Introduction
Stroke is the second leading cause of death
worldwide, of which over 80% cases are ischemic stroke caused by
cerebral infarction (1).
Approximately 6.9 million people had an ischemic stroke annually
(2), and 3.3 million people died
from this terrible disease (3).
Although the stent retrieval technique has clear clinical benefits
in many stroke patients, drug treatment is still the first option
therapy for ischemic stroke (4). The
drug recombinant tissue plasminogen activator (rt-PA) is the only
FDA-approved medication for treating acute ischemic stroke
(5), while several limitations
hamper its utilization clinically (6). There is an unmet requirement for the
development of effective drugs for stroke patients especially those
who are inoperable.
As a new strategy with great potential applications,
gene therapy provides promising approaches to the treatment of
various diseases and has achieved good results (7,8). A
number of studies have indicated the protective roles of
neurotrophic factors, antiapoptosis genes and angiogenic growth
factors in brain infarction (9).
Although great advances in gene therapy, the potential in treating
disorders has fallen short of public expectations.
PR39 is a proline-arginine-rich peptide antibiotic
with 39 amino acids (10). It has
been confirmed that PR39, as the angiogenic Masterswitch protein,
has a protective effect on myocardial ischemia-reperfusion injury
by protective metabolic and survival responses through hypoxia
inducible factor-1 (HIF1)-α stabilization (11). However, PR39 only may be ineffective
for the treatment, because of the existence of blood-brain barrier.
It is urgent to excavate an aptamer to improve the solubility and
activity. Human thioredoxin (hTRX), as a natural human protein with
low immunogenicity, has been used as a frame protein to construct a
gene fusion system (12). hTRX is an
ubiquitous, thiol-mediated protein that protects neurons against a
variety of oxidative stresses and is considered as a promising
target for clinical therapy (13,14). It
is indicated that hTRX gene fusion system could dramatically
improve the activity of the expression products (12).
In our earlier study, we have successfully
constructed recombinant adeno-associated virus human
thioredoxin-PR39 (rAAV/hTRX-PR39), and confirmed its potential role
in preventing cell apoptosis hypoxia-induced (15,16).
Moreover, transfection with rAAV/hTRX-PR39 in chicken embryo model
was shown to promote angiogenesis and cell survival under hypoxic
condition (17). In the present
study, we aimed to explore the potential protective effects of
hTRX-PR39 on acute cerebral infarction.
Materials and methods
Construction of recombinant virus
The pGEM-T-hTRX-PR39 vector containing hTRX-PR39
full-length gene sequence was constructed as previously described
(15). We first generated PR39 cDNA
including EcoR721 and BamHI restriction enzyme sites
and hTRX cDNA including EcoR721 and EcoRI restriction
enzyme sites. Then, the synthesized fragments were cloned into a
pGEM-T vector, respectively. The positive clone was identified
using restriction enzymes, and the cloned amplified fragments were
sequenced by the dideoxy-mediated chain-termination method.
Subsequently, pGEM-T-hTRX and pGEM-T-PR39 were digested by
BamHI and EcoRI, and recombined a vector. Finally, the
recombinant vector pGEM-T-hTRX-PR39 was produced. The viral vector,
plasmid and corresponding cell lines were all purchased from Xi'an
Huaguang Biological Engineering Co., Ltd. (Xi'an, China).
Animal model and experimental
protocol
In this study, 20 female Sprague-Dawley rats
weighting 280-300 g were purchased from Shanghai Slack Laboratory
Animal Co., Ltd. (Shanghai, China; license no. SCXK(HU)2012-0002).
All experiments were approved by the Shandong University
Institutional Animal Care and Use Committee.
Middle cerebral artery occlusion (MCAO) was produced
using the Longa suture-occluded technique (18). The neurological evaluation was
performed 2 h after MCAO with Bederson's test (19). Rats were tail suspended. Then they
showed typical signs and symptoms, such as buckling and elevating
of the contralateral forelimb, shoulder adduction and elbow
extending.
According to random number table method,
experimental rats were divided into three groups: Normal saline
group (Control 1 group, n=4), empty virus group (Control 2 group,
n=4) and rAAV/hTRX-PR39 group (PR39 group, n=12). The catheter was
inserted into the middle cerebral artery via the rat intravenous
administration. Rats were injected with isovolumetric physiological
saline, isovolumetric empty virus fluid and 3×109 pfu
rAAV-hTRX-PR39 fluid, respectively.
Hematoxylin and eosin staining
(H&E)
Three experimental rats in PR39 group and one rat in
each control group were sacrificed at time points of 12 h, 1, 2 and
3 weeks after injection. The rat brains were fixed in formalin and
embedded in paraffin. The ischemic brain tissues were sectioned
into equally spaced (2 mm) coronal blocks. A series of adjacent 4
µm-thick slices were cut from each block. Then, the tissue sections
were counterstained with H&E for histologic examination.
H&E staining results of ischemic tissues were observed and
imaged by microscope (BH2; Olympus, Tokyo, Japan). Six positive
visions (magnification, ×400) of each brain slice were randomly
selected for CD34 positive cells counting.
Immunohistochemistry test
The cerebral tissues were cut into 4 equally spaced
(2 mm) coronal blocks. Immunohistochemistry test was performed with
well-characterized mouse monoclonal antibody against CD34 (1:200;
Proteintech Group Inc., Wuhan, China). Brain sections (4 µm-thick)
were fixed in 4% paraformaldehyde for 30 min at 37°C. Then, the
sections were incubated with primary antibody at 4°C overnight
followed by biotinylated secondary antibody (1:200; Proteintech
Group Inc.) for 2 h at 37°C. Subsequently, sections were incubated
with avidin-biotin complex labeled with alkaline phosphatase (AP),
which then reacted with diaminobenzidine, for 60 min at 37°C.
Omitting primary antibody was considered as a negative control.
Electron microscopy observation
Cerebral slices were fixed in 2.5% (v/v)
glutaraldehyde for 2 h and 1% (v/v) osmic acid for 2-3 h, followed
by dehydration in a graded series of ethanol and 90% (v/v) acetone
for 15-20 min. After embedding and solidifying it in an epoxy
resin, ultra-thin slices were cut at 50-60 nm and stained with 3%
(w/v) uranyl acetate and lead citrate. Electron microscopic images
were ere observed and photographed under a transmission electron
microscopy (TECNAI 10; Philips, Eindhoven, Netherlands).
Statistical analysis
Data are expressed as the mean ± standard deviation.
The two-sample t-test was used for comparisons between groups.
Statistical analysis was carried out by SPSS software (SPSS version
13.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
PR39 ameliorates brain damage after
cerebral ischemia
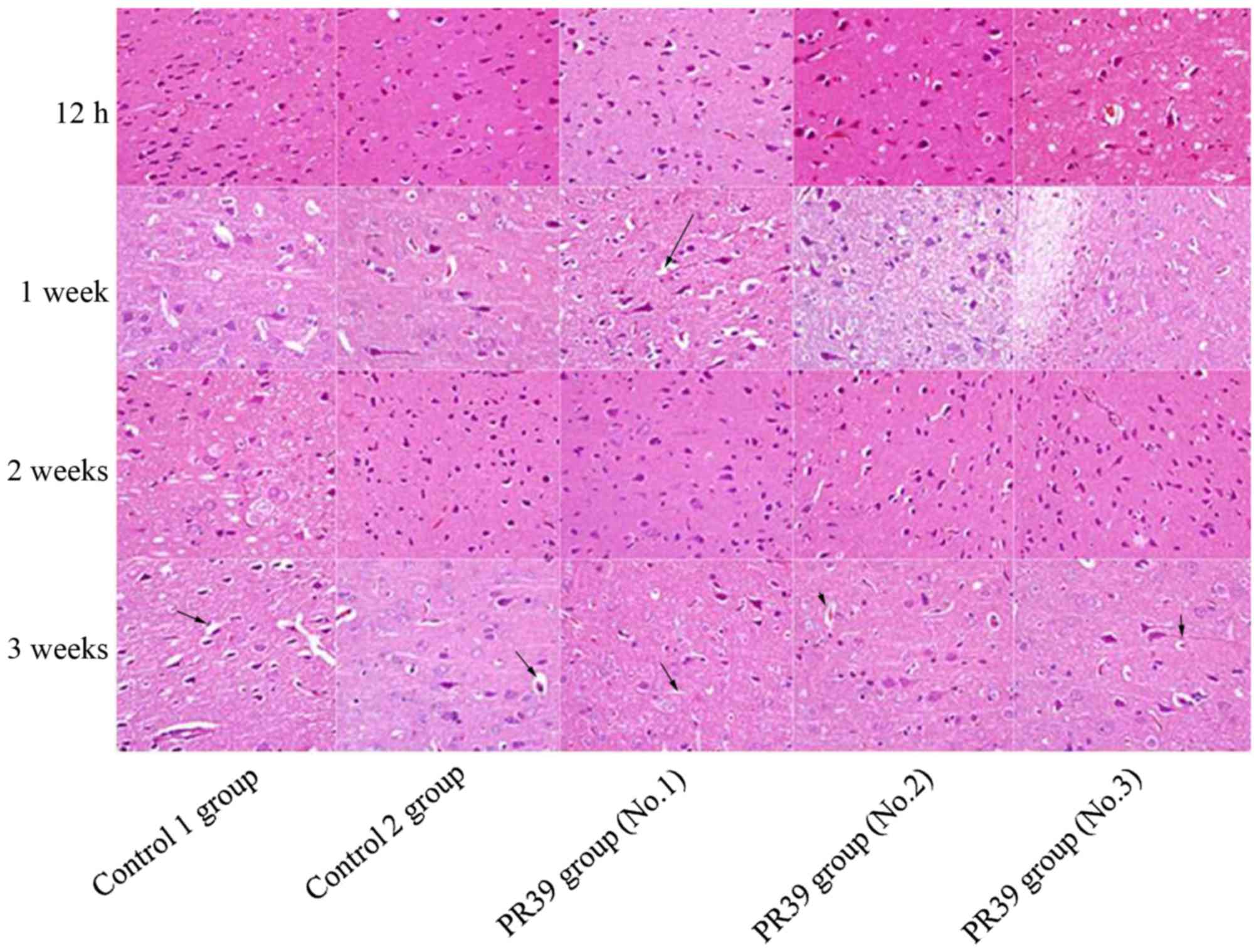
By H&E staining, brain lesion was observed in
the ischemic core of the striatum and cortex in the rats subjected
to MCAO. In two control groups, brain tissues showed gradually
growing cell edema and angioedema over time. At the time points of
12 h and 1 week, brain tissue in all three groups showed same
lesions after MCAO treatment. However, the degree of ischemic brain
edema in the rats treated with rAAV/hTRX-PR39 was alleviated
relative to that in control groups at the time points of 2 and 3
weeks. At 12 h after treatment, brain tissue in all three groups
showed slight lesions. There was no cell degeneration and necrosis.
At 1 week after treatment, the size of nerve cells was still
consistent. Nucleus staining was uniform. While the gap around
nerve cells and blood vessels widened slightly, indicating mild
cell edema and angioedema. At 2 and 3 weeks after treatment, the
degree of tissue edema was aggravated in two control groups, and
the edema appeared in the cerebral medulla and white matter. While
in PR39 group, brain edema was alleviated and nerve cells showed no
obvious morphological degeneration and necrosis. The results were
shown in Fig. 1.
PR39 promotes CD34-positive cells in
ischemic brain
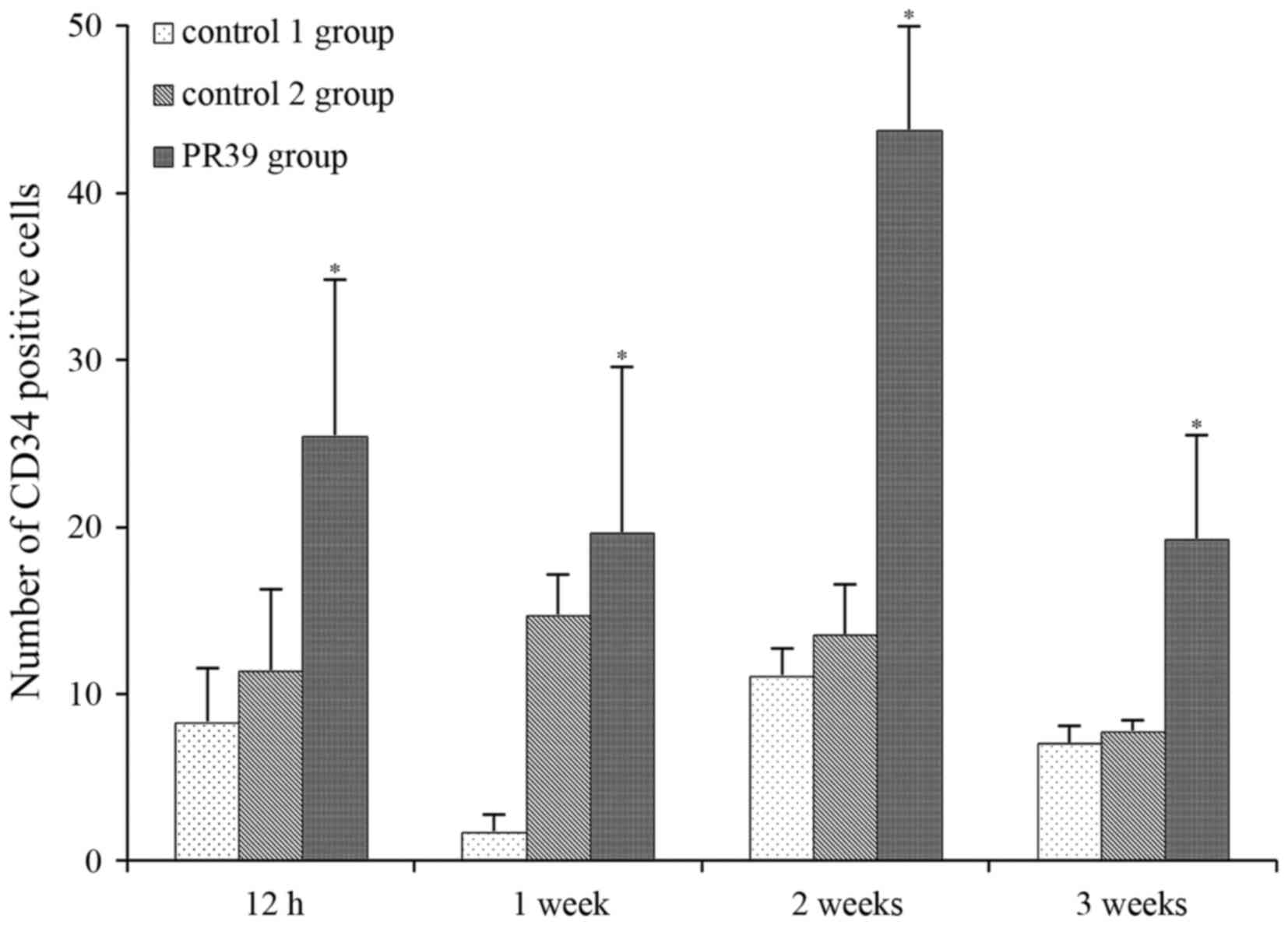
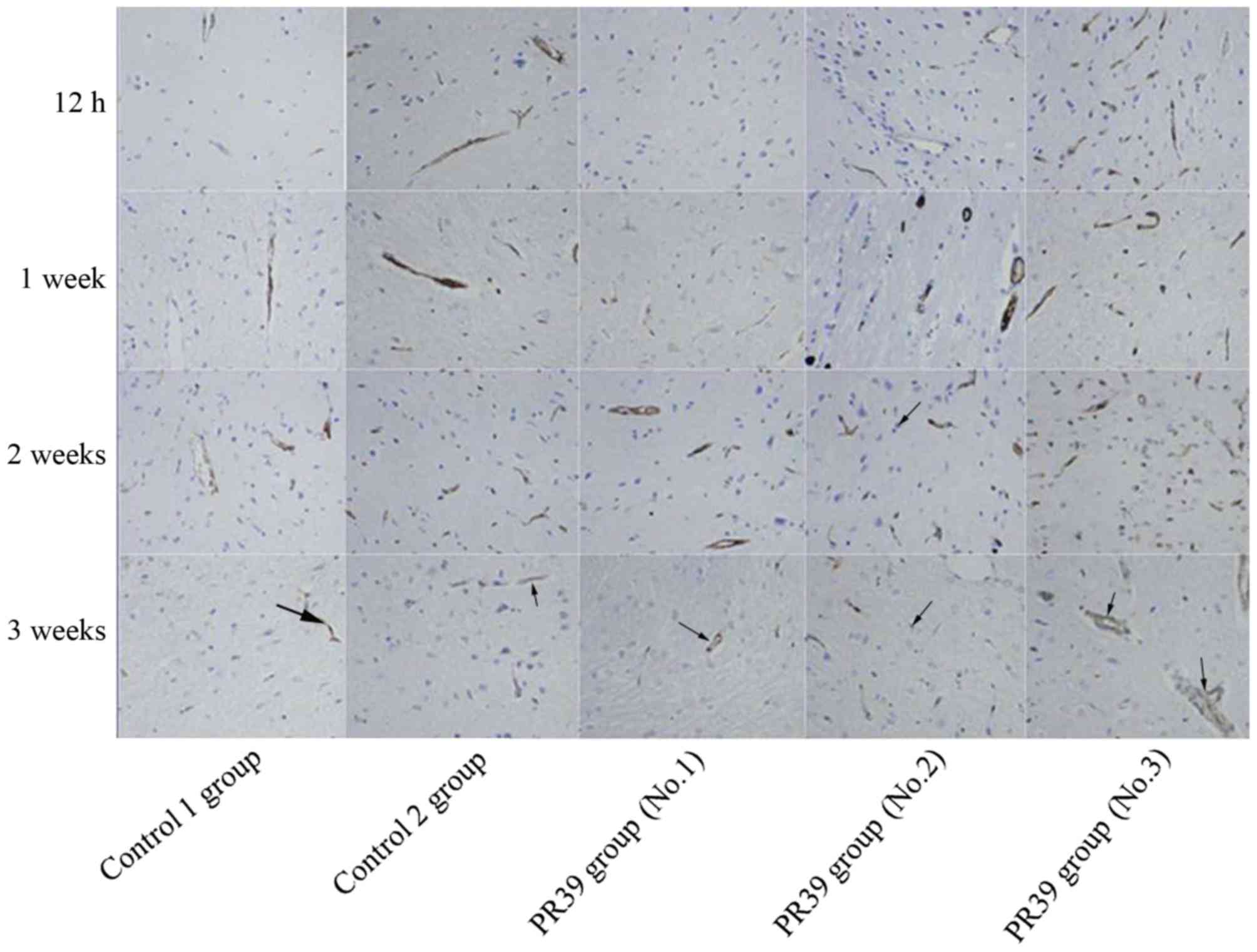
Immunostaining analysis showed that PR39
significantly increased the number of CD34 immunoreactive cells in
ischemic brain tissues at 12 h, 1, 2 and 3 weeks after MCAO
compared to saline control group and empty virus group (P<0.05),
as shown in Fig. 2. CD34-positive
cells were mainly seen around the perivascular in ischemic brain.
The cytoplasm of CD34-positive cells was brown, which had a sharp
contrast with negative cells (Fig.
3).
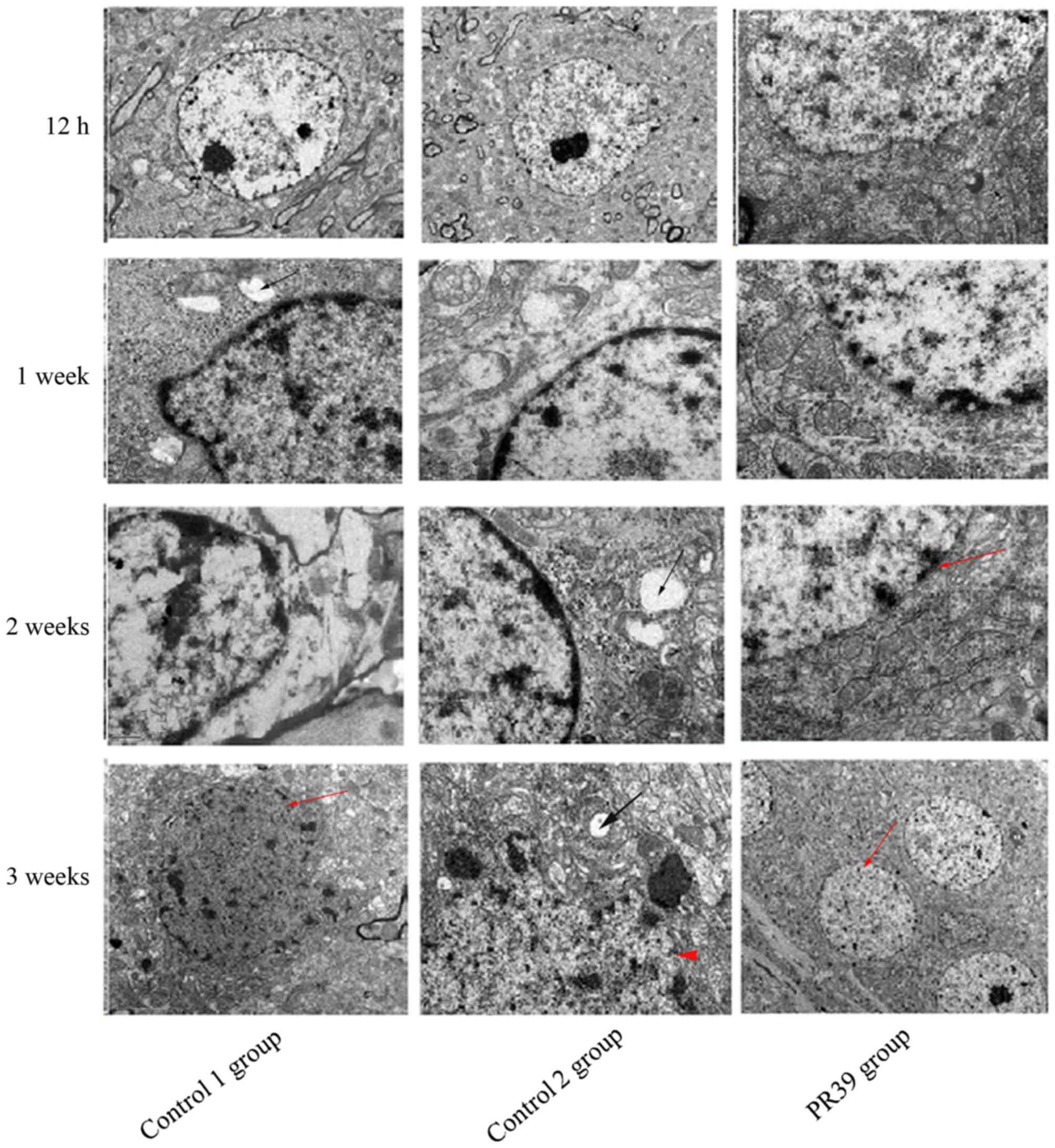
Electron microscopy observation
Ultrastructure of cerebral ultra-thin slices was
examined with electron microscopy (Fig.
4). The neurons structure changes were found in two control
groups (normal saline group and empty virus group). Electron
microscopy showed apoptotic neurons in control groups and the cell
morphology was characterized by irregular and shriveled nucleus and
disruption of nuclei membrane integrity. The cell morphological
structures were similar in normal saline group and empty virus
group, such as mitochondrial swelling and vacuolation. While, cells
treated with PR39 exhibited integrated nucleus membrane and higher
electron density of mitochondria. The vascular structure changes
were also found under electron microscopy in two control groups,
such as the vascular endothelial cell swelling and cavitation
formation. In PR39 group, the structure of vascular endothelial
cells was more complete relative to control groups.
Discussion
This study investigated the neuroprotective effect
of hTRX-PR39 on acute cerebral infarction. Morphological changes of
ischemic brain tissues showed that the damage degree of ischemic
brain in rats treated with rAAV/hTRX-PR39 was slighter than that of
rats in control groups at 2 and 3 weeks. Moreover,
immunohistochemical method found that rAAV/hTRX-PR39 could promote
angiogenesis by detecting the expression of CD34, the most
sensitive endothelial marker with the highest antigen specificity
in nascent microvessels. Our results showed that rAAV/hTRX-PR39
treatment could successfully reduce damage of ischemic brain
tissues and promote angiogenesis in MCAO rats.
In biology, PR39 has multiple biological functions,
such as anti-inflammatory, immunoregulation, stimulating
angiogenesis and apoptosis inhibition (20). Wu et al (21) confirmed the antiapoptotic role in
hypoxic endothelial cells by blocking apoptosis signal transduction
pathway. It can quickly pass through the endothelial cell membrane
by specific binding to SH3 domain structure [p130Cas
(22) and p47phox
(23)]. Moreover, previous study
indicated that PR39 can decrease the ischemic-reperfusion injury by
inhibiting the degradation of IκBα and adhesion molecule (24). In addition, PR39 can block the
degradation of HIF1-α and improve the expression of multiple
angiogenic proteins, such as VEGF, KDR and FLT-1 (17,25–27),
illustrating the crucial role of PR39 in confronting ischemic
damage.
To solve the problem of repeating dosing, rAAV
vector was employed as a carrier for gene transfering. It is
indicated that rAAV shows a series of advantages, such as low
immunogenicity, high transfection efficiency, targeted integration
and long-term stability of gene expression (28). A great number of studies have stated
that rAAV vector is a safe and suitable tool for gene therapy in
ischemic stroke (29,30). So far, rAAV has become the most
common gene vector for the central nervous system disorder. To
improve the activity of the expression products and increase the
solubility of PR39 to pass blood-brain barrier, hTRX was utilized
to construct the gene fusion system in our study. Moreover, hTRX
could inhibit thrombosis by reducing tissue factor activity and
further prevent the injury of the ischemic tissue (31). Consistent with An et al
report, intramyocardial injection of recombinant adeno-associated
viral vector coexpressing pr39/adrenomedullin enhances angiogenesis
and reduces apoptosis in a rat myocardial infarction model
(32). Our study showed that the
gene fusion system rAAV/hTRX-PR39 can effectively ameliorate acute
cerebral infarction-induced ischemic injury by promoting
angiogenesis and inhibiting cell apoptosis. As a limitation in this
report, the intracranial transfection and expression of
rAAV/hTRX-PR39 and rat behavior or brain MR tests have not been
performed, which will be added to the future report.
In conclusion, in this study, rAAV/hTRX-PR39
successfully reduced ischemic brain damage and promoted
angiogenesis, suggesting the underlying application in the
treatment of acute cerebral infarction.
Acknowledgements
The study was supported by the National Natural
Science Foundation of China (grand no. 30970992).
References
|
1
|
O'Donnell MJ, Xavier D, Liu L, Zhang H,
Chin SL, Rao-Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ,
et al: Risk factors for ischaemic and intracerebral haemorrhagic
stroke in 22 countries (the INTERSTROKE study): A case-control
study. Lancet. 376:112–123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Global Burden of Disease Study 2013
Collaborators, : Global, regional, and national incidence,
prevalence, and years lived with disability for 301 acute and
chronic diseases and injuries in 188 countries, 1990-2013: A
systematic analysis for the Global Burden of Disease Study 2013.
Lancet. 386:743–800. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
GBD 2013 Mortality and Causes of Death
Collaborators, : Global, regional, and national age-sex specific
all-cause and cause-specific mortality for 240 causes of death,
1990-2013: A systematic analysis for the Global Burden of Disease
Study 2013. Lancet. 385:117–171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Walcott BP, Boehm KM, Stapleton CJ, Mehta
BP, Nahed BV and Ogilvy CS: Retrievable stent thrombectomy in the
treatment of acute ischemic stroke: Analysis of a revolutionizing
treatment technique. J Clin Neurosci. 20:1346–1349. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brainin M, Teuschl Y and Kalra L: Acute
treatment and long-term management of stroke in developing
countries. Lancet Neurol. 6:553–561. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang W, Li M, Chen Q and Wang J:
Hemorrhagic transformation after tissue plasminogen activator
reperfusion therapy for ischemic stroke: Mechanisms, models, and
biomarkers. Mol Neurobiol. 52:1572–1579. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ooboshi H, Ibayashi S, Takada J, Kumai Y
and Iida M: Brain ischemia as a potential target of gene therapy.
Exp Gerontol. 38:183–187. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moss JA: Gene therapy review. Radiol
Technol. 86:155–184. 2014.PubMed/NCBI
|
|
9
|
Aoki M and Morishita R: Therapeutic
angiogenesis for ischemic diseases. Nihon Rinsho. 64:762–768.
2006.(In Japanese). PubMed/NCBI
|
|
10
|
Gudmundsson GH, Magnusson KP, Chowdhary
BP, Johansson M, Andersson L and Boman HG: Structure of the gene
for porcine peptide antibiotic PR-39, a cathelin gene family
member: comparative mapping of the locus for the human peptide
antibiotic FALL-39. Proc Natl Acad Sci USA. 92:7085–7089. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Muinck ED, Nagy N, Tirziu D, Murakami M,
Gurusamy N, Goswami SK, Ghatpande S, Engelman RM, Simons M and Das
DK: Protection against myocardial ischemia-reperfusion injury by
the angiogenic Masterswitch protein PR 39 gene therapy: the roles
of HIF1alpha stabilization and FGFR1 signaling. Antioxid Redox
Signal. 9:437–445. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Borghouts C, Kunz C, Delis N and Groner B:
Monomeric recombinant peptide aptamers are required for efficient
intracellular uptake and target inhibition. Mol Cancer Res.
6:267–281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamawaki H, Haendeler J and Berk BC:
Thioredoxin: A key regulator of cardiovascular homeostasis. Circ
Res. 93:1029–1033. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoshida T, Nakamura H, Masutani H and
Yodoi J: The involvement of thioredoxin and thioredoxin binding
protein-2 on cellular proliferation and aging process. Ann N Y Acad
Sci. 1055:1–12. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ruan XY, Bi JZ, Liu QY, Zhang SB, Yang GX
and Wang QY: Construction and identification of recombinant
plasmids expressing hTRX-PR39. J Shandong University (Health
Sciences). 47:30–34. 2009.
|
|
16
|
Ruan X, Yuan Z, Du Y, Yang G and Wang Q:
Recombinant adeno-associated virus delivered human thioredoxin-PR39
prevents hypoxia-induced apoptosis of ECV304 cells. Neural Regen
Res. 7:708–713. 2012.PubMed/NCBI
|
|
17
|
Ruan XY, Liang YC, Du B, Lin YT, Guo YD,
Zhao J, Li S, Li JF, Sun QJ and Du YF: Potential role of
recombinant adeno-associated virus human thioredoxin-PR39 in cell
and vascular protection against hypoxia. Exp Ther Med. 9:1605–1610.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bederson JB, Pitts LH, Tsuji M, Nishimura
MC, Davis RL and Bartkowski H: Rat middle cerebral artery
occlusion: Evaluation of the model and development of a neurologic
examination. Stroke. 17:472–476. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ross CR, Ricevuti G and Scovassi AI: The
antimicrobial peptide PR-39 has a protective effect against HeLa
cell apoptosis. Chem Biol Drug Des. 70:154–157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu J, Parungo C, Wu G, Kang PM, Laham RJ,
Sellke FW, Simons M and Li J: PR39 inhibits apoptosis in hypoxic
endothelial cells: Role of inhibitor apoptosis protein-2.
Circulation. 109:1660–1667. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chan YR and Gallo RL: PR-39, a
syndecan-inducing antimicrobial peptide, binds and affects
p130(Cas). J Biol Chem. 273:28978–28985. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi J, Ross CR, Leto TL and Blecha F:
PR-39, a proline-rich antibacterial peptide that inhibits phagocyte
NADPH oxidase activity by binding to Src homology 3 domains of p47
phox. Proc Natl Acad Sci USA. 93:6014–6018. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bao J, Sato K, Li M, Gao Y, Abid R, Aird
W, Simons M and Post MJ: PR-39 and PR-11 peptides inhibit
ischemia-reperfusion injury by blocking proteasome-mediated I kappa
B alpha degradation. Am J Physiol Heart Circ Physiol.
281:H2612–H2618. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li J, Post M, Volk R, Gao Y, Li M, Metais
C, Sato K, Tsai J, Aird W, Rosenberg RD, et al: PR39, a peptide
regulator of angiogenesis. Nat Med. 6:49–55. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carmeliet P, Dor Y, Herbert JM, Fukumura
D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R,
Maxwell P, et al: Role of HIF-1alpha in hypoxia-mediated apoptosis,
cell proliferation and tumour angiogenesis. Nature. 394:485–490.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun L, Hao Y, Nie X, Xu J, Li Z, Zhang W,
Liu Y and Zhang X: Recombinant AAV-PR39-mediated hypoxia-inducible
factor 1α gene expression attenuates myocardial infarction. Int J
Mol Med. 33:171–177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weinberg MS, Samulski RJ and McCown TJ:
Adeno-associated virus (AAV) gene therapy for neurological disease.
Neuropharmacology. 69:82–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li ZJ and Wang RZ: rAAV vector-mediated
gene therapy for experimental ischemic stroke. Neurol India.
56:116–121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chtarto A, Bockstael O, Tshibangu T,
Dewitte O, Levivier M and Tenenbaum L: A next step in
adeno-associated virus-mediated gene therapy for neurological
diseases: Regulation and targeting. Br J Clin Pharmacol.
76:217–232. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang P, Wu Y, Li X, Ma X and Zhong L:
Thioredoxin and thioredoxin reductase control tissue factor
activity by thiol redox-dependent mechanism. J Biol Chem.
288:3346–3358. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
An R, Xi C, Xu J, Liu Y, Zhang S, Wang Y,
Hao Y and Sun L: Intramyocardial injection of recombinant
adeno-associated viral vector coexpressing PR39/adrenomedullin
enhances angiogenesis and reduces apoptosis in a rat myocardial
infarction model. Oxid Med Cell Longev. 2017:12716702017.
View Article : Google Scholar : PubMed/NCBI
|