Introduction
Severe acute pancreatitis (SAP) is an exceptionally
grave disorder of the viscera in the abdominal cavity, in which
pancreatic elastases and a variety of other pro-inflammatory
mediators are released into the portal and systemic circulation,
leading to multiple organ failure and high rates of morbidity and
mortality (1,2). Although the precise mechanisms by which
diverse etiological factors induce an attack remain unclear, once
the disease process is initiated, common inflammatory and repair
pathways are invoked (3).
Significant attention has been dedicated to the investigation of
acute lung injury in the setting of SAP, less attention has been
devoted to pancreatitis-associated liver injury. The liver is also
a major organ involved in the systemic inflammatory response and
subsequent distant organ dysfunction observed during SAP. Previous
studies have demonstrated the importance of the liver in the
development of organ damage associated with SAP, specifically its
role in extra pancreatic organ impairment following the release of
macrophage-derived cytokines that play a critical role in the
pathogenesis of pancreatitis and the subsequent inflammatory
response (4–6). In this regard, the liver is a unique
organ because Kupffer cells are the largest population of
fixed-tissue macrophages and have been demonstrated to release
pro-inflammatory cytokines (e.g., TNF-α, IL-6 and IL-18) in
response to local tissue and multiple organ damage (7). Thus, the pathogenesis of SAP-associated
liver injury needs to be elucidated. Multiple studies have
demonstrated that the Janus kinase/signal transducers and
activators of transcription (JAK/STAT) signaling pathway is
activated by pro-inflammatory cytokines, growth factors and
hormones, and shown the beneficial and protective role of JAK2 and
STAT3 in the inflammatory responses (8,9).
Previous studies have indicated that the soluble factors depend on
hepatic STAT3 during systemic inflammation (10). However, the influence of the systemic
inflammatory response on the JAK2/STAT3 signaling pathway in SAP is
not well understood. In addition, the precise mechanisms of
JAK2/STAT3 pathway inhibitors in SAP in relation to the degree of
pancreatic diseases and liver injury remain largely unknown. The
present study established an SAP rat model, examined the effects of
the JAK2/STAT3 signaling pathway and investigated the possible
protective effects of AG490 against SAP in rats.
Materials and methods
Animals
A total of 64 male Sprague-Dawley rats weighing
between 200–250 g were used in the present study. The animals were
purchased from Institutional Animal Care and Use Committee of
Jinling Hospital (Nanjing, China). All operations were performed
according to international guidelines concerning the care and
treatment of experimental animals. Ethical approval for this study
was obtained from the Ethics Committee for Animal Research at
Jinling Hospital.
Animals were housed in standard conditions, in a
temperature-controlled environment with a 12/12 light/dark cycle.
Rats were randomly divided into four groups: Control (n=8), AG490
groups (n=8), SAP groups (n=24) and SAP-AG490 groups (n=24). The
SAP groups and SAP-AG490 groups were randomly divided into 6, 12
and 18 h time-point groups, with 8 rats in each group. The controls
received injections of 0.9% saline (0.2 ml/min) (11). AG490 groups received injections of
AG490 dissolved in DMSO (30 nmol/ml; cat. no. sc-202046;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) via the vena caualis
at a dose of 8 mg/kg with a single administration at 30 min. SAP
groups was induced by 4% sodium taurocholate in distilled water
(0.1 mg/100 g of body weight; Sigma-Aldrich; Merck KGaA) and
retrograde infused at a rate of 20 µl/min using microinfusion pump
continuously. SAP-AG490 groups were injected with AG490 before SAP
model. The experiment was done half an hour with each rat after
laparotomy. The rats were sequentially sacrificed 6, 12 or 18 h
after SAP induction with an intraperitoneal injection of
pentobarbital (200 mg/kg; Shanghai Xinyu Biotech Co., Ltd., China);
blood, liver and pancreatic tissues were collected for subsequent
analysis.
Measurement of amylase (AMY) and liver
enzymes activities
All blood samples were collected from the post cava
of each animal. The serum AMY, alanine aminotransferase (ALT), and
aspartate aminotransferase (AST) activities were determined in
accordance with the manufacturer's protocols (Beijing Leadman
Biochemistry Technology, Co., Ltd., China).
Analysis of TNF-α, IL-6 and IL-18 in
serum
Serum levels of TNF-α (cat. no. RTA00), IL-6 (cat.
no. CATR6000B) and IL-18 (cat. no. DY992; all R&D Systems
Europe, Ltd., Abingdon, UK) concentrations were measured by ELISA
kits in accordance with the manufacturer's protocols. All samples
were determined in duplicate. The pro-inflammatory cytokines levels
were expressed as pg/ml. Serum samples were collected and stored at
−80°C for subsequent biochemical and cytokine assays.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis of TNF-α, IL-6 and
IL-18 in liver tissue
Total RNA samples were isolated TRIzol reagent (cat.
no. 15596-018; Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) from the rat liver tissues in accordance with the
manufacturer's protocols. Complementary DNA (cDNA) synthesis was
performed using the cDNA reverse transcription kit (cat. no.
AR0063; Wuhan Boster Biological Technology, Ltd., Wuhan, China).
The primers (Sangon Biotech Co., Ltd., Shanghai, China) were
synthesized as follows: TNF-α forward, 5′-TCAGTTCCATGGCCCAGAC-3′
and reverse, 5′-GTTGTCTTTGAGATCCATGCCATT-3′; IL-6 forward,
5′-AATCTGCTCTGGTCTTCTTGGAG-3′ and reverse,
5′-GTTGGATGGTCTTGGTCCTTAG-3′; IL-18 forward,
5′-ACTGTACAACCGGAGTAATACGG-3′ and reverse,
5′-TCCATCTTGTTGTGTCCTGG-3′, β-actin forward,
5′-CTGGCACCACACCTTCTACAATG-3′ and reverse,
5′-AATGTCACGCACGATTTCCCGC-3′). The expression of target genes was
normalized to that of β-actin. The samples were amplified in
triplicate, and the results were calculated using the
2−∆∆Ct method.
Histopathological analysis
Pancreas and liver tissues were rinsed and weighed.
The samples were collected from each rat and fixed in 4% neutral
formalin immediately. The tissues were stained with
hematoxylin-eosin (H&E) by a trained pathologist. The
morphologic evaluation of the tissues was performed by optical
microscopy. The histological assessments of tissues were analyzed
for SAP severity based on edema, inflammation and hemorrhage, and
necrosis. The pathological scores of pancreas grading were
performed as described by Schmidt et al (12). Liver pathological scores were
evaluated according to the method reported by Camargo et al
(13).
Immunohistochemical analysis
The method was used to detect expression of JAK2 and
STAT3 proteins in liver tissue. The rats' livers were fixed with 4%
paraformaldehyde for 24 h and then embedded in paraffin. After
being deparaffinized and pretreated in citrate buffer, sections
were incubated with rabbit anti-rat JAK2 (1:200; cat. no. 3230) and
STAT (1:200; cat. no. 330835; both Cell Signaling Technology, Inc.,
Danvers, MA, USA) primary antibodies with PBS at 4°C overnight.
After three washes with PBS, the liver sections were incubated with
isothiocyanate-conjugated goat anti-rabbit antibody (1:200; cat.
no. BA1010; Wuhan Boster Biological Technology, Ltd.) for 15 min at
room temperature and then rinsed in PBS. The negative control for
the immunostaining were incubated with rabbit IgG without the
specific primary antibody. To measure JAK2 and STAT3 proteins, 5
fields were randomly selected and analyzed using Image-Pro Plus
software version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA)
at a magnification, ×200.
Western blot analysis of JAK2, p-JAK2,
STAT3 and p-STAT3 in liver tissues
Western blot analysis was performed to measure the
JAK2, phosphorylated-JAK2 (p-JAK2; cat. no. 3776), STAT3 and
phosphorylated-STAT3 (p-STAT; cat. no. 39145S; both Cell Signaling
Technology, Inc.) protein expression levels in all the groups.
Total proteins were extracted from the frozen liver samples using
ice-cold RIPA buffer containing protease inhibitors. Protein
concentration was quantified by BCA Protein Assay kit (Beyotime
Institute of Biotechnology, Haimen, China). Then the protein
samples were separated by SDS-PAGE and transferred onto PVDF
membranes. The samples were incubated in 5% nonfat milk in TBS with
0.1% Tween for 1 h, and with primary antibodies (rabbit anti-rat
JAK2, p-Jak2, STAT3 and p-STAT3; 1:1,000) overnight at 4°C. The
blots were washed and incubated with appropriate goat anti-rabbit
antibodies and an enhanced chemiluminescence detection assay used
according to the manufacturer's instructions. β-actin was used as a
loading control. All assays were independently repeated three times
to assure reproducibility of results. The positive target bands
were quantified by Quantity One software (Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Statistical analysis
The statistical analyses were performed using the
Statistical Package for the Social Sciences (SPSS) software version
17.0 (SPSS, Inc., Chicago, IL, USA). Data are expressed as means ±
SD. Differences between groups were compared using one-way analysis
of variance (ANOVA) and followed by Bonferroni's multiple
comparison test. P<0.05 was considered to indicate a
statistically significant difference. All possible comparisons were
made between different groups.
Results
The serum levels of AMY, ALT and
AST
No changes in AMY, ALT or AST were observed in the
control and AG490 groups. The activity levels of AMY, ALT and AST
in the SAP groups increased significantly at 6, 12 and 18 h time
points compared with the control and AG490 groups (P<0.05).
Compared with SAP groups, the activity levels of AMY, ALT and AST
were suppressed in the SAP-AG490 groups at different time points
(P<0.05; Table I).
 | Table I.Serum levels of AMY, ALT and AST in
the different groups (U/L, n=8, x±s). |
Table I.
Serum levels of AMY, ALT and AST in
the different groups (U/L, n=8, x±s).
| Group | AMY | ALT | AST |
|---|
| Control | 545.11±23.52 | 24.29±3.17 | 19.23±2.39 |
| AG490 | 558.22±31.76 | 28.36±6.25 | 21.28±3.74 |
| SAP-6 h |
1,247.45±156.37a |
156.64±17.48a |
276.39±27.44a |
| SAP-12 h |
3,478.26±212.58a |
198.49±21.36a |
396.45±30.27a |
| SAP-18 h |
4,461.64±346.17a |
352.23±29.18a |
478.46±31.57a |
| SAP-AG490-6 h |
1,582.16±125.34a |
117.56±16.37a,b |
324.36±23.78a,b |
| SAP-AG490-12 h |
3,201.56±199.29a |
155.82±24.56a,b |
320.69±29.74a,b |
| SAP-AG490-18 h |
3,719.74±287.63a,b |
287.33±19.63a,b |
386.48±28.83a,b |
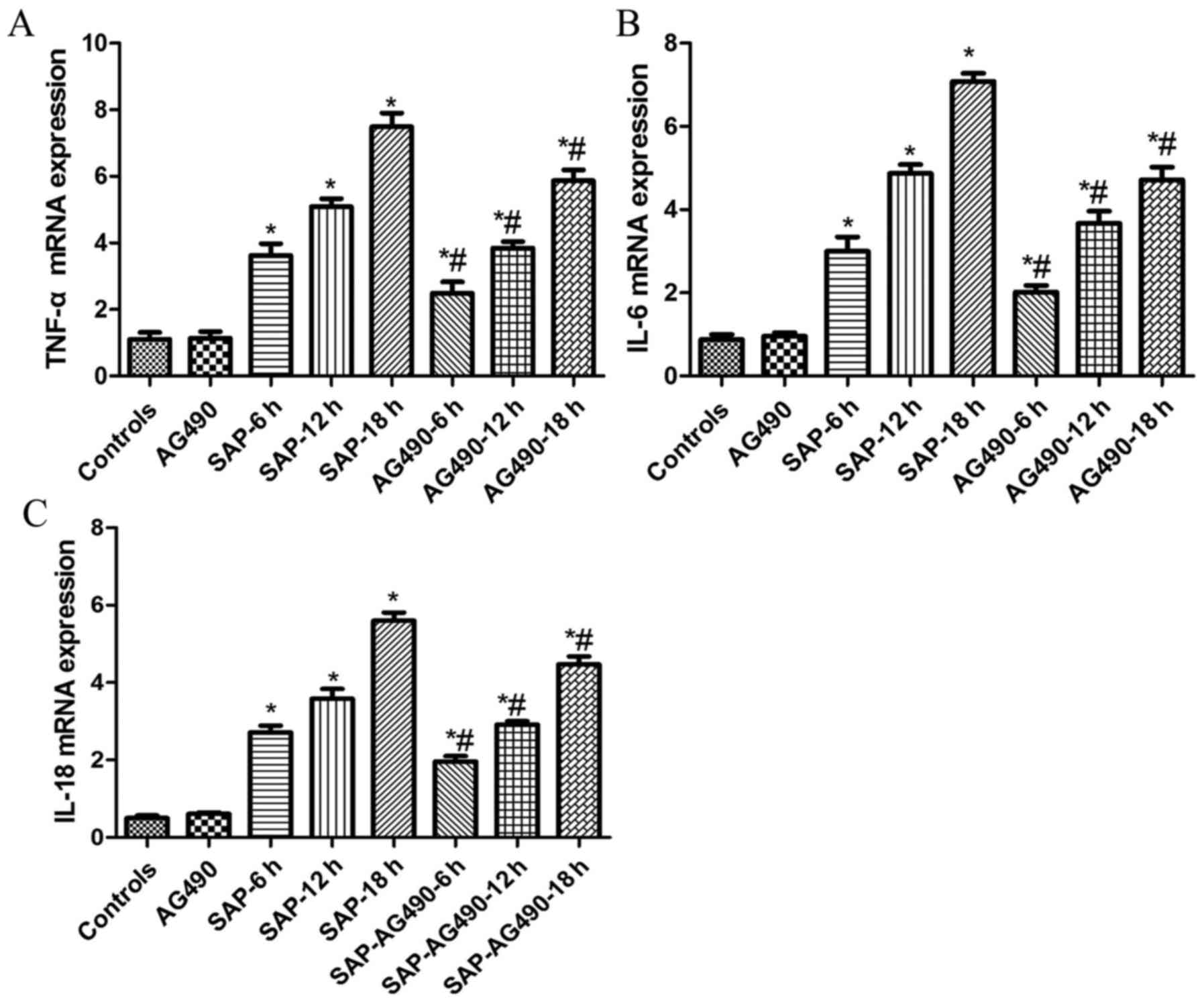
Expression of TNF-α, IL-6 and IL-18
levels in the serum and liver tissues
It is well known that pro-inflammatory cytokines are
important signaling molecules in the development of SAP. To
evaluate the effects of pro-inflammatory cytokine production and to
further determine the mechanisms that underlie reduced injury
following AG490 pretreatment, the expression of TNF-α, IL-6 and
IL-18 in serum and liver tissues were identified by ELISA and
RT-qPCR. The levels of TNF-α, IL-6 and IL-18 were similar in the
control and AG490 groups. As indicated by ELISA and RT-qPCR, these
cytokines increased markedly in the SAP groups compared with the
control and AG490 groups at each time point (P<0.05). It was
then determined whether inhibition of the pro-inflammatory response
by AG490 was mediated through the JAK2/STAT3 signaling pathway.
Compared with SAP groups, these cytokines were suppressed by AG490
pretreatment in the SAP-AG490 groups, but AG490 alone did not have
a significant effect on the cytokine levels in AG490 groups
(P<0.05; Table II, Fig. 1).
 | Table II.Serum levels of TNF-α, IL-6, IL-18 in
the different groups (pg/ml, n=8, x±s). |
Table II.
Serum levels of TNF-α, IL-6, IL-18 in
the different groups (pg/ml, n=8, x±s).
| Group | TNF-α | IL-6 | IL-18 |
|---|
| Control | 60.78±3.81 | 44.23±4.73 | 23.50±2.17 |
| AG490 | 54.12±8.43 | 42.37±5.96 | 24.61±2.99 |
| SAP-6 h |
161.25±10.02a |
337.45±39.64a |
145.05±31.40a |
| SAP-12 h |
253.05±13.24a |
487.47±38.41a |
352.23±20.50a |
| SAP-18 h |
346.78±35.18a |
599.36±51.29a |
468.86±21.32a |
| SAP-AG490-6 h |
150.01±22.25a,b |
310.27±27.68a,b |
122.57±11.63a,b |
| SAP-AG490-12 h |
228.37±16.57a,b |
367.56±38.12a,b |
305.45±27.95a,b |
| SAP-AG490-18 h |
299.13±21.33a,b |
401.92±40.73a,b |
402.37±26.58a,b |
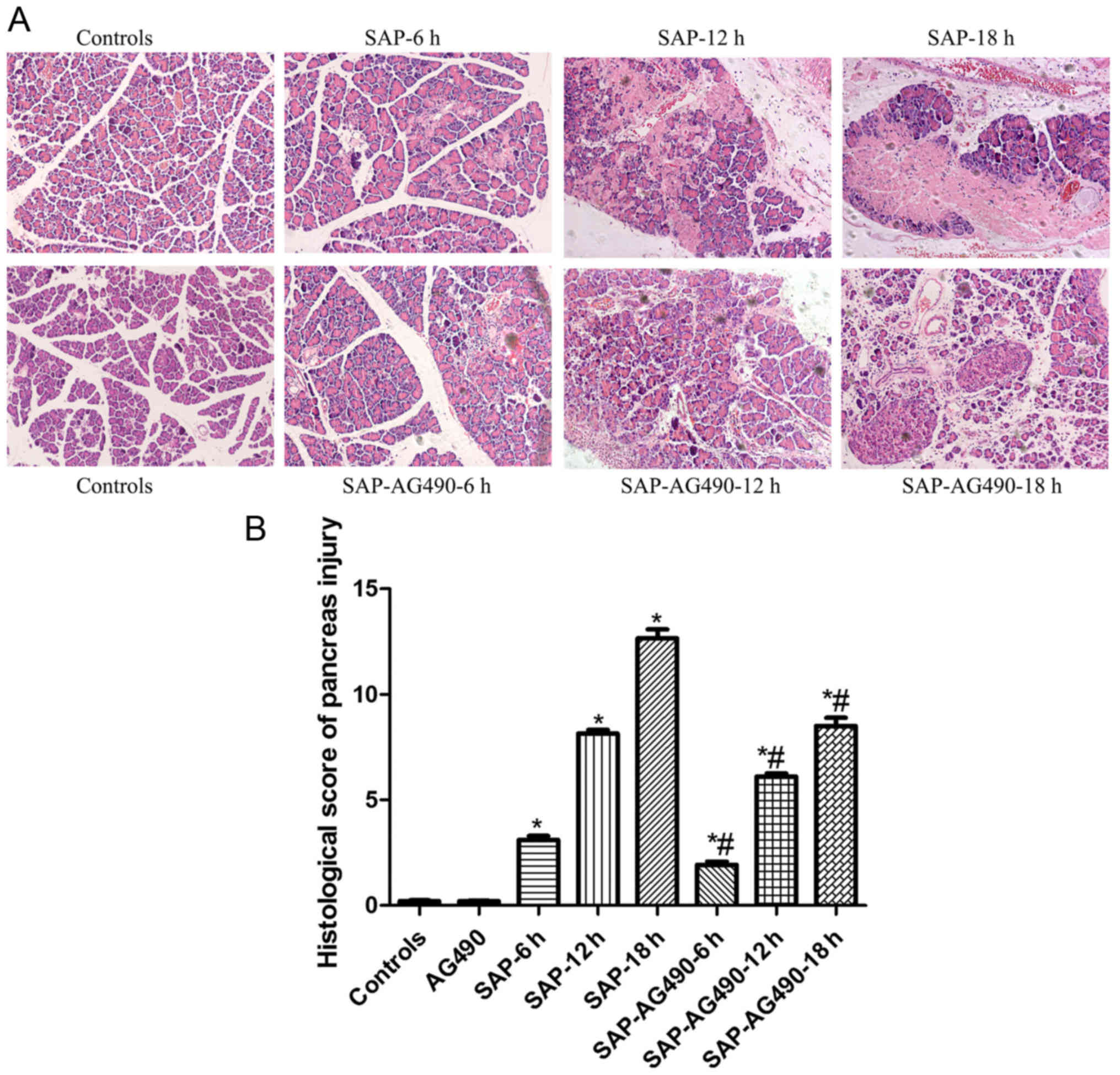
The histopathological alterations of
the pancreas
To determine the changes in the experimental SAP
model, the pathological examination results of the pancreas of rats
at different experimental groups were observed using an optical
microscope. The results indicated that the pancreatic tissue were
normal in the control and AG490 groups (P<0.05). While in the
SAP groups, a large number of inflammatory cells infiltrated the
pancreas. The histopathological changes, pancreatic edema and
substantial necrosis, appeared more pronounced and worsened
significantly with time. There were also differing degrees of
damage in the SAP-AG490 groups, but less severe damage in the SAP
groups (P<0.05; Fig. 2).
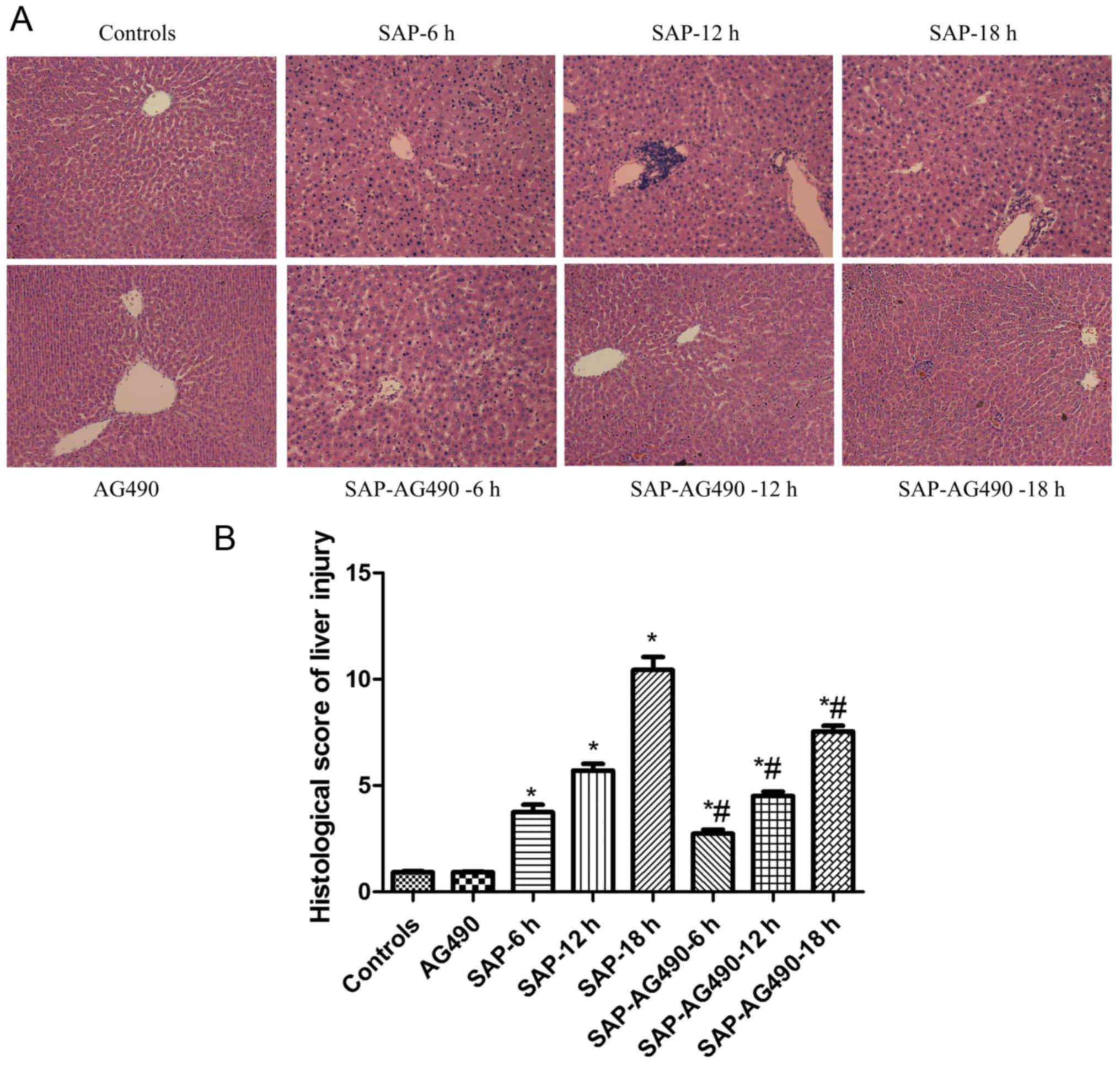
Histopathological alterations of the
liver
The scoring was used to determine the degree of
liver injury. Livers of the control and AG490 groups showed normal
structure. Compared with the control and AG490 groups, liver cell
edema, inflammatory cell infiltration, hepatic spotty necrosis and
coagulation necrosis were detected in the SAP groups. However, the
SAP-AG490 groups exhibited significant attenuation of these
changes, and the edema, necrosis and inflammatory reaction were
significantly reversed compared with the SAP groups (P<0.05;
Fig. 3).
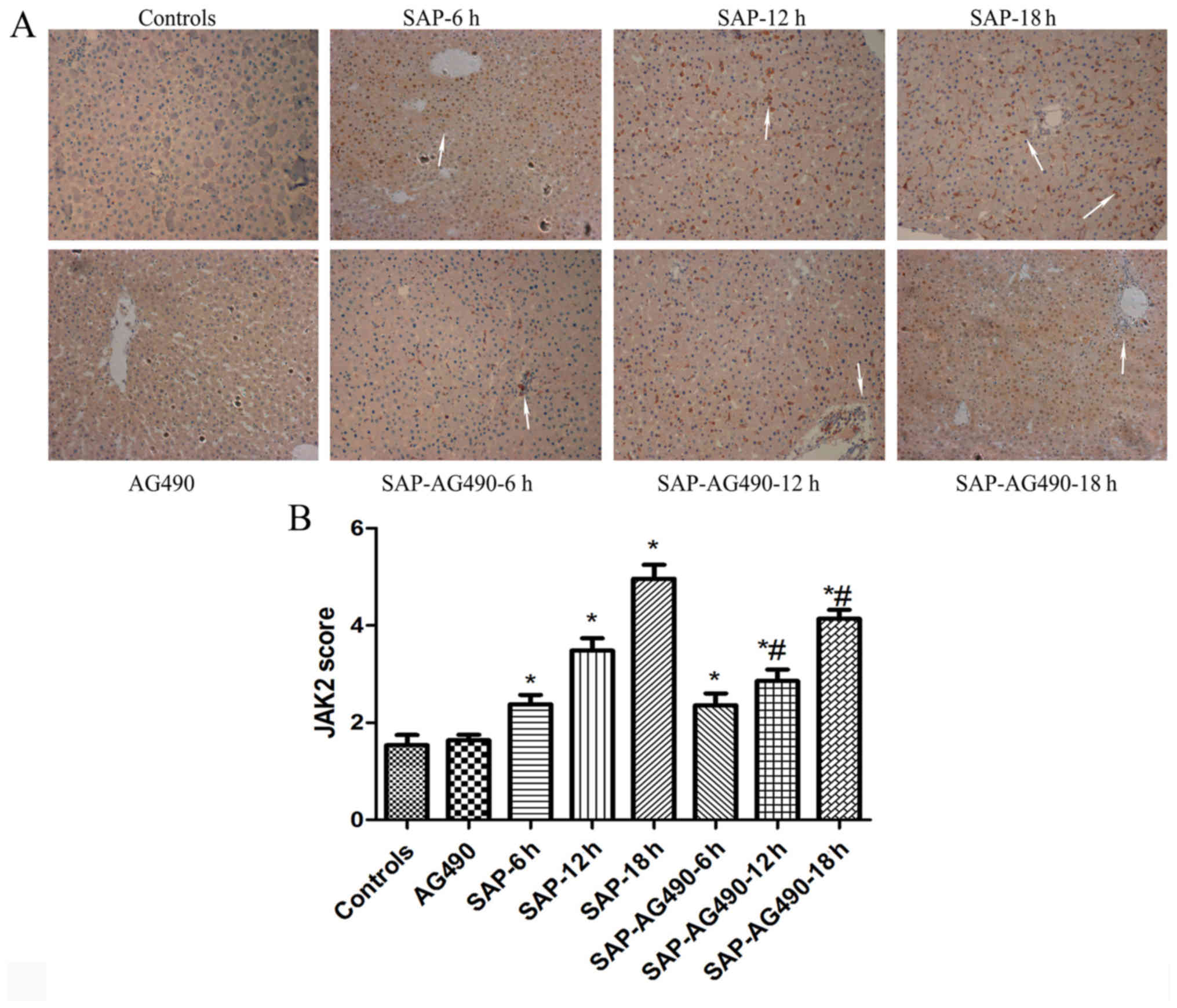
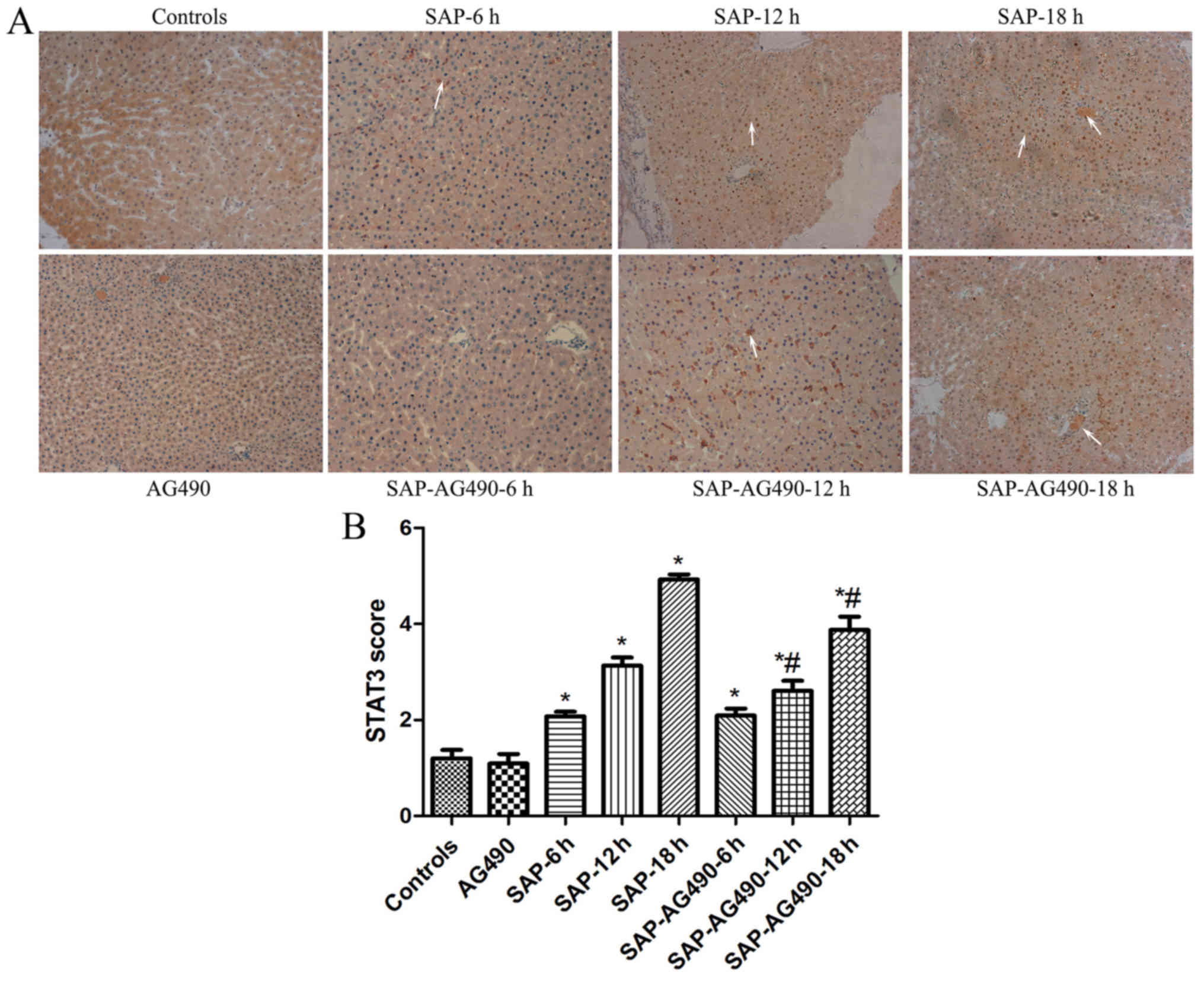
Immunohistochemistry analysis of JAK2
and STAT3
To detect the degree of inflammation in liver
tissues, immunostaining of JAK2 (Fig.
4) and STAT3 (Fig. 5) were
performed in the liver samples at the time points for each group.
In the control and AG490 groups, minimal staining of JAK2 and STAT3
was observed in the liver lining. However, expression of JAK2 and
STAT3 were increased, with JAK2 protein appearing mainly in the
cytoplasm and STAT3 protein appearing mainly in the nucleus. The
proportions of cytoplasmic JAK2 and nuclear STAT3 in SAP groups
were significantly increased compared with the control and AG490
groups. By contrast, the expression of these proteins in SAP-AG490
groups was significantly lower compared with SAP groups at each
time point (P<0.05).
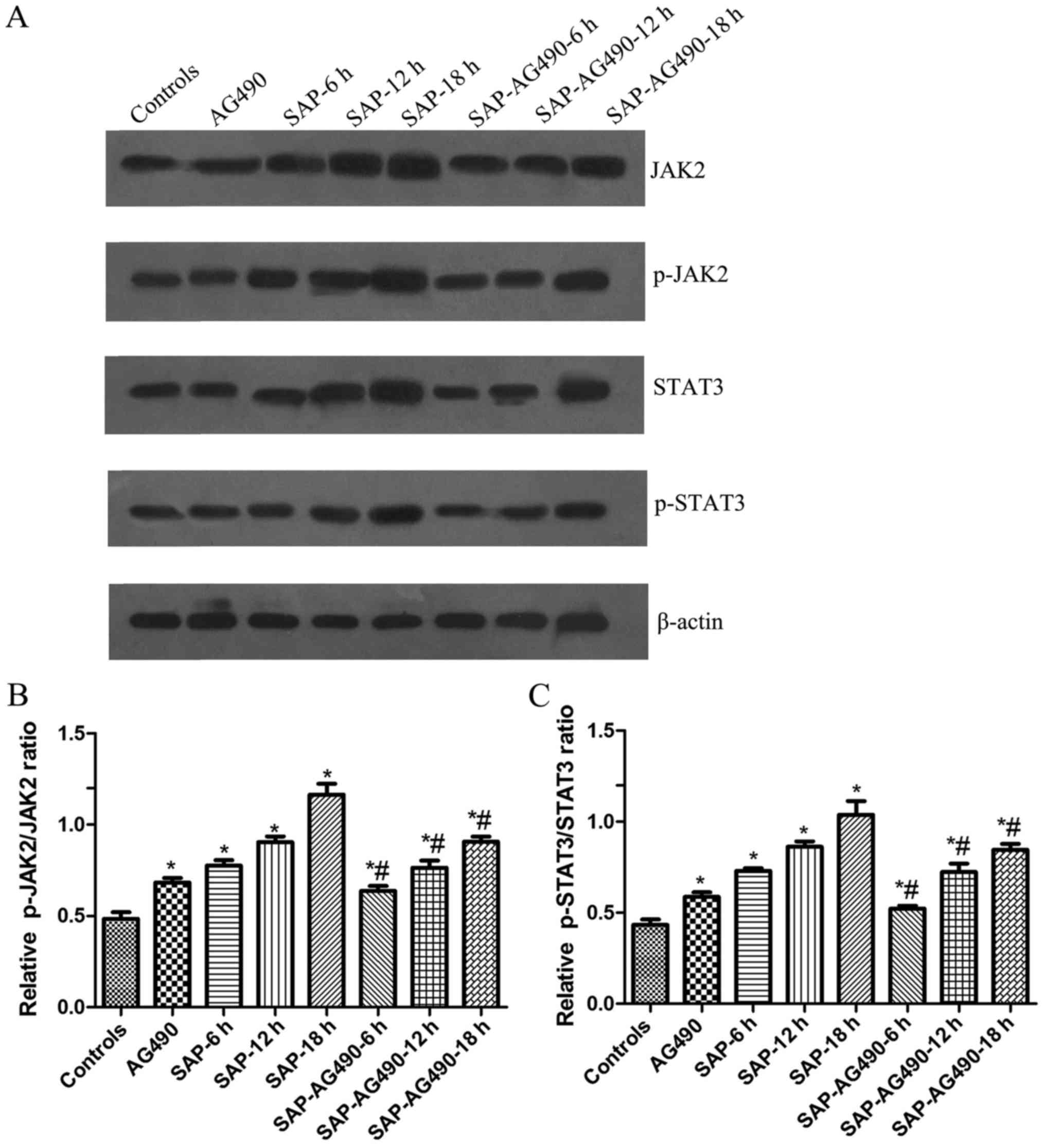
Expression of JAK2, p-JAK2, STAT3 and
p-STAT3 in rat liver tissues determined by western blot
analysis
As shown in Fig. 5,
the activation of JAK2 and STAT3 was examined in liver tissues and
the changes in the p-JAK2 and p-STAT3 levels in these tissues were
measured. Western blot analysis showed that no change was found in
AG490 groups compared with control (P>0.05). The expressions of
JAK2, p-JAK2, STAT3 and p-STAT3 proteins in SAP groups and
SAP-AG490 groups at 6, 12, 18 h were markedly higher compared with
control and AG490 groups (P<0.05). As further confirmation of
the role of the JAK2/STAT3 pathway in the liver tissues, the
SAP-AG490-pretreated groups showed a reduction in the JAK2, p-JAK2,
STAT3 and p-STAT3 levels compared with SAP groups at the same time
point (P<0.05; Fig. 6).
Discussion
SAP is characterized by its acute onset and rapid
progression, leading to clinical multiple organ dysfunction
syndromes with systemic inflammation and high mortality. A high
range of pancreatic injury is found in the early stages of liver
failure. It is associated with the release of digestive enzymes
into the pancreatic interstitium, as well as inflammatory
responses, leading to pancreatitis and subsequent distant organ
dysfunction due to the excessive production and systemic spreading
of inflammatory mediators (4,14,15).
TNF-α, IL-6 and IL-18 have been shown to play important roles in
mediating SAP and liver injury (16–18). In
particular, liver injury is a severe complication of SAP, resulting
in systemic inflammation and an increased risk of mortality
(19). Although previous studies
have suggested that activation of the JAK/STAT pathway up-regulated
the expression of pro-inflammatory cytokines in the progression of
SAP (20–22), the molecular mechanisms underlying
their roles in mediating acute liver injury during the course of
SAP remain poorly understood.
The JAK/STAT signaling pathway is widely involved in
inflammation (23), and numerous
studies have shown that the JAK and STAT family of kinases activate
the cytokine signaling cascade. Among the most important protein
tyrosine kinase families, JAK2 and STAT3 were identified from
studies on the downstream events of cytokine receptor binding,
including the transcriptional activation of genes involved in
inflammatory responses (24,25). Studies have shown that JAK and STAT
activation occurs widely throughout the pancreas and liver in
inflammatory cells (26–29). Specifically, cytokines trigger the
activation of STAT3 mediated by JAK2; additionally, JAK2 plays an
important role in the activation of STAT3, as observed in the
liver. Another study has indicated that TNF-α activated JAK2 and
STAT1 or STAT3 on the development of pancreatic injury in
vitro (30). Blocking the
JAK1/STAT1 signaling pathway prevents the lethal effects of
excessive systemic inflammatory response during SAP (31). However, the influence of the systemic
inflammatory response on the JAK2/STAT3 signaling pathway in SAP
and its relation to the degree of pancreatic diseases is not well
understood. Therefore, we explored these possible modulatory
effects on the pro-inflammatory cytokines (TNF-α, IL-6 and
IL-18)/JAK2 and STAT3 signaling pathway in liver tissues of SAP
rats. The present study found that the expression of TNF-α, IL-6
and IL-18, and the severity of inflammatory response in SAP models
significantly increased compared with the controls. In addition,
the systemic manifestations of SAP were not only caused by local
inflammatory processes but also by the excessive production and
systemic spreading of inflammatory mediators. It was found that the
transcription factors JAK2 and STAT3 were rapidly activated in the
liver following sodium taurocholate injection, with the protein
expressions of JAK2, p-JAK2, STAT3, and p-STAT3 in liver tissues
increased significantly in SAP groups, especially at the 18 h time
point group. This finding possibly indicates that pro-inflammatory
cytokines can regulate the JAK2/STAT3 pathway.
AG490, a member of the tyrphostin family of protein
kinase inhibitors, selectively inhibits the phosphorylation of JAK2
and STAT3. To eliminate these conflicting factors, we investigated
the relationship between the production of pro-inflammatory
cytokines and the activation of JAK2/STAT3 in pancreatic
inflammation in vivo. Previous studies have demonstrated
that TNF-α and IL-6 are primarily produced by macrophages and play
a major role in pancreatitis, and the reduction of these cytokines
in patients may be useful in the treatment of AP-related diseases
(32). In the pancreas, IL-18 is a
pleiotropic cytokine that is secreted primarily by activated
macrophages and Kupffer cells, and which stimulates the release of
other pro-inflammatory cytokines (33–35). It
has been reported that a high level of IL-18 is associated with the
early phase of AP in in vivo models (36). The present study induced AP and
administered pre-treatment with AG490 in different rat models. In
the SAP groups at different time points, the rats exhibited a
marked increase in AMY, ALT and AST concentrations. Moreover, it
was found that histopathological changes in the pancreas and liver
were amplified significantly with the administration of sodium
taurocholate, as indicated by parenchyma necrosis, inflammatory
infiltration and bleeding, suggesting that serious pancreas and
liver injury had occurred, which is consistent with previous
studies (37,38). The present study revealed that
pancreatic and liver injury became dramatically more serious with
time, compared with the pathological changes in SAP rats to varying
degrees. Similarly, the JAK2 inhibitor AG490 effectively inhibited
the activation of JAK2 and STAT3 phosphorylation. Interestingly,
JAK2 and STAT3 levels were affected by the overexpression of TNF-α,
IL-6 and IL-18, as indicated by the high levels of these proteins
in the liver tissues. The decrease in the TNF-α, IL-6 and IL-18
protein expression levels suggested that the anti-inflammatory
effects of AG490 might be mediated through the JAK2/STAT3 signaling
pathway. That IL-6 triggered JAK2 phosphorylation indicated a
tightly restricted signaling pattern for JAK2. Moreover,
pretreatment with AG490 produced prompt and marked decreases in
JAK2/STAT3, which suggested an upstream requirement for JAK2 in
this response, and inhibited JAK2 production by down-regulating
pro-inflammatory cytokines and attenuating the activation of STAT3,
decreasing the severity of pancreas and liver injury, and the
inflammatory response. Thus, there is a strong association between
JAK2/STAT3 activities and the invasive ability of rats, which
suggests that inhibition of this pathway may lead to the
identification of a valid target for the treatment of liver injury
in SAP.
The present study demonstrated that activation of
JAK2/STAT3 gene expression and production of inflammatory
cytokines, such as TNF-α, IL-6 and IL-18, led to
pancreatitis-induced liver injury. Therefore, these results
indicated that strategies to protect liver functions at an early
time point may help prevent multiple system organ failure and could
also aid in determining the pathogenesis and appropriate treatment
options for SAP. Further investigations could facilitate the early
recognition of the development of systemic complications by
clinicians and improve the management of SAP.
Acknowledgements
The present authors would like to thank the
Anesthesiology Department of the Jinling Hospital for their
generosity in supplying the JAK2 antibody.
Funding
The present study was supported by the Army Health
Project (grant no. 14BJZ28).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML, XZ and FW designed the research; ML, XX, XW, BW
and MG performed the research and obtained the data; XX and MG
provided the kits. ML wrote the paper; XZ and FW finalized and
revised the manuscript. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of Jinling Hospital. All operations
were performed according to international guidelines concerning the
care and treatment of experimental animals. Ethical approval for
this study was obtained from the Ethics Committee for Animal
Research at Jinling Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there is no conflict of
interest related to the present study.
References
|
1
|
Shen HN, Lu CL and Li CY: Effect of
diabetes on severity and hospital mortality in patients with acute
pancreatitis: A national population-based study. Diabetes Care.
35:1061–1066. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Banks PA, Bollen TL, Dervenis C, Gooszen
HG, Johnson CD, Sarr MG, Tsiotos GG and Vege SS: Acute Pancreatitis
Classification Working Group: Classification of acute pancreatitis
2012: Revision of the atlanta classification and definitions by
international consensus. Gut. 62:102–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu Y, Liao R, Qiang Z and Zhang C:
Pro-inflammatory cytokine-driven PI3K/Akt/Sp1 signalling and
H2S production facilitates the pathogenesis of severe
acute pancreatitis. Biosci Rep. 37:BSR201604832017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang J, Gallagher SF, Haines K,
Epling-Burnette PK, Bai F, Gower WR Jr, Mastorides S, Norman JG and
Murr MM: Kupffer cell-derived Fas ligand plays a role in liver
injury and hepatocyte death. J Gastrointest Surg. 8:166–174. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ou ZB, Miao CM, Ye MX, Xing DP, He K, Li
PZ, Zhu RT and Gong JP: Investigation for role of tissue factor and
blood coagulation system in severe acute pancreatitis and
associated liver injury. Biomed Pharmacother. 85:380–388. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang J, Fier A, Carter Y, Liu G,
Epling-Burnette PK, Bai F, Loughran TP Jr, Mastorides S, Norman JG
and Murr MM: Liver injury during acute pancreatitis: The role of
pancreatitis-associated ascitic fluid (PAAF), p38-MAPK, and
caspase-3 in inducing hepatocyte apoptosis. J Gastrointest Surg.
7:200–208. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Folch-Puy E: Importance of the liver in
systemic complications associated with acute pancreatitis: The role
of Kupffer cells. J Pathol. 211:383–388. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
O'Shea JJ, Schwartz DM, Villarino AV,
Gadina M, McInnes IB and Laurence A: The JAK-STAT pathway: Impact
on human disease and therapeutic intervention. Annu Rev Med.
66:311–328. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roskoski R Jr: Janus kinase (JAK)
inhibitors in the treatment of inflammatory and neoplastic
diseases. Pharmacol Res. 111:784–803. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakamori R, Takehara T, Ohnishi C, Tatsumi
T, Ohkawa K, Takeda K, Akira S and Hayashi N: Signal transducer and
activator of transcription 3 signaling within hepatocytes
attenuates systemic inflammatory response and lethality in septic
mice. Hepatology. 46:1564–1573. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aho HJ, Koskensalo SM and Nevalainen TJ:
Experimental pancreatitis in the rat. Sodium taurocholate-induced
acute haemorrhagic pancreatitis. Scand J Gastroenterol. 15:411–416.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schmidt J, Rattner DW, Lewandrowski K,
Compton CC, Mandavilli U, Knoefel WT and Warshaw AL: A better model
of acute pancreatitis for evaluating therapy. Ann Surg. 215:44–56.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Camargo CA Jr, Madden JF, Gao W, Selvan RS
and Clavien A: Interleukin-6 protects liver against warm
ischemia/reperfusion injury and promotes hepatocyte proliferation
in the rodent. Hepatology. 26:1513–1520. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kempuraj D, Twait EC, Williard DE, Yuan Z,
Meyerholz DK and Samuel I: The novel cytokine interleukin-33
activates acinar cell proinflammatory pathways and induces acute
pancreatic inflammation in mice. PLoS One. 8:e568662013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Geisler F, Algül H, Riemann M and Schmid
RM: Questioning current concepts in acute pancreatitis: Endotoxin
contamination of porcine pancreatic elastase is responsible for
experimental pancreatitis-associated distant organ failure. J
Immunol. 174:6431–6439. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang Y, An Y, Jiang D, Wu B, Yang Y and
Sun D: TNF-α regulating interleukin-33 induces acute pancreatic
inflammation in rats. Ann Clin Lab Sci. 46:54–59. 2016.PubMed/NCBI
|
|
17
|
Martin MA, Saracibar E, Santamaria A,
Arranz E, Garrote JA, Almaraz A, del Olmo ML, García-Pajares F,
Fernández-Orcajo P, Velicia R, et al: Interleukin 18 (IL-18) and
other immunological parameters as markers of severity in acute
pancreatitis. Rev Esp Enferm Dig. 100:768–773. 2008.(In Spanish).
PubMed/NCBI
|
|
18
|
Zhang XH, Li ML, Wang B, Guo MX and Zhu
RM: Caspase-1 inhibition alleviates acute renal injury in rats with
severe acute pancreatitis. World J Gastroenterol. 20:10457–10463.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wenhong D, Jia Y, Weixing W, Xiaoyan C,
Chen C, Sheng X and Hao J: Zerumbone attenuates the severity of
acute necrotizing pancreatitis and pancreatitis-induced hepatic
injury. Mediators Inflamm. 2012:1565072012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang J, Gallagher SF, Haines K,
Epling-Burnette PK, Bai F, Gower WR Jr, Mastorides S, Norman JG and
Murr MM: Kupffer cell-derived Fas ligand plays a role in liver
injury and hepatocyte death. J Gastrointest Surg. 8:166–174. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen P, Huang L, Zhang Y, Qiao M, Yao W
and Yuan Y: The antagonist of the JAK-1/STAT-1 signaling pathway
improves the severity of cerulein-stimulated pancreatic injury via
inhibition of NF-κB activity. Int J Mol Med. 27:731–738.
2011.PubMed/NCBI
|
|
22
|
Damm J, Harden L, Gerstberger R, Roth J
and Rummel C: The putative JAK-STAT inhibitor AG490 exacerbates
LPS-fever, reduces sickness behavior, and alters the expression of
pro- and anti-inflammatory genes in the rat brain.
Neuropharmacology. 71:98–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu JH and Kim H: Role of janus
kinase/signal transducers and activators of transcription in the
pathogenesis of pancreatitis and pancreatic cancer. Gut Liver.
6:417–422. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang C, Ma R, Sun S, Wei G, Fang Y, Liu R
and Li G: JAK2-STAT3 signaling pathway mediates thrombin-induced
proinflammatory actions of microglia in vitro. J Neuroimmunol.
204:118–125. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Agrawal S, Gollapudi S, Su H and Gupta S:
Leptin activates human B cells to secrete TNF-α, IL-6, and IL-10
via JAK2/STAT3 and p38MAPK/ERK1/2 signaling pathway. J Clin
Immunol. 31:472–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang LY, Chen P, Xu LX, Zhou YF, Zhang YP
and Yuan YZ: Fractalkine upregulates inflammation through CX3CR1
and the Jak-Stat pathway in severe acute pancreatitis rat model.
Inflammation. 35:1023–1030. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gallmeier E, Schäfer C, Moubarak P, Tietz
A, Plössl I, Huss R, Göke B and Wagner AC: JAK and STAT proteins
are expressed and activated by IFN-gamma in rat pancreatic acinar
cells. J Cell Physiol. 203:209–216. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu L, Li H, Zheng SZ, Liu X, Cai H and Cai
BC: Da-Huang-Fu-Zi-Tang attenuates liver injury in rats with severe
acute pancreatitis. J Ethnopharmacol. 150:960–966. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu JH, Kim KH and Kim H: SOCS 3 and
PPAR-gamma ligands inhibit the expression of IL-6 and TGF-beta1 by
regulating JAK2/STAT3 signaling in pancreas. Int J Biochem Cell
Biol. 40:677–688. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Robinson K, Vona-Davis L, Riggs D, Jackson
B and McFadden D: Peptide YY attenuates STAT1 and STAT3 activation
induced by TNF-alpha in acinar cell line AR42J. J Am Coll Surg.
202:788–796. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu JH, Kim KH and Kim H: Suppression of
IL-1beta expression by the Jak 2 inhibitor AG490 in
cerulein-stimulated pancreatic acinar cells. Biochem Pharmacol.
72:1555–1562. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Surbatovic M and Radakovic S: Tumor
necrosis factor-α levels early in severe acute pancreatitis: Is
there predictive value regarding severity and outcome? J Clin
Gastroenterol. 47:637–643. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gallagher SF, Peng Y, Haines K, Baksh K,
Epling-Burnette PK, Yang J and Murr MM: Fas/FasL play a central
role in pancreatitis-induced hepatocyte apoptotis. J Gastrointest
Surg. 9:467–475. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
McCormack D, McDonald D and McFadden D:
Pterostilbene ameliorates tumor necrosis factor alpha-induced
pancreatitis in vitro. J Surg Res. 178:28–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ueda T, Takeyama Y, Yasuda T, Matsumura N,
Sawa H, Nakajima T, Ajiki T, Fujino Y, Suzuki Y and Kuroda Y:
Significant elevation of serum interleukin-18 levels in patients
with acute pancreatitis. J Gastroenterol. 41:158–165. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ning JW, Zhang Y, Yu MS, Gu ML, Xu J,
Usman A and Ji F: Emodin alleviates intestinal mucosal injury in
rats with severe acute pancreatitis via the caspase-1 inhibition.
Hepatobiliary Pancreat Dis Int. 16:431–436. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Segersvärd R, Tsai JA, Herrington MK and
Wang F: Obesity alters cytokine gene expression and promotes liver
injury in rats with acute pancreatitis. Obesity (Silver Spring).
16:23–28. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lv P, Fan LJ, Li HY, Meng QS and Liu J:
Protective effect of thalidomide on liver injury in rats with acute
pancreatitis via inhibition of oxidative stress. Ann Clin Lab Sci.
45:508–514. 2015.PubMed/NCBI
|