Introduction
Radiation treatment, which is an essential
therapeutic tool for pelvic malignancies, can cause both acute and
chronic radiation proctitis. Acute radiation proctitis (ARP) is
defined as occurring within 3 months of radiotherapy and is often
self-limiting. Chronic radiation proctitis (CRP) occurs 3 to 6
months after radiotherapy or even years later (1–4). The
clinical symptoms of CRP include diarrhea, rectal pain, increased
defecate frequency, tenesmus, rectal bleeding, strictures, deep
ulcers and fistulas. Although the symptoms of many CRP patients may
spontaneously, for some patients who are more critically ill, it
may develop to persistent rectal bleeding, deep ulcers and even
fistulas that may deeply affect the patient's quality of life
(5,6). Nowadays, there are no clearly defined
treatment guidelines for CRP and therapeutic modalities include
topical and oral agents, endoscopic treatments, formalin
application, hyperbaric oxygen therapy (HBOT) and surgery. To date,
the majority of clinical trials have focused on controlling rectal
bleeding, which is thought to be a common complication of CRP
(7–9). Although a deep rectal ulcer is not as
common as rectal bleeding in CRP patients, persistent deep rectal
ulcers may result in complications, such as stricture and fistulas.
However, the data available on the treatment of CRP with deep
ulcers is limited and requires further study.
Mesalazine, also known as 5-aminosalicylic acid
(5-ASA), has been extensively studied in inflammatory bowel disease
and the majority of the data has shown that it is a simple, safe
and effective way to treat ulcerative colitis and Crohn's disease.
Numerous studies have been performed to investigate the efficacy of
5-ASA and its precursors in the treatment of CRP but the results
were inconsistent (10–13). Mesalazine is formulated for oral
ingestion and rectal administration. A previous study conducted by
Seo et al (12) demonstrated
that a combination of oral and topical mesalazine may be a safe and
effective treatment of the first episode of CRP, particularly for
hemorrhagic proctitis. Unlike previous studies, the present study
pays more attention to CRP with rectal ulcers. The aim of the
present study was to evaluate the efficacy and safety of topical
mesalazine suppository in the treatment of CRP with rectal
ulcers.
Patients and methods
Subjects
Between January 2010 and December 2015, 10 patients
that were diagnosed with refractory CRP were recruited at The
Second Affiliated Hospital of Guangxi Medical University (Nanning,
China). Exclusion criteria were: i) Patients with other bowel
disease and with a history of prior irradiation to the pelvis or
prior rectal ulcers; ii) patients with relapse or metastasis of a
primary tumor and iii) patients who received surgery as part of the
treatment of cancer. All patients that were enrolled had deep
rectal ulcers and had not responded to previous conventional
therapy, including sucralfate, corticosteroids, antibiotic,
probiotics and traditional Chinese medicine. All patients were
evaluated by the severity of the proctitis by clinical symptoms and
endoscopic examination prior to the treatment. Furthermore, patient
demographics and clinical data, including age, radiotherapy dose
and previous treatment options, were collected. Written informed
consent was obtained from all participants. The study was approved
by the Ethics Committee of The Second Affiliated Hospital of
Guangxi Medical University (Nanning, China).
Treatment methods
All patients were administered 0.5 g topical
mesalazine suppository (Salofalk; Dr. Falk Pharma GmbH, Freiburg,
Germany) twice daily for 6 months. During the 2 weeks prior to
mesalazine suppository, all patients did not receive any treatments
in order to exclude the confounding factors. Patients were followed
up by telephone or by an outpatient department visit at 2, 4, 8, 12
and 24 weeks after the initiation of treatment to collect clinical
data. Endoscopic evaluation was performed before and after
treatment.
Condition assessment criteria
All patients were evaluated by a combination of
clinical symptoms and endoscopic examination. Clinical symptoms
were graded based on the Subjective Objective Management Analysis
(SOMA) scale (Table I). There is no
standard endoscopic evaluation criterion for ulcerative CRP.
Therefore, the following five common endoscopic characteristics
associated with radiation proctitis were evaluated blindly by two
endoscopists, mean scores were reported as the final scores:
Telangiectasia, edema, ulceration, stenosis and necrosis (Table II).
 | Table I.Subjective Objective Management
Analysis assessment criteria for chronic radiation proctitis
symptoms. |
Table I.
Subjective Objective Management
Analysis assessment criteria for chronic radiation proctitis
symptoms.
| Score | Bleeding | Pain | Stool frequency | Tenesmus |
|---|
| 1 | Not obvious | Occasional | 2-4 times/day | Occasional |
| 2 | >2 times/week | Intermittent | 4-8 times/day | Frequent |
| 3 | Often | Sustained | >8 times/day | Sustained |
| 4 | Gross | Refractory | Out of control | Refractory |
 | Table II.Scoring of endoscopic characteristics
for chronic radiation proctitis. |
Table II.
Scoring of endoscopic characteristics
for chronic radiation proctitis.
| Score | Telangiectasia | Congestion/edema | Ulceration | Stenosis | Necrosis |
|---|
| 1 | Focal
telangiectasia | Focal
reddening | Superficial ulcer
<1 cm2 | Lumen diameter,
>2/3 normal range | Yes |
| 2 | Multiple
non-confluent | Diffuse
non-confluent | Superficial ulcer
>1 cm2 | Lumen diameter,
1/3-2/3 normal range | No |
| 3 | Multiple
confluent | Diffuse
confluent | Deep
ulceration | Lumen diameter,
<1/3 normal range | No |
| 4 | – | – |
Perforation/fistula | Obstruction | No |
Statistical analysis
All data were analyzed using SPSS statistical
package version 22.0 (IBM SPSS, Armonk, NY, USA). Continuous
variables were presented as the means with standard deviation or
median (range) according to data distributions. A paired,
two-tailed Student's t-test was performed to compare clinical
symptoms scores and endoscopic examination scores. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patients and treatments
A total of 10 female patients, which underwent
radiotherapy due to cervical cancer and were diagnosed with
refractory CRP, were enrolled in the present study. Demographics
and clinical data of the patients, including radiation dose and
previous treatment options, are listed in Table III. The mean age of all patients
was 48.80 years (range, 35-60 years). The mean interval from the
conclusion of radiotherapy to the onset of symptoms was 7.6 months
(range, 2-12 months), and the mean duration of symptoms before
treatment was 7.9 months (range, 5-15 months). The median
hemoglobin level was 101.3 g/dl (range, 37.9-126.6 g/dl), and only
2 patients required a blood transfusion before the treatment was
initiated.
 | Table III.Characteristics of all patients. |
Table III.
Characteristics of all patients.
| Case | Age (years) | Neoplasm
staging | Hypertension | Diabetes | Smoking
history |
Intervala (months) |
Durationb (months) | HGB (g/l) | Previous
treatmentc |
|---|
| 1 | 42 | IIIA | No | No | No | 4 | 4.0 | 85.0 | 2-6 |
| 2 | 35 | IIIB | No | No | No | 8 | 17.0 | 37.9 | 1-6 |
| 3 | 58 | IIIB | No | No | No | 7 | 2.5 | 126.6 | 1,5 |
| 4 | 46 | IIA | No | No | No | 9 | 3.0 | 124.3 | 4-6 |
| 5 | 41 | IIIB | No | No | No | 7 | 3.0 | 104.5 | 1,2,6 |
| 6 | 49 | IIB | No | No | No | 8 | 2.0 | 100.0 | 4-6 |
| 7 | 52 | IIIB | No | No | No | 2 | 3.0 | 104.2 | 5,6 |
| 8 | 46 | IIB | No | No | No | 9 | 8.0 | 51.4 | 3,4,6 |
| 9 | 59 | IIB | Yes | No | No | 12 | 3.0 | 100.3 | 4,6 |
| 10 | 60 | IIB | No | No | No | 10 | 6.0 | 102.3 | 4,6 |
Symptom score
The SOMA scale was assessed before and 2, 4, 8, 12,
24 weeks after the initiation of treatment. Following treatment,
there were statistically significant improvements in all symptoms.
The mean pre-treatment total symptom score was 8.20 and the
post-treatment mean score was 0.90. A total of 50% (5/10) of the
patients acquired a clinically complete response (post-treatment
total symptom score of 0). Additionally, there were reductions in
the mean scores for rectal bleeding (2.40 vs.0.30; P<0.01),
rectal pain (2.00 vs. 0.50; P<0.01), stool frequency (2.00 vs.
0.10; P<0.01) and tenesmus (1.80 vs. 0.00; P<0.01) before and
24 weeks after the initiation of treatment. The pre- and
post-treatment median symptoms scores are shown in Table IV.
 | Table IV.Symptom scores of patients pre- and
post-treatment. |
Table IV.
Symptom scores of patients pre- and
post-treatment.
| Time (weeks) | Total score | P-value | Rectal
bleeding | P-value | Pain | P-value | Stool
frequency | P-value | Tenesmus | P-value |
|---|
| Baseline | 8.20±3.39 | – | 2.40±0.97 | – | 2.00±0.94 | – | 2.00±1.05 | – | 1.80±1.03 | – |
| 2 | 5.50±3.37 | <0.01 | 1.30±0.95 | 0.003 | 1.70±1.16 | 0.081 | 1.00±0.94 | <0.01 | 1.50±0.97 | 0.081 |
| 4 | 3.60±2.91 | <0.01 | 0.80±0.79 | <0.01 | 1.50±1.27 | 0.052 | 0.40±0.70 | <0.01 | 0.90±0.74 | 0.004 |
| 8 | 1.60±1.90 | <0.01 | 0.40±0.52 | <0.01 | 0.90±0.99 | <0.01 | 0.10±0.32 | <0.01 | 0.20±0.42 | <0.001 |
| 12 | 1.30±1.89 | <0.01 | 0.30±0.48 | <0.01 | 0.80±1.03 | <0.01 | 0.10±0.32 | <0.01 | 0.10±0.32 | <0.001 |
| 24 | 0.90±1.29 | <0.01 | 0.30±0.48 | <0.01 | 0.50±0.71 | <0.01 | 0.10±0.32 | <0.01 | – | <0.001 |
A comparison of the total scores at baseline and at
week 2 revealed that the difference was statistically significant
(P<0.01), demonstrating that the majority of the patients
responded within 2 weeks of treatment initiation. Furthermore, when
comparing other symptom scores during the treatment period, the
data showed that the median time to improvement of rectal bleeding,
stool frequency, tenesmus and rectal pain with mesalazine
suppository treatment was 2, 4 and 8 weeks (Table IV). Furthermore, no patient
experienced worsening of his or her symptoms.
Endoscopic score
A total of 9 patients received endoscopic
examinations before and at 24 weeks after initiation of treatment.
Only 1 patient who suffered vagina-rectum fistula rejected
endoscopic examinations after treatment. After treatment, the mean
pre-treatment total symptom score was 9.22 and with a
post-treatment mean of 5.22 (P<0.01). In total, 2 patients (20%)
experienced complete ulcer healing. Additionally, there were
reductions in the mean scores for telangiectasia (pre vs.
post-treatment, 2.78 vs. 1.89; P=0.009), mucosal edema (2.89 vs.
1.78; P=0.001) and mucosal ulcers (2.44 vs. 0.89; P=0.003).
However, statistical reductions in the median symptom scores were
not observed for stenosis (0.78 vs. 0.67; P=0.347) and necrosis
(0.33 vs. 0.00; P=0.081; Table V).
The patient who suffered vagina-rectum fistula still had a fistula
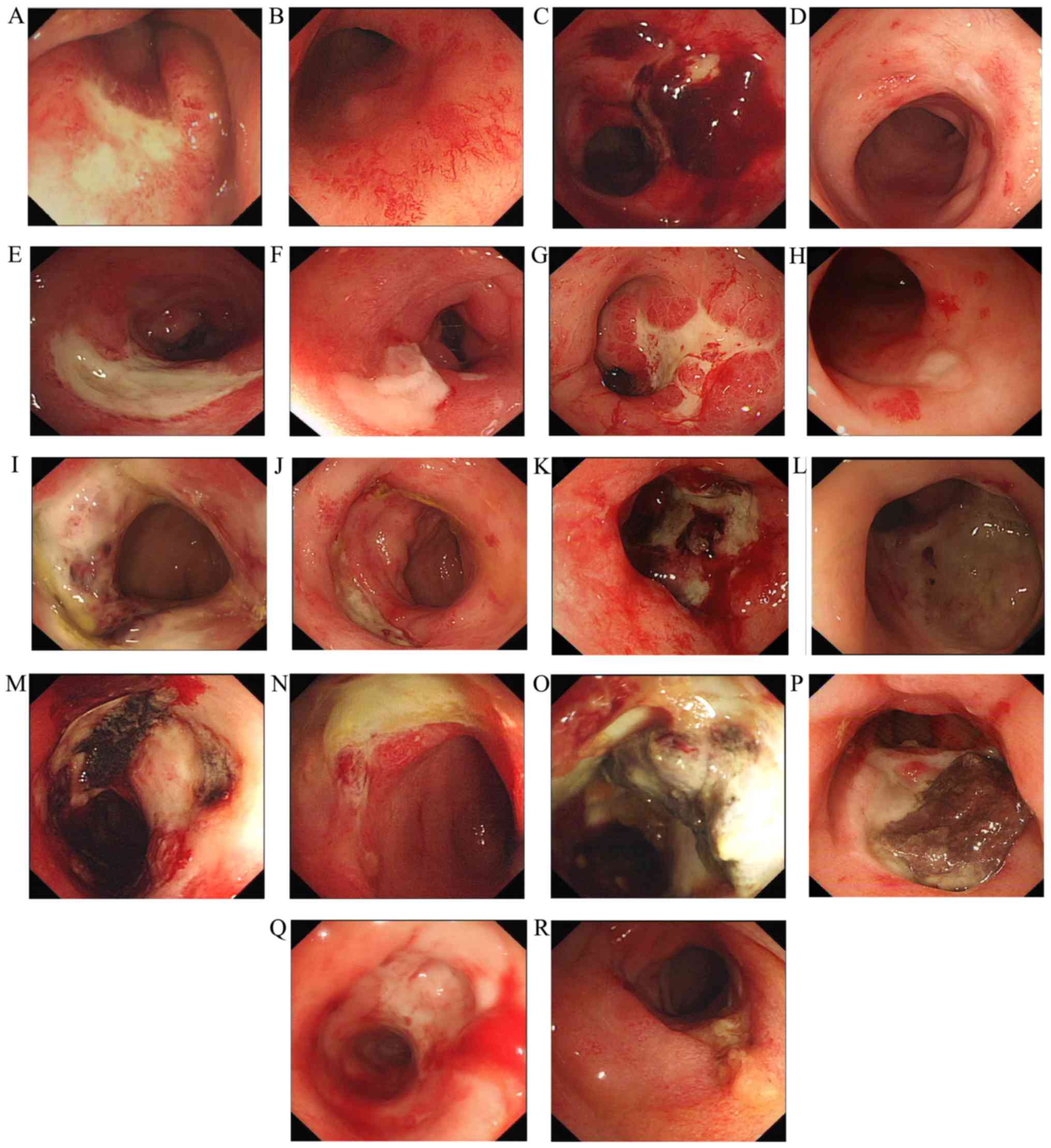
after treatment. The main pathological changes before and after
treatment under endoscopic examination are shown in Fig. 1.
 | Table V.Endoscopic scores of patients pre-
and post-treatment. |
Table V.
Endoscopic scores of patients pre-
and post-treatment.
| Time point | Total score | Telangiectasia | Edema | Ulceration | Stenosis | Necrosis |
|---|
| Baseline | 9.22±1.99 | 2.78±0.44 | 2.89±0.33 | 2.44±1.24 | 0.78±0.44 | 0.33±0.50 |
| 24 weeks | 5.22±1.39 | 1.89±0.60 | 1.78±0.67 | 0.89±1.27 | 0.67±0.50 | – |
| P-value | <0.01 | 0.009 | 0.001 | 0.003 | 0.347 | 0.081 |
Discussion
The present study assessed the efficacy and safety
of the mesalazine suppository in the treatment for CRP patients
complicated with rectal ulcers. The data demonstrated that the
majority of the patients experienced relief of rectal bleeding,
tenesmus, stool frequency and rectal pain symptoms. Furthermore,
endoscopic observations showed that this therapy promotes rectal
ulceration healing in CRP patients.
Radiation proctitis, which is one of the most common
intestinal complications following radiation therapy for pelvic
malignancies, can be divided into acute and chronic radiation
proctitis. Although radiotherapy techniques have been developed and
novel devices exist that spare the rectal exposure, CRP still
occurs in 5-20% of patients after radiotherapy. For mild CRP, they
can be resolved spontaneously without any treatment, whereas severe
symptoms of CRP, including persistent rectal bleeding, deep
ulceration, strictures and fistulas, have a worse prognosis and
require treatment to control symptoms (4,14).
Furthermore, several treatment options, including drug therapy,
endoscopic treatments, formalin application, HBOT and surgery have
been used to relieve symptoms of CRP, but none of them have shown
convincing efficacy (2,15).
Previous studies have focused on hemorrhagic CRP
(7–9), whereas the present study concentrated
on CRP patients with rectal ulcerations. All of the patients
enrolled in the present study had rectal ulcerations and were not
responsive to previous treatment modalities. Furthermore, rectal
ulceration is not a common complication for CRP, but persistent
deep rectal ulceration accompanies evident symptoms and is more
likely to lead to stricture, perforation and fistula. Until
recently, experiments for treatments of deep ulceration CRP were
limited. Although several drugs, including topical sucralfate
(9), intestinal probiotics (16,17),
steroid enemas (18), short chain
fatty acids (19,20), antioxidants (21), antibiotics (22), pentoxifylline (23) synbiotics (17) and traditional Chinese medicine
(24) have been used to relieve
symptoms of CRP, studies revealed that superior results were
observed in patients with mild-to-moderate CRP.
Novel endoscopic therapies, including argon plasma
coagulation (APC) and radiofrequency ablation (RFA), are preferred
alternatives to control persistent rectal bleeding of CRP (25–27).
However, these invasive treatments are not suitable for patients
with deep ulceration. The ulceration lesions involve the intestinal
muscle and even the serosa layers. Furthermore, invasive endoscopic
treatment should be avoided for ulcerative CRP because these
treatments may pose the risk of perforation and fistula (28–31).
Additionally, intrarectal formalin application also exhibited
effective control of rectal bleeding in patients with CRP (26,27).
However, the side effects of formalin application include anal
stenosis, fissures, fecal incontinence and ulceration of the
mucosa; therefore, it was not a good choice for ulcerative CRP
(32,33). Furthermore, although HBOT is reported
to be an effective treatment for CRP, it is not widely applicable
due to its high cost and the specialized equipment required
(34,35). Finally, surgery is thought to be the
last resort for CRP because of its severe postoperative
complications. Therefore, surgery may only be applied for severe
and refractory CRP patients who are not responsive to other medical
and endoscopic approaches (36,37).
Mesalazine, the active component of sulfasalazine,
is considered as an anti-inflammatory drug used to treat
inflammatory bowel disease, including ulcerative colitis and
Crohn's disease. Furthermore, mesalazine suppository is a
bowel-specific formulation that acts locally in the rectum and can
increase drug mucosal concentrations and decrease systemic side
effects. A number of clinical trials have been performed to
investigate the efficacy of 5-ASA and its precursors in the
prevention and treatment of radiation proctitis; however, the
conclusions remain controversial, with some studies reporting
positive results and others showing no effect or even worsening of
the symptoms (10–13). Based on previous results, the present
study attempted to evaluate the efficacy and safety of topical
mesalazine suppository in the treatment of refractory ulcerative
CRP. After 24 weeks of therapy, the clinical symptoms were relieved
and an endoscopic examination showed marked improvements in the
rectal mucosa damage. These results were consistent with the
results of Seo et al (12),
who conducted a prospective study to investigate the combination
therapy with oral and topical mesalazine for patients with a first
episode of radiation. After 4 weeks of treatment, they observed
that there were statistically significant improvements in rectal
bleeding; however, this treatment cannot control the symptoms of
pain, tenesmus and stool frequency. Endoscopic observations,
including telangiectasia, bleeding point and friable mucosa, also
showed a statistically significant improvement after 4 weeks of
combination treatment. The present study found that mesalazine
suppository therapy was able to not only control rectal bleeding
but also relieve the symptoms of tenesmus, stool frequency and
rectal pain in CRP. These contradictory observations may be due to
a difference in the treatment time between the two studies.
Patients in the present study received mesalazine suppository for
24 weeks whereas in the study by Seo et al (12) the patients received only 4 weeks of
combination treatment. Furthermore, the present study revealed that
rectal bleeding and stool frequency could be alleviated after 2
weeks of treatment, while longer treatments of at least 4 weeks
were required to improve the symptoms of tenesmus. Additionally,
rectal pain requires at least 8 weeks of treatment for it to be
relieved. In the present study, a statistically significant
improvement was observed not only in telangiectasia but also in the
mucosal edema and ulcerations after longer mesalazine suppository
treatment.
The present study had various limitations. There
were a small number of cases and no control group. Furthermore, the
period of follow-up was not long enough to evaluate the long-term
efficacies and complications. In conclusion, compared to other
special therapies that are performed in selected centers by skilled
operators, have potential complications and are expensive,
mesalazine suppository may be a feasible and effective treatment
for CRP, particularly in patients with ulcers.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Guangxi
Provincial Department of Health Topics (grant no. 2013176).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CW and JH designed the study. CW, JH, LG and LY
collected the data. LG and LY analyzed the data. CW, LG and LY
prepared the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
participants. The study was approved by the Ethics Committee of the
Second Affiliated Hospital of Guangxi Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ARP
|
acute radiation proctitis
|
|
CRP
|
chronic radiation proctitis
|
|
HBOT
|
hyperbaric oxygen therapy
|
|
5-ASA
|
5-aminosalicylic acid
|
|
APC
|
argon plasma coagulation
|
|
RFA
|
radiofrequency ablation
|
References
|
1
|
Abayomi J, Kirwan J and Hackett A: The
prevalence of chronic radiation enteritis following radiotherapy
for cervical or endometrial cancer and its impact on quality of
life. Eur J Oncol Nurs. 13:262–267. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mendenhall WM, McKibben BT, Hoppe BS,
Nichols RC, Henderson RH and Mendenhall NP: Management of radiation
proctitis. Am J Clin Oncol. 37:517–523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Theis VS, Sripadam R, Ramani V and Lal S:
Chronic radiation enteritis. Clin Oncol (R Coll Radiol). 22:70–83.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanson B, Macdonald R and Shaukat A:
Endoscopic and medical therapy for chronic radiation proctopathy: A
systematic review. Dis Colon Rectum. 55:1081–1095. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ruiz-Tovar J, Morales V, Hervás A,
Sanjuanbenito A, Lobo E and Martínez-Molina E: Late
gastrointestinal complications after pelvic radiotherapy: Radiation
enteritis. Clin Transl Oncol. 11:539–543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wallner K, Sutlief S, Bergsagel C and
Merrick GS: Severe rectal complications after prostate
brachytherapy. Radiother Oncol. 114:272–275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sahakitrungruang C, Thum-Umnuaysuk S,
Patiwongpaisarn A, Atittharnsakul P and Rojanasakul A: A novel
treatment for haemorrhagic radiation proctitis using colonic
irrigation and oral antibiotic administration. Colorectal Dis.
13:e79–e82. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dziki Ł, Kujawski R, Mik M, Berut M, Dziki
A and Trzciński R: Formalin therapy for hemorrhagic radiation
proctitis. Pharmacol Rep. 67:896–900. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McElvanna K, Wilson A and Irwin T:
Sucralfate paste enema: A new method of topical treatment for
haemorrhagic radiation proctitis. Colorectal Dis. 16:281–284. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jahraus CD, Bettenhausen D, Malik U,
Sellitti M and St Clair WH: Prevention of acute radiation-induced
proctosigmoiditis by balsalazide: A randomized, double-blind,
placebo controlled trial in prostate cancer patients. Int J Radiat
Oncol Biol Phys. 63:1483–1487. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sanguineti G, Franzone P, Marcenaro M,
Foppiano F and Vitale V: Sucralfate versus mesalazine versus
hydrocortisone in the prevention of acute radiation proctitis
during conformal radiotherapy for prostate carcinoma. A randomized
study. Strahlenther Onkol. 179:464–470. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seo EH, Kim TO, Kim TG, Joo HR, Park J,
Park SH, Yang SY, Moon YS, Park MJ, Ryu DY and Song GA: The
efficacy of the combination therapy with oral and topical
mesalazine for patients with the first episode of radiation
proctitis. Dig Dis Sci. 56:2672–2677. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Resbeut M, Marteau P, Cowen D, Richaud P,
Bourdin S, Dubois JB, Mere P and N'Guyen TD: A randomized double
blind placebo controlled multicenter study of mesalazine for the
prevention of acute radiation enteritis. Radiother Oncol. 44:59–63.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vanneste BG, Van De Voorde L, de Ridder
RJ, Van Limbergen EJ, Lambin P and van Lin EN: Chronic radiation
proctitis: Tricks to prevent and treat. Int J Colorectal Dis.
30:1293–1303. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sarin A and Safar B: Management of
radiation proctitis. Gastroenterol Clin North Am. 42:913–925. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spyropoulos BG, Misiakos EP, Fotiadis C
and Stoidis CN: Antioxidant properties of probiotics and their
protective effects in the pathogenesis of radiation-induced
enteritis and colitis. Dig Dis Sci. 56:285–294. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nascimento M, Aguilar-Nascimento JE,
Caporossi C, Castro-Barcellos HM and Motta RT: Efficacy of
synbiotics to reduce acute radiation proctitis symptoms and improve
quality of life: A randomized, double-blind, placebo-controlled
pilot trial. Int J Radiat Oncol Biol Phys. 90:289–295. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hille A, Schmidberger H, Hermann RM,
Christiansen H, Saile B, Pradier O and Hess CF: A phase III
randomized, placebo-controlled, double-blind study of misoprostol
rectal suppositories to prevent acute radiation proctitis in
patients with prostate cancer. Int J Radiat Oncol Biol Phys.
63:1488–1493. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pinto A, Fidalgo P, Cravo M, Midões J,
Chaves P, Rosa J, dos Anjos Brito M and Leitão CN: Short chain
fatty acids are effective in short-term treatment of chronic
radiation proctitis: Randomized, double-blind, controlled trial.
Dis Colon Rectum. 42:788–796. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Talley NA, Chen F, King D, Jones M and
Talley NJ: Short-chain fatty acids in the treatment of radiation
proctitis: A randomized, double-blind, placebo-controlled,
cross-over pilot trial. Dis Colon Rectum. 40:1046–1050. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kennedy M, Bruninga K, Mutlu EA, Losurdo
J, Choudhary S and Keshavarzian A: Successful and sustained
treatment of chronic radiation proctitis with antioxidant vitamins
E and C. Am J Gastroenterol. 96:1080–1084. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sahakitrungruang C, Thum-Umnuaysuk S,
Patiwongpaisarn A, Atittharnsakul P and Rojanasakul A: A novel
treatment for haemorrhagic radiation proctitis using colonic
irrigation and oral antibiotic administration. Colorectal Dis.
13:e79–e82. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hille A, Christiansen H, Pradier O,
Hermann RM, Siekmeyer B, Weiss E, Hilgers R, Hess CF and
Schmidberger H: Effect of pentoxifylline and tocopherol on
radiation proctitis/enteritis. Strahlenther Onkol. 181:606–614.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang L, Zhang ZZ, Tu XH, Zou ZD, Liu JH
and Wang Y: Safety and efficacy of Qingre Buyi Decoction in the
treatment of acute radiation proctitis: A prospective, randomized
and controlled trial. Chin J Integr Med. 15:272–278. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pigò F, Bertani H, Manno M, Mirante VG,
Caruso A and Conigliaro RL: Radiofrequency ablation for chronic
radiation proctitis: Our initial experience with four cases. Tech
Coloproctol. 18:1089–1092. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dray X, Battaglia G, Wengrower D, Gonzalez
P, Carlino A, Camus M, Adar T, Pérez-Roldán F, Marteau P and Repici
A: Radiofrequency ablation for the treatment of radiation
proctitis. Endoscopy. 46:970–976. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hortelano E, Gómez-Iturriaga A,
Ortiz-de-Zárate R, Zaballa M, Barturen Á, Casquero F, San-Miguel Í,
Carvajal C, Cacicedo J, Del-Hoyo O, et al: Is argon plasma
coagulation an effective and safe treatment option for patients
with chronic radiation proctitis after high doses of radiotherapy?
Rev Esp Enferm Dig. 106:1–170. 2014.PubMed/NCBI
|
|
28
|
Canard JM, Védrenne B, Bors G, Claude P,
Bader R and Sondag D: Long term results of treatment of hemorrhagic
radiation proctitis by argon plasma coagulation. Gastroenterol Clin
Biol. 27:455–459. 2003.(In French). PubMed/NCBI
|
|
29
|
Karamanolis G, Triantafyllou K, Tsiamoulos
Z, Polymeros D, Kalli T, Misailidis N and Ladas SD: Argon plasma
coagulation has a long-lasting therapeutic effect in patients with
chronic radiation proctitis. Endoscopy. 41:529–531. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Koessler T, Servois V, Mariani P, Aubert E
and Cacheux W: Rectal ulcer: Due to ketoprofen, argon plasma
coagulation and prostatic brachytherapy. World J Gastroenterol.
20:17244–17246. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ravizza D, Fiori G, Trovato C and Crosta
C: Frequency and outcomes of rectal ulcers during argon plasma
coagulation for chronic radiation-induced proctopathy. Gastrointest
Endosc. 57:519–525. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
de Parades V, Etienney I, Bauer P,
Bourguignon J, Meary N, Mory B, Sultan S, Taouk M, Thomas C and
Atienza P: Formalin application in the treatment of chronic
radiation-induced hemorrhagic proctitis-an effective but not
risk-free procedure: A prospective study of 33 patients. Dis Colon
Rectum. 48:1535–1541. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsujinaka S, Baig MK, Gornev R, de la
Garza C, Hwang JK, Sands D, Weiss EG, Nogueras JJ, Efron J, Vernava
AM III and Wexner SD: Formalin instillation for hemorrhagic
radiation proctitis. Surg Innov. 12:123–128. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Allen S, Kilian C, Phelps J and Whelan HT:
The use of hyperbaric oxygen for treating delayed radiation
injuries in gynecologic malignancies: A review of literature and
report of radiation injury incidence. Support Care Cancer.
10:2467–2472. 2012. View Article : Google Scholar
|
|
35
|
Oscarsson N, Arnell P, Lodding P, Ricksten
SE and Seeman-Lodding H: Hyperbaric oxygen treatment in
radiation-induced cystitis and proctitis: A prospective cohort
study on patient-perceived quality of recovery. Int J Radiat Oncol
Biol Phys. 87:670–675. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu XR, Liu XL, Katz S and Shen B:
Pathogenesis, diagnosis, and management of ulcerative proctitis,
chronic radiation proctopathy, and diversion proctitis. Inflamm
Bowel Dis. 21:703–715. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Do NL, Nagle D and Poylin VY: Radiation
proctitis: Current strategies in management. Gastroenterol Res
Pract. 2011:9179412011. View Article : Google Scholar : PubMed/NCBI
|