Introduction
Type 2 diabetes mellitus (DM) is defined as a
metabolic disorder in which patients become hyperglycemic from
defects in insulin secretion or action, as well as from the
hyperglucagonemia that ensues (1).
Diabetic nephropathy (DN) is one of the major chronic microvascular
complications of DM (2), and occurs
in 20–40% of all patients with type 2 DM (2,3). DN is
caused by rising blood glucose and hypertension, obesity, oxidative
stress, and unmitigated inflammation. Repeated or chronic insult to
the kidneys leads to irreversible fibrotic damage of the glomeruli
(glomerulosclerosis) and kidney tubules (tubulointerstitial
fibrosis), and ultimately leads to end-stage renal disease (ESRD)
(4). Due to these potential
complications, DN can also cause a significant financial burden.
Therefore, early diagnosis and management are critical.
Diabetes with microalbuminuria (30 mg/day) is an
early clinical sign of DN; this subsequently progresses to
macroalbuminuria (>300 mg/day) and a decline in glomerular
filtration rate (5). Over the past
20 years, therapies have significantly progressed beyond those that
primarily reduce glucose levels; however, these therapies are also
associated with unwanted side effects, such as weight gain. More
recently developed drug classes can effectively improve glycemia
and promote weight neutrality or even weight loss (6). Dipeptidyl peptidase-4 (DPP-4)
inhibitors belong to these more recent drug classes.
DPP-4 inhibitors are a class of oral
antihyperglycemic treatments for type 2 diabetes. They include
sitagliptin, saxagliptin, vildagliptin, linagliptin, and
teneligliptin. Sitagliptin, the first DPP-4 inhibitor on the
market, was approved in 2006 for patients with type 2 DM (6), and has been widely used for the
treatment of both DM and its complications, particularly DN. A
recent study showed that DPP-4 inhibition in conjunction with
angiotensin II type 1 receptor blockade produced enhanced
renoprotective effects in patients with DN (7). However, whether sitagliptin has
renoprotective properties remains controversial. Hattori's
(8) study suggested that sitagliptin
reduces albuminuria, which is dependent on satisfactory control of
blood sugar. However, other groups discount the presence of a
significant relationship between the change in the UACR and HbA1c,
and claim that sitagliptin reduces blood pressure by increasing
sodium diuresis (9–12). Sitagliptin increases the level of
glucagon-like polypeptide (GLP)-1, GLP-1 decreases salt intake and
increases urinary salt excretion by directly inhibiting the
Na+/H+ exchange at the proximal tubular cells
(11,12). The abovementioned studies did not
measure sodium diuresis and the sample sizes of the
sitagliptin-treated cohorts were too small to be generalized; for
example, Hattori's (8) study
included only 36 subjects. Furthermore, some investigations (such
as that of Kawasaki et al (9)) were non-randomized and uncontrolled
observation studies. Thus, whether sitagliptin truly provides a
benefit remains unconfirmed. To that end, we performed a systematic
review of several randomized controlled trials (RCTs) to
investigate the effect of sitagliptin in patients with type 2
diabetes that also had incipient nephropathy. Moreover, we deem
that sitagliptin truly has renoprotective effect for patients with
early-stage DN.
Materials and methods
Search strategy
Electronic searches of the PubMed, OVID, Cochrane
library, Chinese National Knowledge Infrastructure (CNKI) and
Wanfang databases were performed independently by two investigators
in September 2017. The search terms were: (‘Sitagliptin phosphate’
or ‘sitagliptin’ or ‘sitagliptin phosphate monohydrate’) and
(‘diabetic nephropathies’ or ‘diabetic nephropathy’ or ‘diabetic
kidney diseases’ or ‘diabetic complications’). We filtered the
publications by titles, abstracts and keywords, and assessed the
full-text versions while employing our inclusion and exclusion
criteria (described below). The publication languages were
restricted to English and Chinese. All clinical studies except case
reports were chosen for our analysis. The first reports'
bibliographies, as well as references from selected studies, were
also searched for other relevant publications.
Selection criteria
We collected all relevant articles focusing on the
relationship between sitagliptin and renal function index. The
inclusion criteria were as follows: i) RCTs that compared
sitagliptin to a control group that received routine treatment, or
to drugs other than DPP-4 inhibitors; ii) all participants were
type 2 diabetes patients with incipient nephropathy, with
persistent microalbuminuria (incipient DN) defined as a urinary
albumin-to-creatinine ratio between 30 and 300 mg/g in two morning
spot urine collections sustained over 12 weeks (2); iii) renal function outcomes were
reported in the studies; and iv) the study duration was longer than
eight weeks. The following exclusion criteria were applied: i)
Articles that had no information on renal function or the
biochemical index of type 2 DN; ii) case reports, letters, reviews,
expert opinion, conference abstracts, editorials, and non-English
and non-Chinese language papers; and iii) all articles using cell
lines and/or in vitro/ex vivo studies.
Data extraction
The qualified studies were reviewed by two
investigators independently; differences in interpretation were
resolved by consensus. The following information was recorded for
each study: General data, patient information, test drug
information, course of treatment, and outcome data. The two
researchers identified the articles that satisfied the selection
criteria. Data heterogeneity was assessed to determine whether the
studies could be analyzed. Study characteristics and clinical
examination data were generalized and are described in table
format.
Statistical analysis
Quantitative and qualitative analyses were performed
on corrected data. The RevMan v.5.3 software was downloaded from
the Cochrane collaboration website and used for meta-analysis. The
clinical and methodological heterogeneity of the included studies
were analyzed using the χ2 and I2 tests. The
fixed-effects model was used when P>0.1 and
I2<50%, and each study showed acceptable
heterogeneity. In cases where P<0.1 and I2>50%,
and the study showed essential heterogeneity, we performed subgroup
or sensitivity analysis. However, when many confounding factors
appeared in the studies, the random-effects model was chosen
regardless of the P and I2 values, according to the
Cochrane Handbook (13). The mean
difference (MD) or standard mean difference (SMD) and 95%
confidence intervals (CIs) were used to compare continuous
variables; risk ratios and 95% CIs were used to compare dichotomous
variables (13). Whenever
heterogeneity was significant, we sought to identify its source
using the study-by-study exclusion method. P<0.05 was considered
to indicate a statistically significant difference. Egger's test
and funnel plots were used to detect publication bias.
Assessments of quality of
evidence
Two investigators independently assessed the risk of
bias as recommended by the Cochrane Handbook for Systematic Reviews
of Interventions (13).
Disagreements were resolved by a third reviewer. The quality
appraisal of the literature included: Random sequence generation,
allocation concealment, blinding of participants and personnel,
blinding of outcome assessment, incomplete outcome data, selective
reporting, and other biases. Articles that had clearly described
details and met or surpassed the quality criteria were defined as
low risk; otherwise, they were deemed high-risk. Equivocal articles
in terms of quality criteria were deemed to be of unclear risk.
Results
Study characteristics
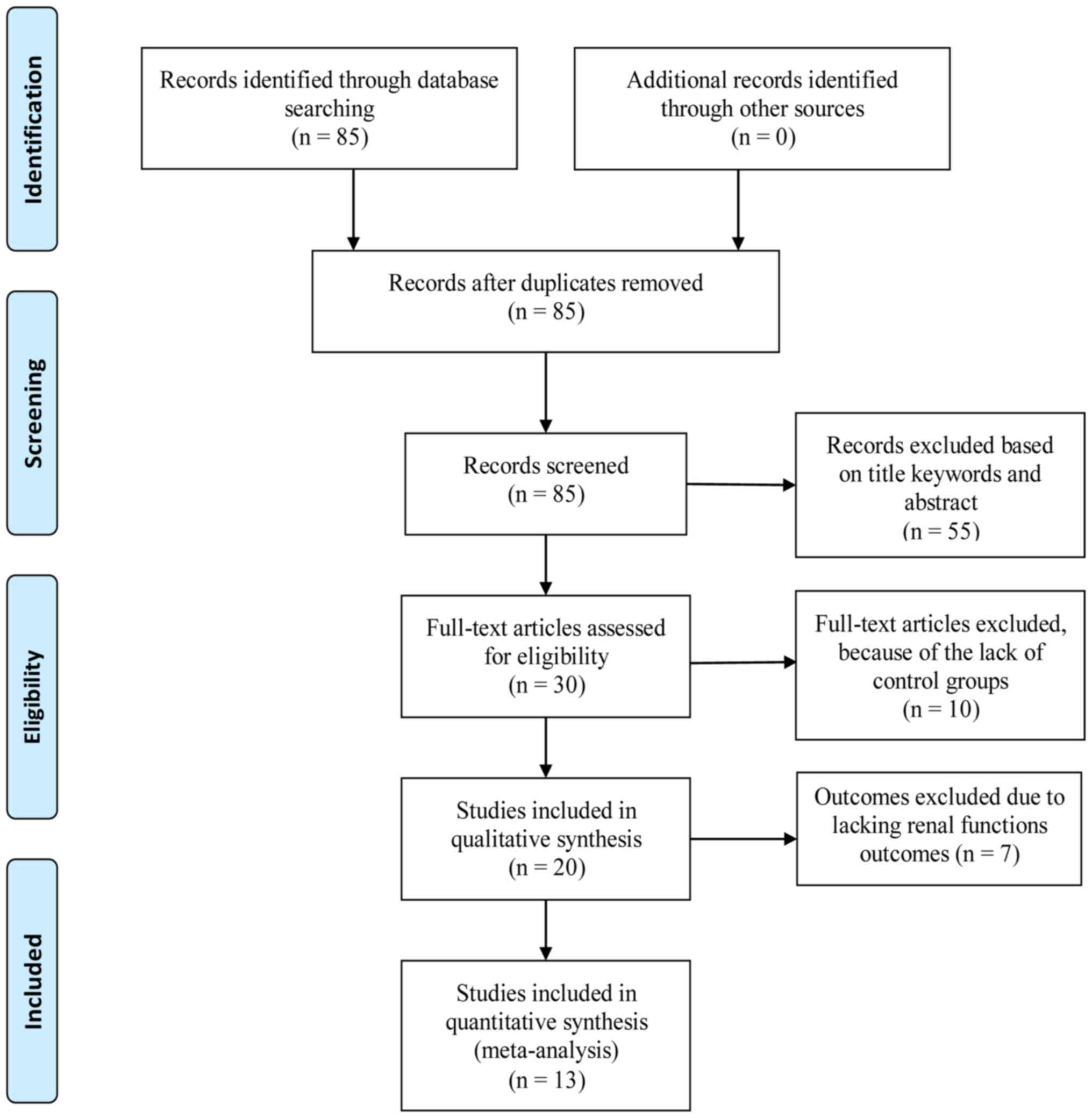
We initially collected 85 articles from the PubMed,
OVID, the Cochrane library, CNKI, and Wanfang databases. Thirty
full-text studies were extracted for more detailed assessment, and
were filtered via their titles and abstracts for final eligibility
assessment. Ten crossover trials without control groups were
excluded, as were seven trials that lacked renal function marker
analysis. Eventually, 13 publications that satisfied the inclusion
criteria were selected for this meta-analysis. The article search
process and study selection are shown in Fig. 1.
Of the total 942 patients included, 481 belonged to
treatment groups, while 461 belonged to control groups receiving
routine treatment such as controlled diet and exercise therapy,
antihypertensive drugs, or other antihyperglycemic treatments
excluding DPP-4 inhibitors. The treatment groups included
sitagliptin combined with routine treatment, antihypertensive
drugs, or other antihyperglycemic treatment. The doses of
sitagliptin used in the included trials differed slightly.
Sitagliptin intake was generally 100 mg per day; only one trial
used 50 mg per day (14). Among the
13 trials, 12 adopted a two-armed parallel group design while one
(Zhang et al (15)) adopted a
three-armed group design. The durations of interventions in the
diabetes trials were different, ranging from eight to 24 weeks. Six
studies lasted 24 weeks, one lasted eight weeks, and the remainder
lasted 12 weeks. Basic information about the involved studies is
shown in Table I.
 | Table I.Characteristics of the included
studies. |
Table I.
Characteristics of the included
studies.
| Stydy, year | Sample size | Testing scheme | Test group | Control group | Duration
(weeks) | Outcomes | (Refs.) |
|---|
| Mori et al,
2014 | 41/44 | RAN | SIT (50 mg qd) Plus
RT | RT | 24 | ACFGH | (14) |
| Zhang et al,
2016 | 29/29 | UNK | SIT (100 mg qd)
Plus IRB | IRB (150 mg
qd) | 12 | GI | (15) |
| Hu et al,
2016 | 80/80 | RAN | SIT (100 mg qd)
Plus RT | RT | 12 | ABCH | (16) |
| Huang et al,
2016 | 30/30 | RAN | SIT (100 mg qd)
Plus GLI | GLI (30 mg qd) | 12 | ABCGJ | (17) |
| Jin et al,
2016 | 40/40 | RAN | SIT (100 mg qd)
Plus RT | RT | 24 | ACFJ | (18) |
| Lan et al,
2016 | 24/24 | RAN | SIT (100 mg qd)
Plus RT | RT | 24 | ABCL | (19) |
| Yang et al,
2015 | 36/36 | RAN | SIT (100 mg qd)
Plus RT | RT | 24 | ACDEILK | (20) |
| Ying et al,
2016 | 32/35 | UNK | SIT (100 mg qd)
Plus RT | RT | 12 | ACL | (21) |
| Zhao et al,
2014 | 35/13 | UNK | SIT (100 mg qd)
Plus VAL | VAL (80 mg qd) | 24 | CI | (22) |
| Huang et al,
2016 | 30/30 | RAN | SIT (100 mg qd)
Plus RT | RT | 12 | ACDEFK | (23) |
| Wang et al,
2015 | 30/30 | RAN | SIT (100 mg qd)
Plus Insulin | INS | 8 | ADEIJ | (24) |
| Han et al,
2015 | 40/40 | RAN | SIT (100 mg qd)
Plus GLI | GLI (30 mg qd) | 12 | ABCDEFIK | (25) |
| Hao et al,
2015 | 34/30 | RAN | SIT (100 mg qd)
Plus RT | RT | 24 | ACDEFGH | (26) |
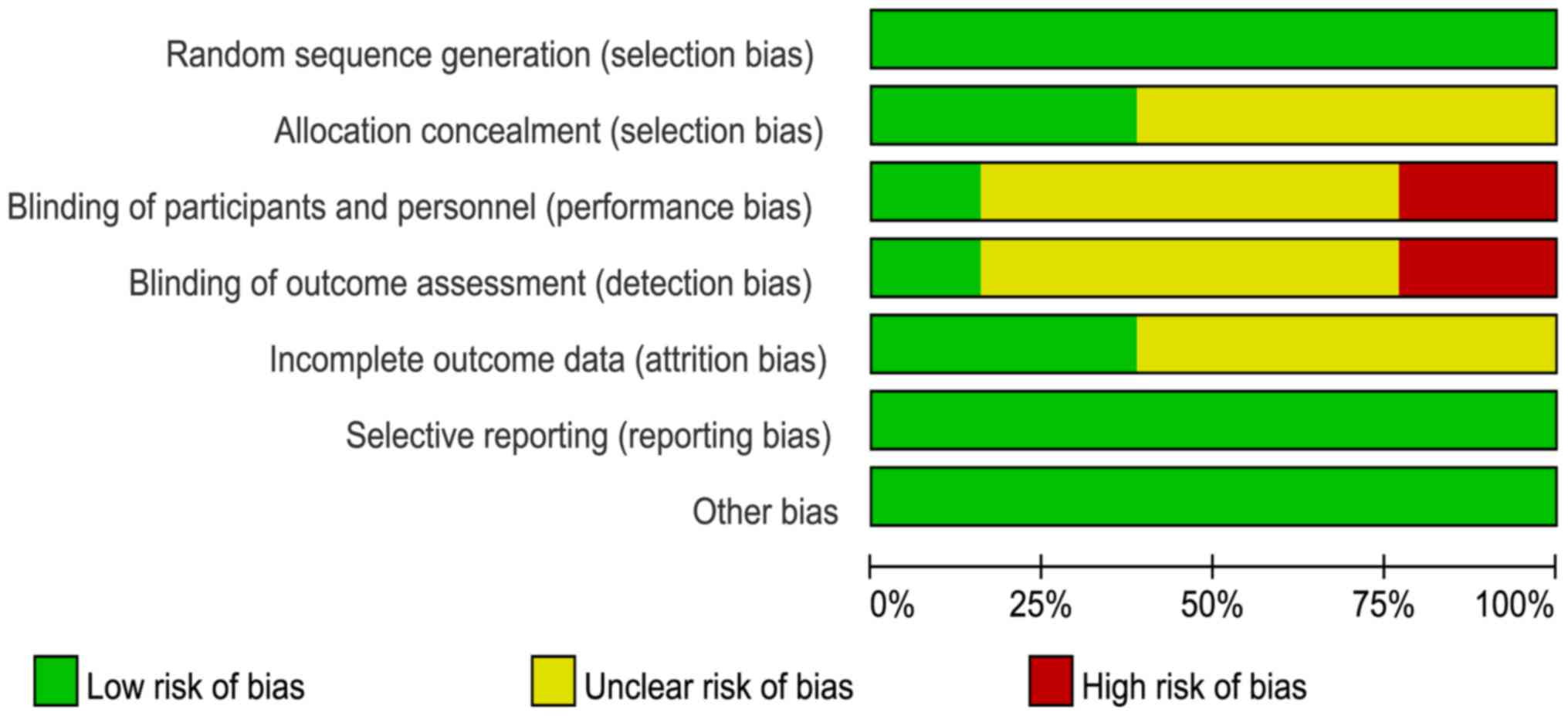
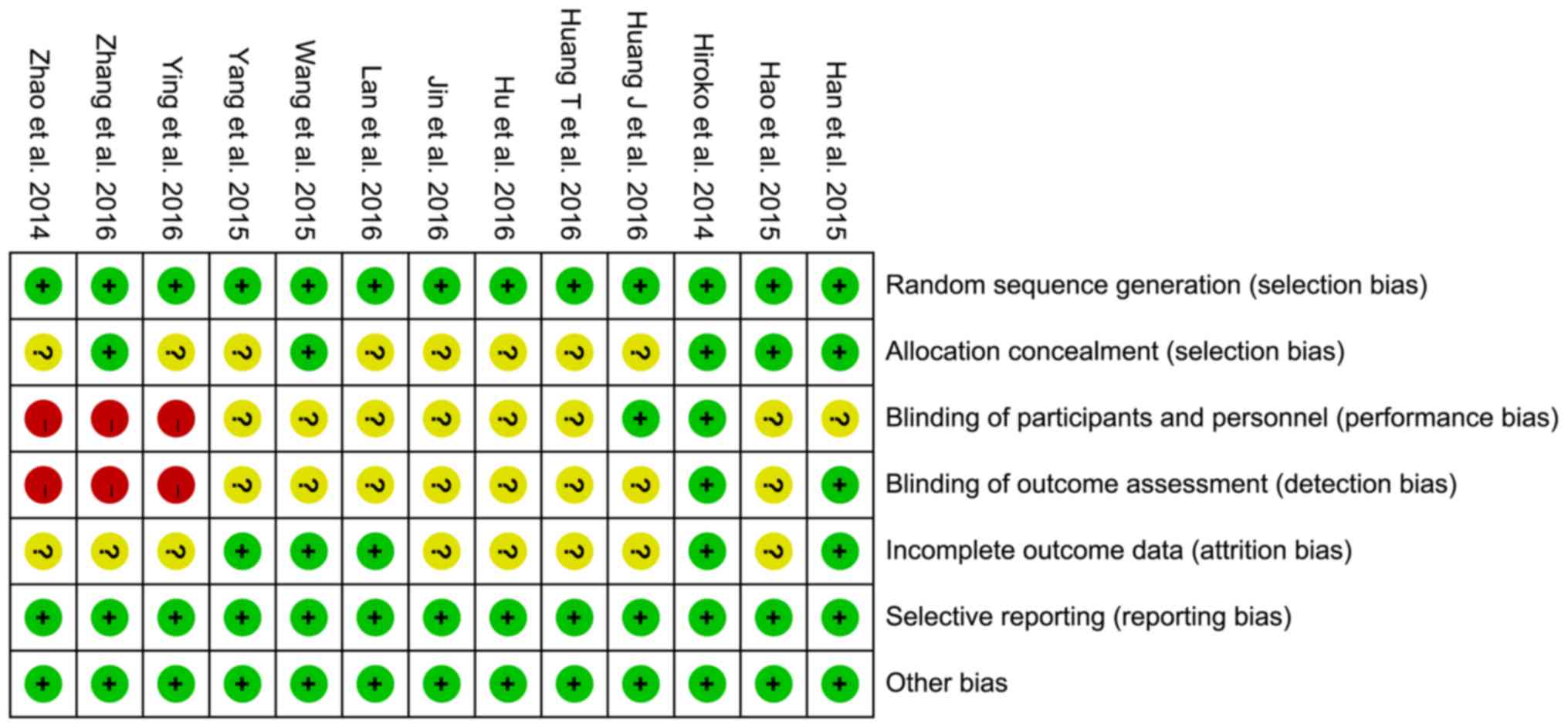
Risk of bias
The risk of bias assessments are shown in Figs. 2 and 3. All trials were randomly designed, but
eight (16–23) were judged to have unclear risks of
bias owing to allocation concealment.
Blinding of participants, personnel, and outcome
assessors were not undertaken in three studies (15,21,22).
Additionally, it was not clear whether the participants and
personnel were blinded in eight trials (16,18–20,23–26).
Furthermore, it was unclear if the outcome assessors were blinded
in eight of the studies (16–20,23,24,26).
Attrition bias was ambiguous in eight of the trials (15–18,21–23,26);
however, all trials showed a low risk of reporting bias or other
biases.
Effect of interventions
Effect on proteinuria and renal
function
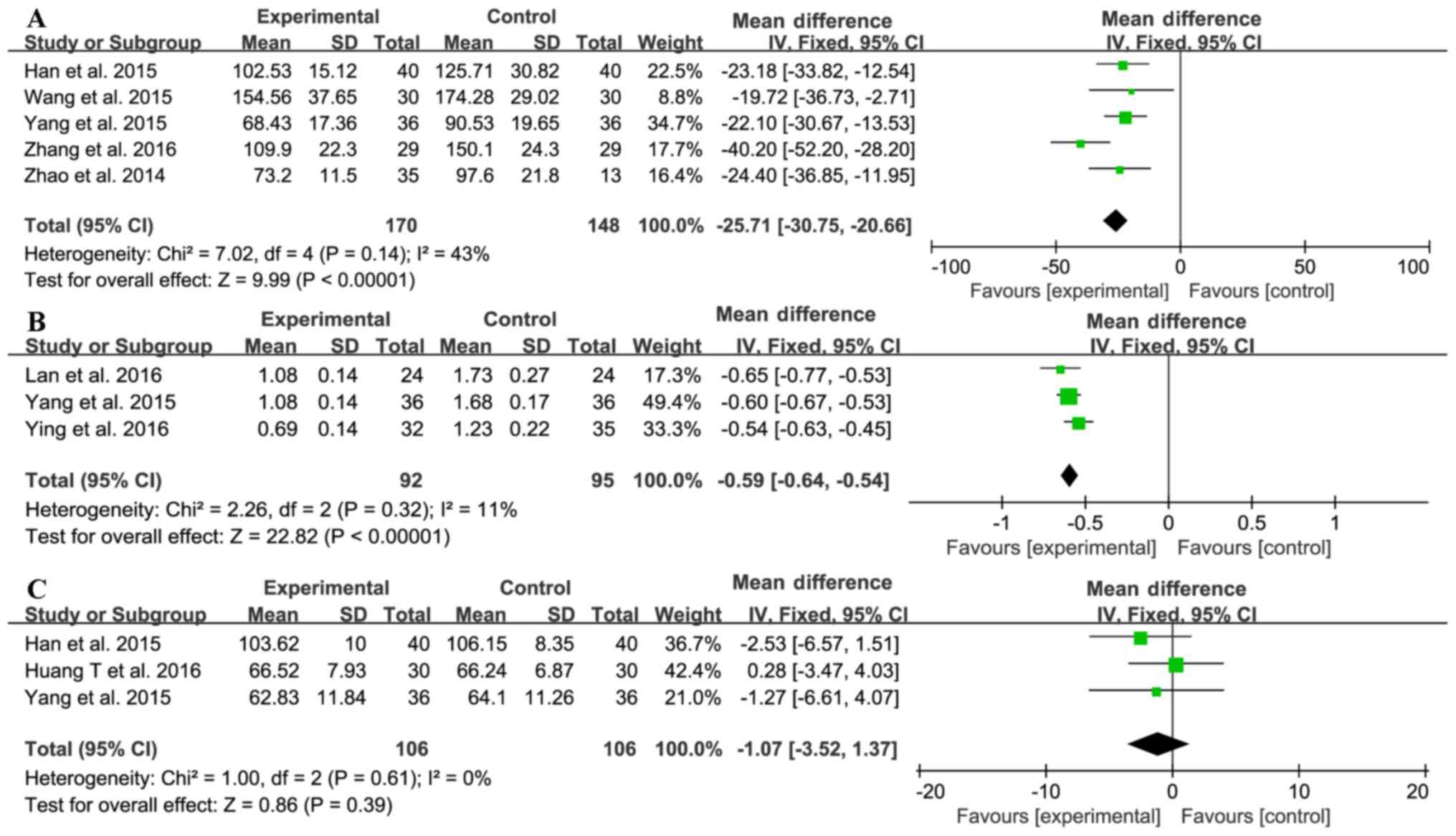
Five trials (15,20,22,24,25)
investigated the effect of sitagliptin on quantitative 24-hour
urinary total protein (UTP) test results (Fig. 4A). The sitagliptin and control groups
comprised 170 and 148 patients, respectively. No significant
heterogeneity was found between the results (χ2=7.02;
I2=43%, P=0.14); hence, a fixed-effect model was used
for statistical analysis. The 24-h UTP was lower in the treatment
group than in the control group (MD=−25.71, 95% CI-30.75 to −20.66,
P<0.00001). Three trials (19–21) were
used to compare serum cystatin C (CysC) levels between the two
groups (Fig. 4B). The treatment
group included 92 patients and the control group included 95
patients. No significant heterogeneity was found between the two
groups (χ2=2.26; I2=11%, P=0.32). Patients
receiving sitagliptin had better CysC levels than the control group
(MD=−0.59, 95% CI-0.64 to-0.54, P<0.00001). Three trials
reported serum creatinine (SCr) levels (20,23,25) with
106 patients in each of the treatment and control groups. These
trials showed homogeneity in the consistency of their results
(χ2=1.00; I2=0%, P=0.61). Therefore, a
fixed-effect model was selected for statistical analysis, which
revealed no difference between the sitagliptin and control groups
in terms of changes in SCr levels (MD=−1.07, 95% CI-3.52 to 1.37,
P=0.39; Fig. 4C). Overall, however,
the results suggested that sitagliptin reduced proteinuria, thus
ameliorating renal function.
Effect on improving inflammation
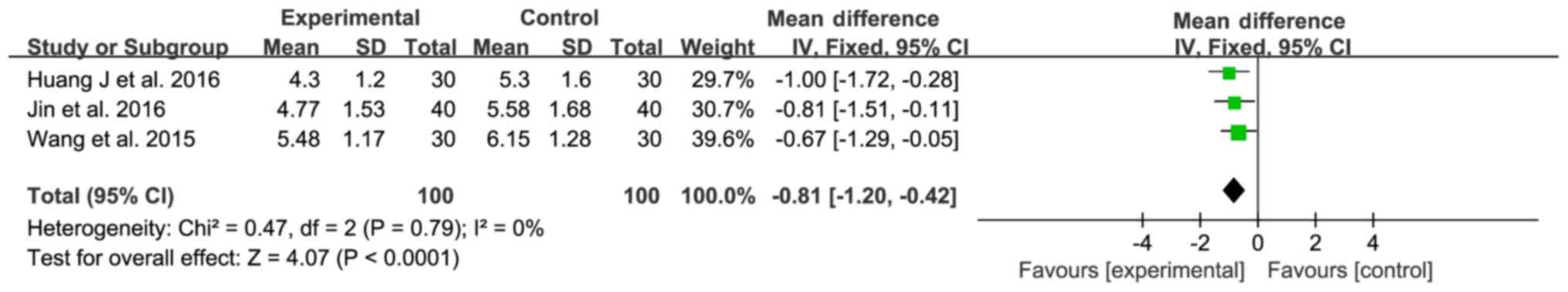
Three trials (17,18,24)
compared C-reactive protein (CRP) levels between the experimental
and control groups (100 patients each); no significant
heterogeneity was observed (χ2=0.47; I2=0%,
P=0.79; Fig. 5) and a fixed-effect
model was used for statistical analysis. The sitagliptin group
expressed lower CRP levels than the control group (MD=−0.81; 95%
CI-1.20 to −0.42; P<0.0001; Fig.
5), indicating that sitagliptin exhibits anti-inflammatory
effects in patients with DN.
Effect on blood glucose and
glycosylated hemoglobin
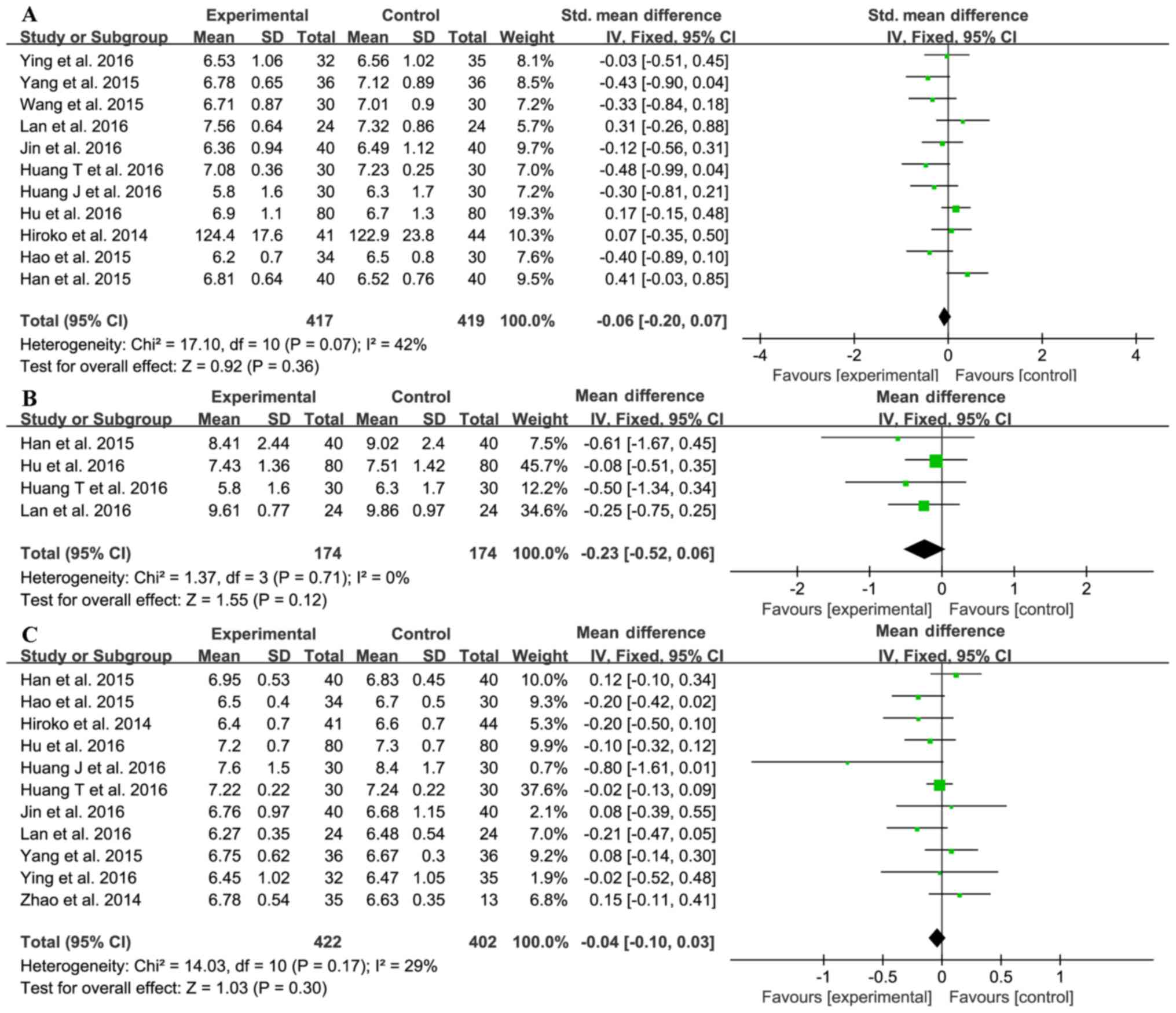
Eleven trials (14,16–21,23–26)
investigated the effect of sitagliptin on fasting blood glucose
(FBG) levels. The sitagliptin and control groups comprised 417 and
419 patients, respectively. The statistical heterogeneity of the
FBG data was unacceptable (χ2=17.10; I2=42%,
P=0.07); therefore, we used the random-effect model for
meta-analysis. The pooled result showed no difference between the
sitagliptin and control groups (SMD=−0.06; 95% CI −0.20 to 0.07;
P=0.36; Fig. 6A).
Four trials (16,19,23,25)
investigated the effect of sitagliptin on postprandial blood
glucose (PBG) with 174 in each of the treatment and control groups;
there was no significant heterogeneity (χ2=1.37;
I2=0%, P=0.71) and a fixed-effect model was used.
However, no differences in FBG between the two groups were detected
(MD=−0.23; 95% CI-0.52 to 0.06; P=0.12; Fig. 6B).
As shown in Fig. 6C,
11 trials (14,16–23,25,26)
investigated the effect of sitagliptin on HbA1c; the heterogeneity
was acceptable (χ2=14.03; I2=29%, P=0.17),
and therefore the fixed-effect model was used. Sitagliptin did not
influence HbA1c levels (MD=−0.04; 95% CI-0.10 to 0.03; P=0.30);
hence, it is unclear if sitagliptin decreases blood glucose
levels.
Effect on blood lipids
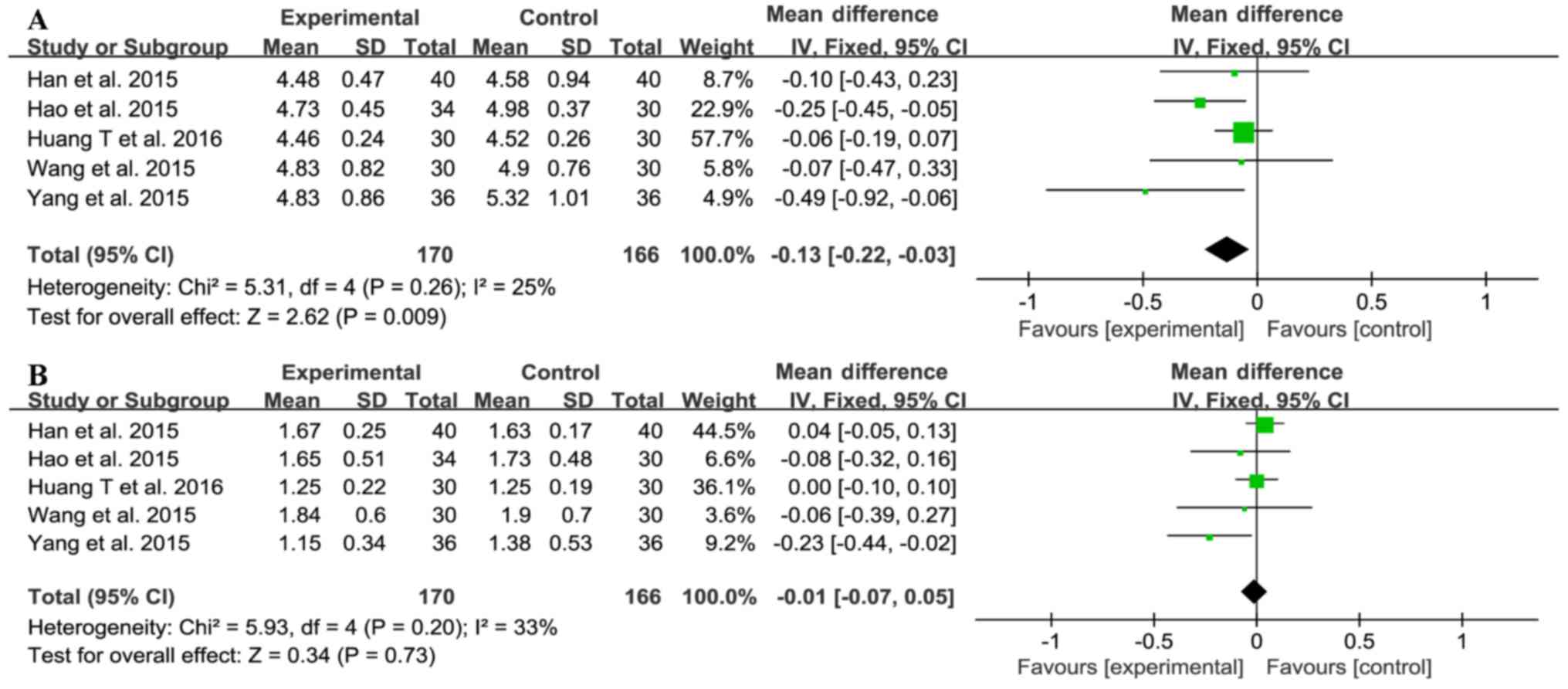
Five trials (20,23–26)
investigated total cholesterol (TC) and triglyceride (TG) levels
(Fig. 7), with 170 subjects in the
treatment group and 166 in the control group. There was no
significant heterogeneity between the TC and TG results (TC:
χ2=5.31, I2=25%, P=0.26; TG:
χ2=5.93, I2=33%, P=0.20), so the fixed-effect
model was used for meta-analysis. Sitagliptin treatment reduced the
level of TC (MD=−0.13, 95% CI-0.22 to-0.03, P=0.009), but not TG
(MD=−0.01, 95% CI-0.07 to 0.05, P=0.73; Fig. 7). These data showed that sitagliptin
does affect blood lipid levels.
Adverse events
Only five trials (15,17,21,22,25)
investigated adverse events, two of which (17,21)
reported no such occurrences. Of the remaining three trials, one
(25) found that patients in the
treatment group presented with hypoglycemia (n=2), dizziness (n=2),
and gastrointestinal tract reaction (n=2); the same conditions were
experienced in patients in their control groups (n=3, n=1, and n=2,
respectively). Another trial (15)
reported a slight cough (n=1) in the control group and
gastrointestinal tract reaction (n=2) in the treatment group. The
final trial (22) reported adverse
effects without specifying their natures. However, there were no
serious adverse events reported in any of these trials. The most
common events were gastrointestinal tract reactions and
hypoglycemia, but these symptoms resolved quickly.
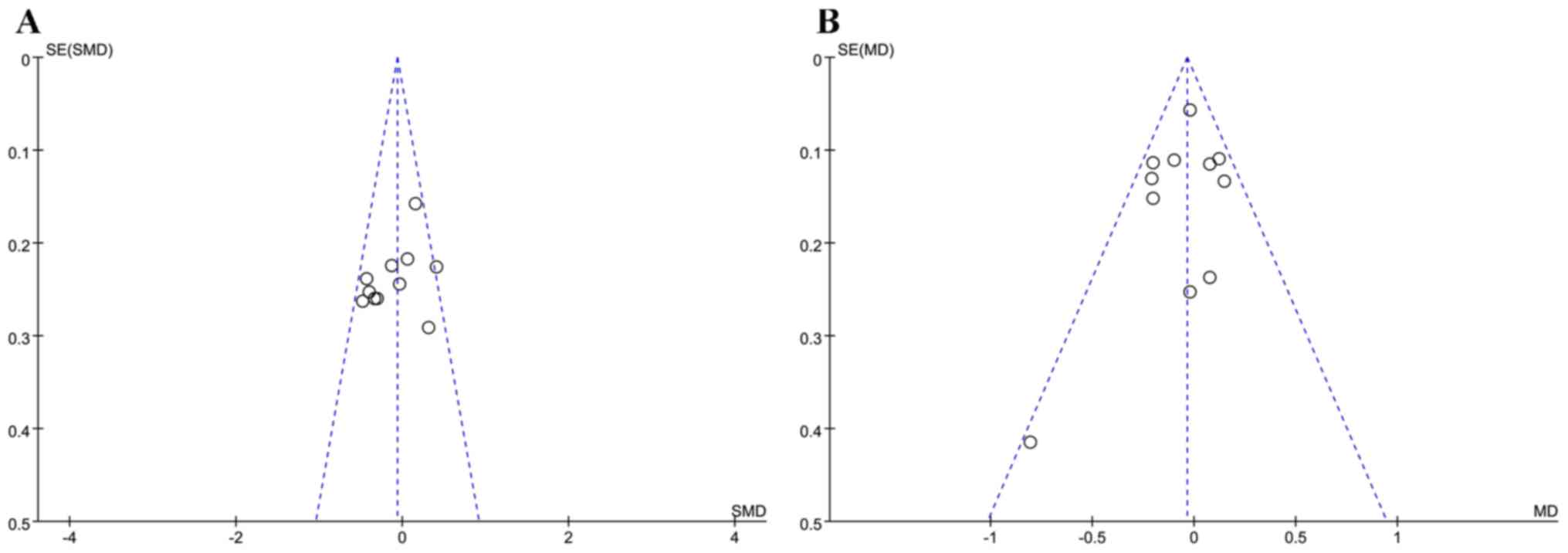
Evaluation of publication bias
In the present study, funnel plots were used to
identify publication bias. Asymmetry is observed in Fig. 8A and B; moreover, one study falls
outside the 95% confidence interval (Fig. 8A). Egger's test of FBG (P=0.164) and
HbAlc (P=0.941) showed that there was no publication bias in the
studies included (Fig. 8A and
B).
Discussion
DN treatment aims to reduce proteinuria. Angiotensin
II receptor blockers (ARBs) are widely used for the improvement of
proteinuria in patients with DN, and provide more favorable
outcomes especially in the incipient DN stage, as shown in the
INNOVATION study (27,28). However, ARBs cannot delay progression
to ESRD, thus rendering renal replacement therapy inevitable.
Therefore, determining whether sitagliptin exhibits a
renoprotective effect is of great scientific value.
Our meta-analysis included 942 patients; most of the
investigated trials adopted a randomized double-blind parallel
control design. For assessing kidney function, 24-h UTP, CysC, and
SCr were chosen as the ideal markers of kidney function because
they are less influenced by age, sex, body mass, and inflammatory
conditions (29,30). Additionally, our data suggested that
sitagliptin may have positive effects on lowering the 24-h UTP,
CysC, and CRP. Although sitagliptin did not appear to affect SCr,
it still offers the benefit of reducing proteinuria, ameliorating
renal function, and producing an anti-inflammatory response in
patients with DN.
Importantly, TGF-β1/Smad3 is known to
mediate fibrosis in DN. A recent study found that sitagliptin
prevented kidney injury, reduced albuminuria, and reduced
inflammation by blocking the TGF-β1/Smad3 signaling
pathway. Wang et al (31)
reported that sitagliptin down-regulated the level of Smad3
phosphorylation by inhibiting TGF-β1, and up-regulated
Smad7 protein expression to prevent the production of collagen IV
and fibronectin and to reduce extracellular matrix accumulation.
Their data inferred that sitagliptin performs anti-fibrosis and
anti-inflammatory functions in the kidney.
With respect to blood glucose and lipid levels,
sitagliptin was found to reduce TC level but appeared to have no
effect on FBG, PBG, HbA1c, or TG. Hence, the role of sitagliptin in
decreasing blood glucose levels remains unclear, which is
inconsistent with prevailing viewpoints that sitagliptin reduces
glucose levels, does not elicit weight gain, and decreases the risk
of hypoglycemia (32,33).
There are some possible reasons for the
discrepancies found in the literature. First, the number of
included articles regarding PBG and TG was less than 10, so the
funnel plot could not evaluate the publication bias. Second,
although there is no publication bias in FBG and HbA1c, as shown by
Egger's test and asymmetry in the funnel plots, we believe that
other biases may have existed in the included studies. Potential
biases in the trials that could have led to incorrect conclusions
regarding sitagliptin include non-disclosure of allocation
concealment, incomplete outcome data, or double-blind analysis
status. Only three of five trials that investigated adverse events
mentioned these potential biases. As conclusions concerning safety
could not be made in our meta-analysis, the safety of sitagliptin
requires more rigorous investigations in future clinical
trials.
Sitagliptin was shown to reduce proteinuria,
ameliorate renal function, and produce an anti-inflammatory effect
in patients with early DN. These findings can be useful for
guidance in the clinical application of sitagliptin.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81573911).
The grantee, JY, was responsible for this article.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WL and JY were involved in conceptualization of the
study and writing of the manuscript. NL and LW curated the data. WX
and LW performed analysis of the data. WX was involved in
acquisition of funding. WL reviewed and edited the manuscript. WL
and QY supervised the study. WX and LW provided the resources. WL
and QY performed the electronic searches on the PubMed, OVID,
Cochrane library, Chinese National Knowledge Infrastructure and
Wanfang databases. NL and JY assessed the quality of evidence and
participated in outlining the inclusion criteria and exclusion
criteria. NL and WL provided final approval of all procedures
performed in the present study.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Unger RH and Orci L: The essential role of
glucagon in the pathogenesis of diabetes mellitus. Lancet. 1:14–16.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tuttle KR, Bakris GL, Bilous RW, Chiang
JL, de Boer IH, Goldstein-Fuchs J, Hirsch IB, Kalantar-Zadeh K,
Narva AS, Navaneethan SD, et al: Diabetic kidney disease: A report
from an ADA Consensus Conference. Diabetes Care. 37:2864–2883.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ahn JH, Yu JH, Ko SH, Kwon HS, Kim DJ, Kim
JH, Kim CS, Song KH, Won JC, Lim S, et al: Prevalence and
determinants of diabetic nephropathy in Korea: Korea national
health and nutrition examination survey. Diabetes Metab J.
38:109–119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Venkatachalam MA, Weinberg JM, Kriz W and
Bidanai AK: Failed tubule recovery, AKI-CKD transition, and kidney
disease progression. J Am Soc Nephrol. 26:1765–1776. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reidy K, Kang HM, Hostetter T and Susztak
K: Molecular mechanisms of diabetic kidney disease. J Clin Invest.
124:2333–2340. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee M and Rhee MK: Sitagliptin for type 2
diabetes: A 2015 update. Expert Rev Cardiovasc Ther. 13:597–610.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fujita H, Taniai H, Murayama H, Ohshiro H,
Hayashi H, Sato S, Kikuchi N, Komatsu T, Komatsu K, Komatsu K, et
al: DPP-4 inhibition with alogliptin on top of angiotensin II type
1 receptor blockade ameliorates albuminuria via up-regulation of
SDF-1α in type 2 diabetic patients with incipient nephropathy.
Endocr J. 61:159–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hattori S: Sitagliptin reduces albuminuria
in patients with type 2 diabetes. Endocr J. 58:69–73. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kawasaki I, Hiura Y, Tamai A, Yoshida Y,
Yakusiji Y, Ikuno Y, Okada M, Ueno H, Tanaka N, Yamagami K, et al:
Sitagliptin reduces the urine albumin-to-creatinine ratio in type 2
diabetes through decreasing both blood pressure and estimated
glomerular filtration rate. J Diabetes. 7:41–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mistry GC, Maes AL, Lasseter KC, Davies
MJ, Gottesdiener KM, Wagner JA and Herman GA: Effect of
sitagliptin, a dipeptidyl peptidase-4 inhibitor, on blood pressure
in nondiabetic patients with mild to moderate hypertension. J Clin
Pharmacol. 48:592–598. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ogawa S, Ishiki M, Nako K, Okamura M,
Senda M, Mori T and Ito S: Sitagliptin, a dipeptidyl peptidase-4
inhibitor, decreases systolic blood pressure in Japanese
hypertensive patients with type 2 diabetes. Tohoku J Exp Med.
223:133–135. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gutzwiller JP, Tschopp S, Bock A, Zehnder
CE, Huber AR, Kreyenbuehl M, Gutmann H, Drewe J, Henzen C, Goeke B
and Beglinger C: Glucagon-like peptide 1 induces natriuresis in
healthy subjects and in insulin-resistant obese men. J Clin
Endocrinol Metab. 89:3055–3061. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Higgins JPT and Green S: Cochrane handbook
for systematic reviews of interventions version 5.1.2 [updated
March 2011]. The Cochrane Collaboration; 2011, http://handbook.cochrane.org/April
8–2018
|
|
14
|
Mori H, Okada Y, Arao T and Tanaka Y:
Sitagliptin improves albuminuria in patients with type 2 diabetes
mellitus. J Diabetes Investig. 5:313–319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang K, Ren Q, Wu T and Du J: Effects of
sitagliptin used alone or combined with irbesartan on changes of
interlukin-18 in early diabetic nephropathy patients. Med J Air
Force. 1:56–58. 2016.(In Chinese).
|
|
16
|
Hu S, Bai X, Li X, Li S and Lv S: Effect
of sitagliptin on microalbuminuria of patients with type 2 diabetes
mellitus. Prog Mod Biomed. 16:5324–5326. 2016.(In Chinese).
|
|
17
|
Huang J, Xu H, Lin Y, Lin B, Wei Y, Yuan B
and Chen X: Effect of sitagliptin in combination with atorvastatin
on urinary albumin-to-creatinine ratio in type 2 diabetic patients
with early nephropathy. Acad J Guangzhou Med Univ. 44:51–53.
2016.(In Chinese).
|
|
18
|
Jin J, Sun H, Xu Z, Lin Z and Tao K:
Sitagliptin influence on clinical indicators in elderly patients
with diabetic nephropathy. Chin J Gerontol. 36:79–81. 2016.(In
Chinese).
|
|
19
|
Lan L and Yan Z: Protective effects of
DPP-4 inhibitors on kidney in patients with type 2 diabetes. Chin
Foreig Med Res. 14:1–3. 2016.(In Chinese).
|
|
20
|
Yang T, Li D, Zhao J, Liu L and Hu L:
Effects of sitagliptin on renal function in patients with early
type 2 diabetic nephropathy. Prog Mod Biomed. 19:3690–3693.
2015.(In Chinese).
|
|
21
|
Ying J, Zhang X, Chen J and Chen G:
Effects of sitagliptin on renal function and
gamma-glutamyltransferase in diabetes combined with kidney injury.
Chin J New Drugs Clin Rem. 35:273–276. 2016.(In Chinese).
|
|
22
|
Zhao C, Guo H, Dai H, Tian J and Zhao Y:
Curative effects of sitagliptin with valsartan on incipient type 2
diabetic nephropathy. Pract Pharm Remed. 11:1420–1423. 2014.(In
Chinese).
|
|
23
|
Huang T: The effects of urine PCX
excretion for type 2 diabetic patients with early nephropathy with
treatment of sitagliptin. Shanxi Med Univ. 7-10:2016.(In
Chinese).
|
|
24
|
Wang X, Zhang H, Zhang X, Zhang R, Shi M,
Lyu S and Gao P: Effects of sitagliptin on serum chemerin in early
diabetic patients with nephropathy. Acta Acad Med Xuzhou.
35:825–828. 2015.(In Chinese).
|
|
25
|
Han H: Sitagliptin for type 2 diabetes
early kidney disease curative effect observation. Shanxi Med Univ.
5-9:2015.(In Chinese).
|
|
26
|
Hao Q, Liu Y and Fei D: The effect of
sitagliptin on type 2 diabetes mellitus complicated with early
diabetic kidney disease patients. J Taishan Med Coll. 36:989–991.
2015.(In Chinese).
|
|
27
|
Aschner P, Chan J, Owens DR, Picard S,
Wang E, Dain MP, Pilorget V, Echtay A and Fonseca V; EASIE
investigators, : Insulin glargine versus sitagliptin in
insulin-naive patients with type 2 diabetes mellitus uncontrolled
on metformin (EASIE): A multicentre, randomised open-label trial.
Lancet. 379:2262–2269. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Katsuno T, Ikeda H, Ida K, Miyagawa J and
Namba M: Add-on therapy with the DPP-4 inhibitor sitagliptin
improves glycemic control in insulin-treated Japanese patients with
type 2 diabetes mellitus. Endocr J. 60:733–742. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lopez-Giacoman S and Madero M: Biomarkers
in chronic kidney disease, from kidney function to kidney damage.
World J Nephrol. 4:57–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Javanmardi M, Azadi NA, Amini S and Abdi
M: Diagnostic value of cystatin C for diagnosis of early renal
damages in type 2 diabetic mellitus patients: The first experience
in Iran. J Res Med Sci. 20:571–576. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang D, Zhang G, Chen X, Wei T, Liu C,
Chen C, Gong Y and Wei Q: Sitagliptin ameliorates diabetic
nephropathy by blocking TGF-β1/Smad signaling pathway. Int J Mol
Med. 41:2784–2792. 2018.PubMed/NCBI
|
|
32
|
Tsurutani Y, Omura M, Matsuzawa Y, Saito
J, Higa M, Taniyama M and Nishikawa T; SINGLE-Y investigation
group, : Efficacy and safety of the dipeptidyl peptidase-4
inhibitor sitagliptin on atherosclerosis, β-cell function, and
glycemic control in Japanese patients with Type 2 diabetes mellitus
who are treatment naïve or poorly responsive to antidiabetes
agents: A multicenter, prospective observational, uncontrolled
study. Curr Ther Res Clin Exp. 84:26–31. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Arjona Ferreira JC, Marre M, Barzilai N,
Guo H, Golm GT, Sisk CM, Kaufman KD and Goldstein BJ: Efficacy and
safety of sitagliptin versus glipizide in patients with type 2
diabetes and moderate-to-severe chronic renal insufficiency.
Diabetes Care. 36:1067–1073. 2013. View Article : Google Scholar : PubMed/NCBI
|