Introduction
Permanent pacemaker implantation has become an
important treatment for a variety of organic arrhythmias, but about
50–75% of patients are required to receive nuclear magnetic
resonance imaging (MRI) scans after implantation (1). MRI does not involve radiation and has
multi-directional imaging as well as excellent contrast resolution
of soft tissues, so it can provide relatively more comprehensive
information about tissue perfusion, function and metabolism.
Besides, it can also clearly show the heart, blood vessels and body
cavity without contrast agents, and its cardiac function evaluation
also has a higher application value (2). Components and materials of conventional
cardiac pacemakers contain ferromagnetic substance, so under the
action of high field strength of static fields, the pulse generator
and pacing electrodes can be turned or shifted (3); radio frequency fields interfere with
magnetic components to partly or fully disable them in ways such as
closing reeds, changing the frequency of pacemakers, resetting
programs or inhibiting pace-making (4). Thermal energy generated at the junction
of the top of electrodes and myocardial tissues injures the
myocardia, thus contributing to the formation of scars or
perforations, and it also affects the perception and
program-controlled functions of the device, thus inducing
arrhythmia (5). On the area around
15 cm of the pulse generator, there appear heavy artifacts
(6). In 2008, the first EnRhythm MRI
SureScan IPG pacemaker and CapSureFix MRI pacing electrode (Type:
5086MRI) of Medtronic were approved by the European Conformity (CE)
and U.S. Food and Drug Administration (FDA) and clinical safety
studies have been conducted (7,8). Up to
now, the largest sample study was conducted for 723 patients from
66 heart centers in the United States. About 13% of patients were
followed up for 1 year and received MRI scans for neurological
diseases (33%), spinal diseases (16%), cancers (11%), joint
injuries (11%) and other causes (29%), and 47% of patients did not
receive MRI and CT scans before pacemaker implantation. There
appeared no serious symptoms and pacemaker dysfunctions in any of
the patients (9). The aim of this
study was to evaluate the safety of MRI scans in pacemaker
implantation and present precautions.
Patients and methods
Patient information
A total of 86 patients undergoing this pacemaker
implantation who were admitted to Huanggang Hospital (Huanggang,
China) from June 2006 to March 2017 were continuously selected.
Among them, there were 45 males and 41 females aged from 45 to 78
years with the mean age of 56.5±14.3 years. The duration of
operation was 46–82 min with the mean value of 65.3±19.8 min. The
basic types of cardiovascular diseases: Severe slow rhythm (49
cases), persistent atrial fibrillation after radiofrequency
ablation (12 cases), severe ventricular rhythm (15 cases) and
severe coronary heart disease or heart failure with arrhythmia (10
cases). There were 35 cases of pacemaker-dependent type and 51
cases of pacemaker-independent type. Pacing modes: AAI (A, atrial
paced; A, atrial sensed; I, pacing is inhibited if beat is sensed)
(38 cases), VVI (V, ventricle paced; V, ventricle sensed; I, pacing
is inhibited if beat is sensed) (25 cases) and DDR (D, both atrial
and ventricle paced; D, both atrial and ventricle sensed; R, rate
responsive) (23 cases); there were 26 cases accompanied with
hypertension, 12 cases with diabetes and 20 cases with stroke; and
there were 30 cases who had received MRI scans before the pacemaker
implantation and 56 cases with no such history. Education duration
was 9–15 years with the average value of 11.2±3.5 years. The study
was approved by the Ethics Committee of Ezhou Central Hospital
(Hubei, China) and patients signed written informed consents.
Study methods
The study was completed by the same team for MRI
scans and nursing. They informed patients risks in scans and signed
safety questionnaires. The whole process was accompanied by
specialist physicians. Before scans, the pacemaker was adjusted to
the MRI-compatible mode [AOO (A, atrial paced; O, none sensed; O,
none-rate responsive), VOO (V, ventricle paced; O, none sensed; O,
none-rate responsive), DOO (D, both atrial and ventricle paced; O,
none sensed; O, none-rate responsive) and OFF] with the pacing
threshold ≤2.0 V/0.4 m/sec. The low resistance value was 200–1,500
Ω and the high resistance value was 30–100 Ω. First aid equipment
and drugs were prepared. The low field strength was (<1.5T), and
the gradient switching rate was ≤200 T/m/sec. Synchronized
electrocardiogram (ECG) monitoring was conducted. Body radiation
absorption rate was ≤2.0 W/kg; the radiation rate of the head was
≤3.2 W/kg; the duration of scan in pacemaker implantation for the
first time was ≥6 weeks. The type of the MRI scanner was Siemens
Sonata superconductive 1.5T, and we selected different scan
sequences according to different inspection sites.
Observation indexes
Follow-up duration was 2–80 months with the mean
value of 40.5±15.6 months. Mean time of first MRI scan, scan times
and mean scan duration after pacemaker implantation were recorded.
Causes were examined, and according to all the symptoms and
abnormalities of pacemakers appearing in patients after the
examination, patients were divided into the discomfort group and
the normal group, and possible causes of discomfort were
analyzed.
Statistical analysis
SPSS 20.0 (IBM Corp., Armonk, NY, USA) was used for
statistical analysis. Measurement data were represented as mean ±
standard deviation. Risk factors affecting MRI scans were analyzed
by multivariate logistic regression analysis and screened by using
progressive retraction method. P<0.05 was considered to indicate
a statistically significant difference.
Results
Mean time of the first MRI scan, scan
times and mean scan duration
After implantation (2–15 months), the first MRI scan
was conducted with the mean time of 6.8±2.3 months; scan times was
1–5 times with the average value of 2.2±0.9 times; scan duration
was 35–68 min with the average value of 45.6±12.3 min.
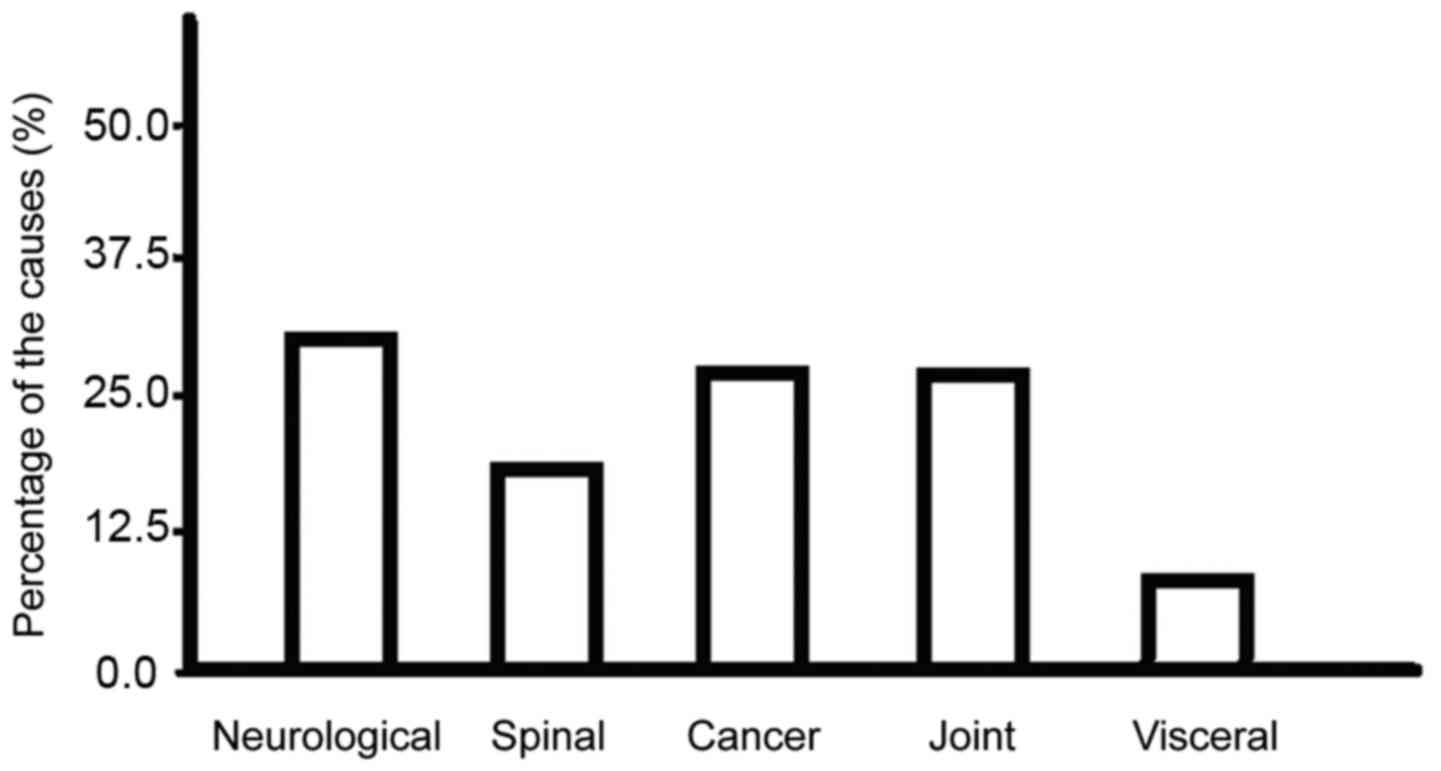
Causes
The main causes include neurological diseases (such
as stroke, neurodegenerative diseases, spinal cord lesions and
craniocerebral trauma), spinal diseases (such as disc herniation
and traumatic fracture), cancers (such as bone tumors, brain tumors
and breast tumors), joint injuries (such as femoral head necrosis,
knee cruciate ligament injury and hip dislocation) and visceral
system scans (hepatobiliary pancreas, prostate, ovary and uterus)
(Fig. 1).
Discomfort symptoms and pacemaker
abnormalities
There appeared discomfort symptoms in 12 patients
among 86 patients, such as excessive anxiety, significantly higher
blood pressure (10% higher than the base value), significantly
faster heart rate (10% faster than the base value); there appeared
pacemaker abnormalities in a total of 10 patients, such as
increased impedance (20% greater than the base value),
abnormalities in perception or pacemakers (appeared pace-making and
self-rhythm), disorders in perception or pacemakers (severe heart
rate overrun or arrest), hypotension or blackness, no syncope and
disturbance of consciousness. Safe scans could be achieved by
psychological counseling, debugging pacemaker parameters, changing
MRI scan sequences and other methods without pacemaker or wire
shifting or other changes.
Risk factors affecting MRI scans
Data such as patient baseline data as well as MRI
scan and pacemaker para-meters were included in the multivariate
logistic regression analysis model. As independent variables,
appearances of discomfort symptoms and pacemaker abnormalities were
selected as dependent variables for screening. The final results
showed that the basic types of cardiovascular diseases, dependence
on pacemakers, duration of education, pacemaker threshold,
impedance and MRI scan time were correlated with the occurrence of
adverse outcomes (p<0.05) (Table
I).
 | Table I.Analysis of risk factors affecting MRI
scans. |
Table I.
Analysis of risk factors affecting MRI
scans.
| Factors | β | Wald | P-value | OR | 95% CI |
|---|
| Basic types of
cardiovascular diseases | 0.123 | 5.236 | 0.018 | 1.625 | 1.128–2.659 |
| Dependence on
pacemakers | 0.285 | 4.625 | 0.029 | 1.147 | 1.007–3.065 |
| Duration of
education | −0.547 | 4.121 | 0.033 | 0.522 | 0.147–0.863 |
| Pacemaker
threshold | 0.352 | 4.554 | 0.031 | 1.085 | 1.021–2.659 |
| Impedance | 0.427 | 4.329 | 0.035 | 1.072 | 1.011–2.954 |
| MRI scan time | 0.441 | 4.859 | 0.025 | 1.326 | 1.124–2.836 |
Discussion
The pacemaker has small volume, light weight and
high magnetization resistance, good magnetic field stability and
other advantages (10). Replacing
pacemaker switches with Hall sensors increased magnetic field
stability; replacing traditional ferromagnetic wires with steel
fiber wires reduced wire impedance; shield protection for internal
power supply circuits reduced the content of pacemaker
ferromagnetic materials; the mode exclusive for MRI was started
during the scan, and resumed after the scan. Under the high field
strength (3T and 8T) and high specific absorption rate (SAR) (3.90
W/kg), the scan was performed for a long time (210 min), and the
results showed that the image quality was not reduced and there
appeared no obvious discomfort in patients and no obvious
dysfunction in the pacemaker (11).
The study included relatively more subjects, so the
basic types of cardiovascular diseases were complex, including
pacemaker-dependent and pacemaker-independent types and pacing
modes were different. Duration of the first MRI scan was 2–15
months, and the perception, pacing function and electrode impedance
of the pacemaker tended to reach a steady state for 1 to 2 weeks
after operation, which needed to be programmed to be adjusted to
the optimal state (12). Sequences
needed to be scanned were different for different parts undergoing
MRI scans, thus posing different levels of influence on the
pacemaker working state, which has not yet been specifically
analyzed. A total of 12 cases (14.0%) showed significant discomfort
symptoms, 10 cases (11.6%) showed pacemaker abnormalities, and the
incidence rate was 25.6%. The study distinguished between patient
discomfort and pacemaker abnormality, and the results showed that
patient discomfort was often associated with poor communication and
great psychological fluctuation, while pacemaker abnormality was
more likely to be associated with MRI interference (13). For patients with neurological
disorders, tumors, and osteoarthritis, it was advisable to select
an MRI-compatible pacemaker, and for those who did not need MRI
scan for the time being, using MRI-compatible pacing electrodes in
patients in advance could retain the right to receive MRI scans in
the future (8). Data and results of
present studies are still limited to the scan under the 1.5T or
less field strength, and there still exists no large sample data
about that under 3.0T field strength (14). Therefore, clinical selection of
intended population still needs to be cautious.
Through screening, the study further showed that the
basic types of cardiovascular diseases, dependence on pacemakers,
duration of education, pacing threshold, impedance and MRI scan
time were related to the occurrence of adverse outcomes. Patients
with severe ventricular arrhythmia (15 cases) and severe coronary
heart disease or heart failure with arrhythmia were more likely to
be examined with adverse events after postoperative radiofrequency
ablation of persistent atrial fibrillation among patients with the
basic types of cardiovascular diseases. Possible causes might be
poor cardiac rhythm and dependence on pacemaker (15,16).
Patients dependent on pacemakers tended to have high pacing
thresholds and relatively higher impedance (17). The longer the duration of education
was, the more clearly the pacemaker maintenance and MRI scan
precautions would be, which could reduce the incidence rate of
adverse events. And the longer the duration of MRI scans was, the
higher the probability of resulted uncontrollable pacing
abnormalities would be (18,19). This study provided an important
reference for guiding clinical MRI and pre-scan preparation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MX, NZ and BF conceived and designed the study. MX,
YQ and PM were responsible for the collection and analysis of the
patient data. NZ, HP and YZ interpreted the data and drafted the
manuscript. BF revised the manuscript critically for important
intellectual content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Ezhou Central Hospital (Hubei, China) and patients signed written
informed consents.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jung W, Zvereva V, Hajredini B and Jäckle
S: Safe magnetic resonance image scanning of the pacemaker patient:
Current technologies and future directions. Europace. 14:631–637.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nordbeck P, Ertl G and Ritter O: Magnetic
resonance imaging safety in pacemaker and implantable cardioverter
defibrillator patients: How far have we come? Eur Heart J.
36:1505–1511. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Y, Han Z, Sun Y, Li A, Zhang W, Li A
and Liu S: Endoscopic polypectomy for pacemaker patients: Is it
safe? ANZ J Surg. 85:834–837. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mesubi O, Ahmad G, Jeudy J, Jimenez A, Kuk
R, Saliaris A, See V, Shorofsky S and Dickfeld T: Impact of ICD
artifact burden on late gadolinium enhancement cardiac MR imaging
in patients undergoing ventricular tachycardia ablation. Pacing
Clin Electrophysiol. 37:1274–1283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Klein-Wiele O, Garmer M, Urbien R, Busch
M, Kara K, Mateiescu S, Grönemeyer D, Schulte-Hermes M, Garbrecht M
and Hailer B: Feasibility and safety of adenosine cardiovascular
magnetic resonance in patients with MR conditional pacemaker
systems at 1.5 Tesla. J Cardiovasc Magn Reson. 17:1122015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Levine GN, Gomes AS, Arai AE, Bluemke DA,
Flamm SD, Kanal E, Manning WJ, Martin ET, Smith JM, Wilke N, et al:
American Heart Association Committee on Diagnostic and
Interventional Cardiac Catheterization; American Heart Association
Council on Clinical Cardiology; American Heart Association Council
on Cardiovascular Radiology and Intervention: Safety of magnetic
resonance imaging in patients with cardiovascular devices: an
American Heart Association scientific statement from the Committee
on Diagnostic and Interventional Cardiac Catheterization, Council
on Clinical Cardiology, and the Council on Cardiovascular Radiology
and Intervention: endorsed by the American College of Cardiology
Foundation, the North American Society for Cardiac Imaging, and the
Society for Cardiovascular Magnetic Resonance. Circulation.
116:2878–2891. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferreira AM, Costa F, Tralhão A, Marques
H, Cardim N and Adragão P: MRI-conditional pacemakers: Current
perspectives. Med Devices (Auckl). 7:115–124. 2014.PubMed/NCBI
|
|
8
|
Shinbane JS, Colletti PM and Shellock FG:
Magnetic resonance imaging in patients with cardiac pacemakers: Era
of ‘MR Conditional’ designs. J Cardiovasc Magn Reson. 13:632011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wollmann CG, Thudt K, Kaiser B,
Salomonowitz E, Mayr H and Globits S: Safe performance of magnetic
resonance of the heart in patients with magnetic resonance
conditional pacemaker systems: the safety issue of the ESTIMATE
study. J Cardiovasc Magn Reson. 16:302014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sutton R, Kanal E, Wilkoff BL, Bello D,
Luechinger R, Jenniskens I, Hull M and Sommer T: Safety of magnetic
resonance imaging of patients with a new Medtronic EnRhythm MRI
SureScan pacing system: Clinical study design. Trials. 9:682008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nazarian S, Hansford R, Roguin A, Goldsher
D, Zviman MM, Lardo AC, Caffo BS, Frick KD, Kraut MA, Kamel IR, et
al: A prospective evaluation of a protocol for magnetic resonance
imaging of patients with implanted cardiac devices. Ann Intern Med.
155:415–424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roguin A, Schwitter J, Vahlhaus C,
Lombardi M, Brugada J, Vardas P, Auricchio A, Priori S and Sommer
T: Magnetic resonance imaging in individuals with cardiovascular
implantable electronic devices. Europace. 10:336–346. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nazarian S, Beinart R and Halperin HR:
Magnetic resonance imaging and implantable devices. Circ Arrhythm
Electrophysiol. 6:419–428. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gimbel JR: Unexpected pacing inhibition
upon exposure to the 3T static magnetic field prior to imaging
acquisition: What is the mechanism? Heart Rhythm. 8:944–945. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Beinart R and Nazarian S: Effects of
external electrical and magnetic fields on pacemakers and
defibrillators: From engineering principles to clinical practice.
Circulation. 128:2799–2809. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tandri H, Zviman MM, Wedan SR, Lloyd T,
Berger RD and Halperin H: Determinants of gradient field-induced
current in a pacemaker lead system in a magnetic resonance imaging
environment. Heart Rhythm. 5:462–468. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Calcagnini G, Triventi M, Censi F, Mattei
E, Bartolini P, Kainz W and Bassen HI: In vitro investigation of
pacemaker lead heating induced by magnetic resonance imaging: Role
of implant geometry. J Magn Reson Imaging. 28:879–886. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mollerus M, Albin G, Lipinski M and Lucca
J: Cardiac biomarkers in patients with permanent pacemakers and
implantable cardioverter-defibrillators undergoing an MRI scan.
Pacing Clin Electrophysiol. 31:1241–1245. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nordbeck P, Weiss I, Ehses P, Ritter O,
Warmuth M, Fidler F, Herold V, Jakob PM, Ladd ME, Quick HH, et al:
Measuring RF-induced currents inside implants: Impact of device
configuration on MRI safety of cardiac pacemaker leads. Magn Reson
Med. 61:570–578. 2009. View Article : Google Scholar : PubMed/NCBI
|