Introduction
Atherosclerosis (AS) is frequently observed in
middle-aged and aged people. It is a common cardiovascular disease,
mainly manifested as increased blood lipid. The incidence of AS is
increasing year by year with a high recurrence rate (1,2). The
pathogenesis of AS is complicated. It can be caused by a variety of
reasons, mainly by lipid infiltration, increase of inflammatory
response and endothelial cell injury (3). The treatment of AS is currently
dominated by medication. The progression of the disease is
inhibited through the reduction of the patient's blood lipid and
the control of risk factors for the occurrence of AS; however, the
treatment of AS faces great challenges. Patients need lifelong
medication due to high recurrence rate and fatality rate of the
disease. Therefore, it is urgent to illuminate the pathogenesis of
AS and seek new treatment methods (4). A large number of studies have shown
that the protein in the Wnt signaling pathway is highly expressed
in a variety of cardiovascular diseases, and it has a certain
relationship with multiple cardiovascular diseases (5,6).
Therefore, the study on the role of Wnt signal pathway in AS has
been the direction of feasible studies of AS-targeted
treatments.
Dickkopf1 (DKK1) protein is the target protein of
Wnt/β-catenin signaling pathway and plays an important role in the
progression of multiple cardiovascular diseases. For example,
myocardial infarction can affect the expression levels of various
inflammatory factors and vascular endothelial growth factor (VEGF)
in patients with myocardial infarction and influence angiogenesis,
thus exerting impacts on the progression of cardiovascular diseases
(7,8). However, the role of Wnt signaling
pathway in the progression of AS and its relationship with
angiogenesis have not been studied yet. This study was conducted to
investigate the role of Wnt signaling pathway via AS model of rats
and explore the relationship between Wnt signaling pathway and
angiogenesis in rats with AS.
Materials and methods
Animals and grouping
Twenty-four adult male Sprague-Dawley (SD) rats were
purchased from the Animal Experimental Center (Guangdong, China).
The experimental animals (production license no. SCXK2013-012; use
license no. SYXK2014-008) weighed 250±10 g. They were raised in a
specific pathogen-free (SPF) animal room at a constant temperature
(25°C). They could eat and drink freely. High-fat fodder and
sustaining fodder were purchased from the Animal Experimental
Center. The aforementioned rats were divided into control group and
model group with 12 rats in each group. The rats in the control
group were fed with sustaining fodder, while those in the model
group were fed with high-fat fodder. Eight weeks later, the level
of blood lipid in the serum and the pathological changes in the
aorta were detected to judge whether the model was successful. All
the animals were operated strictly as per the regulations for
experimental animals in the health guidance for the care and use of
experimental animals specified by the National Institute. The study
was approved by the Ethics Committee of The Second Affiliated
Hospital of Fujian Medical University (Fujian, China).
Materials and instruments
TRIzol kits, reverse transcription kits,
electrochemiluminescence (ECL) solution (all from Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), enzyme-linked
immunosorbent assay-interleukin-6 (ELISA-IL-6) kits, ELISA-tumor
necrosis factor-α (TNF-α) kits (Wuhan Boster Biological Technology,
Ltd., Wuhan, China), rabbit anti-Wnt1, rabbit anti-β-catenin,
rabbit anti-DKK1, rabbit anti-VEGF, rabbit
anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH), horseradish
peroxidase-labeled anti-rabbit secondary antibody (all from Cell
Signaling Technology, Inc., Danvers, MA, USA), polymerase chain
reaction (PCR) instrument, gel electrophoresis and transmembrane
systems (both from Applied Biosystems; Thermo Fisher Scientific,
Inc.), pipettors (Gilson, Inc., Middleton, WI, USA), ultraviolet
imaging system (Biometra GmbH, Göttingen, Germany), fully automatic
biochemical analyser (Toshiba, Tokyo, Japan) and electronic scales
(BP121S; Sartorius AG, Göttingen, Germany). Other relevant
instruments and reagents are illustrated in relevant sections.
Rabbit polyclonal Wnt1 antibody (dilution 1/500; cat. no. ab15251);
rabbit monoclonal β-catenin antibody (dilution 1/500; cat. no.
ab32572); rabbit monoclonal DKK1 antibody (dilution 1/500; cat. no.
ab109416); rabbit monoclonal VEGF antibody (dilution 1/500; cat.
no. ab32152); rabbit polyclonal GAPDH antibody (dilution 1:500,
cat. no. ab37168) and secondary goat anti-rabbit (HRP) IgG antibody
(dilution 1/2,000; cat. no. ab6721) were all purchased from Abcam
(Cambridge, MA, USA).
Detection of the level of blood
lipid
After the rats in the model group were fed with
high-fat diet and those in the control group were fed with common
diet for 8 weeks, blood was taken from the carotid artery after
12-hour fasting. The blood was put into different centrifugal
tubes, and they were allowed to stand for 30 min. Then they were
centrifuged for 10 min at 3,500 × g. The supernatant was taken to
obtain the serum and the plasma, respectively, which were stored at
−80°C for standby. The serum of the rats in each group was taken to
detect the levels of total cholesterol (TC), low-density
lipoprotein (LDL-C) and triglycerides (TG) using a fully automatic
biochemical analyser.
Aortic slices and observation
The rats were sacrificed immediately after the blood
was drawn. The chest was opened, and the blood vessels from the
aortic valve to the bifurcation part of the abdominal aorta were
cut and removed. The blood vessels were incised after the
connective tissues and the fat outside the blood vessels were
isolated completely under the microscope. Phosphate-buffered saline
(PBS) was used to wash away the residual blood, and a freezing
microtome was used to make aortic slices. The slices were treated
under the following conditions, respectively: xylene, anhydrous
ethanol, 95% ethanol, 90% ethanol, 80% ethanol and 70% ethanol.
They were washed with ultrapure water after the aforementioned
treatments were completed. Then they were stained with hematoxylin
for 10 min and washed with ultrapure water again. They were treated
with 1% ammonia water, washed with ultrapure water, and stained
with eosin for 2 min. The slices were dehydrated, made transparent
and sealed after they were washed with water. The pathological
changes of aortic blood vessels of the rats in each group were
observed under a microscope, and the ratio of intima thickness to
media thickness of the aorta was calculated.
Detection of the level of inflammatory
factors
The contents of IL-6 and TNF-α were detected using
ELISA kits after the plasma of the rats in each group was obtained
via centrifuging. The standard curves of IL-6 and TNF-α were
prepared respectively for the subsequent quantification tests. A
total of 100 µl plasma sample of each group diluted 10 times with
sample diluent was added to the sample well. The plates were placed
at the constant temperature (37°C) for reaction for 60 min after
they were sealed with plate-sealing membrane. The plates were
patted to clear liquid inside, and corresponding biotin-labelled
antibodies were added. Then the plates were sealed, and reacted for
60 min at 37°C. The plates were washed with scrubbing solution 3
times (1 min/time) after the liquid in the plates were dried by
patting. A total of 100 µl affinity-peroxidase complex was added,
and the plates were sealed and reacted for 30 min at 37°C. The
plates were washed with scrubbing solution 5 times (2 min/time)
after the liquid in the plates were dried by patting. A total of
100 µl reaction-terminating solution was added to stop reaction,
and the optical density of each well at 450 nm were measured with a
microplate reader (BioTek Instruments, Inc., Winooski, VT, USA).
The levels of IL-6 and TNF-α in the plasma were calculated via
standard curves.
Semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
The samples taken from the carotid artery of the
rats in each group were added to TRIzol kits at a ratio of 100 mg:1
ml. They were homogenated with an ultrasonic homogenizer until
there was no visible debris. The supernate was taken after the
samples were centrifuged at 4°C at 10,500 × g. Ribonucleic acid
(RNA) was extracted according to the instructions for
RNA-extraction kits. The detection showed that the concentration
and the purity of RNA were relatively good, and they could be used
for the subsequent tests. RT-PCR was conducted to obtain
complementary DNA (cDNA) using RT-PCR kits with RNA specimen as the
template. Using this as a template, thermu aquaticus (Taq) PCR, Taq
buffer solution, deoxy-ribonucleoside triphosphate (dNTP) mixture
and distillation-distillation H2O (ddH2O)
were further added for PCR amplification on a PCR instrument.
Finally, the product was placed on a quantitative PCR instrument to
detect mRNA expression of the target gene. The reaction system
volume was in total 25 µl, pre-denaturation at 95°C for 5 min,
denaturation at 95°C for 30 sec, annealing at 60°C for 45 sec,
extension at 72°C for 3 min, with 35 cycles, and then extension at
72°C for 5 min. PCR products were stored at 4°C. The sequences of
Wnt1, β-catenin and DKK1 primers are indicated in Table I. The primers were synthesized by
Tiangen Biotech Co., Ltd. (Beijing, China).
 | Table I.Sequences of qPCR primers. |
Table I.
Sequences of qPCR primers.
|
| Sequence |
|---|
| Wnt | F:
5′-TGGAATTGCAACACCCTGGA-3′ |
|
| R:
5′-TTGGGCGCTTCCCATCTTCTT-3′ |
| β-catenin | F:
5′-GACACGCCACAGGACTACAAGAA-3′ |
|
| R:
5′-CGTATCCACCAGAGTGAAAAGAA-3′ |
| DKK1 | F:
5′-ATGAGGCACGCTATGTGCTG-3′ |
|
| R:
5′-CTCGAGGTAAATGGCTGTGGTC-3′ |
| GAPDH | F:
5′-GACATCAAGAAGGTGGTGAAGC-3′ |
|
| R:
5′-TGTCATTGAGAGCAATGCCAGC-3′ |
Western blot analysis
After the aortic tissues of the rats in each group
were cut into pieces, they were added to radio immunoprecipitation
assay (RIPA) lysis buffer at a ratio of 100 mg:1 ml. The resulting
solutions were placed on an ice box, and homogenate processing was
conducted using an ultrasonic homogenizer until there was no
visible tissue. They were centrifuged at 10,500 × g for 10 min at
4°C. The supernatant (that is, total protein sample) was taken. The
concentration of total protein in the aorta of the rats in each
group was measured using BCA protein assay kits. Sample-loading
buffer solution at the same concentration was prepared, and boiled
for 15 min to inactivate the protein. The samples were added to the
sample-loading wells after 10% separation gel and 4% concentration
gel were prepared. Sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) was conducted at 80 V. Membrane
transference was conducted at 100 V to transfer the protein to
polyvinylidene fluoride (PVDF) membranes (IPVH00010; EMD Millipore,
Billerica, MA, USA) after the electrophoresis was completed. Skim
milk powder (5%) was prepared, and sealed for 2 h. After the target
strip was cut, antibodies of Wnt1, β-catenin, DKK1, VEGF and GAPDH
(dilution at 1:1,000) were prepared. The target strips were placed
in corresponding antibodies, and incubated overnight at 4°C. They
were washed with Tris-buffered saline with Tween-20 (TBST) 3 times
(10 min/time) after incubation. Horseradish peroxidase-labeled
anti-rabbit secondary antibody (Cell Signaling Technology, Inc.)
(dilution at 1:5,000) was prepared. The strips were washed with
TBST 3 times (5 min/time) after they were incubated for 2 h at room
temperature. ECL solution was prepared and dripped on each strip.
The strips were placed in a dark box for pressing, and placed in
developing solution and fixing solution for development. They were
scanned after drying to analyze the gray value of each protein
strip. Wnt1/GAPDH, β-catenin/GAPDH, DKK1/GADH and VEGF/GAPDH were
used to compare the expression level of each protein.
Statistical analysis
The data of the study are expressed as means ±
standard deviation, and the data were analyzed by Statistical
Product and Service Solutions (SPSS) 19.0 (IBM Corp., Armonk, NY,
USA). t-test was conducted for the comparison between two groups.
Pearson analysis was used to examine the correlation between VEGF
and Wnt1 expression. P<0.05 was considered to indicate a
statistically significant difference.
Results
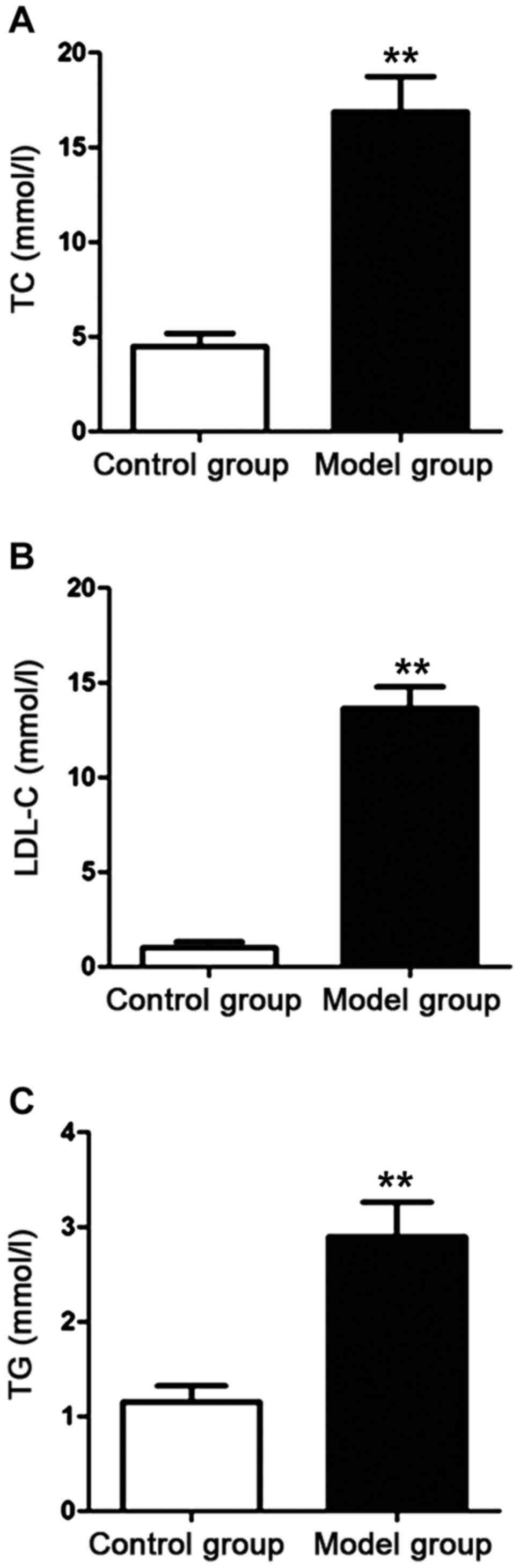
Detection of the level of blood lipid
in the rats
The serum was obtained via isolation after the rat
model was established. Fully automatic biochemical analyzer was
used to detect the contents of TC, LDL-C and TG in the serum of the
rats in the control group and the model group. The results are
shown in Fig. 1. Compared with those
of the rats in the control group, the contents of TC, LDL-C and TG
of the rats in the model group were increased significantly. The
differences were statistically significant (P<0.01).
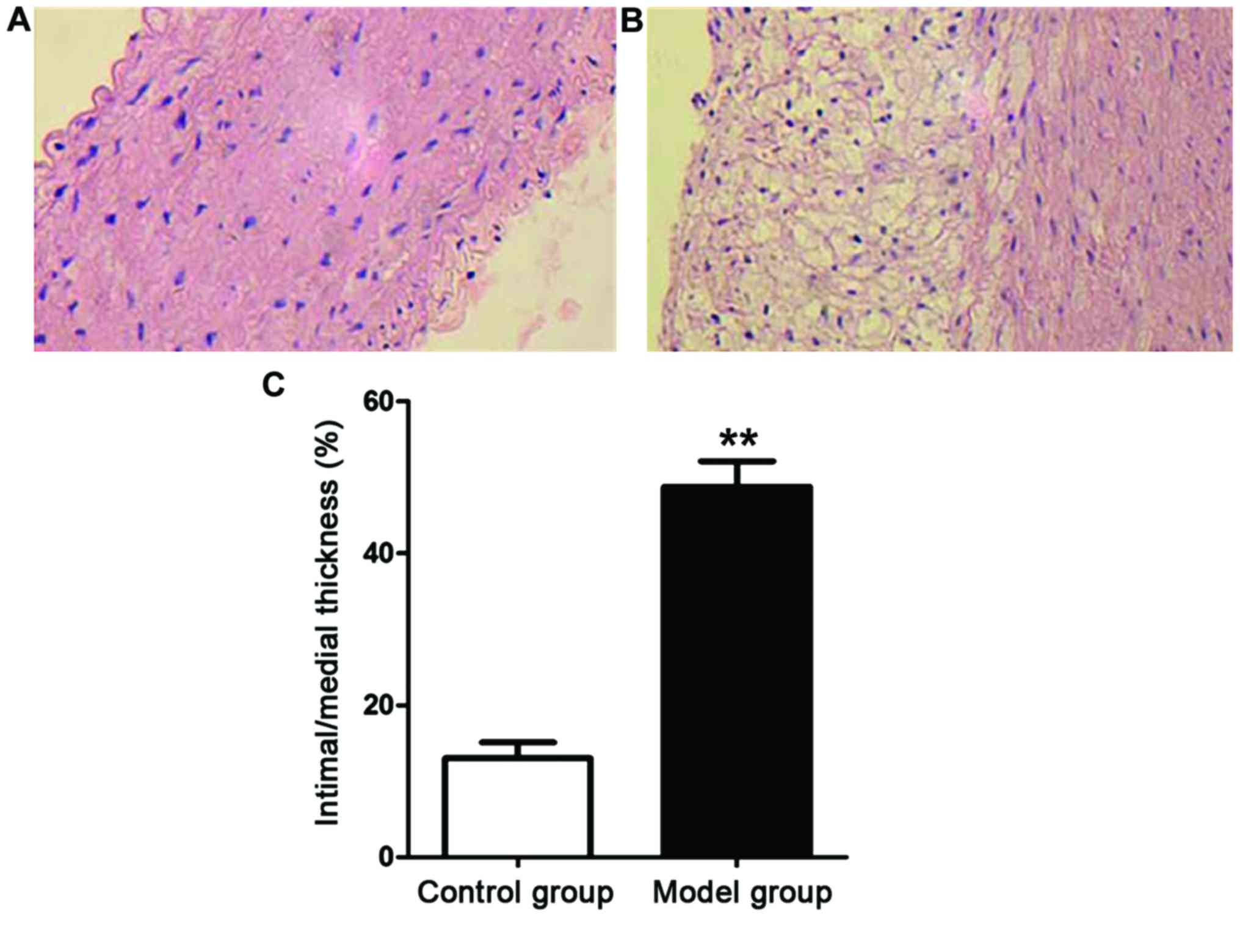
Pathological changes in the aorta of
the rats
Aortic slices of the rats in each group were
prepared. The pathological changes in the aorta of the rats in each
group were observed under an optical microscope (Fig. 2). The aortic intima of the rats in
the control group was smooth on surface without the formation of
lipid plaque by naked eye, while that of the rats in the model
group was rough with significant thickening and the formation of
lipid plaque. The ratio of intima thickness to media thickness of
the rats in the model group was notably higher than that of the
rats in the control group (P<0.01).
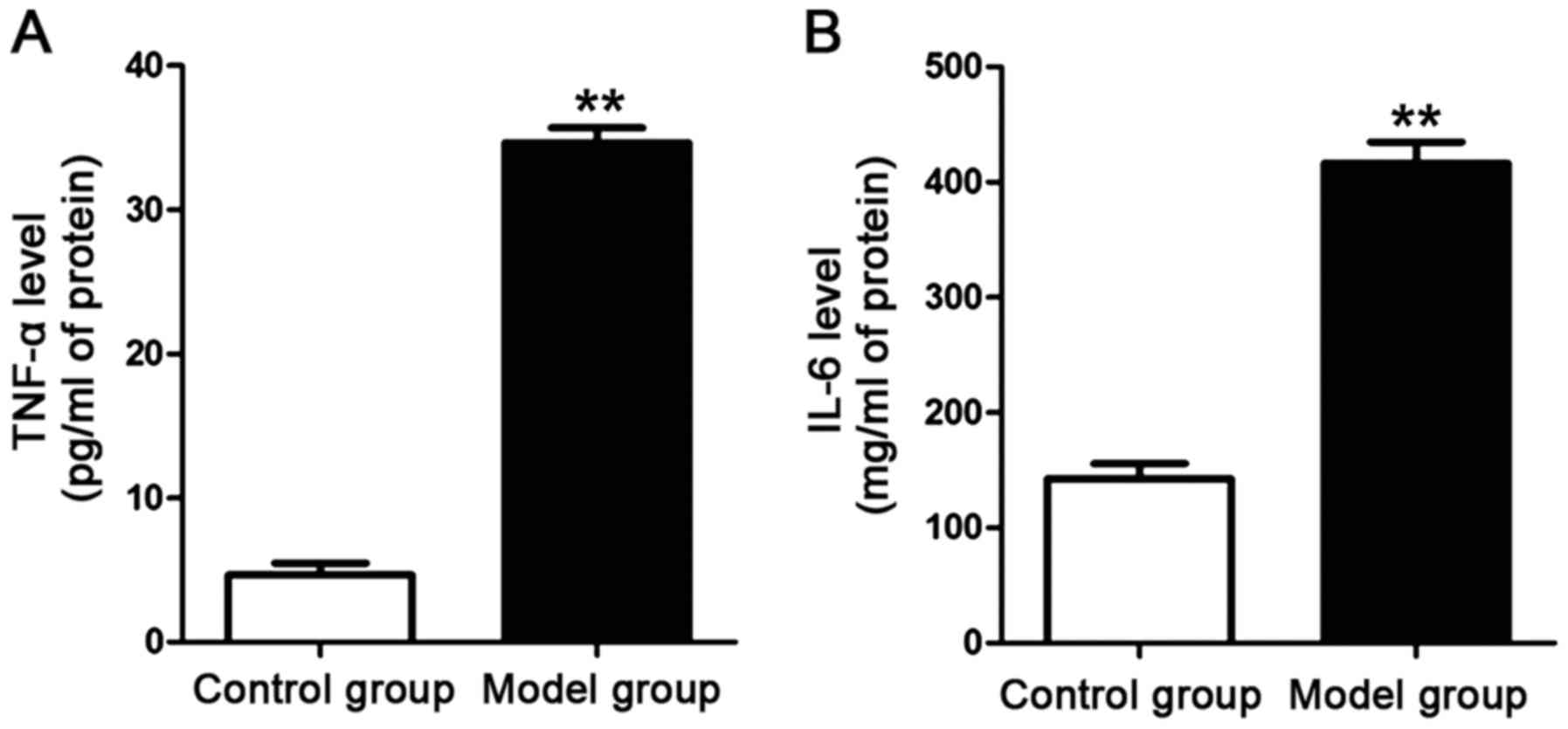
Detection of the contents of
inflammatory factors in the plasma of the rats
The plasma was obtained via isolation after the rat
model was established. ELISA kits for IL-6 and TNF-α were used to
detect the contents of IL-6 and TNF-α in the plasma of the rats in
each group. The results are shown in Fig. 3. The contents of IL-6 and TNF-α in
the plasma of the rats in the model group were significantly higher
than those of the rats in the control group. The differences were
statistically significant (P<0.01).
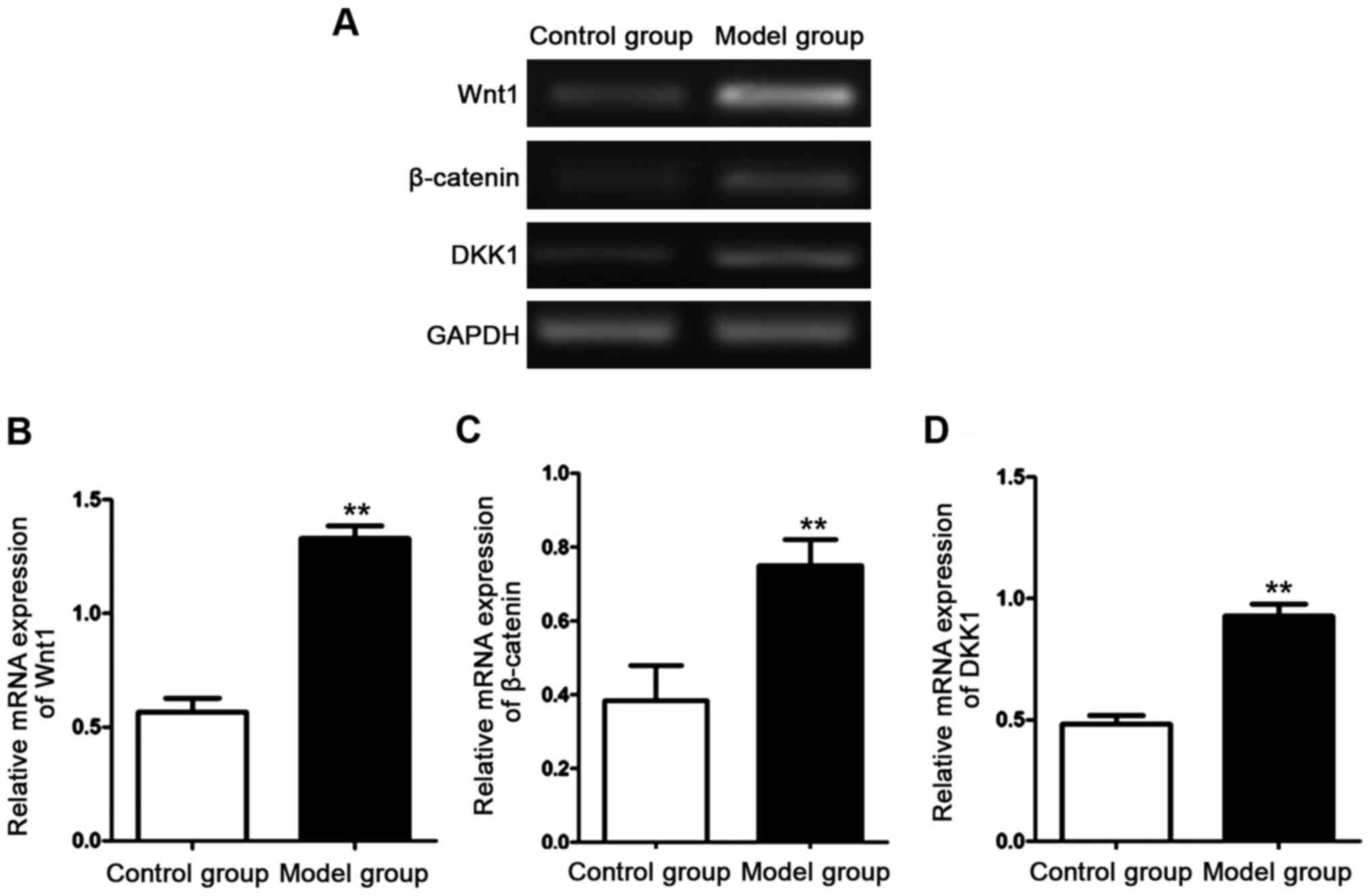
Detection of mRNA expression
levels
The mRNA expression levels of Wnt1, β-catenin and
DKK1 in the aorta of the rats in each group were detected with
semi-quantitative RT-PCR after RNA in the aortic tissues of the
rats in each group was extracted. The results are shown in Fig. 4. The mRNA expression levels of Wnt1,
β-catenin and DKK1 in the aorta of the rats in the model group were
significantly higher than those of the rats in the control group.
The differences were statistically significant (P<0.01).
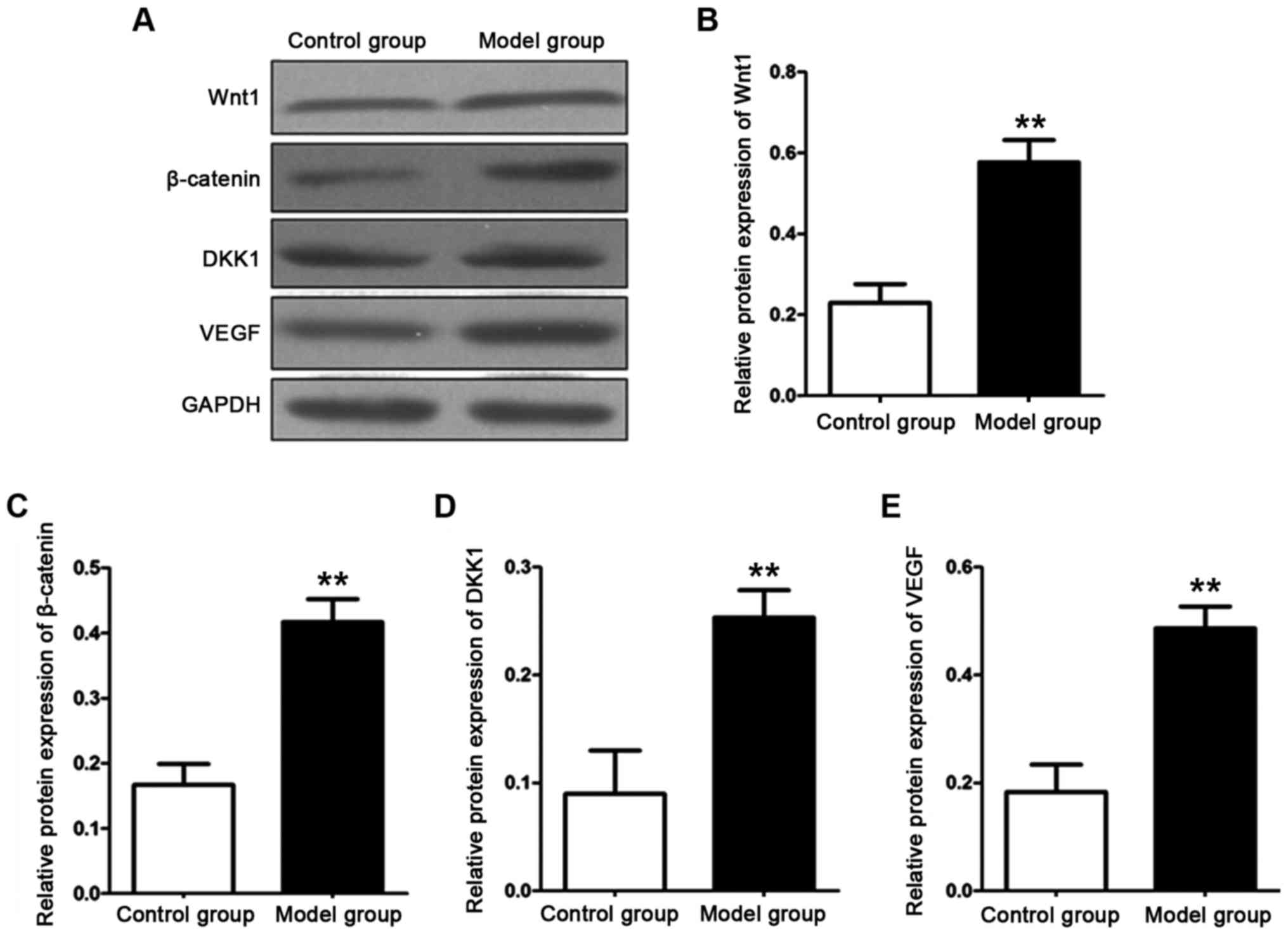
Detection of relative protein
expression levels
The protein expression levels of Wnt, β-catenin,
DKK1 and VEGF in the aorta of the rats in each group were detected
with western blot analysis after total protein in the aortic
tissues of the rats in each group was extracted. The results are
shown in Fig. 5. The protein
expression levels of Wnt1, β-catenin, DKK1 and VEGF in the aorta of
the rats in the model group were significantly higher than those of
the rats in the control group. The differences were statistically
significant (P<0.01).
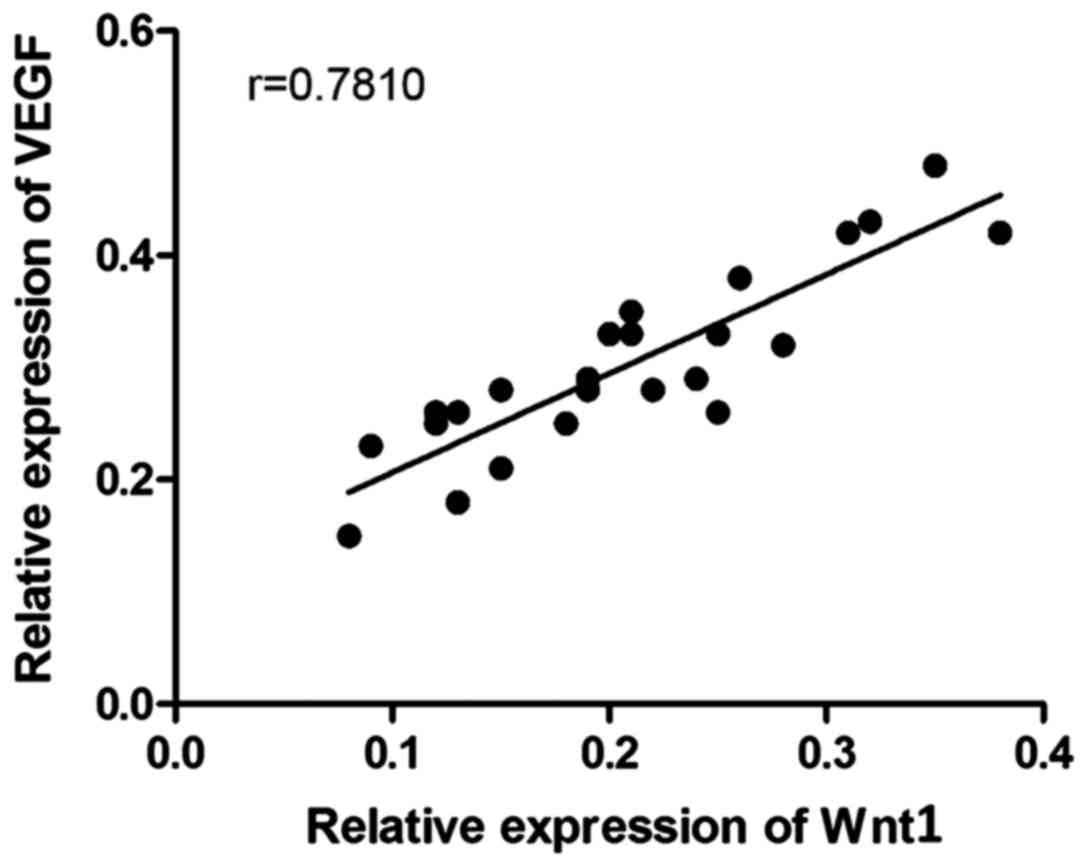
Correlation analysis
The correlation analysis between the expression
level of Wnt1 and that of VEGF of the rats in the control group and
the model group was conducted. The result is shown in Fig. 6. The protein expression level of Wnt1
in the aorta of the rats was positively correlated with that of
VEGF (P<0.01, r=0.7810).
Discussion
Atherosclerosis is the pathogenetic basis of a
variety of cardiovascular diseases. It shows a dynamic evolution.
Vascular wall injury is caused by the precipitation of the lipid in
the vascular wall. The cells of the vascular smooth muscle continue
to proliferate under the combined action of various reasons and
continue to transfer to the site where the vascular medial plaque
is formed, thus aggravating the formation of AS (9,10).
Vascular intimal injury caused by AS will result in the release of
various inflammatory factors, which provides necessary
environmental factors for the continuous development and
deterioration of AS (11,12). The formation of AS will lead to
ischemia or necrosis of the tissues whose blood and oxygen are
supplied by this part, and the body will establish collateral
circulation in case of ischemia or necrosis of the tissues to
resist poor environments. Some of the blood vessels show
compensated regeneration (13). The
process is called angiogenesis which occurs in various
cardiovascular diseases (14,15).
Recently, a large number of studies found that Wnt signaling
pathway is involved in the proliferation and apoptosis process of a
variety of cells, affecting the occurrence and development of
multiple physiological processes. Studies have shown that the
protein expression levels of β-catenin and DKK1 are significantly
increased during the formation of vascular endothelial cells, which
indicated that Wnt signaling pathway participates in regulating the
formation of vascular endothelial cells (16). A study (17) showed that the endothelial injury will
activate Wnt signaling pathway, resulting in increased expression
of β-catenin protein, further aggravating the infiltration of
inflammatory factors, and promoting the formation of vascular
plaque.
AS model of rats was established in this study; the
detection showed that the contents of TC, TG and LDL-C in the serum
of the rats in AS model were increased significantly, the vascular
wall of the rats in the model group was thickened obviously, and
that the ratio of intima thickness to media thickness was increased
significantly. The aforementioned results indicated that the AS
model of rats was established successfully in this study. It was
previously (18) shown that the
reduction of LDL-C can effectively prevent the occurrence of
multiple cardiovascular diseases. The regulation of the lipid level
in the serum is an important means for the prevention and treatment
of AS clinically. The occurrence of AS is accompanied with the
release of various inflammatory factors. IL-6 and TNF-α, as common
pro-inflammatory factors, are generated by the activation of T
cells and fibroblasts. This study revealed that the contents of
IL-6 and TNF-α in the plasma of the rats in the model group were
significantly increased. The release of inflammatory factors
induced by AS provided important environmental basis for the
activation of Wnt signaling pathway. This study also showed that
mRNA expression levels of Wnt signaling pathway-related proteins in
the aortic blood vessels of the rats in AS model group were
increased remarkably, and the expression levels of corresponding
proteins were also higher than those of normal rats. The above
results indicated that the activation of Wnt signaling pathway in
AS model is involved in the regulation of AS progression. The
activation of Wnt signaling pathway will result in the generation
of a large amount of free β-catenin, cause dysfunction of vascular
endothelium, and destroy the barrier function of vascular
endothelium, thus further aggravating the development of AS
(19). As an important protein of
angiogenesis, VEGF plays an irreplaceable role in labeling
angiogenesis (20). This study
suggested that the expression level of VEGF of the rats in AS model
group was increased significantly, which was positively correlated
with the expression level of Wnt1. The above results suggested that
Wnt signaling pathway also has the effect of regulating
angiogenesis, but how it regulates the expression of angiogenic
proteins and how it influences angiogenesis were not researched
deeply in this study.
In conclusion, AS can result in increased level of
blood lipid, promote the release of inflammatory factors, and
activate Wnt signaling pathway in the aorta. The activation of Wnt
signaling pathway can also aggravate the progression of AS and
promote angiogenesis, but the specific molecular mechanism needs to
be studied further.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL conceived and designed the study, and drafted the
manuscript. JD collected, analyzed and interpreted the experimental
data, and revised the manuscript critically for important
intellectual content. Both authors read and approved the final
study.
Ethics approval and consent to
participate
The study was approved by the Animal Ethics
Committee of Fujian Medical University Animal Center (Fujian,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dolan H, Crain B, Troncoso J, Resnick SM,
Zonderman AB and Obrien RJ: Atherosclerosis, dementia, and
Alzheimer disease in the Baltimore Longitudinal Study of Aging
cohort. Ann Neurol. 68:231–240. 2010.PubMed/NCBI
|
|
2
|
Yamamoto S, Zhong J, Yancey PG, Zuo Y,
Linton MF, Fazio S, Yang H, Narita I and Kon V: Atherosclerosis
following renal injury is ameliorated by pioglitazone and losartan
via macrophage phenotype. Atherosclerosis. 242:56–64. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rose H, Low H, Dewar E, Bukrinsky M, Hoy
J, Dart A and Sviridov D: The effect of HIV infection on
atherosclerosis and lipoprotein metabolism: A one year prospective
study. Atherosclerosis. 229:206–211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alberts-Grill N, Denning TL, Rezvan A and
Jo H: The role of the vascular dendritic cell network in
atherosclerosis. Am J Physiol Cell Physiol. 305:C1–C21. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bauer AJ and Martin KA: Coordinating
regulation of gene expression in cardiovascular disease:
Interactions between chromatin modifiers and transcription factors.
Front Cardiovasc Med. 4:192017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gajjala PR, Sanati M and Jankowski J:
Cellular and molecular mechanisms of chronic kidney disease with
diabetes mellitus and cardiovascular diseases as its comorbidities.
Front Immunol. 6:3402015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garcia-Martín A, Reyes-Garcia R,
García-Fontana B, Morales-Santana S, Coto-Montes A, Muñoz-Garach M,
Rozas-Moreno P and Muñoz-Torres M: Relationship of Dickkopf1 (DKK1)
with cardiovascular disease and bone metabolism in Caucasian type 2
diabetes mellitus. PLoS One. 9:e1117032014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Register TC, Hruska KA, Divers J, Bowden
DW, Palmer ND, Carr JJ, Wagenknecht LE, Hightower RC, Xu J, Smith
SC, et al: Plasma Dickkopf1 (DKK1) concentrations negatively
associate with atherosclerotic calcified plaque in
African-Americans with type 2 diabetes. J Clin Endocrinol Metab.
98:E60–E65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Witztum JL and Lichtman AH: The influence
of innate and adaptive immune responses on atherosclerosis. Annu
Rev Pathol. 9:73–102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Geng YJ and Jonasson L: Linking immunity
to atherosclerosis: Implications for vascular pharmacology - a
tribute to Göran K. Hansson. Vascul Pharmacol. 56:29–33. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taghavie-Moghadam PL, Butcher MJ and
Galkina EV: The dynamic lives of macrophage and dendritic cell
subsets in atherosclerosis. Ann N Y Acad Sci. 1319:19–37. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Patel KM, Strong A, Tohyama J, Jin X,
Morales CR, Billheimer J, Millar J, Kruth H and Rader DJ:
Macrophage sortilin promotes LDL uptake, foam cell formation, and
atherosclerosis. Circ Res. 116:789–796. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fontes JA, Rose NR and Čiháková D: The
varying faces of IL-6: From cardiac protection to cardiac failure.
Cytokine. 74:62–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Orbay H, Hong H, Zhang Y and Cai W:
PET/SPECT imaging of hindlimb ischemia: Focusing on angiogenesis
and blood flow. Angiogenesis. 16:279–287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bogdanovich S, Kim Y, Mizutani T, Yasuma
R, Tudisco L, Cicatiello V, Bastos-Carvalho A, Kerur N, Hirano Y,
Baffi JZ, et al: Human IgG1 antibodies suppress angiogenesis in a
target-independent manner. Signal Transduct Target Ther. 1:458–463.
2016. View Article : Google Scholar
|
|
16
|
Xu Y, Chen B, George SK and Liu B:
Downregulation of MicroRNA-152 contributes to high expression of
DKK1 in multiple myeloma. RNA Biol. 12:1314–1322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mozos I and Marginean O: Links between
vitamin D deficiency and cardiovascular diseases. BioMed Res Int.
2015:1092752015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Taghavie-Moghadam PL, Gjurich BN, Jabeen
R, Krishnamurthy P, Kaplan MH, Dobrian AD, Nadler JL and Galkina
EV: STAT4 deficiency reduces the development of atherosclerosis in
mice. Atherosclerosis. 243:169–178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Caliceti C, Nigro P, Rizzo P and Ferrari
R: ROS, Notch, and Wnt signaling pathways: Crosstalk between three
major regulators of cardiovascular biology. BioMed Res Int.
2014:3187142014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou H, Binmadi NO, Yang YH, Proia P and
Basile JR: Semaphorin 4D cooperates with VEGF to promote
angiogenesis and tumor progression. Angiogenesis. 15:391–407. 2012.
View Article : Google Scholar : PubMed/NCBI
|