Introduction
Kimura disease is a rare lymphoproliferative disease
of unknown origin that occurs in the head and neck (1). A majority of Kimura disease cases have
been reported in China, Japan and other Southeast Asian countries
and rarely in European countries (1,2). The
cause of Kimura disease is unclear, but it may be associated with
autoimmunity, insect bites or infections and allergies caused by
parasites (3). As Kimura disease
often results in an increase of lesions, peripheral blood
eosinophils and elevated serum immunoglobulin E (IgE) levels, it is
considered the result of inflammation caused by immune dysfunction
(4). IgE-mediated type I allergy
caused by abnormal regulation of CD4+ cells may describe
the major pathogenic mechanism (5).
A majority of reports on Kimura disease focus on
clinical findings from China, Korea and Southeast Asian countries,
with few computerized tomography (CT) reports published (3,4).
Gopinathan and Tan (6) examined CT
features of 13 patients with Kimura disease and divided Kimura
disease into two categories: Type I and type II. Type I exhibited a
clear boundary and marked homogeneous enhanced nodules. In the
study, lesions were of uniform density and had clear boundaries,
with moderate or marked homogeneous enhancement. The degree of
enhancement in the arterial and venous phase was similar. Type I
Kimura disease nodules had an intact capsule, pointing to lymph
node swelling in the head and neck region, particularly in the
parotid gland. Type II Kimura disease exhibited unclear boundaries
around nodules and mild inhomogeneous enhancement with an uneven
structure. In addition, increased hospitalization times were
recorded for these patients. Gopinathan and Tan (6) reported 30.70% of cases with diffuse
masses. Boundaries of these lesions were unclear, demonstrated an
ambiguous fat layer and increased density. There was no obvious
cystic degeneration, necrosis or calcification, and lesions
exhibited moderate heterogeneous enhancement. No obvious capsules
were observed in Type II nodules. In the study, boundaries of
patients with type I and type II Kimura disease nodules and lesions
were unclear. In these cases, lesions were characterized by a large
amount of eosinophils and inflammatory cell infiltration inside and
outside of capsules. Gopinathan and Tan (6) concluded that further studies are needed
to determine whether an unclear boundary represented the transition
from type I to type II Kimura disease nodules.
Clinical symptoms of Kimura disease include a
painless soft tissue mass, with peripheral lymphadenopathy or
lymphadenectasis in the neck and submandibular region (7). Kimura disease may be misdiagnosed as
malignant tumors based on imaging and clinical symptoms (8). A precise preoperative diagnosis is
important. Postoperative recurrence of Kimura disease is high to
62% (9). Kimura disease is sensitive
to hormone therapy, surgery, radiotherapy, steroid therapy,
intravenous immunoglobulin and cyclosporin A (6). In the current study, CT manifestations
and clinical pathological features of patients with Kimura disease
were analyzed retrospectively to improve the understanding of the
disease.
Materials and methods
General information
Clinical, imaging and pathological data of 12
patients with Kimura disease (males, n=11; females, n=1) diagnosed
by postoperative pathology or open biopsy at Cangzhou Central
Hospital (Cangzhou, China) between May 2011 and May 2015 were
analyzed (Table I). Patients were
aged from 26–71 years, with a mean age of 43.9 years. The course of
the disease ranged from 1 week-20 years. There were 7 patients with
unilateral disease and 5 patients with bilateral disease. A total
of 10 patients held an initial diagnosis. Kimura disease recurred
in 1 patient with a 20-year history of the disease and 1 patient
with a 5-year history of the disease. There were no known causes of
unilateral or bilateral parotid, submandibular painless nodules or
masses in the neck. In all cases, nodules had grown slowly
following the onset of symptoms and initial diagnosis. Pruritus in
the lesion area was exhibited in 1 patient with thickening of the
skin surface and pigmentation. In all patients, blood eosinophilia
exceeded 0.63×109/l [normal count,
0.02–0.52×109/l (10);
Table I]. Terms were defined as
follows: Soft, referring to lymphangioma or abscess; hard,
referring to the hardness of bone tissue and medium, an
intermediate stage.
 | Table I.Clinical imaging and pathological data
of patients with Kimura disease. |
Table I.
Clinical imaging and pathological data
of patients with Kimura disease.
|
|
|
|
| Eosinophil
granulocyte count on admission |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Sex | Age (years) | Clinical
presentation | CT features | Absolute
×109/l | % | Treatment | Diagnostic
method |
|---|
| F | 71 | Painless right
parotid gland mass with pruritus | Diffuse mass of right
parotid gland mass with right VIII, II, | 0.64 | 6.1 | Right parotid
resection, postoperative radiotherapy | Postoperative
pathology I b and IV a lymphadenectasis |
| M | 30 | Painless and
low-growing mass in bilateral face for 20 years | Diffuse mass of
bilateral parotid gland with bilateral VIII, II and I b
lymphadenectasis | 4.33 | 38.1 | Radiotherapy | Open biopsies,
pathology of parotid gland mass under general anesthesia |
| M | 65 | Low-growing right
parotid gland mass for 2 weeks | Right parotid gland
mass with right VIII, II, II, V and IV a lymphadenectasis | 3.04 | 40.2 | Excision of right
parotid gland mass | Postoperative
pathology |
| M | 35 | Painless right neck
mass for 3 months | Bilateral I b, II
and III lymphadenectasis | 0.75 | 11.0 | Excision of right
neck mass | Postoperative
pathology |
| M | 26 | Painless bilateral
face mass for 2 years with more obvious mass in left face | Diffuse mass of
bilateral parotid gland with bilateral I a, I b, II, III, VIII and
Xa lymphadenectasis | 3.17 | 41.3 | Excision of left
parotid gland mass, postoperative radiotherapy | Open biopsies,
pathology of left parotid gland mass under local anesthesia |
| M | 34 | Painless right
parotid gland mass for 15 days | Irregular mass in
the rear of right auricle with right X a, VIII and II
lymphadenectasis | 0.74 | 9.5 | Excision of right
parotid gland mass, postoperative radiotherapy | Postoperative
pathology |
| M | 51 | Painless and
slow-growing mass in right postauricular region for a month | Left I b, II, VIII
and IX lymphadenectasis | 0.8 | 21.1 | Excision of left
parotid gland mass | Postoperative
pathology |
| M | 44 | Painless right face
mass for 5 years; excision of mass was performed 2.5 years ago, new
mass was observed 1 year following surgery | Diffuse mass of
right parotid gland with II, III and V lymphadenectasis | 3.14 | 17.3 | Excision of right
parotid gland mass, postoperative radiotherapy | Postoperative
pathology |
| M | 34 | Right neck mass for
15 days and right face mass for a week | Right VIII, II, I b
and III lymphadenectasis | 1.87 | 22.1 | Excision of right
parotid gland mass and right neck mass, postoperative
radiotherapy | Postoperative
pathology |
| M | 45 | Painless right
submaxillary tumor with progressive enlargement for 1 year | Right I b and
bilateral II lymphadenectasis | 0.63 | 12.4 | Excision of
submaxillary gland and submaxillary tumor | Postoperative
pathology |
| M | 42 | Painless bilateral
face mass for 13 years; reoccurrence of left parotid gland
following excision | Diffuse mass of
bilateral parotid gland with bilateral VIII and II
lymphadenectasis | 2.68 | 23.8 | Excision of
bilateral parotid gland | Postoperative
pathology |
| M | 50 | Left face enclosed
mass for 20 years; excision of mass was performed 10 years ago with
relapse | Subcutaneous
diffuse soft tissue mass of the left cheek with I b
lymphadenectasis | 1.45 | 20.2 | Excision of left
face mass, postoperative radiotherapy | Postoperative
pathology |
CT examination
All patients underwent a CT scan and a
contrast-enhanced scanning of the head and neck using a GE Light
Speed 64-slice spiral CT scanner (tube voltage, 120 kV; tube
current, 250 mA; thickness, 5 mm; layer spacing, 5 mm; pitch,
1.375; conventional 2.5-mm thin-slice reconstruction). Ultravist
was used for contrast-enhanced scanning. A high-pressure injector
was used for intravenous bolus injection of the contrast agent,
with an injection rate of 3.5 ml/sec through the elbow. The degree
of enhancement of the lesions was judged by the difference in CT
values. A value of 10–20 Hounsfield units (HU) was defined as mild
enhancement and a value of 20–40 HU was considered moderate
enhancement. Marked enhancement was at >40 HU.
Pathological examination
Color of the masses, texture and borders were
analyzed. Specimens were fixed with 4% neutral formalin solution at
room temperature for 6 h, followed by conventional dehydration,
paraffin embedding and hematoxylin and eosin staining. The
thickness of the sections was 4 µm. Sections were treated with 1%
periodic acid solution for 10 min at room temperature, washed with
PBS for 5 min, treated with Schiff solution for 10 min and washed
with PBS for further 10 min. Section were stained with hematoxylin
for 1 min at 60°C. Sections were observed by light microscope
(magnification, ×100).
Results
CT features
Out of 12 patients with Kimura disease, 6 cases
involved the parotid gland (3 unilateral and 3 bilateral), 12 cases
involved lymphadenectasis, 1 case involved the rear of the right
auricle and lymphadenectasis, 1 case involved the subcutaneous
diffuse soft tissue mass of the left cheek and lymphadenectasis and
6 cases involved the parotid gland and lymphadenectasis (Table I).
Parotid gland lesions
Pathological tissues of parotid gland lesions were
obtained from postoperative specimen (n=4) or open biopsy (n=2).
The parotid gland volume was increased and a focal nodular or
diffuse mass was observed. In 3 cases, multiple lesions were
present. Lesions had a uniform density and clear boundaries, with
moderate or marked homogeneous enhancement. The degree of
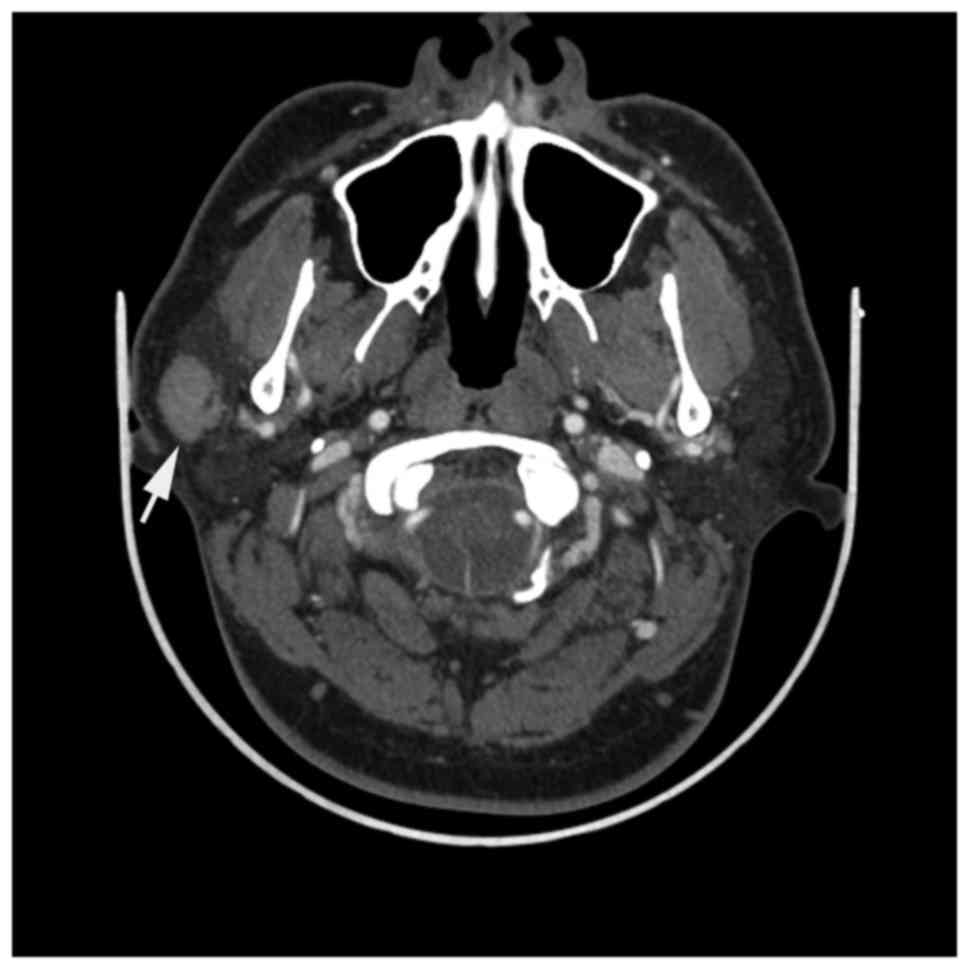
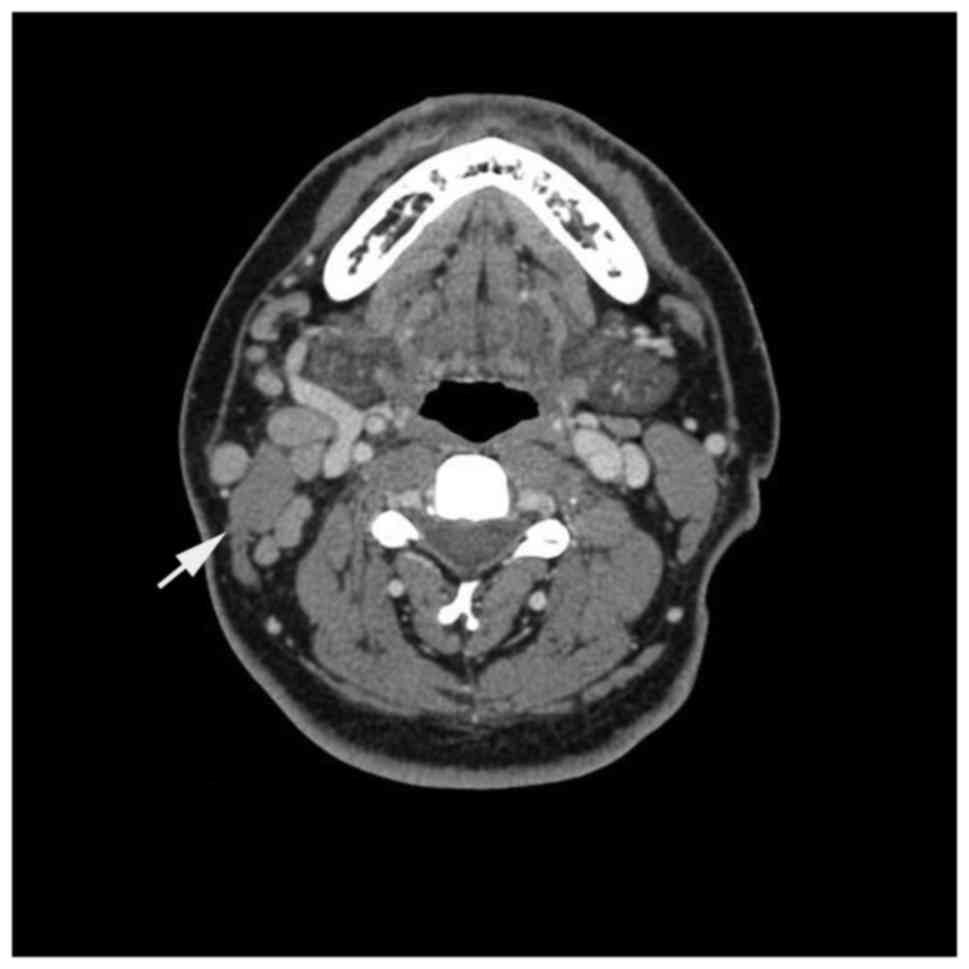
enhancement in arterial and venous phases was similar (Fig. 1). A diffuse mass was present in 5
patients. The boundaries of the lesions were unclear, with an
undefined fat layer and increased density. There was no visible
cystic degeneration, necrosis or calcification and mild or moderate
heterogeneous enhancement was observed (Fig. 2A). Interstitial fibrosis and hyaline
degeneration of the capillary wall were further observed in
Fig. 2A.
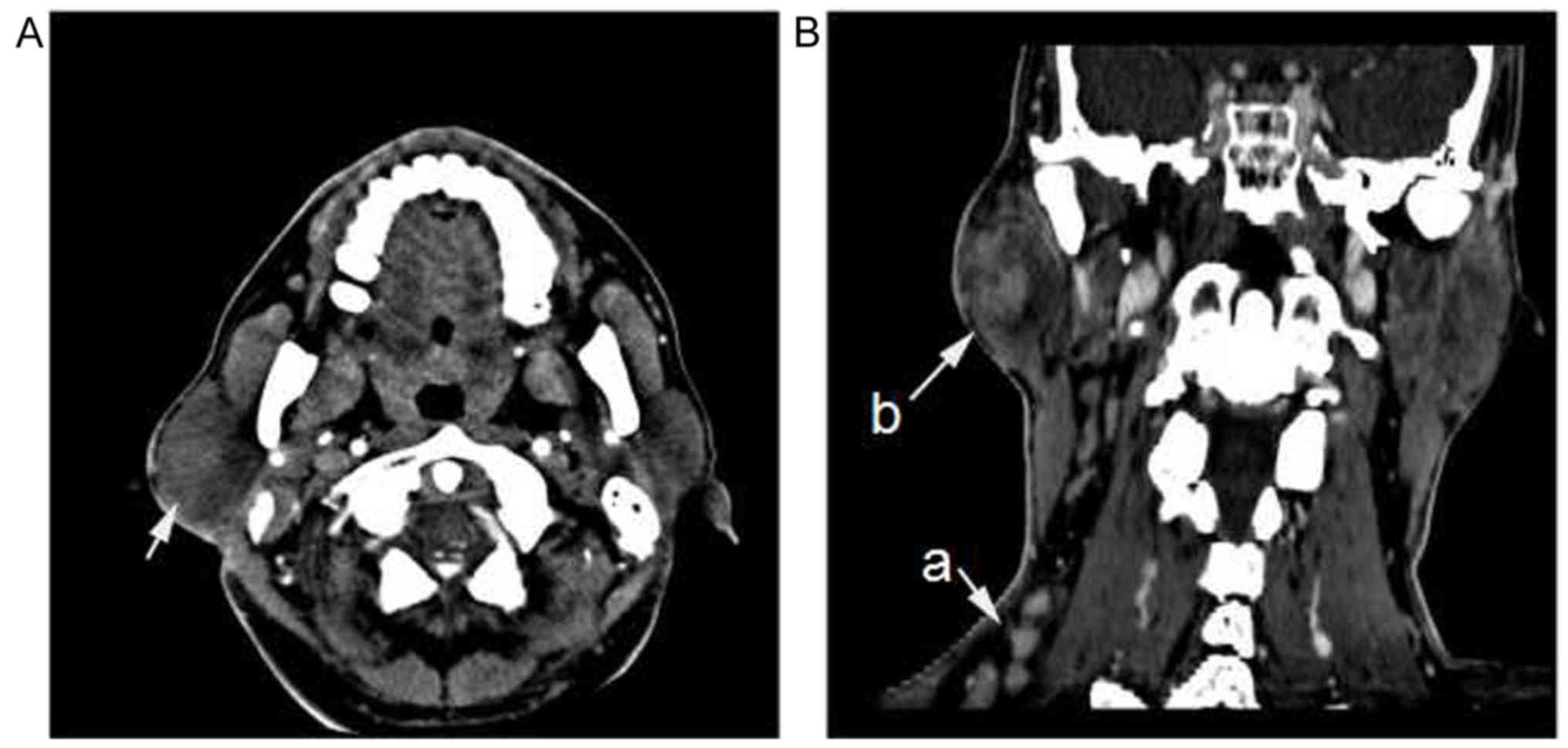
Cervical lymph node lesions
A total of 2 patients had varying numbers of
cervical lymph node lesions in neck region. Pathological tissues of
cervical lymph node lesions were obtained from the postoperative
specimen. In patients with ipsilateral neck masses, the boundaries
of the masses were clear and exhibited homogeneous enhancement,
which was increased compared with cases of parotid lesions
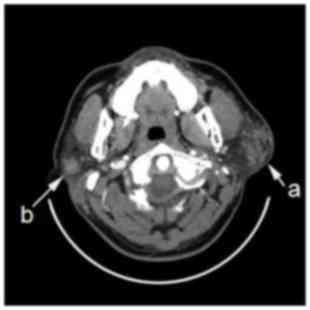
(Fig. 2B). Fig. 3 visualizes unclear boundaries of the
lesions, with moderate or marked homogeneous enhancement. The short
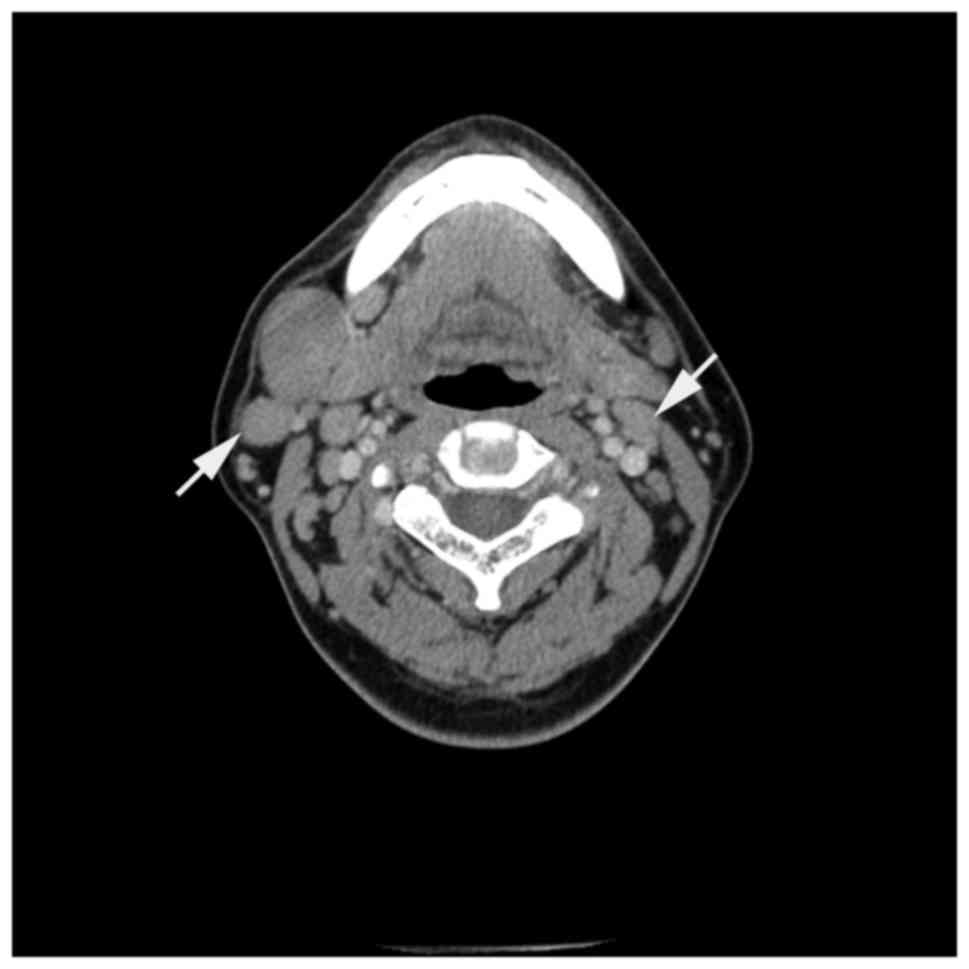
diameter of the largest lymph node was 23.9 mm. Figs. 4 and 5
present the clear boundaries of lesions with a uniform density.
There was no marked liquefaction necrosis, calcification or fusion,
with moderate or marked homogeneous enhancement. There was 1 case
of disease recurrence in a patient with a 10-year history of the
disease. The boundary of the parotid mass of the case of recurrence
was unclear, with mild or moderate heterogeneous enhancement.
General pathological features
All lesions were soft or medium hard and the cut
surface of the lesion was gray or gray-yellow. Lymph nodes had a
complete capsule. In contrast, boundaries of parotid mass lesions
were unclear and had incomplete capsules.
Microscopic analysis features
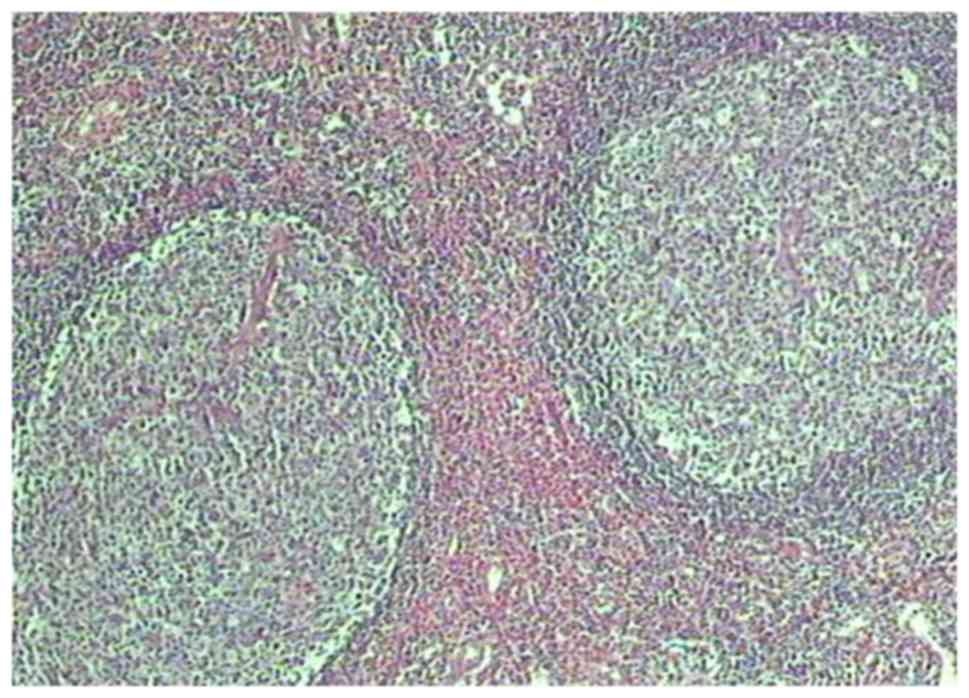
Lesions were mainly composed of lymphoid hyperplasia
and lymphoid follicles and had enlarged germinal centers. They were
characterized by capillary proliferation and inflammatory changes,
with marked eosinophil infiltration. In addition, eosinophilic
microabscesses were observed. As compared with focal nodular
lesions, diffuse mass lesions contained more fibrillar components
and less vascular hyperplasia. Nodular lesions were characterized
by enhanced vascular hyperplasia and fibrillar components. Nodular
lesions revealed a large number of lymphoid follicles and enlarged
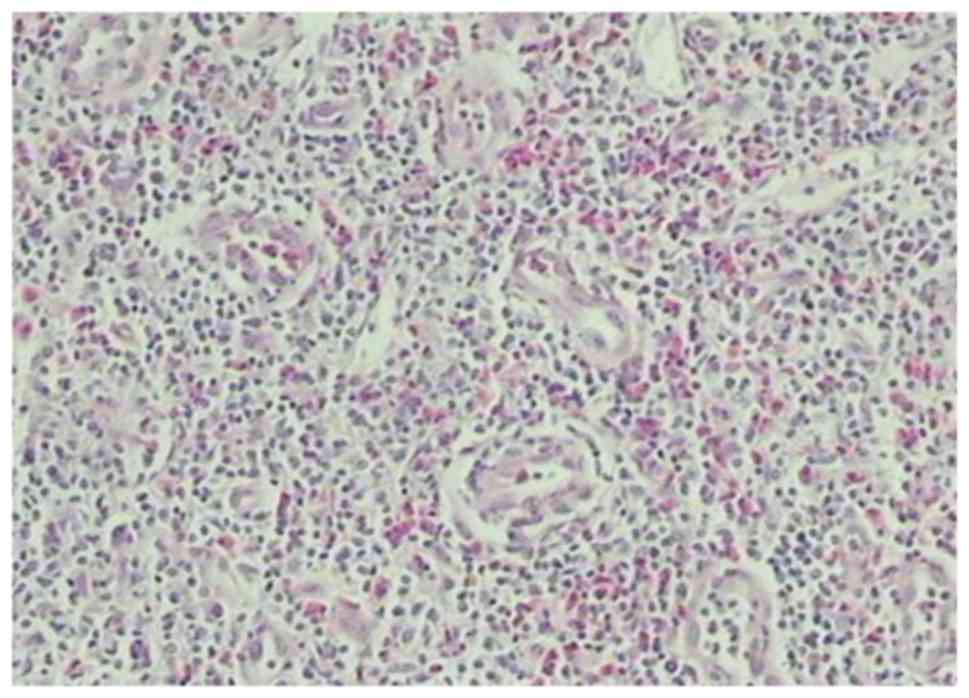
germinal centers (Fig. 6). A large
number of capillary hyperplasia with eosinophilic infiltration was
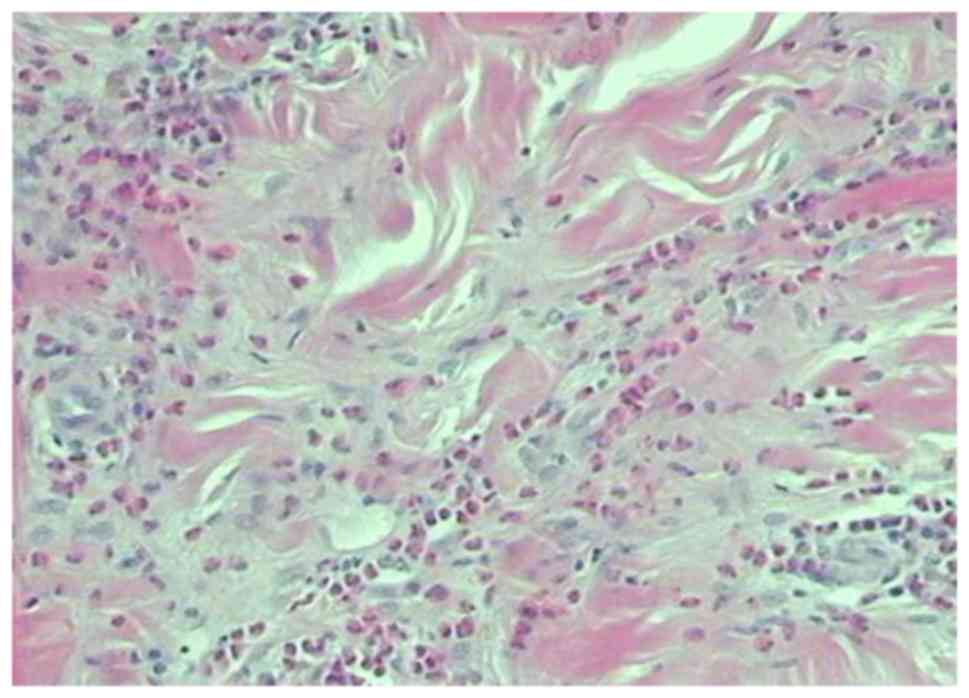
observed in Fig. 7 and in Fig. 8 nodular lesions demonstrated more
fibrillar components with eosinophilic infiltration.
Discussion
Kimura disease is a rare lymphoproliferative disease
of unknown origin. Kimura disease is characterized by
lymphoproliferative formation of lymphoid follicles and
eosinophilic granulomas in soft tissue (11). Kimura disease may occur at any age,
although the peak age is 20–40 years; the majority of cases occur
in middle-aged males (12,13). The male to female ratio has been
reported to be as high as 14:1 (14). Kimura disease primarily occurs in
head and neck regions and may affect major salivary glands and
lymph nodes, especially in the parotid region (15,16).
There are a few reports of Kimura disease cases involving the
groin, limbs, eyelid, tongue, auricle, hard palate or throat
(17,18). The onset age (middle aged, 35–50
years), sex (>90% males) and site of pathological changes, with
a majority in the head and neck regions potentially affecting major
salivary glands and lymph nodes, in the current study were
consistent with the literature (12,13).
Ultrasound-guided core needle biopsy increased
specimen adequacy and diagnosis to reduce the need and dependence
on an onsite cytopathologist (19).
For Kimura disease, magnetic resonance (MR) imaging is useful for
determining lesion morphology, anatomical distribution, enhancement
pattern and degree of intralesional vascularity (20). It has been reported that well-defined
nodular masses or ill-defined plaque-like infiltrative masses from
CT scans are often associated with lymphadenectasis (21). A majority of lesions is observed in
the parotid gland (22). On MR
images, masses exhibited variable signal intensity and visualized
vascular structures (10). In the
current study, 25.0% of patients (3/12) had bilateral involvement,
which differed from the prevalence of bilateral involvement in
previous report (23,24).
Diagnosis of Kimura disease is based on the analysis
of pathological specimens. Pathological features of Kimura disease
include lymphoid tissue hyperplasia; lymphoid follicle formation;
an active follicular germinal center; hyaline degeneration;
infiltration of tissue, plasma and mast cells; and lymphatic sinus
fibrosis (25,26). Characteristic changes include
infiltration of mature eosinophils in follicular, capsular and
extracapsular regions and formation of eosinophilic microabscesses
(27). Takeishi et al
(28) reported that a majority of
neck lumps consisted of fibrous tissue hyperplasia in patients with
long disease course. As the degree of enhancement is reduced in the
presence of fewer vessels in neck region, the current study
speculated that different degrees of enhancement in patients with
Kimura disease may be associated with the degree of proliferation
of fibrous tissue and blood vessels. In a follow-up study of
patients with eosinophilic lymphogranulomas neck nodules, Lim et
al (29) reported that
lesion-enhanced areas exhibited a trend toward gradual narrowing.
Such narrowing may be associated with fibrosis and sclerosis of
postcapillary venules. In the present study, CT and pathological
analysis revealed capillary proliferation within lesions in
patients with a short disease course. It was considered that this
proliferation may be the pathological basis of homogeneous
enhancement of the lesions. Capillary proliferation was not
observed in patients with a long disease course (≥10 years).
Interstitial fibrosis and hyaline degeneration of the capillary
wall were further observed. These features may be the pathological
basis of mild to moderate enhancement in head and neck lesions.
Dynamic enhanced CT revealed characteristics of progressive
strengthening, indicating vascular proliferation and fiber
components in lesions. In 1 patient with neck lymph and parotid
gland involvement, the degree and mode of enhancement were
differed. In this patient, as the course of the disease progressed,
the degree of vascular proliferation and interstitial fibrosis in
the parotid gland and lymph nodes were inconsistent. Further
studies are required to clarify the underlying mechanisms.
There are many diseases of the parotid gland,
including benign tumors, malignant tumors and inflammatory lesions,
making a differential diagnosis of parotid gland nodules difficult.
A careful analysis of clinical imaging features is necessary for an
accurate diagnosis (29).
Pleomorphic adenomas and adenolymphomas are the most common benign
tumors. Pleomorphic adenomas are often single, with delayed
enhancement (30). Adenolymphomas
are usually found in elderly males. Adenolymphomas are often
discovered during a superficial parotidectomy and exhibit marked
enhancement in early stages. The density decreases rapidly in cases
of delayed diagnosis and density may be heterogeneous in some cases
(30). In the present study,
low-density areas were observed in lesions. In addition, cervical
lymph nodes were not swollen, which was different from low-density
Kimura disease involving cervical lymph nodes. Diagnosis may be
combined with clinical manifestations and elevated eosinophils in
peripheral blood.
Hemorrhagic necrosis and cystic degeneration are
common in lesions in primary malignant tumors of the parotid gland
and neck metastases (31). In
contrast, liquefaction necrosis is uncommon in Kimura disease.
Bilateral lymph nodes are swollen in cases of lymphoma and lymph
node fusion is frequently observed. It may be differentiated from
Kimura disease, which exhibits a clear boundary and no marked
fusion trend (32). In addition,
patients with lymph node fusion, calcification and local necrosis
present general symptoms, including fever and fatigue (33). Using a combination of clinical
examinations and molecular laboratory tests, Kimura disease may be
identified more easily.
According to previous research, Kimura disease
outcomes are positive following comprehensive treatment, which may
consist of surgery, radiotherapy and adrenal hormone therapy
(34). Diagnosis of Kimura disease
strongly relies on pathology and imaging features. Kimura disease
is commonly found in head and neck regions of middle-aged males,
who exhibit a painless mass, with or without itchy skin and
pigmentation, in addition to an increased peripheral blood
eosinophil count.
Diagnosis of Kimura disease in head and neck regions
may be improved based on lesions with clear or unclear boundaries,
homogeneous or heterogeneous enhancement with or without
lymphadenectasis and by the presence of peripheral blood
eosinophilia. All of which describe the pathological basis for the
diagnosis of Kimura disease. Future research aims to increase the
number of patients to allow a more systematic approach for
diagnosis of Kimura disease.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ and LY designed the study, recruited patients,
analyzed data and drafted the manuscript. W-Z, J-NM and C-QZ
collected the cases, analyzed the data and revised the manuscript.
All authors reviewed and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Ethics
Committee of Cangzhou Central Hospital and all patients provided
written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gao Y, Chen Y and Yu GY: Clinicopathologic
study of parotid involvement in 21 cases of eosinophilic
hyperplastic lymphogranuloma (Kimura's disease). Oral Surg Oral Med
Oral Pathol Oral Radiol Endod. 102:651–658. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lanjewar DN, Bhosale A and Iyer A:
Spectrum of dermatopathologic lesions associated with HIV/AIDS in
India. Indian J Pathol Microbiol. 45:293–298. 1996.
|
|
3
|
de Castro HA Jr, Lasmar MT, de Souza EA,
Figueiredo JA, Nogueira BD and Tardelli FC: Renal epitelial
neoplasia associated with Kimura disease. Arch Esp Urol.
63:547–549. 2010.(In English, Spanish). PubMed/NCBI
|
|
4
|
Nonaka M, Sakitani E, Ono E, Yamamura Y,
Seo Y, Shibata N, Pawankar R and Yoshihara T: Basophils are
increased and express increased levels of interleukin-4 in the
parotid lesions of kimura disease. Asia Pac Allergy. 7:221–226.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nonaka M, Sakitani E and Yoshihara T:
Anti-IgE therapy to Kimura's disease: A pilot study. Auris Nasus
Larynx. 41:384–388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gopinathan A and Tan TY: Kimura's disease:
Imaging patterns on computed tomography. Clin Radiol. 64:994–999.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang Y, Hua Q, Ren J, Zeng F, Sheng J,
Liao H, Zhang Z and Guan H: Eosinophilic hyperplastic
lymphogranuloma: Clinical diagnosis and treatment experience of 41
cases. Am J Otolaryngol. 38:626–629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsuo T, Tanaka T and Kinomura M:
Nephrotic syndrome during the tapering of oral steroids after
pathological diagnosis of Kimura disease from a lacrimal gland
mass: Case report and review of 10 Japanese patients. J Clin Exp
Hematop. 57:147–152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang R, Ban XH, Mo YX, Lv MM, Duan XH,
Shen J, Li JP, Liu XW and Xie CM: Kimura's disease: The CT and MRI
characteristics in fifteen cases. Eur J Radiol. 80:489–497. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin YY, Jung SM, Ko SF, Toh CH, Wong AM,
Chen YR, Chan SC, Cheung YC and Ng SH: Kimura's disease: Clinical
and imaging parameters for the prediction of disease recurrence.
Clin Imaging. 36:272–278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang DY, Mao JH, Zhang Y, Gu WZ, Zhao SA,
Chen YF and Liu AM: Kimura disease: A case report and review of the
chinese literature. Nephron Clin Pract. 111:c55–c61. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen C, Chen K, Huang X, Wang K and Qian
S: Concurrent eosinophilia and IgG4-related disease in a child: A
case report and review of the literature. Exp Ther Med.
15:2739–2748. 2018.PubMed/NCBI
|
|
13
|
Iguchi Y, Inoue T, Shimono M, Yamamura T,
Shigematsu T and Takahashi S: Kimura's disease and its relation to
angiolymphoid hyperplasia with eosinophilia: Report of three cases
and review of the literature. J Oral Pathol. 15:132–137. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arshad AR: Kimura's disease of parotid
gland presenting as solitary parotid swelling. Head Neck.
25:754–757. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sawaimul K, Iqbal B and Kambale T:
Kimura's disease embedding radial artery: A very rare presentation.
J Cancer Res Ther. 11:10312015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ye P, Wei T, Yu GY, Wu LL and Peng X:
Comparison of local recurrence rate of three treatment modalities
for kimura disease. J Craniofac Surg. 27:170–174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang H and Zheng Z: One case of parotid
eosinophilic lymphogranuloma. Lin Chung Er Bi Yan Hou Tou Jing Wai
Ke Za Zhi. 28:830–831. 2014.(In Chinese). PubMed/NCBI
|
|
18
|
Zhang JZ, Zhang CG and Chen JM:
Thirty-five cases of Kimura's disease (eosinophilic
lymphogranuloma). Br J Dermatol. 139:542–543. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koh H, Kamiishi N, Chiyotani A, Takahashi
H, Sudo A, Masuda Y, Shinden S, Tajima A, Kimura Y and Kimura T:
Eosinophilic lung disease complicated by Kimura's disease: A case
report and literature review. Intern Med. 51:3163–3167. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Naveed M, Siddiqui AA, Kowalski TE, Loren
DE, Khalid A, Soomro A, Mazhar SM, Yoo J, Hasan R, Yalamanchili S,
et al: A Multicenter comparative trial of a novel EUS-guided core
biopsy needle (SharkCore) with the 22-gauge needle in patients with
solid pancreatic mass lesions. Endosc Ultrasound. 7:34–40. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baba A, Ojiri H, Dogru M, Tanaka Y,
Takahashi S, Mogami T, Kobashi Y, Yamazoe S, Nozawa Y, Ogino N, et
al: An unusual clinical presentation of kimura disease manifesting
with a typical cephalocervical lesion and an atypical subcutaneous
hip mass lesion. Intern Med. 55:1017–1020. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Tang Z, Feng X, Zeng W, Tang W, Wu
L and Jin L: Preliminary study of diffusion-weighted imaging and
magnetic resonance spectroscopy imaging in Kimura disease. J
Craniofac Surg. 25:2147–2151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park SW, Kim HJ, Sung KJ, Lee JH and Park
IS: Kimura disease: CT and MR imaging findings. AJNR Am J
Neuroradiol. 33:784–788. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iwai H, Nakae K, Ikeda K, Ogura M,
Miyamoto M, Omae M, Kaneko T and Yamashita T: Kimura disease:
Diagnosis and prognostic factors. Otolaryngol Head Neck Surg.
137:306–311. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuroda K, Kashiwagi S, Teraoka H,
Kinoshita H, Nanbara M, Noda E, Chikugo T, Hirakawa K and Ohira M:
Kimura's disease affecting the axillary lymph nodes: A case report.
BMC Surg. 17:632017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Uysal IO, Eryilmaz MA, Salk I and
Abasiyanik F: Kimura disease in the parotid gland. J Craniofac
Surg. 22:337–338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu ZF, Yong F, Yu T, Chen YY, Gao Q, Zhou
T, Pan AZ and Wu RH: Different histological subtypes of parotid
gland tumors: CT findings and diagnostic strategy. World J Radiol.
5:313–320. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takeishi M, Makino Y, Nishioka H, Miyawaki
T and Kurihara K: Kimura disease: Diagnostic imaging findings and
surgical treatment. J Craniofacial Surg. 18:1062–1067. 2007.
View Article : Google Scholar
|
|
29
|
Lim WE, Tan NG and Tan KP: Radiological
features in a patient with Kimura's disease. Singapore Med J.
38:125–128. 1997.PubMed/NCBI
|
|
30
|
Bastos JT, Rocha CRMD, Silva PMCE, Freitas
BMP, Cassia FF and Avelleira JCR: Angiolymphoid hyperplasia with
eosinophilia versus Kimura's disease: A case report and a clinical
and histopathological comparison. An Bras Dermatol. 92:392–394.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu L, Chen RG, Li XQ and Wang J: Kimura
disease and epithelioid hemangioma: A comparative study of 12
cases. Zhonghua Bing Li Xue Za Zhi. 34:353–357. 2005.(In Chinese).
PubMed/NCBI
|
|
32
|
Mantsopoulos K, Goncalves M, Koch M, Iro H
and Agaimy A: Submandibular gland pleomorphic adenoma:
Histopathological capsular characteristics and correlation with the
surgical outcome. Ann Diagn Pathol. 34:166–169. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu S, Zhang Z, Bao Q, Su J, Liu M, Shi Q
and Cai W: Diffusion kurtosis imaging in the differential diagnosis
of parotid gland disease and parotid adenolymphoma: Preliminary
results. Dentomaxillofac Radiol. 16:201703882018. View Article : Google Scholar
|
|
34
|
Chen Y, Wang J, Xu F, Zeng C and Liu Z:
Clinicopathological features and prognosis of kimura's disease with
renal involvement in Chinese patients. Clin Nephrol. 85:332–339.
2016. View
Article : Google Scholar : PubMed/NCBI
|