Introduction
UV radiation is a malignant radiation that causes
inflammation in skin, aging, changes in immune system, and even
severe skin tumors (1). Hirano et
al (2) reported that
approximately 500,000 people in the world suffered from different
degrees of tissue damage in 2015 due to UV radiation. Since UV
radiation is an unavoidable natural light radiation, studies on UV
radiation in the clinic has attracted increasing attention. One of
the main reasons for the development of cataract is UV radiation
(3,4), and lens epithelial cell (LEC) in the
human body can be easily affected by UV radiation (5,6). LEC
strongly regulate the material metabolism of lens. LECs are
extremely sensitive and fragile and apoptosis of LECs can be easily
induced by UV rays, which is one of the most common pathogenesis of
cataract (7). Meyer et al
(8) have shown that apoptotic
factors p53, Bax, and Bcl-2 are closely related to the occurrence
and development of cataracts, while correlations between UV
radiation and these apoptotic factors are unknown. In this study,
UV irradiation SD rat model was established to explore the
correlations between UV radiation and apoptotic factors in LECs.
Our study provided references for future studies and clinical
practices.
Materials and methods
Experimental animals
Sixty SPF 6-week-old Sprague Dawley (SD) rats (30
males and 30 females) were provided by the Animal Experimental
Center of Central South University. Rearing conditions were: Room
temperature (26°C), humidity 75%, 5 rats in a cage, normal
illumination, and free access to water.
Methods
SD rats were subjected to UV irradiation model
construction according to the methods described by Ji et al
(9). Rats were randomly divided into
2 groups including control group (n=12) and model group (n=48).
Rats in control group were normally fed, while rats in model group
were subjected to UV radiation on eyeballs. All rats in model group
were treated with compound tropicamide eye drops to achieve
mydriasis. Anesthesia was performed using chloral hydrate via
intraperitoneal injection at a dose of 300~350mg/kg, 10 min after
drug administration, and eyeballs of rats were irradiated with an
UV lamp (300 to 350 nm, 1.0×103 µW/cm2) for
15 min. Twelve rats in model group were sacrificed at day 1, 3, 5,
and 7, and eyeballs were dissected and lens was collected. After
rinsing with physiological saline, lens capsule was isolated and 1
ml of homogenate was added. Total RNA was extracted from each group
using TRIzol reagent and transcribed into cDNA using a reverse
transcription kit (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). RT-qPCR assay was performed and reaction system
was configured according to the kit. Primers were designed and
synthesized by Sangon Co., Ltd., (Shanghai, China). Sequences of
primers are listed in Table I. PCR
reaction conditions: 95°C for 10 min, followed by 45 cycles of 95°C
for 15 sec, 65°C for 30 sec, and 72°C for 30 sec. β-actin was used
as endogenous control and data were processed using
2−∆∆Cq method (10)
(Table I). The study was approved by
the Ethics Committee of Renmin Hospital of Wuhan University (Wuhan,
China).
 | Table I.Sequences of primers used in PCR
reactions. |
Table I.
Sequences of primers used in PCR
reactions.
| Genes | Reverse sequence | Forward sequence |
|---|
| p53 |
5′-GCTGAGTATCTGGACGACAGG-3′ |
5′-AGCGTGATGATGGTAAGGATG-3′ |
| Bcl-2 |
5′-GAGCGTCAACAGGGAGATGT-3′ |
5′-CAGCCAGGAGAAATCAAACAG-3′ |
| Bax |
5′-ACGCATCCACCAAGAAGC-3′ |
5′-GCCACACGGAAGAAGACCT-3′ |
| β-actin |
5′-CCCATCTATGAGGGTTACGC-3′ |
5′-TTTAATGTCACGCACGATTTC-3′ |
Observation indicators
Twelve rats in th model group and 3 rats in the
control group were sacrificed at day 1, 3, 5 and 7, and the
expression of p53, Bax and Bcl-2 in LECs was detected by RT-qPCR.
Correlation of expression of p53, Bax and Bcl-2 with time after UV
irradiation was analyzed.
Statistical analysis
SPSS 22.0 statistical software (IBM Corp., Armonk,
NY, USA) was used process all the data. The data were expressed as
mean ± standard deviation. Comparison between multiple groups was
done using one-way ANOVA test followed by post hoc test (Least
Significant Difference). Pairwise t-test was used for comparison
between 2 groups. Correlation analysis was performed using linear
correlation analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Modeling results
Of the 48 rats in the model group, 46 were
successfully modeled and the success rate of modeling was 95.83%.
One rat died at day 5 and 7, respectively. Therefore, we have 12
rats in control group, 12 rats in model group on day 1, 12 rats in
model group on day 3, 11 rats in model group on day 5, and 11 rats
in model group on day 7.
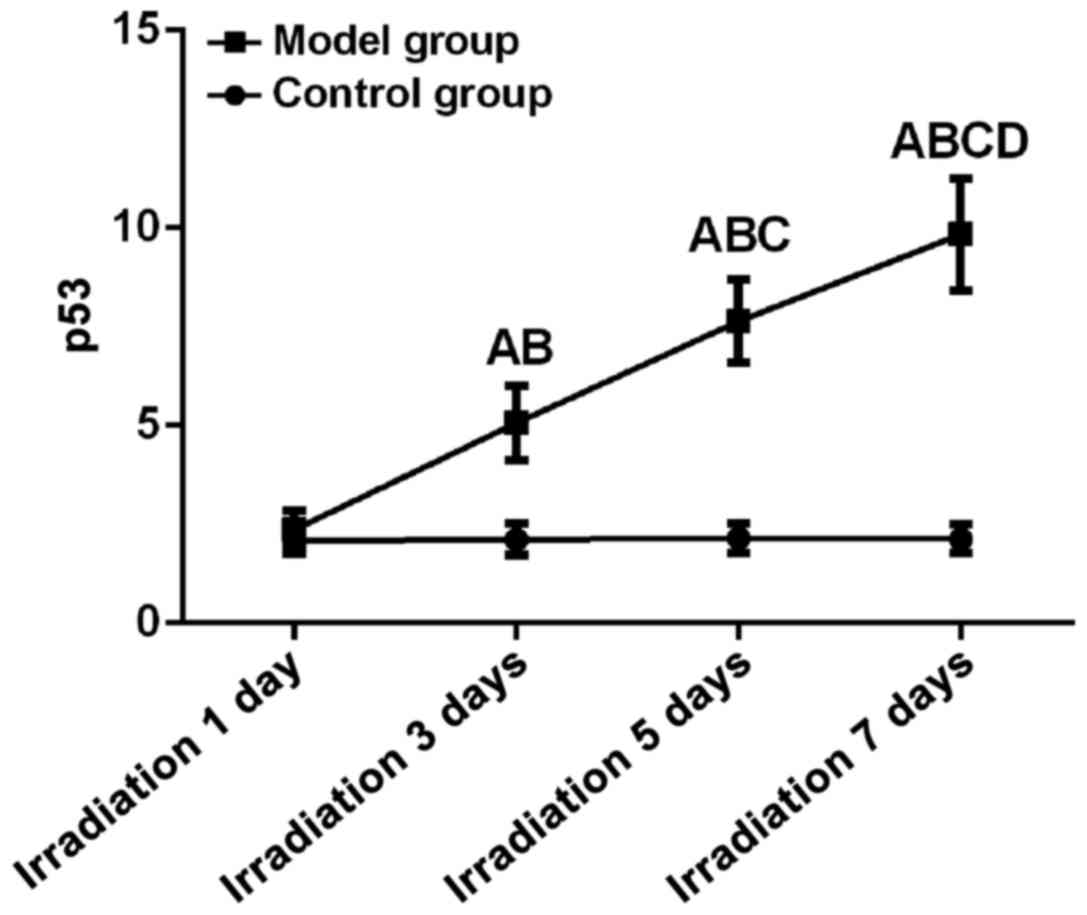
p53 expression
Differences between control and model group at all
time points were statistically significant (F=148.62, P<0.01).
Expression level of p53 began to rise significantly on day 3.
Expression level of p53 on day 7 (9.84±1.42) was significantly
higher than that on day 5, 3 and 1 and that in control (P<0.05).
Expression level of p53 on day 5 (7.64±1.05) was significantly
higher than that on day 3 and 1 and that in control (P<0.05).
Expression level of p53 on day 3 (5.07±0.94) was significantly
higher than that on day 1 and that in control (P<0.05). There
was no significant difference between expression level of p53 on
day 1 (2.37±0.48) and that in control group at the same time
(P>0.05) (Fig. 1).
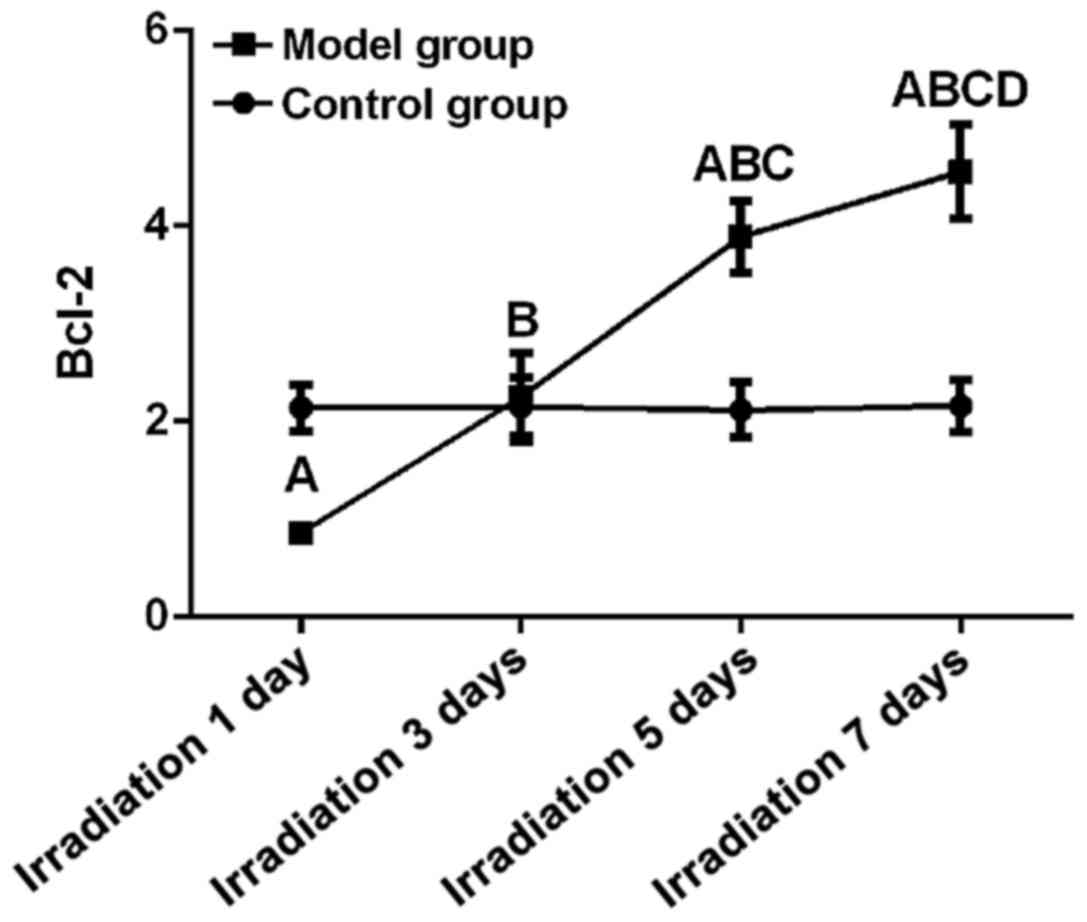
Bcl-2 expression
Differences between control and model group at all
time points were statistically significant (F=179.94, P<0.01).
Expression level of Bcl-2 on day 7 (4.56±0.48) was significantly
higher than that on day 5, 3 and 1 and that in control (P<0.05).
Expression level of Bcl-2 on day 5 (3.89±0.37) was significantly
higher than that on 3 and day 1 and that in control (P<0.05).
Expression level of Bcl-2 on day 3 (2.25±0.45) was significantly
higher than that on day 1 but showed no significant differences to
control group at the same time point (P<0.05). Expression level
of Bcl-2 on day 1 (2.37±0.48) was significantly lower than that in
control group at the same time (P>0.05). Linear correlation
analysis showed that relative expression level of Bcl-2 was
positively correlated with UV irradiation time (r=0.90, P<0.05)
(Fig. 2).
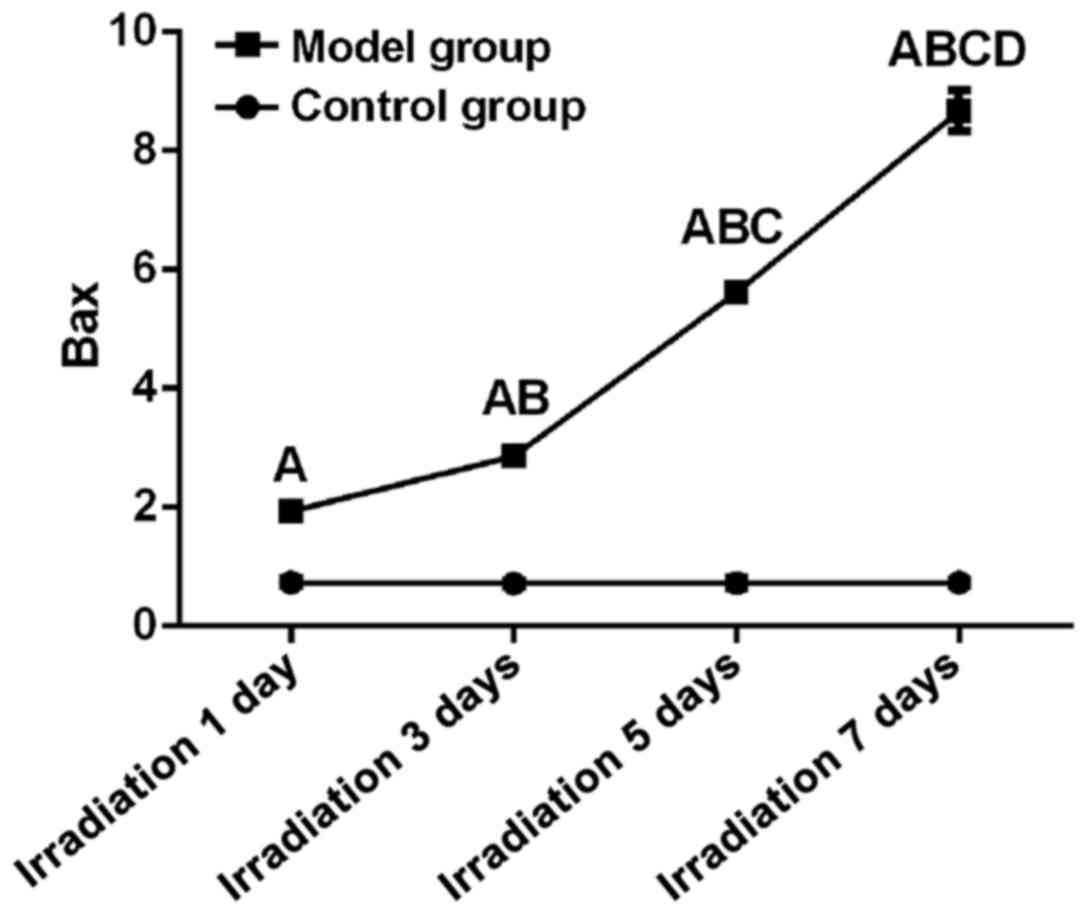
Bax expression
Differences between control and model group at all
time points were statistically significant (F=4378.04, P<0.01).
Expression level of Bax on day 7 (8.67±0.34) was significantly
higher than that on day 5, 3 and 1 and that in control (P<0.05).
Expression level of Bax on day 5 (5.62±0.08) was significantly
higher than that on day 3 and 1 and that in control (P<0.05).
Expression level of Bax on day 3 (2.86±0.05) was significantly
higher than that on day 1 but showed no significant differences to
control group at the same time point (P<0.05). Expression level
of Bax on day 1 (1.94±0.10) was significantly lower than that in
control group at the same time (P>0.05). Linear correlation
analysis showed that relative expression level of Bax was
positively correlated with UV irradiation time (r=0.95, P<0.05)
(Fig. 3).
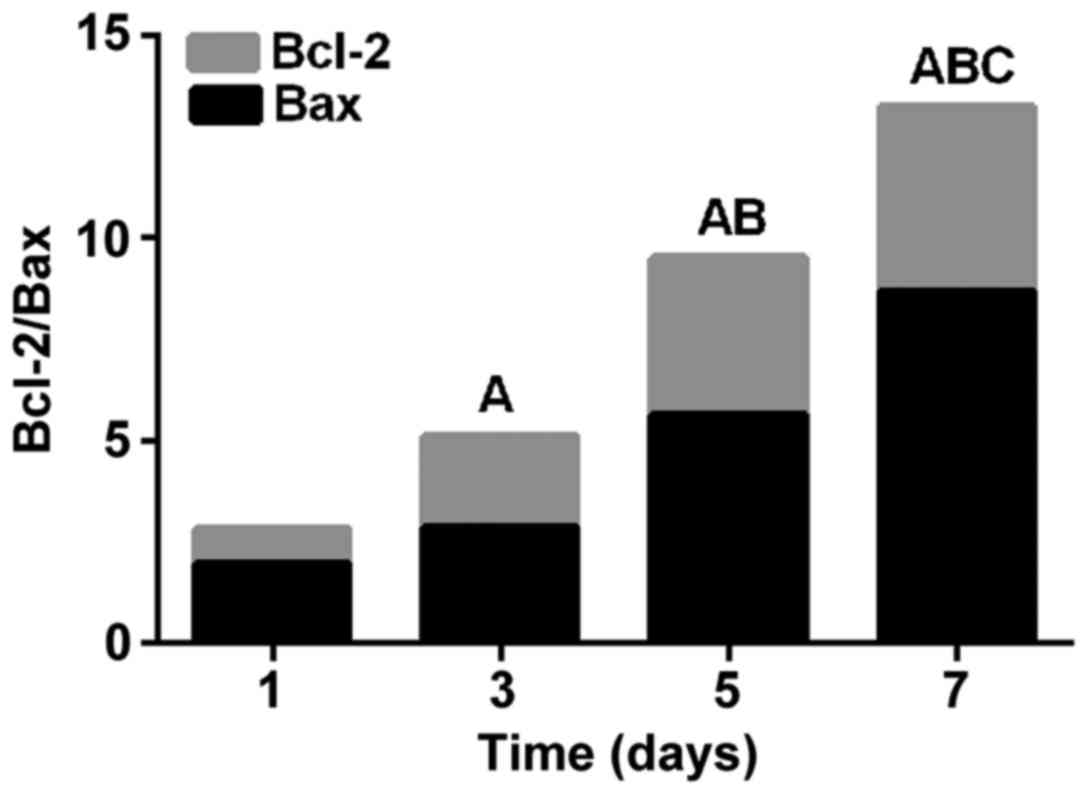
Bcl-2/Bax ratio
Bcl-2/Bax was highest on day 7 (P<0.05), followed
by day 5 (P<0.05), day 3 (P<0.05), and day 1 (P<0.05).
Bcl-2/Bax significantly increased with prolonged time after UV
exposure (Fig. 4).
Discussion
LEC belongs to the subcapsular monolayer epithelial
cells and is the basis for maintaining normal function of the lens
(11). In addition, LEC is the
driving force for the growth and differentiation of the lens, and
is important for repair of the internal damage of the lens and
stability of the internal environment (12). Once the LEC is damaged, a series of
systemic changes will appear in the patient's body, causing a
series of eye diseases, such as eyeball congestion and cataract
(12,13). One of the factors that can easily
cause LEC damage is UV. Numerous studies have shown (14–16) that
UV radiation is closely related to the occurrence of cataract. LEC
apoptosis is the initiation of cataract, in which p53 has a strong
ability to regulate cell growth (17). Proteins encoded by the two genes,
Bcl-2 and Bax, can form dimers and their ratio is
also one of the determinants of cell survival (18). Changes of LEC apoptotic factors under
UV irradiation are not yet clear. In the present study, a rat model
of UV irradiation was established to detect the expression of p53,
Bax, and Bcl-2 in rat LEC. The aim was to study the effect of UV on
the apoptosis of LEC, so as to provide references for clinical
treatment of this disease.
Results of this experiment showed that relative
expression levels of p53, Bax, and Bcl-2 in LECs of the model group
were basically higher than those in control group, and p53, Bax,
and Bcl-2 expression levels were positively correlated with the
time after UV irradiation. p53 expression level increased with
prolonged time after UV irradiation, suggesting that the longer the
time after UV irradiation, the more serious the LEC apoptosis.
Studies have shown that p53 is positively correlated with the
severity of DNA damage (19), and
increased expression level of p53 protein can accelerate cell
proliferation and makes DNA damage more serious. In this study,
expression of Bax in model group was significantly higher than that
in control group, and Bcl-2 expression level in LEC was positively
correlated with UV irradiation time, but the relative expression
level was lower in the model group than in control group at day 1.
When expression level of Bax is decreased and expression level of
Bcl-2 is increased, Bcl-2 will interact with Bax to inhibit
apoptosis of cells. When expression level of Bax is increased and
expression level of Bcl-2 is decreased, Bcl-2 will interact with
Bax to prommote cell apoptosis (20). The condition was the same as the
former one on day 1, indicating that LEC is undergoing apoptosis.
Therefore, UV may increase apoptosis of LEC by increasing the ratio
of Bax/Bcl-2. Positive correlation between p53, Bax, Bcl-2 and
UV-irradiation time indicates that when DNA is damaged, expression
of p53 protein will be up-regulated, and the ratio of Bax/Bcl-2 is
increased to induce LEC apoptosis, which affects patient's lens and
induces a series of diseases.
Our study established a UV irradiation rat model to
detect the expression of apoptosis factors in LECs. We explored the
effect of UV on apoptotic factors in LECs. However, clinical
studies are needed to further confirm our conclusions in humans. In
summary, expression levels of p53, Bax, and Bcl-2 in LECs of the
model group were positively correlated with UV irradiation time,
suggesting that UV may induce LEC apoptosis by increasing the
regulation of the expression of p53, Bax, and Bcl-2.
Acknowledgements
The authors would like to thank Professor Yin Shen
and Dr Yanxin Meng for their technical guidance and advice on the
experiments in this study.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL designed the study and wrote the manuscript. JL
and YX constructed the UV irradiation model and analyzed the
relevant observation indicators. Both authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Renmin Hospital of Wuhan University (Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bornman JF, Barnes PW, Robinson SA,
Ballaré CL, Flint SD and Caldwell MM: Solar ultraviolet radiation
and ozone depletion-driven climate change: Effects on terrestrial
ecosystems. Photochem Photobiol Sci. 14:88–107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hirano S, Hosokawa T, Yoshida N, Omukai K
and Yorke HW: Primordial star formation under the influence of far
ultraviolet radiation: 1540 cosmological haloes and the stellar
mass distribution. Mon Not R Astron Soc. 448:568–587. 2015.
View Article : Google Scholar
|
|
3
|
McColl N, Auvinen A, Kesminiene A, Espina
C, Erdmann F, de Vries E, Greinert R, Harrison J and Schüz J:
European Code against Cancer 4th edition: Ionising and non-ionising
radiation and cancer. Cancer Epidemiol. 39 Suppl 1:S93–100. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Olsen CM, Wilson LF, Green AC, Bain CJ,
Fritschi L, Neale RE and Whiteman DC: Cancers in Australia
attributable to exposure to solar ultraviolet radiation and
prevented by regular sunscreen use. Aust NZJ Public Health.
39:471–476. 2015. View Article : Google Scholar
|
|
5
|
Terrell AM, Anand D, Smith SF, Dang CA,
Waters SM, Pathania M, Beebe DC and Lachke SA: Molecular
characterization of mouse lens epithelial cell lines and their
suitability to study RNA granules and cataract associated genes.
Exp Eye Res. 131:42–55. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen P, Chen JZ, Shao CY, Li CY, Zhang YD,
Lu WJ, Fu Y, Gu P and Fan X: Treatment with retinoic acid and lens
epithelial cell-conditioned medium in vitro directed the
differentiation of pluripotent stem cells towards corneal
endothelial cell-like cells. Exp Ther Med. 9:351–360. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu L, Yu R, Shi Y, Dai Y, Zeng Z, Guo X,
Ji Q, Wang G and Zhong J: Transduced protein transduction domain
linked HSP27 protected LECs against UVB radiation-induced damage.
Exp Eye Res. 120:36–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meyer K, Buettner S, Ghezzi D, Zeviani M,
Bano D and Nicotera P: Loss of apoptosis-inducing factor critically
affects MIA40 function. Cell Death Dis. 6:e18142015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ji Y, Cai L, Zheng T, Ye H, Rong X, Rao J
and Lu Y: The mechanism of UVB irradiation induced-apoptosis in
cataract. Mol Cell Biochem. 401:87–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang HM, Li GX, Zheng HS and Wu XZ:
Protective effect of resveratrol on lens epithelial cell apoptosis
in diabetic cataract rat. Asian Pac J Trop Med. 8:153–156. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen Y, Dong LF, Zhou RM, Yao J, Song YC,
Yang H, Jiang Q and Yan B: Role of long non-coding RNA MIAT in
proliferation, apoptosis and migration of lens epithelial cells: A
clinical and in vitro study. J Cell Mol Med. 20:537–548. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Liu S, Zhang F, Jiang P, Wu X and
Liang Y: Expression of the microRNAs hsa-miR-15a and hsa-miR-16-1
in lens epithelial cells of patients with age-related cataract. Int
J Clin Exp Med. 8:2405–2410. 2015.PubMed/NCBI
|
|
14
|
Varma SD, Kovtun S and Hegde KR: Role of
ultraviolet irradiation and oxidative stress in cataract
formation-medical prevention by nutritional antioxidants and
metabolic agonists. Eye Contact Lens. 37:233–245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wright F and Weller RB: Risks and benefits
of UV radiation in older people: More of a friend than a foe?
Maturitas. 81:425–431. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kontomaris SV, Yova D, Stylianou A and
Balogiannis G: The effects of UV irradiation on collagen D-band
revealed by atomic force microscopy. Scanning. 37:101–111. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kruiswijk F, Labuschagne CF and Vousden
KH: p53 in survival, death and metabolic health: A lifeguard with a
licence to kill. Nat Rev Mol Cell Biol. 16:393–405. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jia G, Wang Q, Wang R, Deng D, Xue L, Shao
N, Zhang Y, Xia X, Zhi F and Yang Y: Tubeimoside-1 induces glioma
apoptosis through regulation of Bax/Bcl-2 and the ROS/Cytochrome
C/Caspase-3 pathway. Onco Targets Ther. 8:303–311. 2015.PubMed/NCBI
|
|
19
|
Jiang L, Kon N, Li T, Wang SJ, Su T,
Hibshoosh H, Baer R and Gu W: Ferroptosis as a p53-mediated
activity during tumour suppression. Nature. 520:57–62. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hajiahmadi S, Panjehpour M, Aghaei M and
Shabani M: Activation of A2b adenosine receptor regulates ovarian
cancer cell growth: Involvement of Bax/Bcl-2 and caspase-3. Biochem
Cell Biol. 93:321–329. 2015. View Article : Google Scholar : PubMed/NCBI
|