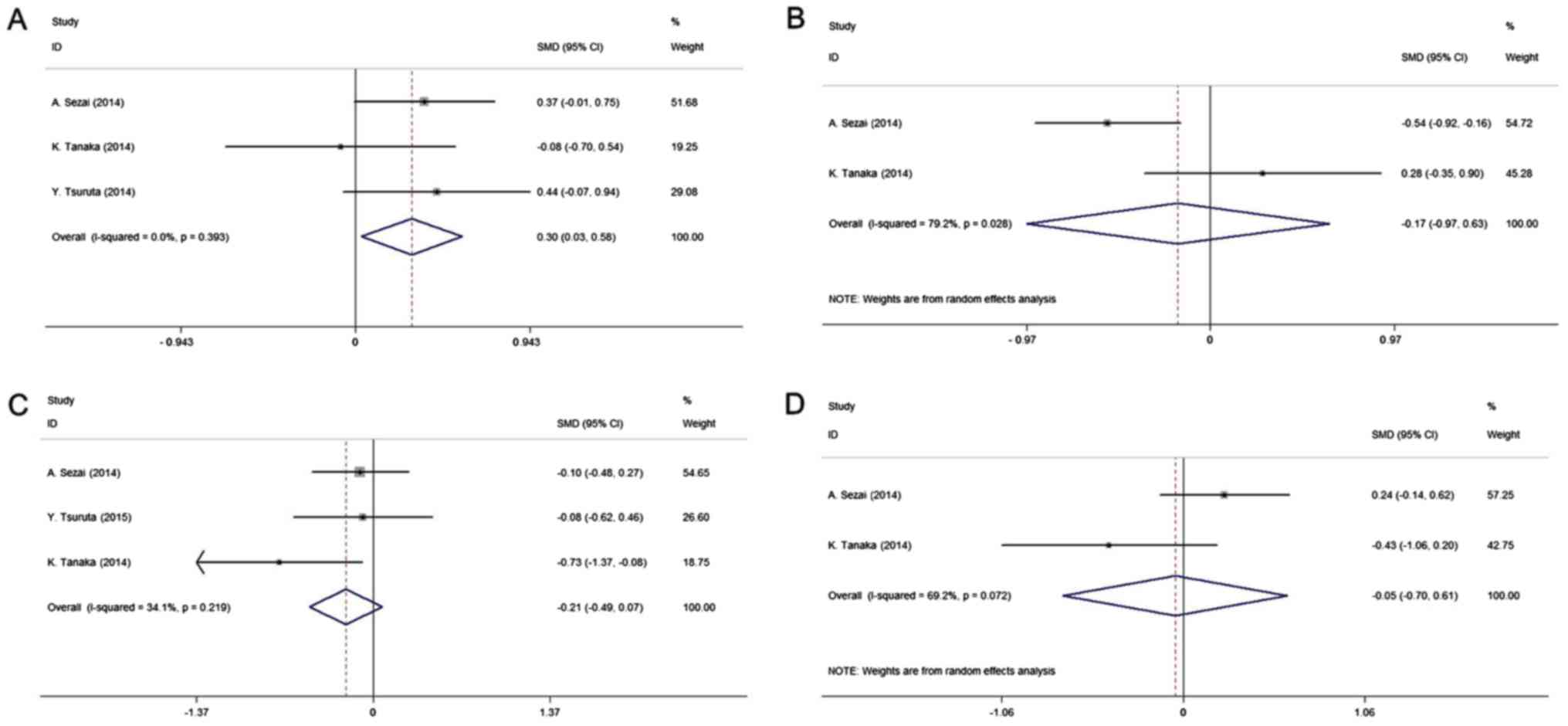
All studies included in the quantitative analysis
were compared with allopurinol. The pooled results of four studies
(14,20–22)
indicated that febuxostat may prevent the kidney function from
deteriorating. There was a significantly higher increase in the
eGFR in the intervention group compared with that in the control
group (SMD, 0.30; 95% CI, 0.031–0.58; P-value of SMD=0.029;
I2, 0.0%; Fig. 3A),
whereas no significant difference in Scr levels was obtained
between the two groups (SMD, −0.17; 95% CI, −0.97–0.63; P-value of
SMD=0.67; I2, 79.2%; Fig.
3B). The present study further analyzed the effect of
febuxostat on lipid metabolism among the pooled cohort. Compared
with the control treatment, the results indicated no significant
effect of febuxostat on lipid metabolism, including LDL and HDL
[LDL: SMD, −0.21; 95% CI, −0.49–0.07; P-value of SMD=0.13;
I2, 34.1% (Fig. 3C); HDL:
SMD, −0.05; 95% CI, −0.70–0.61; P-value of SMD=0.89; I2,
69.2% (Fig. 3D)].
Sensitivity analysis and publication
bias
The results of the leave-one-out sensitivity
analyses indicated that none of the studies excluded affected the
pooled results, which confirmed that the significance of
differences obtained between the two groups was the overall effect
of all studies included. Analysis of publication bias revealed that
among the studies included in the present meta-analysis, no
publication bias was present according to Egger's linear regression
and Begg's rank correlation test (Egger's test: t=0.18, P=0.85;
Begg's test: z=0.12, P=0.90).
Discussion
Hyperuricemia is a common metabolic complication in
patients with CKD and in renal transplant recipients. Studies
demonstrating the efficacy and safety of febuxostat in UA-lowering
therapy among patients with CKD and in renal transplant recipients
have reported conflicting results (18,23).
Considering the potential influence of febuxostat on renal
function, which has caused the greatest concern of clinicians
regarding its use in patients with CKD and kidney transplantation,
the data of clinical trials on patients with CKD and renal
transplant recipients were combined in the present study. In the
present systematic review and meta-analysis, it was revealed that
febuxostat significantly reduced serum UA levels and attenuated the
decrease in renal function without causing any lipid
metabolism-associated disorders in patients with CKD and in renal
transplant recipients.
Febuxostat is an established and effective xanthine
oxidative inhibitor alternative to allopurinol, the long-standing
gold standard, which remains the most widely prescribed
urate-lowering therapy for gout (24). Consistent with the well-established
outcomes, the present study indicated that short-term
administration (1–6 months) of febuxostat significantly decreased
the serum concentrations of UA in patients with CKD and in renal
transplant recipients when compared to allopurinol. Furthermore,
there was no statistically significant difference in the 12-month
administration subgroup, even if the clinical efficacy of
febuxostat for reducing UA levels was higher than that of the
control group, which received allopurinol. Furthermore, there was
significant heterogeneity among the two studies included in the
12-month subgroup analysis, most likely due to the diversity of the
included patients, who were patients with CKD and renal transplant
recipients, respectively, and due to methodological differences.
Consequently, the relatively high heterogeneity may have
contributed to the results of the 12-month subgroup analysis. More
importantly, the guidelines for the administration of febuxostat
for chronic gout recommend dosages of 40–80 mg once daily, with a
target serum UA concentration of <6 mg/dl (25,26). In
the present study, it was observed that the average dosage of
febuxostat used in the studies included was ~40 mg/day, and the
mean serum level of UA after febuxostat treatment for 12 months was
5.89 mg/dl, indicating that febuxostat administration at 40 mg/day
over 12 months may achieve the target serum UA concentration in
patients with CKD and in renal transplant recipients. Finally, the
present study did not perform any subgroup analysis based on the
patients with CKD or renal transplant recipients due to the limited
amount of studies. In accordance with our clinical experience, no
differences in the treatment regimen were observed between the
patients with CKD or renal transplant recipients. However, in
patients with CKD, the dosage should be reduced according to their
impaired renal function.
Febuxostat is generally well-tolerated and offers
the potential advantage of not requiring any dose adjustment in
patients with mild to moderate renal impairment (15,27).
Consistent with these studies, no obvious difference in the effects
of febuxostat and the gold-standard regimen of allopurinol or
placebo on the decrease in renal function was observed, which
supports the administration of febuxostat to patients with CKD and
to renal transplant recipients. The most frequently reported
adverse events during these clinical trials were liver dysfunction,
hyperlipidemia and gastrointestinal disturbance (28,29). The
present meta-analysis study further investigated the effects of
febuxostat on lipid metabolism, including the extent of changes in
serum LDL and HDL levels. No significant difference was observed
between the intervention and control groups, further supporting the
administration of the well-established and well-tolerated
febuxostat. However, other adverse events, including diarrhea and
joint signs and symptoms, still remain to be further
investigated.
Of note, the present study has several limitations.
The individual studies involved in the systematic review differed
in terms of study design, participant ethnicity, inclusion criteria
and usage of febuxostat and the administration of the control group
(placebo or allopurinol), which significantly contributes to
substantial heterogeneity. Doe to the limited number of patients
included in the present meta-analysis, it was not possible to
further investigate the long-term efficacy of febuxostat on
hyperuricemia and certain frequent adverse events in patients with
CKD and in renal transplant recipients.
In conclusion, the present meta-analysis study
indicated that febuxostat has significant clinical efficacy in
reducing serum UA concentrations in the short-term, and has
well-tolerated effects on renal function and lipid metabolism in
patients with CKD and in renal transplant recipients. These results
should be interpreted with caution due to the observational design
of the included studies. A well-designed, large-scale, controlled
study is required for further clarification.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL contributed to the study design and preparation
of the manuscript. KL contributed to the study design and
statistical analysis. QS contributed to the statistical analysis
and preparation of the manuscript. YW contributed to the
statistical analysis. JM contributed to the study design and data
collection. ZX contributed to the statistical analysis and data
collection. ZS contributed to the interpretation of data and the
preparation of the manuscript. The final version of the manuscript
has been read and approved by all authors, and each author believes
that the manuscript represents honest work.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CKD
|
chronic kidney disease
|
|
UA
|
uric acid
|
|
eGFR
|
estimated glomerular filtration
rate
|
|
Scr
|
serum creatinine
|
|
LDL
|
low-density lipoprotein
|
|
HDL
|
high-density lipoprotein
|
|
NOS
|
Newcastle-Ottawa quality assessment
scale
|
|
SMD
|
standard mean difference
|
|
95% CI
|
95% confidence interval
|
References
|
1
|
Woo KT, Choong HL, Wong KS, Tan HB and
Chan CM: The contribution of chronic kidney disease to the global
burden of major noncommunicable diseases. Kidney Int. 81:1044–1045.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hasegawa S, Jao TM and Inagi R: Dietary
metabolites and chronic kidney disease. Nutrients. 9:E3582017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Niizuma S, Iwanaga Y, Yahata T and
Miyazaki S: Renocardiovascular biomarkers: From the perspective of
managing chronic kidney disease and cardiovascular disease. Front
Cardiovasc Med. 4:102017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee SM, Lee AL, Winters TJ, Tam E, Jaleel
M, Stenvinkel P and Johnson RJ: Low serum uric acid level is a risk
factor for death in incident hemodialysis patients. Am J Nephrol.
29:79–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weiner DE, Tighiouart H, Elsayed EF,
Griffith JL, Salem DN and Levey AS: Uric acid and incident kidney
disease in the community. J Am Soc Nephrol. 19:1204–1211. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li L, Yang C, Zhao Y, Zeng X, Liu F and Fu
P: Is hyperuricemia an independent risk factor for new-onset
chronic kidney disease? A systematic review and meta-analysis based
on observational cohort studies. BMC Nephrol. 15:1222014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takano Y, Hase-Aoki K, Horiuchi H, Zhao L,
Kasahara Y, Kondo S and Becker MA: Selectivity of febuxostat, a
novel non-purine inhibitor of xanthine oxidase/xanthine
dehydrogenase. Life Sci. 76:1835–1847. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chinchilla SP, Urionaguena I and
Perez-Ruiz F: Febuxostat for the chronic management of
hyperuricemia in patients with gout. Expert Rev Clin Pharmacol.
9:665–673. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frampton JE: Febuxostat: A review of its
use in the treatment of hyperuricaemia in patients with gout.
Drugs. 75:427–438. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li S, Yang H, Guo Y, Wei F, Yang X, Li D,
Li M, Xu W, Li W, Sun L, et al: Comparative efficacy and safety of
urate-lowering therapy for the treatment of hyperuricemia: A
systematic review and network meta-analysis. Sci Rep. 6:330822016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun Y, Li L, Zhou TY and Lu W: A
model-based meta-analysis to compare urate-lowering response rate
of febuxostat and allopurinol in gout patient. Yao Xue Xue Bao.
49:1674–1683. 2014.(In Chinese). PubMed/NCBI
|
|
12
|
Faruque LI, Ehteshami-Afshar A, Wiebe N,
Tjosvold L, Homik J and Tonelli M: A systematic review and
meta-analysis on the safety and efficacy of febuxostat versus
allopurinol in chronic gout. Semin Arthritis Rheum. 43:367–375.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Omori H, Kawada N, Inoue K, Ueda Y,
Yamamoto R, Matsui I, Kaimori J, Takabatake Y, Moriyama T, Isaka Y
and Rakugi H: Use of xanthine oxidase inhibitor febuxostat inhibits
renal interstitial inflammation and fibrosis in unilateral ureteral
obstructive nephropathy. Clin Exp Nephrol. 16:549–556. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sezai A, Soma M, Nakata K, Osaka S, Ishii
Y, Yaoita H, Hata H and Shiono M: Comparison of febuxostat and
allopurinol for hyperuricemia in cardiac surgery patients with
chronic kidney disease (NU-FLASH trial for CKD). J Cardiol.
66:298–303. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shekelle PG, Newberry SJ, FitzGerald JD,
Motala A, O'Hanlon CE, Tariq A, Okunogbe A, Han D and Shanman R:
Management of gout: A systematic review in support of an american
college of physicians clinical practice guideline. Ann Intern Med.
166:37–51. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luís Â, Domingues F and Pereira L: Can
cranberries contribute to reduce the incidence of urinary tract
infections? A systematic review with meta-analysis and trial
sequential analysis of clinical trials. J Urol. 198:614–621. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Beddhu S, Filipowicz R, Wang B, Wei G,
Chen X, Roy AC, DuVall SL, Farrukh H, Habib AN, Bjordahl T, et al:
A Randomized controlled trial of the effects of Febuxostat therapy
on adipokines and markers of kidney fibrosis in asymptomatic
hyperuricemic patients with diabetic nephropathy. Can J Kidney
Health Dis. 3:20543581166753432016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sofue T, Inui M, Hara T, Nishijima Y,
Moriwaki K, Hayashida Y, Ueda N, Nishiyama A, Kakehi Y and Kohno M:
Efficacy and safety of febuxostat in the treatment of hyperuricemia
in stable kidney transplant recipients. Drug Des Devel Ther.
8:245–253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsuruta Y, Kikuchi K, Tsuruta Y, Sasaki Y,
Moriyama T, Itabashi M, Takei T, Uchida K, Akiba T, Tsuchiya K and
Nitta K: Febuxostat improves endothelial function in hemodialysis
patients with hyperuricemia: A randomized controlled study.
Hemodial Int. 19:514–520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tanaka K, Nakayama M, Kanno M, Kimura H,
Watanabe K, Tani Y, Hayashi Y, Asahi K, Terawaki H and Watanabe T:
Renoprotective effects of febuxostat in hyperuricemic patients with
chronic kidney disease: A parallel-group, randomized, controlled
trial. Clin Exp Nephrol. 19:1044–1053. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsuruta Y, Mochizuki T, Moriyama T,
Itabashi M, Takei T, Tsuchiya K and Nitta K: Switching from
allopurinol to febuxostat for the treatment of hyperuricemia and
renal function in patients with chronic kidney disease. Clin
Rheumatol. 33:1643–1648. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Viggiano D, Gigliotti G, Vallone G,
Giammarino A, Nigro M and Capasso G: Urate-lowering agents in
asymptomatic hyperuricemia: Role of urine sediment analysis and
musculoskeletal ultrasound. Kidney Blood Press Res. 43:606–615.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grassi D, Pontremoli R, Bocale R, Ferri C
and Desideri G: Therapeutic approaches to chronic hyperuricemia and
gout. High Blood Press Cardiovasc Prev. 21:243–250. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uloric (febuxostat) tablets (prescribing
information). https://www.ulorichcp.com/Takeda Pharaceuticals
North America; Deerfield, IL: 2009 June 1st–2018
|
|
26
|
Gray CL and Walters-Smith NE: Febuxostat
for treatment of chronic gout. Am J Health Syst Pharm. 68:389–398.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Borghi C and Perez-Ruiz F: Urate lowering
therapies in the treatment of gout: A systematic review and
meta-analysis. Eur Rev Med Pharmacol Sci. 20:983–992.
2016.PubMed/NCBI
|
|
28
|
Becker MA, Schumacher HR Jr, Wortmann RL,
MacDonald PA, Eustace D, Palo WA, Streit J and Joseph-Ridge N:
Febuxostat compared with allopurinol in patients with hyperuricemia
and gout. N Eng J Med. 353:2450–2461. 2005. View Article : Google Scholar
|
|
29
|
Schumacher HR Jr, Becker MA, Wortmann RL,
Macdonald PA, Hunt B, Streit J, Lademacher C and Joseph-Ridge N:
Effects of febuxostat versus allopurinol and placebo in reducing
serum urate in subjects with hyperuricemia and gout: A 28-week,
phase III, randomized, double-blind, parallel-group trial.
Arthritis Rheum. 59:1540–1548. 2008. View Article : Google Scholar : PubMed/NCBI
|