Introduction
Heart failure refers to a group of clinical
syndromes caused by ventricular filling and/or impaired ejection
capacity due to various cardiac structures and/or functional
diseases, which is the end stage of various cardiovascular diseases
(1). At present, scholars worldwide
have agreed that cardiac remodeling is the root cause of the
development and progression of heart failure (2,3). Thus,
inhibition of cardiac remodeling can improve cardiac function and
long-term prognosis of patients with heart failure. Under the
guidance of this theory, the clinical treatment mode of heart
failure has also been transformed from the simple improvement of
hemodynamics to the treatment that aims to improve the cardiac
remodeling (4,5), such as the inhibition of
renin-angiotensin-aldosterone system (RAAS) and sympathetic nervous
system. At present, the clinical drugs used to inhibit RAAS and
sympathetic nervous system mainly include the β-receptor blocker,
angiotensin converting enzyme inhibitor, angiotensin receptor
inhibitor and aldosterone receptor antagonist. The wide application
of these drugs helps significantly decrease the fatality rate and
hospitalization rate of heart failure. But the morbidity and
mortality of heart failure are still high, which are still the main
causes of disability and death of cardiovascular diseases,
seriously threatening human health (6,7).
Therefore, research on new anti-heart failure drugs is still in the
ascendant.
The fundamental feature of heart failure is cardiac
remodeling, which is the change in myocardial structure, function
and phenotype due to a series of complex molecular and cellular
mechanisms, manifested as pathological hypertrophy of myocardial
cells, myocardial cell apoptosis, and excessive fibrosis or
increased degradation of extracellular matrix (8–10).
Myocardial cell apoptosis is the main reason for the continuous
loss of myocardial contraction units in the development and
progression of heart failure, which is related to the progressive
decrease of cardiac function, and involved in the basic
pathological and physiological processes of heart failure.
Therefore, the inhibition of myocardial cell apoptosis has
important clinical significance for the reduction of cardiac
remodeling and improvement of cardiac function. Recent studies have
shown that endoplasmic reticulum stress (ERS) is associated with
heart failure, and ERS-mediated myocardial cell apoptosis is an
important aspect of cardiac remodeling (11,12),
thus inhibiting the ERS-mediated myocardial cell apoptosis is of
great importance for improvement of cardiac function.
Berberine is the main active ingredient of
traditional Chinese medicine, Coptis chinensis, mainly used
clinically in the treatment of bacterial infection of
gastrointestinal tract. The latest study has found that berberine
has not only a good anti-dysentery activity, but also a variety of
cardiovascular pharmacological activity, such as antiarrhythmic
effect, anti-heart failure, antihypertensive effect and regulation
of blood lipids (13–16). Clinical studies have confirmed that
berberine has the function to enhance myocardial contractility and
improve left ventricular function (17). Berberine increasingly shows a certain
function in the treatment of cardiovascular disease, especially
heart failure, but its mechanism of anti-heart failure remains
unclear.
This study hypothesized that the myocardial cell
apoptosis caused by persistent ERS response was involved in the
cardiac remodeling of heart failure, and berberine could reduce the
myocardial cell apoptosis and improve cardiac remodeling through
inhibiting ERS response, thereby improving the cardiac function.
The rat model of heart failure after myocardial infarction was
established, followed by berberine intervention, so as to study its
effects on ERS-mediated myocardial cell apoptosis and its
correlation with cardiac function, thereby investigating the
mechanism of berberine improving the cardiac function of heart
failure rats.
Materials and methods
Experimental animal and myocardial
infarction model
Fifty female Wistar rats weighing 200–220 g were fed
in the animal center for one week to adapt to the environment.
After fasting for 4–6 h, under anesthesia via inhalation of ether,
the rats were fixed on the operating table in a supine position;
the chest hair was shaved and disinfected; and a purse was made in
the chest to be opened using a no. 10 suture, and the pericardium
was quickly opened to expose the heart. The coronary artery was
found in the pulmonary arterial cone and left atrium; then the
anterior descending coronary artery was ligatured using a no. 0
suture and the heart was placed back to the chest; the air and
blood in the chest were squeezed out, and the purse and chest were
quickly closed. The thoracotomy lasted for <30 sec in total. The
steps for the Sham group were the same as those in the model group,
except for stringing and no ligation. After operation, the rats
received intraperitoneal injection of penicillin (200,000 U/kg) for
7 days to prevent infection. This study was approved by the Animal
Ethics Committee of Fujian Medical University Animal Center
(Fujian, China).
Grouping and administration
At 4 weeks after the myocardial infarction model was
established, the rats were randomly divided into three groups:
Sham-operation group (Sham group), myocardial infarction control
group (Sal group) and berberine treatment group (Ber group). The
control group and experimental group received the gavage with the
same amount of normal saline and berberine (20 mg/kg) for 4
weeks.
Hemodynamic detection
After administration, rats received the
intraperitoneal injection of 3% pentobarbital sodium for
anesthesia, and left ventricular intubation via the right common
carotid artery. The pressure transducer was connected to the
carrier amplifier, the left ventricular end-systolic pressure
(LVSP) and left ventricular end-diastolic pressure (LVEDP) were
recorded using the 8-channel physiological recorder; the electric
signal was input to the differentiator to trace the maximum
ascending velocity of left ventricular pressure (+dp/dt) and the
maximum descending velocity of left ventricular pressure
(-dp/dt).
Determination of plasma BNP level
Before the treatment of rats, 0.5 ml serum was taken
from the rat, and 0.5 ml tissue lysis buffer was added, followed by
tissue homogenate at 4°C and centrifugation at 4°C for 10 min
(1,750 × g); the supernatant was taken and centrifuged again at 4°C
for 30 min (10,500 × g), and then recycled. The specific procedures
were carried out according to the instructions of the kit.
Pathomorphological detection of
myocardial tissue
The pathomorphological changes in myocardium were
evaluated via H&E staining. The pathomorphological changes in
myocardial tissue were observed via an optical microscope (BX-42,
Olympus Corporation, Tokyo, Japan). The degree of myocardial
fibrosis was analyzed via Massons staining: pale blue for collagen
fibers, pink for muscle fibers, red blood cells and cytoplasm, and
blue brown for nuclei.
Detection of apoptotic cells
TUNEL staining was applied to myocardial tissues,
followed by counting the number of apoptotic cells under an optical
microscope and then images were captured.
Immunohistochemistry
The expression levels of Bcl-2, Bax and caspase-3
were detected by immunohistochemistry. After the slices were
treated according to the standard procedure, Bcl-2, Bax and
caspase-3 primary antibody diluents were added for reaction at room
temperature for 3 h. Then the secondary antibody was used for 30
min incubation, followed by color development using the
hypersensitivity kit according to the supplier's protocol. Rabbit
polyclonal Bcl-2 antibody (1:100; cat. no. ab59348), rabbit
monoclonal Bax antibody (1:100; cat. no. ab32503), rabbit
polyclonal caspase-3 antibody (1:100; cat. no. ab13847) and
secondary goat anti-rabbit (HRP) IgG antibody (1:1,000; cat. no.
ab6721) were all purchased from Abcam (Cambridge, MA, USA).
Protein extraction and western blot
analysis
The heart was taken from the rat and the total
protein was extracted according to the standard procedure, followed
by protein quantification via BCA method. Endoplasmic reticulum
stress-related proteins were detected by western blot analysis. The
concentration of separation gel (15%) and the size of
electrophoresis voltage were adjusted according to the molecular
weight of target protein, followed by color development via
chemiluminesence kit (Merck Millipore, Burlington, MA, USA) and
semi-quantitative analysis via gray value. Primary rabbit
polyclonal GRP78 antibody (1:500; cat. no. ab21685); rabbit
monoclonal CHOP antibody (1:500; cat. no. ab179823); rabbit
polyclonal caspase-12 antibody (1:500; cat. no. ab62484); rabbit
polyclonal GAPDH antibody (1:500, cat. no. ab37168) and secondary
goat anti-rabbit (HRP) IgG antibody (1:2,000; cat. no.. ab6721)
purchased from Abcam (Cambridge, MA, USA), were used for Western
blot analysis.
RNA extraction and RT-PCR
According to the standard procedure, RNA in
myocardial tissues was extracted, and the primer design and
synthesis were completed by Shanghai Sangon Co. (Shanghai, China).
Total RNA was taken to synthesize complementary deoxyribonucleic
acid (cDNA) using the RT Revert Aid First Strand cDNA Synthesis kit
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). PCR was
performed after reverse transcription according to the instructions
of Invitrogen kit (Invitrogen, Carlsbad, CA, USA). ImageJ software
(version X; Media Cybernetics, Silver Spring, MD, USA) was employed
for determination of bands grey values. Primer sequences used in
this study was according to previous reports (11,12).
Statistical analysis
Data were analyzed using SPSS 17.0 statistical
software (Chicago, IL, USA). Student's t-test was used for the
comparison between the two groups, and one-way analysis of variance
followed by post hoc test (Least Significant Difference) was used
for the comparison of correlation among groups. P<0.05 suggested
that the difference was statistically significant.
Results
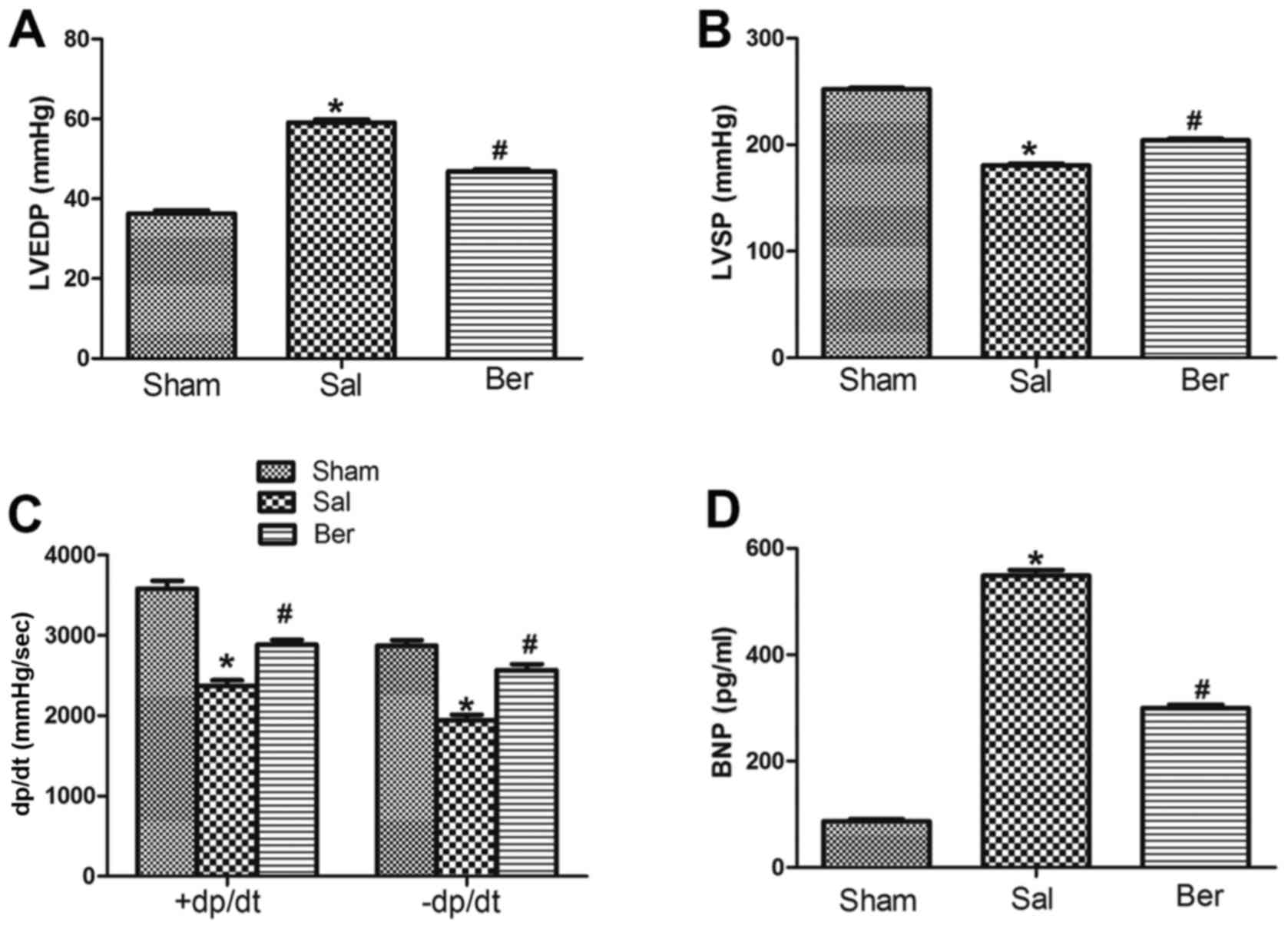
Berberine improves the cardiac
function of rats after myocardial infarction
Compared with those in Sham group, LVEDP in Sal
group was significantly increased, but LVSP and ±dp/dt were
significantly decreased at 8 weeks after myocardial infarction.
After berberine treatment, LVEDP was decreased and LVSP and ±dp/dt
were increased. At the end of experiment, plasma BNP levels in the
Sham, Sal and Ber groups were measured. Compared with that in Sham
group, BNP level in Sal group was significantly increased. By
contrast, it was found that berberine significantly decreased the
BNP level (Fig. 1).
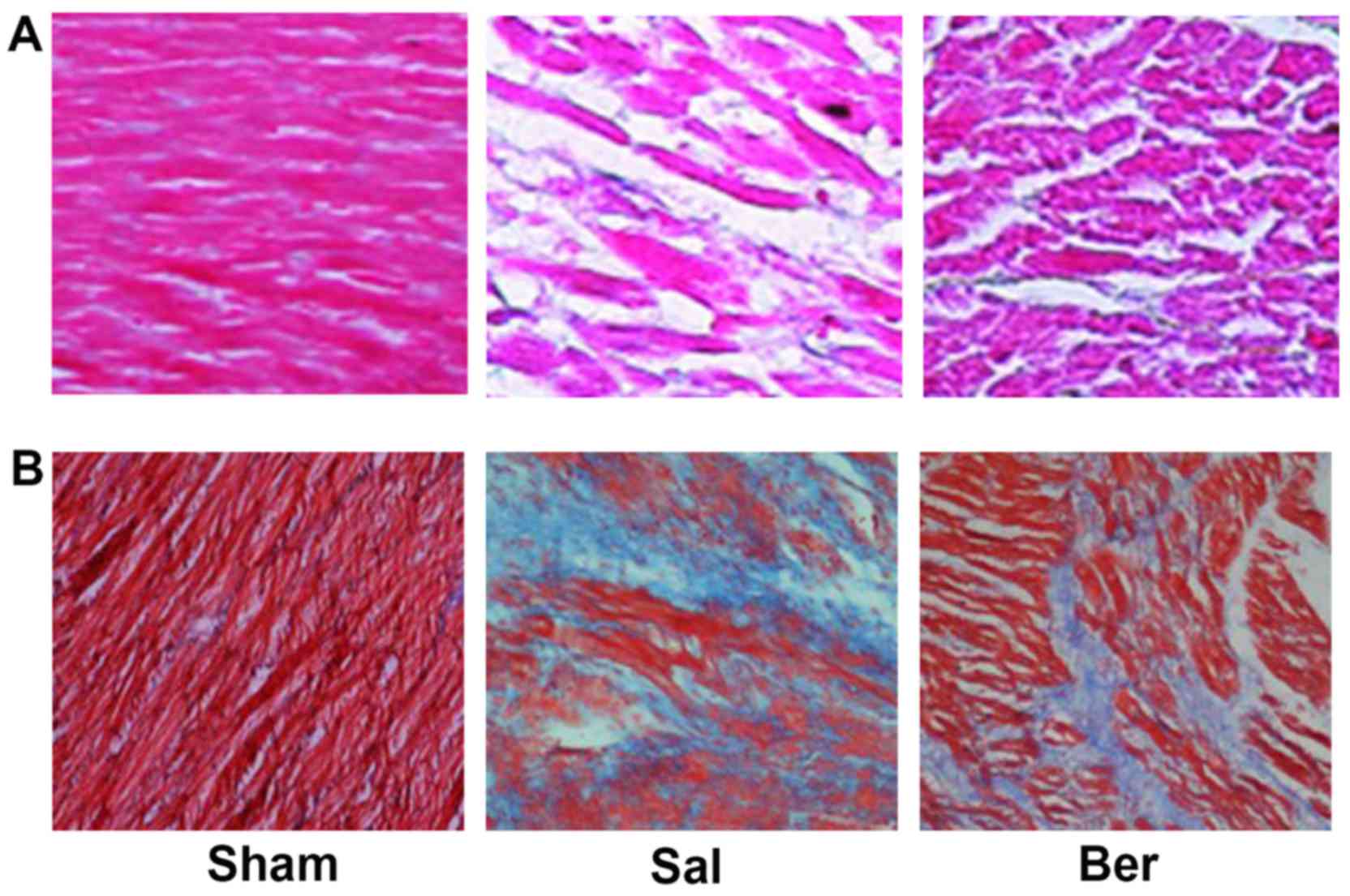
Berberine improves the myocardial
fibrosis after myocardial infarction
H&E staining showed that the myocardial cells in
Sham group were neatly arranged with clear transverse striations.
The myocardial cells in Sal group were swollen with irregular shape
and disordered arrangement, and the gap of myocardial cells was
filled with a large number of fibrous tissues, and the transverse
striations were blurry and broken. After berberine treatment,
myocardial cells were neatly arranged with decreased degree of
fibroplasias (Fig. 2A). Massons
staining showed that myocardial cells dominated in the Sham group,
and there were no obvious myocardial interstitial and collagen
components. In Sal group, there were obvious collagen components
with a small number of myocardial cells. After berberine treatment,
the number of myocardial cells and nuclei was significantly
increased, but the collagen component was significantly decreased
(Fig. 2B).
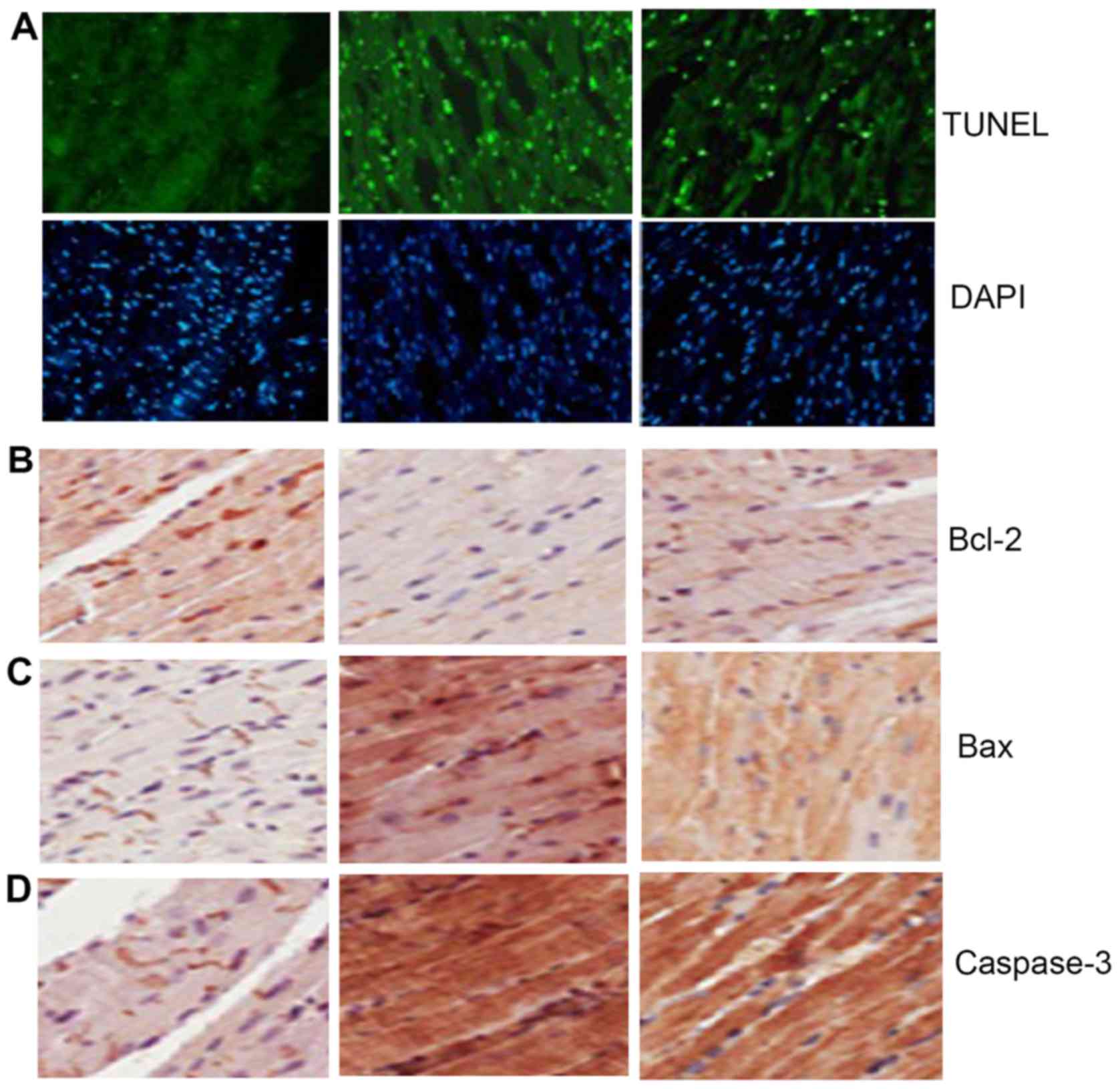
Berberine reduces cell apoptosis after
myocardial infarction
TUNEL staining showed that compared with that in
Sham group, the myocardial cell apoptosis in Sal group was
significantly increased, suggesting that berberine can inhibit
myocardial cell apoptosis of heart failure (Fig. 3A). Immunohistochemical detection
showed yellow brown staining region in the three groups of
myocardial tissues, indicating that the protein expression in
Bcl-2, Bax and caspase-3 existed in the three groups of myocardial
tissues. Compared with those in Sham group, Bcl-2 in Sal group was
significantly decreased, but Bax and caspase-3 were significantly
increased, suggesting that berberine can increase the expression of
Bcl-2 and decrease the expression of Bax and caspase-3 (Fig. 3B-D).
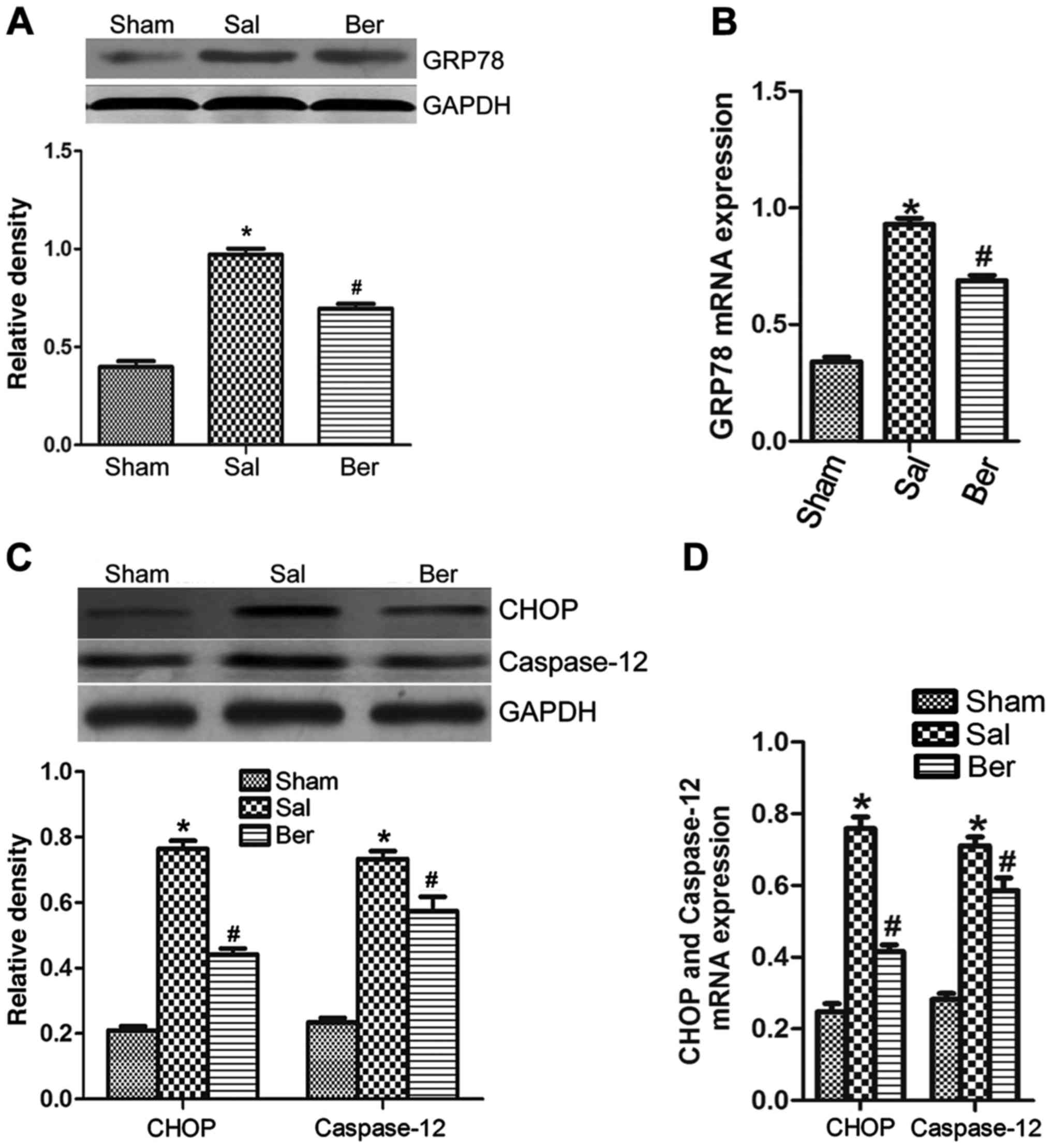
Berberine alleviates ERS response
after myocardial infarction
The results of western blot analysis showed that the
expression of GRP78 (Fig. 4A and B),
CHOP and caspase-12 (Fig. 4C and D)
in myocardium was increased significantly after myocardial
infarction, suggesting that berberine can significantly decrease
the expressions of GRP78, CHOP and caspase-12. The results of
RT-PCR further proved that the mRNA expression in GRP78, CHOP and
caspase-12 were increased after myocardial infarction, suggesting
that berberine can significantly reduce the mRNA expression in
GRP78, CHOP and caspase-12 (Fig.
4).
Discussion
Myocardial infarction leads to heart failure and to
changes in cardiac hemodynamics. LVEDP is an index reflecting the
left ventricular preload, which depends on the returned blood
volume before ventricular systole and cardiac ejection function.
The maximum ascending velocity (+dp/dt) of left ventricular
pressure during isovolumetric contraction phase reflects the
maximum velocity of tension changes in ventricular wall in systole.
It is affected by cardiac afterload and myocardial contractility,
which is an important index to evaluate myocardial contractility.
The maximum descending velocity (-dp/dt) of left ventricular
pressure during isovolumetric relaxation phase is mainly affected
by the preload of myocardial relaxation. ±dp/dt will be decreased
and LVEDP will be increased when myocardial systolic and diastolic
function is inhibited (18). In this
experiment, compared with that in Sham group, LVEDP in Sal group
was significantly increased and ±dp/dt was decreased, indicating
that the myocardial systolic and diastolic function of rats with
heart failure after myocardial infarction was impaired. Berberine
can reduce LVEDP, improve LVSP and ±dp/dt and decrease the plasma
BNP level, indicating that berberine can improve the cardiac
function of heart failure after myocardial infarction.
Cardiac remodeling occurs after myocardial
infarction, which is manifested as the disordered arrangement of
myocardial cells and significant increase of myocardial fibrosis in
heart morphology (19). In this
study, myocardial cells were arranged disorderly and myocardial
fibrosis was obvious with cardiac remodeling in non-infarcted zone
in Sal group. Berberine can significantly inhibit the left
ventricular myocardial fibrosis of heart failure and reduce cardiac
remodeling.
Previous findings showed that, at 1–6 h after
myocardial infarction, the frequency of myocardial cell apoptosis
away from the infarcted zone was significantly correlated with
LVEDP, reflecting the severity of left ventricular insufficiency
(20). In addition, the nitric oxide
synthase gene deletion via gene knockout showed that the number of
apoptotic myocardial cells was decreased and LVEF was increased
after myocardial infarction, suggesting that the improvement of
cardiac function in heart failure is closely related to the
decrease of frequency of myocardial cell apoptosis away from the
infarcted zone, and the myocardial cell apoptosis away from the
infarcted zone may be one of the important reasons for cardiac
remodeling and development of heart failure after myocardial
infarction (20). In heart failure,
the hypoxia-ischemia of myocardial cells, oxidative stress,
inflammatory factors, mechanical load and neuroendocrine factors,
can induce myocardial cell apoptosis (21,22), but
which factor dominates in heart failure remains unclear, and the
specific mechanism of myocardial apoptosis and relationship need to
be further studied. At present, animal experiments and autopsy of
patients with heart failure have shown that myocardial cell
apoptosis exists in heart failure, but the proportion of apoptosis
varies in different studies. TUNEL staining is a sensitive index of
evaluating apoptosis. In this study, the level of myocardial cell
apoptosis was evaluated by TUNEL staining. It was found that the
proportion of myocardial cell apoptosis in non-infarcted zone in
heart failure after myocardial infarction was much higher than that
in Sham group, and such a change was related to the severity of
cardiac remodeling; and the increased level of myocardial cell
apoptosis in Sal group was related to the increased levels of
plasma BNP and LVEDP and the decreased levels of LVSP and ±dp/dt,
which was consistent with the results in most literature,
suggesting that myocardial cell apoptosis is involved in the
cardiac remodeling in heart failure after myocardial infarction,
deteriorating the cardiac function.
Bcl-2 and Bax are the most important members of
Bcl-2 family, which are involved in the regulation of apoptosis
(23): The decreased protein
expression of Bcl-2, the increased protein expression of Bax and
the downregulation of Bcl-2/Bax protein expression level induce
cell apoptosis. In this study, it was found that Bcl-2 protein
expression was decreased in heart failure after myocardial
infarction, and Bax protein expression was increased, which was
consistent with increased myocardial cell apoptosis. After
berberine treatment, Bcl-2 protein expression was increased, Bax
protein expression was decreased and the apoptosis level was
decreased, suggesting that berberine inhibits the myocardial cell
apoptosis by upregulating the expression of Bcl-2/Bax. Caspase-3 is
a member of caspase family, which is an important downstream
effector molecule in the apoptosis process and the executor of
apoptosis (24). The results of this
experiment showed that caspase-3 protein expression in myocardial
tissue of heart failure after myocardial infarction was increased,
but caspase-3 protein expression was decreased after berberine
intervention, suggesting that the inhibitory effect of berberine on
caspase-3 expression is involved in the regulation of apoptosis,
thus promoting cell survival.
Previous findings have confirmed ERS and ERS-induced
myocardial cell apoptosis existed in the pathological process of
heart failure, which is manifested as the increased GRP78
expression (25,26) of ERS response and the activation of
ERS-mediated CHOP and caspase-12 pathways (12,27). The
rat model of heart failure after myocardial infarction was
established in this experiment. WB detection showed that compared
with that in Sham group, GRP78 protein expression was increased;
RT-PCR showed mRNA expression in GRP78 was increased, indicating
that ERS response is activated in heart failure after myocardial
infarction, and protein and mRNA expression of CHOP and caspase-12
are increased. TUNEL staining showed that the number of apoptotic
myocardial cells in heart failure was increased, indicating that
ERS response promotes myocardial cell apoptosis through the above
apoptotic signaling pathways, which is consistent with the result
of previous studies. The protein and mRNA expression of GRP78, CHOP
and caspase-12 were downregulated by berberine, and the ratio of
positive cells in TUNEL staining was decreased compared with that
in Sal group, indicating that berberine inhibits the myocardial
cell apoptosis through inhibiting the ERS response in heart failure
after myocardial infarction and ERS-induced CHOP and caspase-12
apoptosis signaling pathways.
In conclusion, berberine can inhibit the myocardium
cell apoptosis of heart failure after myocardial infarction, and
its mechanism may be realized through affecting the ERS in
myocardial tissue of heart failure after myocardial infarction and
CHOP and caspase-12 apoptotic signaling pathway, upregulating
Bcl-2/Bax expression and downregulating caspase-3 expression, thus
inhibiting cardiac remodeling and protecting the cardiac
function.
Acknowledgements
Not applicable.
Funding
This study was supported by the Medical Innovation
Project of Fujian Provice of China (2014-XB-30) and the Natural
Science Foundation of Fujian Provice of China (2018J01406).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL, KC and XD conceived and designed the study. WL,
GH and GL were responsible for the analysis of the animal data. WS,
LC and YF interpreted the western blot and PCR data and drafted the
manuscript. YL revised the manuscript critically for important
intellectual content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Animal Ethics
Committee of Fujian Medical University Animal Center (Fujian,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Deng J and Zhong Q: Advanced research on
the microRNA mechanism in heart failure. Int J Cardiol. 220:61–64.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu L, Wu J and Kennedy DJ: Regulation of
cardiac remodeling by cardiac Na(+)/K(+)-ATPase isoforms. Front
Physiol. 7:3822016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karaye KM, Lindmark K and Henein MY: One
year survival in nigerians with peripartum cardiomyopathy. Heart
Views. 17:55–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sung MM and Dyck JR: Therapeutic potential
of resveratrol in heart failure. Ann NY Acad Sci. 1348:32–45. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao Z, Luo J, Ma L, Luo X and Huang L:
Effect of granulocyte colony stimulating EPC on cardiac function
and myocardial energy expenditure in patients with heart failure
after myocardial infarction. Int J Clin Exp Med. 8:16578–16584.
2015.PubMed/NCBI
|
|
6
|
Bonnefoy E and Kirkorian G: Mortality of
myocardial infarction. Ann Cardiol Angeiol (Paris). 60:311–316.
2011.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roig T, Márquez MA, Hernández E, Pineda I,
Sabartés O, Miralles R and Inzitari M: Geriatric assessment and
factors associated with mortality in elderly patients with heart
failure admitted to an acute geriatric unit. Rev Esp Geriatr
Gerontol. 48:254–258. 2013.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alpert MA, Omran J and Bostick BP: Effects
of obesity on cardiovascular hemodynamics, cardiac morphology, and
ventricular function. Curr Obes Rep. 5:424–434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suarez G and Meyerrose G: Heart failure
and galectin 3. Ann Transl Med. 2:862014.PubMed/NCBI
|
|
10
|
Kinoshita T, Ishikawa Y, Arita M,
Akishima-Fukasawa Y, Fujita K, Inomata N, Suzuki T, Namiki A,
Mikami T, Ikeda T, et al: Antifibrotic response of cardiac
fibroblasts in hypertensive hearts through enhanced TIMP-1
expression by basic fibroblast growth factor. Cardiovasc Pathol.
23:92–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park CS, Cha H, Kwon EJ, Sreenivasaiah PK
and Kim DH: The chemical chaperone 4-phenylbutyric acid attenuates
pressure-overload cardiac hypertrophy by alleviating endoplasmic
reticulum stress. Biochem Biophys Res Commun. 421:578–584. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xin W, Li X, Lu X, Niu K and Cai J:
Involvement of endoplasmic reticulum stress-associated apoptosis in
a heart failure model induced by chronic myocardial ischemia. Int J
Mol Med. 27:503–509. 2011.PubMed/NCBI
|
|
13
|
Chen K, Li G, Geng F, Zhang Z, Li J, Yang
M, Dong L and Gao F: Berberine reduces ischemia/reperfusion-induced
myocardial apoptosis via activating AMPK and PI3K-Akt signaling in
diabetic rats. Apoptosis. 19:946–957. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang W, Li K, Guan F, Yao F, Yu Y, Zhang
M, Hatch GM and Chen L: Berberine pretreatment confers
cardioprotection against ischemia-reperfusion injury in a rat model
of type 2 diabetes. J Cardiovasc Pharmacol Ther. 21:486–494. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Doggrell SA: Berberine - a novel approach
to cholesterol lowering. Expert Opin Investig Drugs. 14:683–685.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu JC, Chan P, Chen YJ, Tomlinson B, Hong
SH and Cheng JT: The antihypertensive effect of the berberine
derivative 6-protoberberine in spontaneously hypertensive rats.
Pharmacology. 59:283–289. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Imenshahidi M and Hosseinzadeh H: Berberis
vulgaris and berberine: An update review. Phytother Res.
30:1745–1764. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seara FAC, Maciel L, Barbosa RAQ,
Rodrigues NC, Silveira ALB, Marassi MP, Carvalho AB, Nascimento JHM
and Olivares EL: Cardiac ischemia/reperfusion injury is inversely
affected by thyroid hormones excess or deficiency in male Wistar
rats. PLOS ONE. 13:e1903552018. View Article : Google Scholar
|
|
19
|
Meijers WC, van der Velde AR,
Pascual-Figal DA and de Boer RA: Galectin-3 and post-myocardial
infarction cardiac remodeling. Eur J Pharmacol. 763:115–121. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sam F, Sawyer DB, Chang DL, Eberli FR,
Ngoy S, Jain M, Amin J, Apstein CS and Colucci WS: Progressive left
ventricular remodeling and apoptosis late after myocardial
infarction in mouse heart. Am J Physiol Heart Circ Physiol.
279:H422–H428. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li S, Feng J, Wei S, Qian X, Cao J and
Chen B: Delayed neutrophil apoptosis mediates intermittent
hypoxia-induced progressive heart failure in pressure-overloaded
rats. Sleep Breath. 20:95–102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barajas-Espinosa A, Basye A, Angelos MG
and Chen CA: Modulation of p38 kinase by DUSP4 is important in
regulating cardiovascular function under oxidative stress. Free
Radic Biol Med. 89:170–181. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang W, Shi HY and Zhang M: Maspin
overexpression modulates tumor cell apoptosis through the
regulation of Bcl-2 family proteins. BMC Cancer. 5:502005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun Y, Lin Y, Li H, Liu J, Sheng X and
Zhang W: 2,5-Hexanedione induces human ovarian granulosa cell
apoptosis through BCL-2, BAX, and CASPASE-3 signaling pathways.
Arch Toxicol. 86:205–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gupta MK, Tahrir FG, Knezevic T, White MK,
Gordon J, Cheung JY, Khalili K and Feldman AM: GRP78 interacting
partner Bag5 responds to ER stress and protects cardiomyocytes from
ER stress-induced apoptosis. J Cell Biochem. 117:1813–1821. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu L, Wang HJ, Xin Q, Zhou XM, Zhao YJ,
Huang X and Zhao M: The potential effects of endoplasmic reticulum
stress on the apoptosis of myocardial cells from mice with heart
failure induced by acute viral myocarditis caused by B3 Coxsackie
virus. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 30:461–464. 2014.(In
Chinese). PubMed/NCBI
|
|
27
|
Fu HY, Minamino T, Tsukamoto O, Sawada T,
Asai M, Kato H, Asano Y, Fujita M, Takashima S, Hori M, et al:
Overexpression of endoplasmic reticulum-resident chaperone
attenuates cardiomyocyte death induced by proteasome inhibition.
Cardiovasc Res. 79:600–610. 2008. View Article : Google Scholar : PubMed/NCBI
|