Introduction
Traumatic brain injury (TBI) is a major cause of
death and disability that disproportionately affects young adults
(1). The persistence of symptoms for
>3 months following the onset of mild TBI is known as
post-concussion syndrome (PCS). The incidence of PCS following the
onset of TBI is ~15% after 3 months and 3-5% after 1 year (2). Common post-concussion symptoms include
headaches, balance problems, sleep disturbance, fatigue,
forgetfulness, poor concentration, irritability and anxiety
(3). There are currently few
established therapies available to treat patients with persistent
PCS.
Hyperbaric oxygenation (HBO) therapy is currently
used to treat acute and chronic ischemic injuries. HBO has well
established theoretical underpinnings and is able to treat
dive-related injuries, soft tissue injuries and carbon monoxide
poisoning (4). The results of
several studies have provided inconclusive evidence for the
efficacy of HBO therapy in treating patients with PCS. Previous
studies lacking control groups compared data pre- and post-HBO and
found that HBO has a beneficial effect (5,6).
However, a selection of prospective randomized trials did not prove
the therapeutic effectiveness of HBO in PCS following mild TBI
(7,8).
TBI is categorized into two phases: The primary
insult and ensuing secondary reaction. A variable degree of
irreversible primary damage to the neurological tissue occurs at
the onset of injury (9). Secondary
pathologies of TBI include ischemia, edema, hypoxia and other
biochemical and inflammatory processes (10,11).
Local hypoxia and ischemia may lead to the initiation of neuronal
cell death. The use of HBO to treat TBI is based on the fact that
hypoxia may serve an important role in causing secondary injury
(12).
Previous studies in animals have demonstrated that
HBO may have beneficial effects on brain injury. HBO limits the
growth of cerebral contusions (13),
increases the contused hippocampus vascular density (14), decreases the extent of secondary cell
death and reactive neuroinflammation (15), preserves mitochondrial integrity and
inhibits the mitochondrial apoptotic pathway (16). As an adjunctive treatment for
patients with TBI, HBO may reduce the risk of mortality and improve
the final Glasgow Coma Scale score (17). However there is little evidence that
the prognosis of survivors improves following HBO (18,19).
The present study conducted a systematic review and
meta-analysis of the current literature to examine the benefit of
HBO therapy in the treatment of patients with PCS. The current
available clinical evidence was presented in order to provide a
foundation for future research.
Methods
The present systematic review was conducted
according to the Preferred Reporting Items for Systematic Reviews
and Meta-Analyses guidelines (20)
and was registered on the PROSPERO database (crd.york.ac.uk/PROSPERO; registration no. CRD
42016032620).
Study retrieval and screening
Studies were identified through by searching the
Cochrane (cochranelibrary.com), EBSCOhost
(search.ebscohost.com), Embase
(embase.com/login), Ovid MEDLINE (ovidsp.ovid.com), PubMed (ncbi.nlm.nih.gov/pmc) and Web of Science (login.webofknowledge.com) databases. Database
searches were limited to peer-reviewed scholarly journal articles
published in English from inception up until the first week of
2016. Keywords, medical sub headings and an all fields search were
conducted using the terms ‘hyperbaric oxygenation’ and
‘post-concussion syndrome’ to obtain articles meeting the
eligibility criteria.
The results were analyzed independently by two
reviewers. Three additional studies were identified from the
reference lists of the retrieved studies, review articles and
textbooks. All hits obtained with the search strategies were
imported into EndNote version ×7 (Clarivate Analytics,
Philadelphia, PA, USA) and duplicates were subsequently
removed.
The titles and abstracts of the remaining studies
were screened by two reviewers independently to assess their
eligibility. The full texts of potentially eligible studies were
retrieved and assessed according to the inclusion criteria by the
same two reviewers. Disagreements between the reviewers regarding
the eligibility of titles/abstracts or full texts were resolved in
a consensus meeting. In the case where consensus was not reached, a
third reviewer was asked to make the final decision.
Eligibility criteria
The studies that satisfied all the following
criteria were eligible for inclusion in the present review: i)
Full-text articles published in a peer-reviewed scientific journal;
ii) randomized controlled trials (RCTs) aimed at assessing the
effectiveness of HBO in PCS; and iii) articles written in English.
Exclusion criteria included studies that were not written in
English, not RCTs or conducted in patients with mild brain injury
who were not diagnosed with PCS.
Methodological quality assessment
The Cochrane Risk of Bias tool (21) was used to assess the methodological
quality of the included studies in terms of sequence generation,
allocation concealment, blinding, incomplete outcome data,
selective outcome reporting and other sources of bias.
Data extraction
The following data were extracted from the full
texts of the studies included in the present review: Author, year
published, study design, population, sample size, patient age and
sex, intervention and comparison, outcome and outcome administered
time. Data were extracted by one reviewer and reviewed a second
time by a different reviewer to ensure accuracy.
Statistical analysis
Review Manager 5.3 (The Nordic Cochrane Centre, The
Cochrane Collaboration; ims.cochrane.org/revman) was used for data analysis.
Pre- and post-intervention data were analyzed and compared. The
weighted mean difference (MD) was pooled for continuous outcomes
using the same measurement and the standardized mean difference
(SMD) was calculated for continuous outcomes with different
measurements. Statistical heterogeneity was detected using the Q
statistic and I2>50% indicated high heterogeneity. Using a 95%
confidence interval (CI) a fixed effect model was used where there
was no evidence of significant heterogeneity between studies and a
random effects model when such heterogeneity was high. P<0.05
was considered to indicate a statistically significant
difference.
Study selection and participants
Study selection and
characteristics
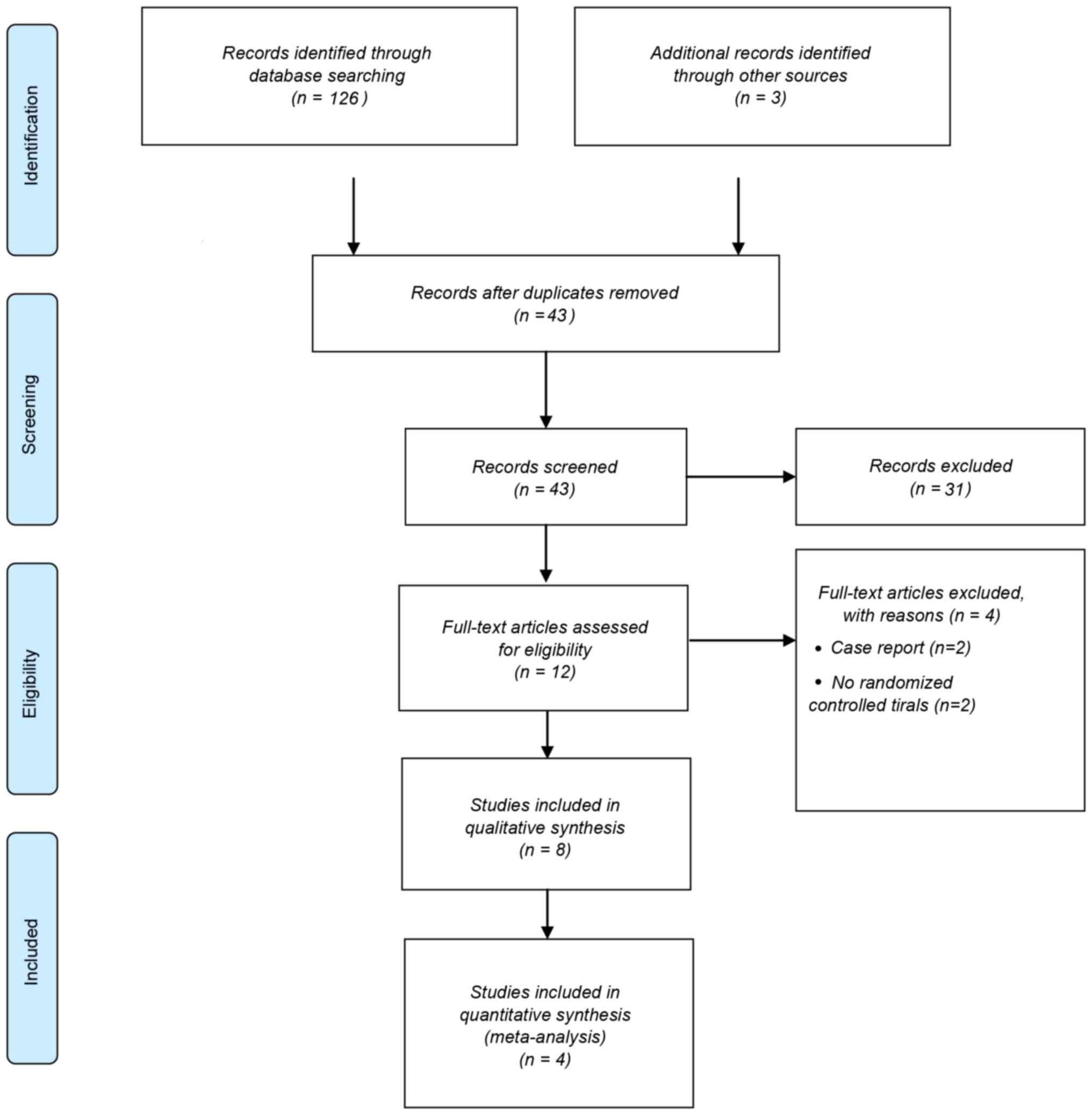
A total of 126 articles were identified through
database searching and 43 of these were screened following the
removal of duplicates (Fig. 1).
These included 13 comments and reviews (22–34), 9
letters to the editor and their matching responses (35–43), two
conference abstracts (44,45), one paper on study design (2), one animal experiment (46) and one study on side effects (47). These studies were not RCTs and were
therefore excluded. Concussion was not the focus of four of the
studies screened (48–51) and an additional four studies
(5,6,19,52) were
not RCTs. Therefore following the exclusion of the aforementioned
studies, eight RCTs (4,7,53–57) were
suitable for inclusion in the current review (Fig. 1). Four of these (7,53–55)
reported different aspects of the same study and another two
(4,42) reported on the same trial; therefore
these articles were evaluated together. A total of four articles
were therefore used in the subsequent meta-analysis (4,8,55,56).
Characteristics of the included studies are presented in Table I.
 | Table I.Characteristics of the enrolled
studies. |
Table I.
Characteristics of the enrolled
studies.
| Authors (year) | Study design | Population | Sample size
(n) | Mean age
(years) | M/F (n) | Intervention and
comparison | Outcome | Outcome
administered time | (Refs.) |
|---|
| Miller et al
(2015) | RCT | Military service
members | 72 | 31 | 69/3 | HBO group, 1.5 ATA
of 100% oxygen, 40×60 min sessions over 8 weeks; Sham group, room
air pressurized to 1.2 ATA, 40×60 min sessions over 8 weeks;
Standard care group, no supplemental chamber procedures | RPQ NSI PCL | Baseline;
post-intervention | (8) |
| Cifu et al
(2014) | RCT | Military service
members | 60 | 23 | 60/0 | Sham air group,
10.5% oxygen (balance 89.5% nitrogen) at 2.0 ATA; 1.5-ATA oxygen
group, 75% oxygen (balance 25% nitrogen) at 2.0 ATA; 2.0-ATA oxygen
group, pure oxygen (0% nitrogen) at 2.0 ATA | RPQ PCL | Baseline;
immediately post-intervention | (55) |
| Boussi-Gross et
al (2013) | RCT | Patients with mild
traumatic brain injury | 56 | 44 | 24/32 | HBO group, 100%
oxygen at 1.5 ATA 40×60 min sessions over 2 months; Crossover
group, a 2 month control period followed by HBO therapy | EQ-5D Cognitive
Outcomes SPECT | Baseline;
immediately post-intervention; post 2-month control period
(crossover group) | (56) |
| Wolf et al
(2012) | RCT | Military service
members | 50 | 28 | 48/2 | HBO group, 2.4 ATA
of 100% oxygen; Sham group, room air at 1.3 ATA Both 30×90 min
sessions | PCL ImPACT | Baseline; each
exposure interval; 6 weeks post-intervention | (4) |
Participants
A total of 238 patients were enrolled in the studies
included in the present review. The majority of these were members
of the military service (4,8,55) apart
from the participants of one study, who were patients with mild,
traumatic brain injury (56). The
age range of all participants was 23-44 years and there were 201
males and 37 females.
Intervention
Trial designs varied among the studies included in
the present review (Table I). A
crossover control without a sham group was used in one study
(56). Patients in the treatment
group underwent 40 HBO sessions for 60 min with 100% oxygen at 1.5
atmospheres absolute (ATA). Patients in the crossover group
underwent the same HBO therapy following a 2 month control period
of no treatment. HBO and sham groups were used in two of the other
studies (4,8) and one study also included a standard
care group with no supplemental chamber procedures (8). In one study, patients in the HBO group
underwent a series of 30 hyperbaric chamber compressions at 2.4 ATA
with 100% oxygen, once each day for 90 min over an 8 week period,
whereas participants in the sham group breathed air at 1.2-1.3 ATA
(4). In the other study, patients in
the HBO group underwent a series of 40 60 min hyperbaric chamber
compressions at 1.5 ATA with 100% oxygen over 8 weeks and
participants in the sham group breathed air at 1.2 ATA (8). In a study by Cifu et al
(55), subjects breathed 1 of 3
pre-assigned oxygen fractions for 60 min, including 10.5% oxygen,
75% oxygen or 100% oxygen all at 2.0 ATA, resulting in an exposure
to oxygen equivalent to breathing surface air, 100% oxygen at 1.5
ATA or 100% oxygen at 2.0 ATA, respectively.
Clinical outcomes
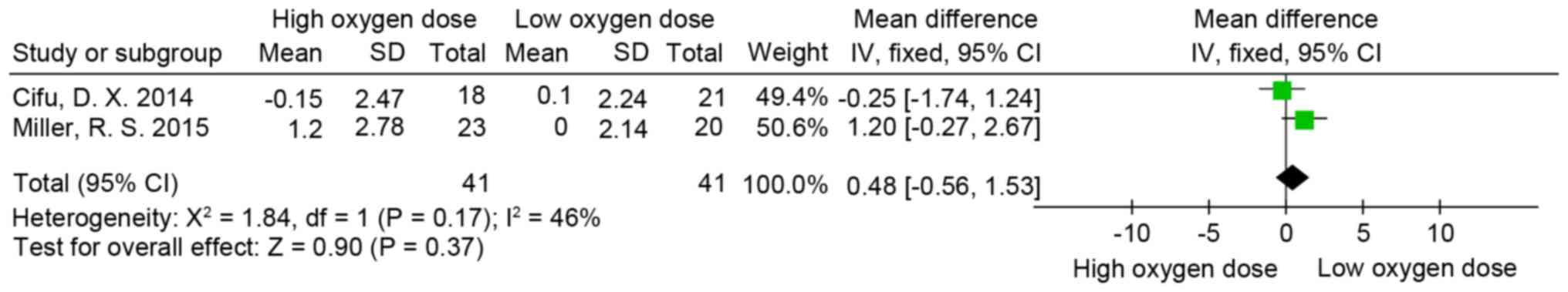
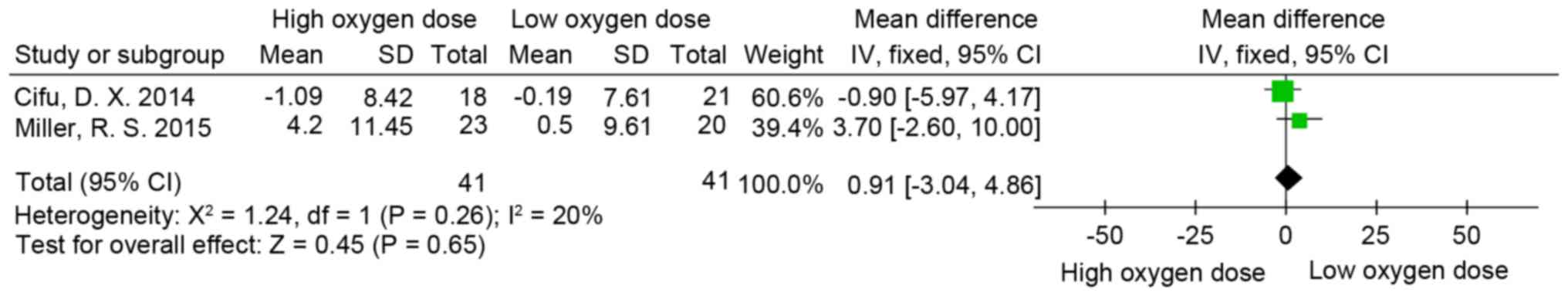
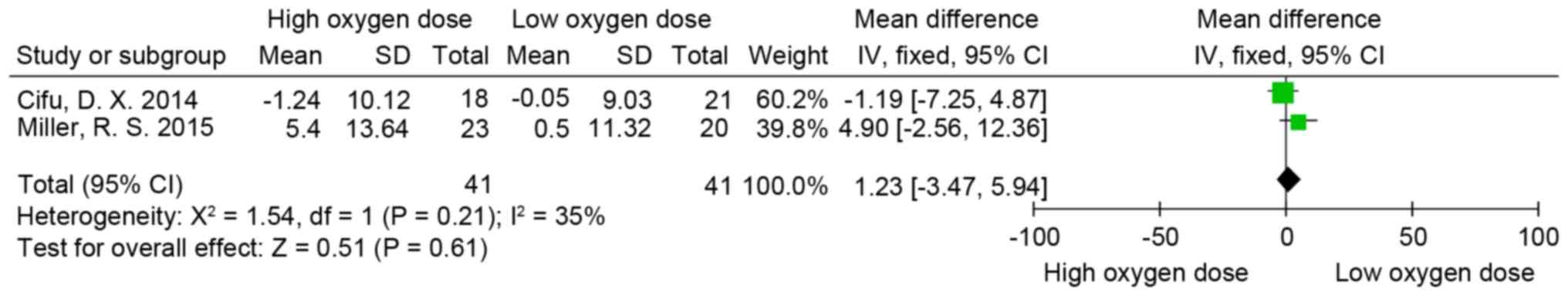
Rivermead post-concussion symptoms
questionnaire (RPQ)
RPQ (58) scores were
measured in two of the trials included in the present review
(8,55). The psychometric properties of the RPQ
suggest that it is most appropriately scored and analyzed using two
subscales. These subscales consist of items 1-3, which constitute
the RPQ-3 score and the remaining 13 items constitute the RPQ-13
score (59,60). Following measurement of the
individual doses of oxygen in partial pressures and concentration
of oxygen multiplied by time for each treatment, the oxygen
equivalent patients of the two studies were pooled into one group,
thus generating two new groups: A high oxygen dose group and the
low oxygen dose group. The difference in RPQ-3 (MD=0.48; 95% CI,
−0.56-1.53; P>0.05; I2=46%; Fig.
2), RPQ-13 (MD=0.91; 95% CI, −3.04-4.86; P>0.05; I2=20%;
Fig. 3) and RPQ-total scores
(MD=1.23; 95% CI, −3.47-5.94; P>0.05; I2=35%; Fig. 4) was not significant between the low
and high oxygen dose groups.
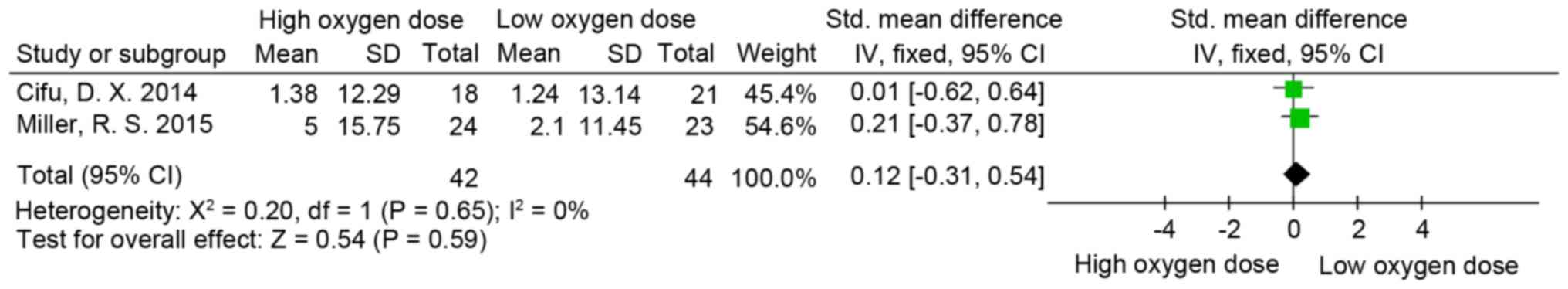
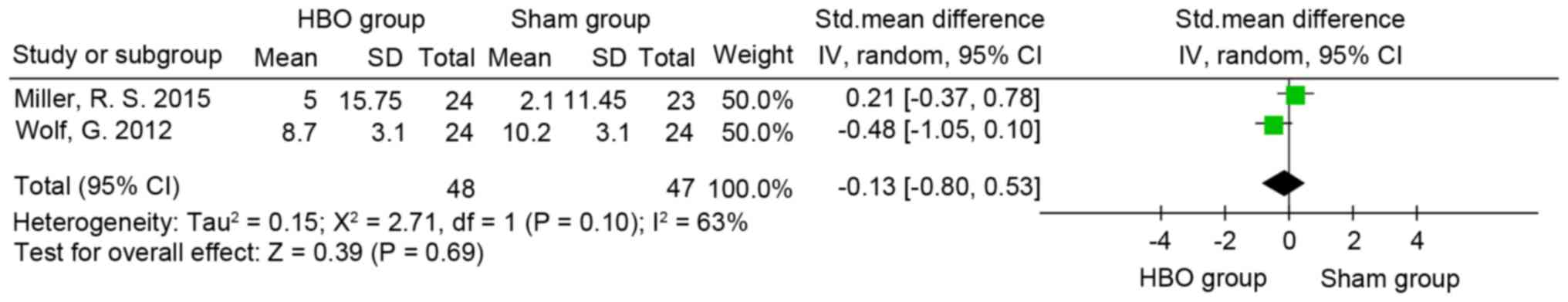
Post-Traumatic Stress Disorder
Checklist (PCL)
A total of 3 out of 4 studies included PCL in their
results (4,8,55). Two
(4,52) used the military version (60) and one (8) used the civilian version (61). The results indicated that the
difference between the high and low oxygen dose groups (SMD=0.12;
95% CI, −0.31-0.54; P>0.05; I2=0%; Fig. 5) and HBO and sham groups (SMD, −0.13;
95% CI, −0.8 to 0.53; P>0.05; I2=63%; Fig. 6) was not significant.
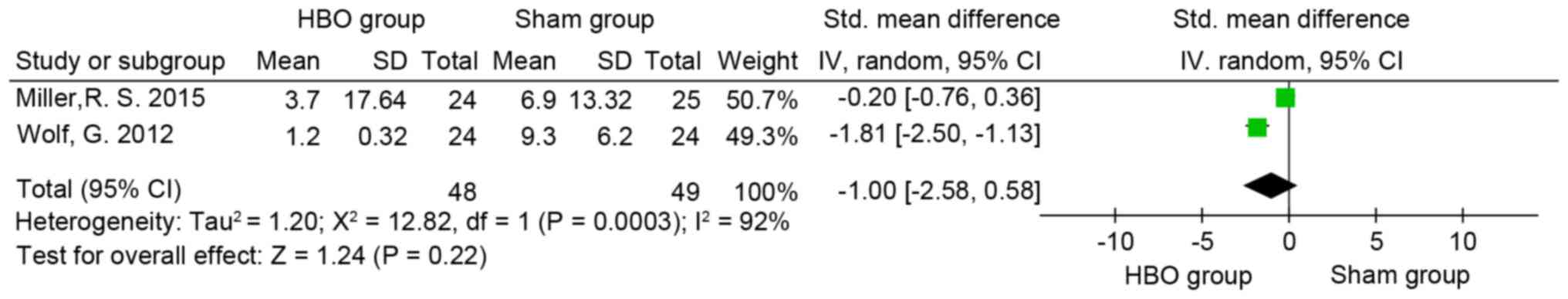
Neurobehavioral symptom
assessment
The neurobehavioral symptoms of participants were
monitored in two trials (4,8) using the Neurobehavioral Symptom
Inventory (NSI) (8,62) and Immediate Post-Concussion
Assessment and Cognitive Testing (4,63),
respectively. The difference between the neurobehavioral symptoms
of subjects in the HBO and sham groups was not significant (SMD=−1;
95% CI, −2.58-0.58; P>0.05; I2=92%; Fig. 7).
Health-related quality of life
(QOL)
Miller et al (8) demonstrated an improvement in
health-related QOL outcomes, including physical functioning, bodily
pain, social functioning and emotionality on the 36-Item Short Form
Health Survey (SF-36) in the sham group compared with the HBO group
and in the HBO group compared with the standard care group. QOL was
evaluated in another trial using the EuroQuol five dimensions
(EQ-5D) questionnaire (56). The
EQ-5D questionnaire scores significantly improved following HBO
therapy in the treated and crossover groups compared with the
control group (P<0.05). However, no improvements were observed
in the EQ-5D score in the crossover group following the control
period. These results suggest that HBO therapy may improve QOL
compared with the no supplemental chamber procedure, but not
compared with the sham group. The standard deviation of SF-36 total
score change was not available, thus a meta-analysis on QOL was not
performed.
Cognitive function
There were insufficient data to conduct a
meta-analysis on cognitive function. The crossover design study
demonstrated significant improvements in cognitive function in the
groups following HBO but no significant improvement following the
control period (56). Single-photon
emission computed tomography (SPECT) imaging also revealed elevated
brain activity with cognitive improvements (56). Exposure to 1.5 or 2.0 ATA did not
improve cognitive function compared with the sham air intervention
in one study (7). Wolf et al
(42) demonstrated that the
difference in cognitive function between the sham and treatment
groups undergoing HBO therapy at 2.4 ATA was not significant.
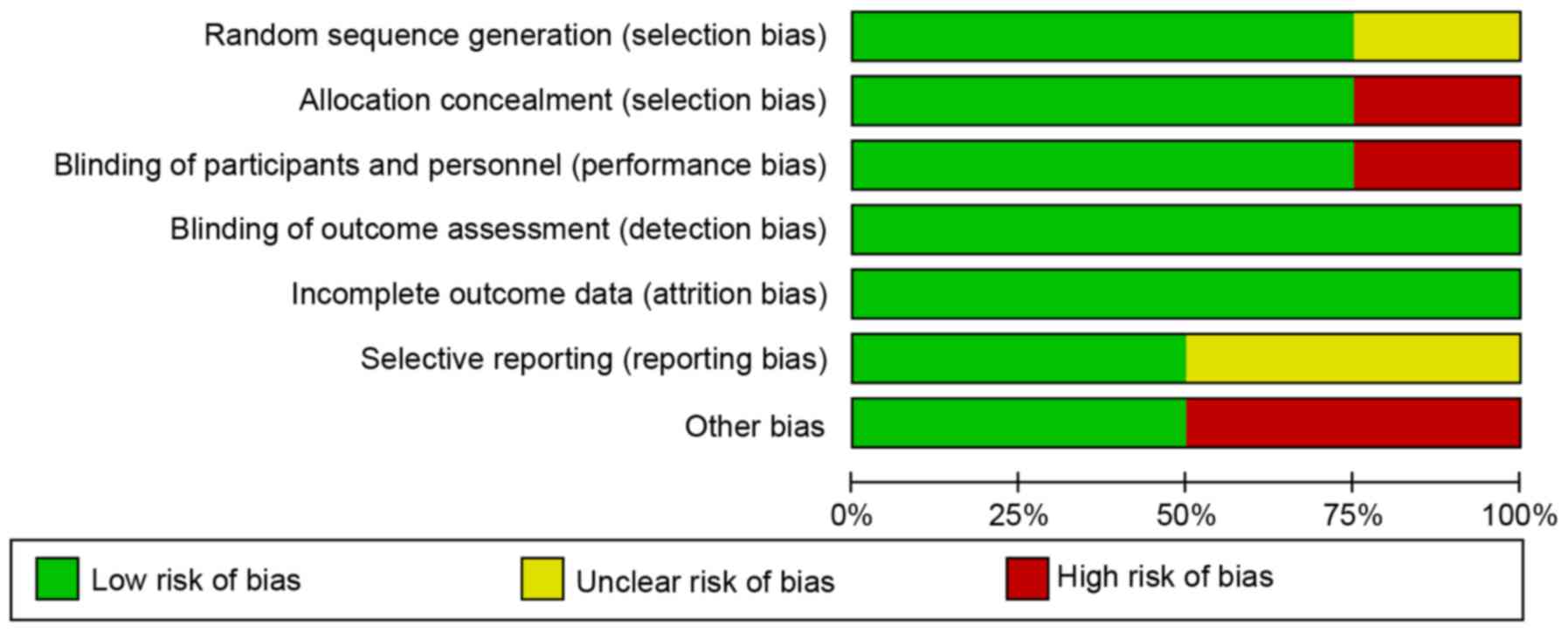
Quality and risk of bias
There was a low risk of bias in blinding outcome
assessment and incomplete outcome data in the studies included in
the present review (Fig. 8).
However, the crossover study exhibited high risk regarding the
blinding participants and personnel, as well as allocation
concealment. A high risk of other bias was identified in two
studies, including regarding the sham group as a control. The
randomization method was unclear in 1 out of 4 studies. Therefore,
the risk of bias among all studies was classed as medium due to
selection, reporting and performance biases (Fig. 8).
Discussion
PCS is a term used to describe the complex and
controversial physical, cognitive and emotional symptoms associated
with mild brain injury. PCS persists for weeks or months in the
majority of patients and <25% of patients may experience
prolonged PCS in which symptoms last for >6 months (64). These patients are at high risk of
emotional and cognitive dysfunction in which they may be unable to
perform ordinary daily activities and maintain work
responsibilities, as well as normal social relationships (65,66).
Currently, there is limited evidence that multifaceted
rehabilitation programs that include psychotherapy improve the
management of persistent symptoms in PCS (67). Based on previous studies
investigating other neurological conditions, it has been suggested
that HBO therapy may be a potential treatment for chronic PCS
(5,6,52).
The results of the present systematic review
identified no improvement in RPQ score or symptoms of PCS between
low and high oxygen dose groups and no significant difference in
the improvement of neurobehavioral symptoms between HBO and sham
groups. Thus, there is no evidence that HBO therapy is effective at
treating patients with PCS. However, HBO therapy is a combination
of increased pressure and increased pressure of oxygen above
ambient atmospheric pressure and the sham designs used in the
studies included in the present review may not have tested an
extensive enough range of pressurized air doses (37,57).
The effects of air pressure have been investigated
since the early 20th century; however, they have been more actively
studied since the 1990s. The majority of studies performed so far
have been cell culture experiments (57). A follow-up study by Mulkey et
al (68) suggests that neuronal
barosensitivity occurs at pressures of 100 mmHg (1.13 ATA).
Furthermore, it has been demonstrated that even a small increase in
partial pressure to 1.05 ATA at an altitude of 402 m below sea
level may induce noticeable physiological effects, such as improved
pulmonary function and blood oxygen saturation (69–71). A
room air pressure of 1.2 or 1.3 ATA may not be appropriate for sham
controls, as this may lead to significant increases in tissue
oxygenation (72). Thus, the use of
21% oxygen at 1.14-1.5 ATA in for sham controls in clinical trials
as an alternative to observation or crossover controls may lead to
false acceptance of the null hypothesis, due to the biological
activity that occurs under these conditions (29). Consequently, studies that included a
sham group were identified as having high bias (4,7,8,55). The
minimum pressure at which patients sense an increase in air
pressure is 1.3 ATA. Controlled experiments testing the effects of
HBO therapy must therefore ensure that pressure and oxygen
concentrations are not above base levels in the control groups to
meet the true definition of a sham (73). However, this may result in inherent
ethical and logistic difficulties in handling the sham control in
HBO trials.
Objective and precise assessment methods are another
challenge in evaluating the efficacy of HBO therapy in patients
with PCS. To the best of our knowledge, validated outcome measures
for intervention trials in PCS have not yet been established. In
the majority of the studies included in the present review,
outcomes were evaluated using RPQ, PCL or NSI. All of these
assessments are well established; however, they are all subjective
performance evaluations (58,60–62).
The RPQ has several limitations in its implementation and ability
to accurately reflect test-taker experience (74). The interpretation and accuracy of the
RPQ and other methods varies widely due to self-administration and
the confounding variables involved due to its sensitivity to
covariates, including subjective patient memory, social
desirability, stress, personality factors and the willingness of
patients to reveal problems (74).
The studies included in the present review relied on
self-administration assessments, which is a limitation. SPECT
imaging was used in one randomized, crossover controlled trial and
revealed elevated brain activity with cognitive improvements
following HBO therapy in the treated and crossover groups (56). This is consistent with the results of
previous studies (5,6). Although the use of SPECT imaging may
not be sensitive enough to detect abnormalities in patients with
PCS, it is an objective assessment method that may provide evidence
supporting the use of HBO or sham interventions and allows a
greater refinement of HBO treatment for patients with PCS.
A study of HBO therapy used to treat sub-acute
moderate to severe TBI at 2.0 ATA reported a 9% seizure rate
(75). However, serious side effects
from HBO therapy are rare in patients with chronic and mild TBI and
a previous study demonstrated that patients with TBI treated with
HBO do not experience any marked side effects (76). Two trials in the current study
reported that adverse events occurred during HBO therapy, which
were equally distributed between the HBO and sham groups (8,47)
included in the present review. Serious adverse events, including
pulmonary barotraumas, pulmonary edema or seizure were not
observed.
There are several differences between blast-related
and sports-related PCS. Patients with blast mild TBI have usually
experienced two episodes of head trauma, often within sec of each
other. The magnitude of head acceleration is stronger than that of
a sports-related concussion and the entire body is exposed to the
blast. Consequently, blast TBI is caused by multiple, interwoven
mechanisms of systemic, local and cerebral responses to blast
exposure (77). The majority of
patients included in the present review were from the military,
thus, the results are not representative of patients diagnosed with
sports-related concussion.
Previous studies have demonstrated that there is an
optimal therapeutic time frame for HBO treatment in neonatal rats
with hypoxic-ischemic brain damage (78–80).
However, to the best of our knowledge, the optimal timing of
treatment for patients with brain injury remains unknown. Efrati
and Ben-Jacob (23) suggested that
HBO therapy may begin either at the degenerative or regenerative
stages and is usually safe 1 month following acute injury. Subset
analysis of isolated mild TBI demonstrated a trend toward harm from
HBO at 2.4 ATA, suggesting that HBO at 2.4 ATA may actually have a
negative impact on isolated mild TBI symptoms (81), however, the optimal effective doses
of pressure and oxygen concentration in HBO therapy remain unclear,
as does the optimal duration of treatment. The majority of the
results of the studies included in the present review were obtained
following 30-40 sessions of HBO therapy. It is hypothesized that
additional sessions of HBO therapy may be beneficial (43) however, to the best of our knowledge,
no data are available on the upper time limit at which no further
improvements occur. The long-term effects of HBO therapy are not
well studied. In the studies included in the current review, the
time point for outcome assessment was usually <1 week following
completion of treatment. One previous study identified that
treating blast-related PCS 3 months following compression had no
significant effect (53). Thus,
further studies are required to develop understanding of the
optimal number, duration and long-term effects of treatment
sessions and the optimum time frame following injury onset for
initiating HBO therapy.
Case reports and phase I clinical trials have
demonstrated that HBO is an effective therapy for correctly
diagnosed PCS (5,6) however, the results of RCTs included in
the current review were not consistent with this. HBO therapy may
improve the symptoms of patients with increased levels of oxygen
concentration and pressure. The results of the current
meta-analysis indicated that increased doses of oxygen had no
effect on PCS. The efficacy of pressurization remains elusive, as
the symptoms of patients in the HBO and sham groups were improved
with no difference in improvements observed between them. It is
therefore essential to develop a sham that controls for
pressurization or oxygen concentration separately in RCTs.
Although it remains unknown whether the symptoms of
PCS improve following HBO therapy, it has been demonstrated that
HBO therapy does not cause any serious side effects; thus, it has
been recommended that patients with PCS should undergo HBO therapy,
until future studies are completed. However, it should be noted
that HBO treatment is costly and potentially dangerous thus, its
use must be evidence-based (82).
The use of genuine sham controls in studies on HBO therapy is not
feasible or cost effective and the optimal therapeutic window and
oxygen dose of HBO therapy remain unknown. Therefore future studies
should be conducted on a large scale or in cohorts to produce more
useful results. The approval of HBO therapy on a tentative basis
would allow for studies to be conducted on a large patient
population.
There were several limitations of the current
meta-analysis. Although a comprehensive search of six databases was
conducted, only four studies consisting of 238 patients in total
were included in the present systematic review. The small number of
included studies limited the statistical power of detection.
Additionally, the sample set of patients used is not representative
of patients with sport-related PCS due to the small number of
studies included, which primarily included members of the military
that had experienced blast-induced PCS. A comparison of all groups
was not conducted due to the heterogeneity between different
trials. In addition, the study design of the sham group may lead to
high bias. Therefore, more rigorous reviews are required to assess
the effects and safety of HBO therapy in patients with PCS.
In conclusion, the present systematic review
demonstrated that HBO therapy was not associated with significant
improvements in patients with PCS. Large scale observation or
cohort studies are required to provide information for the design
and execution of a large clinical trial consisting of proper
treatment, control and sham groups. This future trial may
subsequently provide the evidence required for the efficacy of HBO
therapy in the treatment of patients with PCS.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Author's contributions
ToW made substantial contributions to the conception
and design of the study. YD and XHH searched and analyzed the data
independently. In the case where consensus was not reached, TaW was
asked to make the final decision and interpret the data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
All authors declare no conflicts of interest.
Glossary
Abbreviations
Abbreviations:
|
HBO
|
hyperbaric oxygenation
|
|
ATA
|
atmospheres absolute
|
|
RPQ
|
Rivermead Post-Concussion Symptoms
Questionnaire
|
|
NSI
|
Neurobehavioral Symptom Inventory
|
|
PCL
|
Post-Traumatic Stress Disorder
Checklist
|
|
EQ-5D
|
EuroQoL Group's 5-dimension
questionnaire
|
References
|
1
|
Hyder AA, Wunderlich CA, Puvanachandra P,
Gururaj G and Kobusingye OC: The impact of traumatic brain
injuries: A global perspective. NeuroRehabilitation. 22:341–353.
2007.PubMed/NCBI
|
|
2
|
Weaver LK, Cifu D, Hart B, Wolf G and
Miller S: Hyperbaric oxygen for post-concussion syndrome: Design of
department of defense clinical trials. Undersea Hyperb Med.
39:807–814. 2012.PubMed/NCBI
|
|
3
|
King NS: Post-concussion syndrome: Clarity
amid the controversy? Br J Psychiatry. 183:276–278. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wolf G, Cifu D, Baugh L, Carne W and
Profenna L: The effect of hyperbaric oxygen on symptoms after mild
traumatic brain injury. J Neurotrauma. 29:2606–2612. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harch PG, Fogarty EF, Staab PK and Van
Meter K: Low pressure hyperbaric oxygen therapy and SPECT brain
imaging in the treatment of blast-induced chronic traumatic brain
injury (post-concussion syndrome) and post traumatic stress
disorder: A case report. Cases J. 2:65382009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harch PG, Andrews SR, Fogarty EF, Amen D,
Pezzullo JC, Lucarini J, Aubrey C, Taylor DV, Staab PK and Van
Meter KW: A phase I study of low-pressure hyperbaric oxygen therapy
for blast-induced post-concussion syndrome and post-traumatic
stress disorder. J Neurotrauma. 29:168–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Walker WC, Franke LM, Cifu DX and Hart BB:
Randomized, Sham-Controlled, feasibility trial of hyperbaric oxygen
for service members with postconcussion syndrome: Cognitive and
psychomotor outcomes 1 week postintervention. Neurorehabil Neural
Repair. 28:420–432. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miller RS, Weaver LK, Bahraini N,
Churchill S, Price RC, Skiba V, Caviness J, Mooney S, Hetzell B,
Liu J, et al: Effects of hyperbaric oxygen on symptoms and quality
of life among service members with persistent postconcussion
symptoms: A randomized clinical trial. JAMA Intern Med. 175:43–52.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Loane DJ, Stoica BA and Faden AI:
Neuroprotection for traumatic brain injury. Handb Clin Neurol.
127:343–366. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fiskum G: Mitochondrial participation in
ischemic and traumatic neural cell death. J Neurotrauma.
17:843–855. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roth TL, Nayak D, Atanasijevic T, Koretsky
AP, Latour LL and McGavern DB: Transcranial amelioration of
inflammation and cell death after brain injury. Nature.
505:223–228. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Warriner RA III and Hopf HW: The effect of
hyperbaric oxygen in the enhancement of healing in selected problem
wounds. Undersea Hyperb Med. 39:923–935. 2012.PubMed/NCBI
|
|
13
|
Palzur E, Vlodavsky E, Mulla H, Arieli R,
Feinsod M and Soustiel JF: Hyperbaric oxygen therapy for reduction
of secondary brain damage in head injury: An animal model of brain
contusion. J Neurotrauma. 21:41–48. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harch PG, Kriedt C, Van Meter KW and
Sutherland RJ: Hyperbaric oxygen therapy improves spatial learning
and memory in a rat model of chronic traumatic brain injury. Brain
Res. 1174:120–129. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vlodavsky E, Palzur E and Soustiel JF:
Hyperbaric oxygen therapy reduces neuroinflammation and expression
of matrix metalloproteinase-9 in the rat model of traumatic brain
injury. Neuropathol Appl Neurobiol. 32:40–50. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Palzur E, Zaaroor M, Vlodavsky E, Milman F
and Soustiel JF: Neuroprotective effect of hyperbaric oxygen
therapy in brain injury is mediated by preservation of
mitochondrial membrane properties. Brain Res. 1221:126–133. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Teasdale G and Jennett B: Assessment of
coma and impaired consciousness. A practical scale. Lancet.
2:81–84. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bennett MH, Trytko B and Jonker B:
Hyperbaric oxygen therapy for the adjunctive treatment of traumatic
brain injury. Cochrane Database Syst Rev.
12:CD0046092012.PubMed/NCBI
|
|
19
|
Tal S, Hadanny A, Berkovitz N, Sasson E,
Ben-Jacob E and Efrati S: Hyperbaric oxygen may induce angiogenesis
in patients suffering from prolonged post-concussion syndromedue to
traumatic brain injury. Restor Neurol Neurosci. 33:943–951.
2015.PubMed/NCBI
|
|
20
|
Moher D, Liberati A, Tetzlaff J and Altman
DG; PRISMA Group, : Preferred reporting items for systematic
reviews and meta-analyses: The PRISMA Statement. Int J Surg.
8:336–341. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Higgins JP and Green S: Cochrane Handbook
for Systematic Reviews of Interventions: Cochrane Book Series. John
Wiley and Sons, Ltd.; Chichester, UK: 2008,
doi:10.1002/9780470712184.ch1. View Article : Google Scholar
|
|
22
|
Bennett M: Hyperbaric oxygen therapy no
better than sham in improving post-concussion symptoms following
mild traumatic brain injury. Diving Hyperb Med.
43:1732013.PubMed/NCBI
|
|
23
|
Efrati S and Ben-Jacob E: Reflections on
the neurotherapeutic effects of hyperbaric oxygen. Expert Rev
Neurother. 14:233–236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Davis MC, Shoja MM, Tubbs SR and
Griessenauer CJ: Hyperbaric oxygen therapy for chronic
post-concussive syndrome. Med Gas Res. 4:82014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harch PG: Hyperbaric oxygen in chronic
traumatic brain injury: Oxygen, pressure, and gene therapy. Med Gas
Res. 5:92015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hoge C and Jonas WB: The ritual of
hyperbaric oxygen and lessons for the treatment of persistent
postconcussion symptoms in military personnel. JAMA Intern Med.
175:53–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mychaskiw G: Known knowns, known unknowns
and unknown unknowns: The science and the passion of HBO2 therapy
and traumatic brain injury: An editorial perspective. Undersea
Hyperb Med. 40:371–372. 2013.PubMed/NCBI
|
|
28
|
Brkic P, Sanja P, Danijela K and Jovanović
T: Hyperbaric oxygenation as an adjuvant therapy for traumatic
brain injury: A review of literature. Periodicum Biologorum.
116:29–36. 2014.
|
|
29
|
Figueroa XA and Wright JK: Clinical
results in brain injury trials using HBO2 therapy: Another
perspective. Undersea Hyperb Med. 42:333–351. 2015.PubMed/NCBI
|
|
30
|
Guedes VA, Song S, Provenzano M and
Borlongan CV: Understanding the pathology and treatment of
traumatic brain injury and posttraumatic stress disorder: A
therapeutic role for hyperbaric oxygen therapy. Expert Rev
Neurother. 16:61–70. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu Q, Manaenko A, Guo Z, Huang L, Tang J
and Zhang JH: Hyperbaric oxygen therapy for post concussion
symptoms: Issues may affect the results. Med Gas Res. 5:102015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
McCrary BF, Weaver L, Marrs K, Miller RS,
Dicks C, Deru K, Close N and DeJong M: Hyperbaric oxygen (HBO) for
post-concussive syndrome/chronic TBI product summary. Undersea
Hyperb Med. 40:443–467. 2013.PubMed/NCBI
|
|
33
|
Mitchell SJ and Bennett MH: Unestablished
indications for hyperbaric oxygen therapy. Diving Hyperb Med.
44:228–234. 2014.PubMed/NCBI
|
|
34
|
Canadian Agency for Drugs and Technologies
in Health (CADTH), : Hyperbaric Oxygen Therapy for Adults with
Mental Illness: A Review of the Clinical Effectiveness. CADTH Rapid
Response Reports. CADTH; Ottawa, ON, Canada: 2014
|
|
35
|
Armistead-Jehle P and Lee D: Response to
the Harch Group's ‘A phase I study of low-pressure hyperbaric
oxygen therapy for blast-induced post-concussion syndrome and
post-traumatic stress disorder’. J Neurotrauma. 29:2513–2515. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Harch PG, Andrews SR, Fogarty EF, Amen D,
Pezzullo JC, Lucarini J, Aubrey C, Taylor DV, Staab PK, Van Meter
KW; Response to the letter to the editor by Armistead-Jehle and Lee
on Harch, ; et al: A Phase I Study of Low-Pressure Hyperbaric
Oxygen Therapy for Blast-Induced Post-Concussion Syndrome and
Post-Traumatic Stress Disorder. J Neurotrauma. 29:2516–2519. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Harch PG: Department of defense trials for
hyperbaric oxygen and TBI: Issues of study design and questionable
conclusions. Undersea Hyperb Med. 40:469–470. 2013.PubMed/NCBI
|
|
38
|
Hoge CW and Jonas WB: Hyperbaric oxygen
treatment for persistent postconcussion symptoms-reply. JAMA Intern
Med. 175:12412015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Marois P, Mukherjee A and Ballaz L:
Hyperbaric oxygen treatment for persistent postconcussion
symptoms-A Placebo effect? JAMA Intern Med. 175:1239–1240. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Miller RS, Weaver LK and Brenner LA:
Hyperbaric oxygen treatment for persistent postconcussion
symptoms-reply. JAMA Intern Med. 175:1240–1241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Weaver LK, Cifu D, Hart B, Wolf G and
Miller RS: Reply: Department of Defense trials for hyperbaric
oxygen and TBI: Issues of study design and questionable
conclusions. Undersea Hyperb Med. 40:471–472. 2013.PubMed/NCBI
|
|
42
|
Wolf EG, Baugh LM, Kabban CM, Richards MF
and Prye J: Cognitive function in a traumatic brain injury
hyperbaric oxygen randomized trial. Undersea Hyperb Med.
42:313–332. 2015.PubMed/NCBI
|
|
43
|
Wortzel HS, Arciniegas DB, Anderson CA,
Vanderploeg RD and Brenner LA: A phase I study of low-pressure
hyperbaric oxygen therapy for blast-induced post-concussion
syndrome and post-traumatic stress disorder: A neuropsychiatric
perspective. J Neurotrauma. 29:2421–2424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Miller RS, Brenner L, Churchill S, et al:
A phase II, randomized, sham-controlled trial hyperbaric oxygen for
post-concussion syndrome: Impact of intervention on symptoms and
well being. J Neurotrauma. 30:A562013.
|
|
45
|
Walker W, Cifu D, West S, Sima A, Graham
C, Hart B, Franke LM and Carne W: Hyperbaric oxygen for blast
related post-concussion syndrome: 3-month outcomes. Brain Injury.
28:6682014.
|
|
46
|
Hu Q, Liang X, Chen D, Chen Y, Doycheva D,
Tang J, Tang J and Zhang JH: Delayed hyperbaric oxygen therapy
promotes neurogenesis through reactive oxygen
species/hypoxia-inducible factor-1α/β-catenin pathway in middle
cerebral artery occlusion rats. Stroke. 45:1807–1814. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wolf EG, Prye J, Michaelson R, Brower G,
Profenna L and Boneta O: Hyperbaric side effects in a traumatic
brain injury randomized clinical trial. Undersea Hyperb Med.
39:1075–1082. 2012.PubMed/NCBI
|
|
48
|
Deng J, Lei C, Chen Y, Fang Z, Yang Q,
Zhang H, Cai M, Shi L, Dong H and Xiong L: Neuroprotective
gases-fantasy or reality for clinical use? Prog Neurobiol.
115:210–245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Eovaldi B and Zanetti C: Hyperbaric oxygen
ameliorates worsening signs and symptoms of post-traumatic stress
disorder. Neuropsychiatr Dis Treat. 6:785–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Morries LD, Cassano P and Henderson TA:
Treatments for traumatic brain injury with emphasis on transcranial
near-infrared laser phototherapy. Neuropsychiatr Dis Treat.
11:2159–2175. 2015.PubMed/NCBI
|
|
51
|
Raji CA, Tarzwell R, Pavel D, Schneider H,
Uszler M, Thornton J, van Lierop M, Cohen P, Amen DG and Henderson
T: Clinical utility of spect neuroimaging in the diagnosis and
treatment of traumatic brain injury: A systematic review. PLoS One.
9:e910882014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wright JK, Zant E, Groom K, Schlegel RE
and Gilliland K: Case report: Treatment of mild traumatic brain
injury with hyperbaric oxygen. Undersea Hyperb Med. 36:391–399.
2009.PubMed/NCBI
|
|
53
|
Cifu DX, Walker WC, West SL, Hart BB,
Franke LM, Sima A, Graham CW and Carne W: Hyperbaric oxygen for
blast-related postconcussion syndrome: Three-month outcomes. Ann
Neurol. 75:277–286. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cifu DX, Hoke KW, Wetzel PA, Wares JR,
Gitchel G and Carne W: Effects of hyperbaric oxygen on eye tracking
abnormalities in males after mild traumatic brain injury. J Rehabil
Res Dev. 51:1047–1056. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cifu DX, Hart BB, West SL, Walker W and
Carne W: The effect of hyperbaric oxygen on persistent
postconcussion symptoms. J Head Trauma Rehabil. 29:11–20. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Boussi-Gross R, Golan H, Fishlev G, Bechor
Y, Volkov O, Bergan J, Friedman M, Hoofien D, Shlamkovitch N,
Ben-Jacob E and Efrati S: Hyperbaric oxygen therapy can improve
post concussion syndrome years after mild traumatic brain
injury-randomized prospective trial. PLoS One. 8:e799952013.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Harch PG: Hyperbaric oxygen therapy for
post-concussion syndrome: Contradictory conclusions from a study
mischaracterized as sham-controlled. J Neurotrauma. 30:1995–1999.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Eyres S, Carey A, Gilworth G, Neumann V
and Tennant A: Construct validity and reliability of the Rivermead
Post-Concussion symptoms questionnaire. Clin Rehabil. 19:878–887.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bliese PD, Wright KM, Adler AB, Cabrera O,
Castro CA and Hoge CW: Validating the primary care posttraumatic
stress disorder screen and the posttraumatic stress disorder
checklist with soldiers returning from combat. J Consult Clin
Psychol. 76:272–281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Rice VJ, Overby C, Boykin G, Jeter A and
Villarreal J: How do I handle my life now? Coping and the post
traumatic stress disorder checklist-military version. Proc Hum
Factors Ergonomics Soc Ann Meet. 58:1252–1256. 2014. View Article : Google Scholar
|
|
61
|
Gardner PJ, Knittel-Keren D and Gomez M:
The posttraumatic stress disorder checklist as a screening measure
for posttraumatic stressdisorder in rehabilitation after burn
injuries. Arch Phys Med Rehabil. 93:623–628. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
King PR, Donnelly KT, Donnelly JP, Dunnam
M, Warner G, Kittleson CJ, Bradshaw CB, Alt M and Meier ST:
Psychometric study of the neurobehavioral symptom inventory. J
Rehabil Res Dev. 49:879–888. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ibarra S: Immediate post-concussion
assessment and cognitive testing. Encyclopedia of Clinical
Neuropsychology. Kreutzer JS, DeLuca J and Caplan B: Springer; New
York: pp. 1297–1299. 2011, View Article : Google Scholar
|
|
64
|
Kashluba S, Paniak C, Blake T, Reynolds S,
Toller-Lobe G and Nagy J: A longitudinal, controlled study of
patient complaints following treated mild traumatic brain injury.
Arch Clin Neuropsychol. 19:805–816. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bohnen N, Jolles J and Twijnstra A:
Neuropsychological deficits in patients with persistent symptoms
six months after mild head injury. Neurosurgery. 30:692–696. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Bazarian JJ, McClung J, Shah MN, Cheng YT,
Flesher W and Kraus J: Mild traumatic brain injury in the United
States, 1998-2000. Brain Inj. 19:85–91. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Al Sayegh A, Sandford D and Carson AJ:
Psychological approaches to treatment of postconcussion syndrome: A
systematic review. J Neurol Neurosurg Psychiatry. 81:1128–1134.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Mulkey DK, Henderson RA III, Putnam RW and
Dean JB: Pressure (< or=4 ATA) increases membrane conductance
and firing rate in the rat solitary complex. J Appl Physiol (1985).
95:922–930. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Gabizon I, Shiyovich A, Novack V,
Khalameizer V, Yosefy C, Moses SW and Katz A: Impact of descent and
stay at a Dead sea resort (low altitude) on patients with systolic
congestive heart failure and an implantable cardioverter
defibrillator. Isr Med Assoc J. 13:402–407. 2011.PubMed/NCBI
|
|
70
|
Goldbart AD, Cohen AD, Weitzman D and Tal
A: Effects of rehabilitation winter camps at the Dead Sea on
European cystic fibrosis patients. Isr Med Assoc J. 9:806–809.
2007.PubMed/NCBI
|
|
71
|
Falk B, Nini A, Zigel L, Yahav Y, Aviram
M, Rivlin J, Bentur L, Avital A, Dotan R and Blau H: Effect of low
altitude at the Dead Sea on exercise capacity and cardiopulmonary
response to exercise in cystic fibrosis patients with moderate to
severe lung disease. Pediatr Pulmonol. 41:234–241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Collet JP, Vanasse M, Marois P, Amar M,
Goldberg J, Lambert J, Lassonde M, Hardy P, Fortin J, Tremblay SD,
et al: Hyperbaric oxygen for children with cerebral palsy: A
randomised multicentre trial. HBO-CP Research Group. Lancet.
357:582–586. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Sham. September 13–2013http://www.merriam-webster.com/medical/sham
|
|
74
|
Potter S, Leigh E, Wade D and Fleminger S:
The rivermead post concussion symptoms questionnaire: A
confirmatory factor analysis. J Neurol. 253:1603–1614. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lin JW, Tsai JT, Lee LM, Lin CM, Hung CC,
Hung KS, Chen WY, Wei L, Ko CP, Su YK and Chiu WT: Effect of
hyperbaric oxygen on patients with traumatic brain injury. Acta
Neurochir Suppl. 101:145–149. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Mychaskiw G II and Stephens PL: Hyperbaric
oxygen, mild traumatic brain injury, and study design: An elusive
target. J Neurotrauma. 30:1681–1682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Peskind ER, Brody D, Cernak I, McKee A and
Ruff RL: Military- and sports-related mild traumatic brain injury:
Clinical presentation, management, and long-termconsequences. J
Clin Psychiatry. 74:180–188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Liu XH, Zhao YL, Ma QM, Zhou XH and Wang
Y: Optimal therapeutic window of hyperbaric oxygenation in neonatal
rat with hypoxic-ischemic brain damage. Zhonghua Er Ke Za Zhi.
44:177–181. 2006.(In Chinese). PubMed/NCBI
|
|
79
|
Liu XH, Yan H, Xu M, Zhao YL, Li LM, Zhou
XH, Wang MX and Ma L: Hyperbaric oxygenation reduces long term
brain injury and ameliorates behavioral function by suppression of
apoptosis in a rat model of neonatal hypoxia-ischemia. Neurochem
Int. 62:922–930. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhu M, Lu M, Li QJ, Zhang Z, Wu ZZ, Li J,
Qian L, Xu Y and Wang ZY: Hyperbaric oxygen suppresses
hypoxic-ischemic brain damage in newborn rats. J Child Neurol.
30:75–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Scorza KA, McCarthy W, Miller RS, Carne W
and Wolf G: Hyperbaric oxygen effects on PTSD and mild TBI
symptoms: A subset analysis. Undersea Hyperb Med (UHMS Annual
Meeting abstracts). 5:1066–2936. 2013.http://archive.rubicon-foundation.org/10677
|
|
82
|
Hooker JS: Hyperbaric oxygen therapy:
Using evidence-based medicine to heal injured brain tissue. N C Med
J. 77:69–70. 2016.PubMed/NCBI
|