Introduction
Immunoglobulin A nephropathy (IgAN) was first
described and named by two French scholars, Berger and Hinglais, in
1968 (1). IgAN is typically
characterized by IgA deposits in the mesangial area of the
glomeruli. Glomerular IgA in biopsy specimens from IgAN patients
almost always belong to the IgA1 subclass and are generally
polymeric. These abnormal IgA1 peptides show a defect in the
galactose molecule. Levels of defective glycosylated IgA1 are
higher in the blood of IgAN patients in comparison to normal
individuals (2). Recently, defective
IgA1 has been considered to be an important factor for the
development and progression of IgAN. It has been reported that
(3) Fas mRNA expression and
podocyte apoptosis increased markedly upon exposure to conditioned
media produced by cultured mesangial cells incubated with
aggregated IgA1 (aIgA1) from IgAN patients. Both aIgA1 from IgAN
patients and the conditioned media from mesangial cells incubated
with aIgA1 from IgAN patients inhibited the expression of podocyte
nephrin, which is a necessary protein for the proper functioning of
the renal filtration barrier (4).
The IgA1-immune complex in IgAN patients may increase the
production of chemokine (C-X-C motif) ligand 1 (CXCL1) and
transforming growth factor-beta (TGF-β) from mesangial cells
(5). In turn, CXCL1 and TGF-β may
exert a synergistic effect on podocytes, inducing podocyte
dysfunction and even death. Thus, it can be concluded that the
podocyte plays an important role in IgAN. Clinical findings have
also consolidated this point of view. Podocytes were found to
detach from the glomerular basal membrane (6) and were detected in the urine of IgAN
patients. Most importantly, the amount of podocytopenia has been
found to be closely associated with the severity of glomerular
pathological lesions (7). Autophagy
is especially important for the maintenance of podocytes, which
have only a limited capacity for regeneration (8). For example, aging mice with
podocyte-specific deletion of autophagy-related genes exhibited
proteinuria and glomerulopathy (9).
Triptolide is a common Chinese herb often used as an
immunosuppressant in glomerulonephritis (10). However, its exact mechanism of action
remains unclear. The present study was designed to explore the
renal protective effect of triptolide on podocyte autophagy upon
treatment with the conditioned media from mesangial cells incubated
with aIgA1 from IgAN patients.
CD63, also known as lysosomal-associated membrane
protein-3, is a widely used marker of secretory lysosomes, and
positively correlates with the formation of autolysosomes. As a
soluble protein in mammalian cells, microtubule-associated protein
light chain 3 (LC3) exists in the two forms of LC3-I and LC3-II.
LC3-II, the conjugated product of LC3-I and phosphatidyl
ethanolamine (PE), is a necessary molecule during the formation of
autophagosome. Hence an elevated LC3-II or LC3-II/LC3-I ratio is
considered as the typical and standard marker for activation of
autophagy. At present, the PI3K/Akt signalling pathway has been
shown to be involved in multiple physiological and pathological
processes and is one of the most important pathways regulating
autophagy. The phosphorylation of mTOR, a major suppressor of
autophagy, can be promoted by phosphorylated Akt (p-Akt).
Therefore, the above were selected as the autophagic markers of
reference in our study.
Materials and methods
Cell culture and grouping
We used a conditionally immortalized mouse podocyte
cell line (MPC5, Tong-Pai Bio-tech Co., Ltd., Shanghai, China). The
aIgA1 purification protocol has been described in detail previously
(11). Of the 35 patients in this
study, who were admitted to Zhejiang Provincial Peope's Hospital
(Hangzhou, China) from January 1, 2013 to December 31, 2014, 18
were male and 17 were female. Five healthy male subjects were also
enrolled in this study. The study has obtained human research
ethics approval from the ethics committee of Zhejiang Provincial
People's Hospital and all subjects provided written consent
(KY2014011).
All experiments were performed on podocytes
differentiated for 10–14 passages. MPC5 cells were exposed to media
conditioned by mouse mesangial cells (MSC1097, Tong-Pai Bio-tech
Co., Ltd.), which had been cultured for 24 h without additional
treatment (control group, CON) or in the presence of the following:
1) aIgA1 (100 mg/l) from IgAN patients (IgANs group); or 2) aIgA1
(100 mg/l) from healthy subjects (HEAs group); 3) triptolide (10
ng/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and aIgA1
(100 mg/l) from IgAN patients (TRI group), in RPMI 1640 medium.
After co-culture for 24 h, cells were collected for further
experiments. We selected 100 mg/l aIgA1and 10 ng/ml triptolide to
treat the cells just according to previous researches (12,13).
Western blot analysis
Podocytes were lysed in a lysis buffer at 4°C. After
being centrifuged at 12,000 rpm for 5 min, the media containing
cellular proteins were collected. Subsequently, a 6–15% sodium
dodecyl sulphate polyacrylamide gel was run under standard
electrophoresis conditions, with 30 µg of total protein loaded in
each lane. Separated proteins were transferred to a nitrocellulose
membrane (Merck KGaA) at 200 mA for 2 h and blocked with 5% non-fat
milk powder at room temperature for 2 h. Membranes with proteins
were then incubated with primary antibodies (LC3, p62, p-Akt, Akt,
mTOR, and p-mTOR: Rabbit polyclonal antibodies, dilution 1:1,000;
GAPDH: Rabbit polyclonal antibody, dilution 1:5,000; Wuhan Sanying
Biotechnology, Wuhan, China) at 4°C overnight. The secondary
antibodies (horseradish peroxidase-conjugated secondary antibody,
goat anti-rabbit, dilution 1:3,000, Wuhan Sanying Biotechnology)
were subsequently added and incubated for 1 h. Finally, all blots
were developed using ECL Chemiluminescence Reagent (Merck KGaA).
The levels of the target protein relative to the control GAPDH were
detected by densitometric scanning using Gel-Pro Application.
Immunofluorescence analysis
Fifty cells in three independent experiments were
counted. Cells were cultured on coverslips in 12 well plates at a
density of 5×104 cells per well. Podocytes were washed
with sterile PBS and fixed in 4% paraformaldehyde for 10 min at
37°C. Afterwards, they were permeabilized with PBS containing 1%
Triton X-100. After being washed twice with PBS, cells were blocked
with 3% bovine serum albumin in PBS for 30 min and a primary
antibody (p62, CD63, or LC3-II, Abcam, Cambridge, MA, USA) was
added in blocking buffer overnight at a 1:200 dilution. Alexa Fluor
488-conjugated Affinipure anti-goat (1:500, Tong-Pai Bio-tech Co.,
Ltd.) was used as the secondary antibody. After being
counterstained with 4,6-diamidino-2-phenylindole (Santa Cruz
Biotechnology, Dallas, TX, USA) for 10 min, podocytes were viewed
and imaged by a laser-scanning confocal microscope (PerkinElmer,
Inc., and Olympus Corporation, Waltham, MA, USA).
Apoptosis assays
Apoptosis was determined using an FITC-Annexin V/PI
Apoptosis Detection Kit, according to the manufacturer instructions
(Shanghai BeiBo Biotech, Shanghai, China). Cells were collected and
resuspended in binding buffer. Subsequently, cells were incubated
with Annexin V for 10 min at room temperature and 5 µl of propidium
iodide was added before analysis. Cells were analysed by the FAC
Scan flow cytometer (BD Biosciences, Franklin Lanes, NJ, USA).
Statistical analysis
All experiments were performed a minimum of three
times. SPSS 19.0 statistical software was used for the data
processing. Data were compared by repeated measurement analysis of
variance followed by the least significant difference post hoc test
and are presented as means ± SEM. P-value <0.05 was required for
the results to be considered statistically significant.
Results
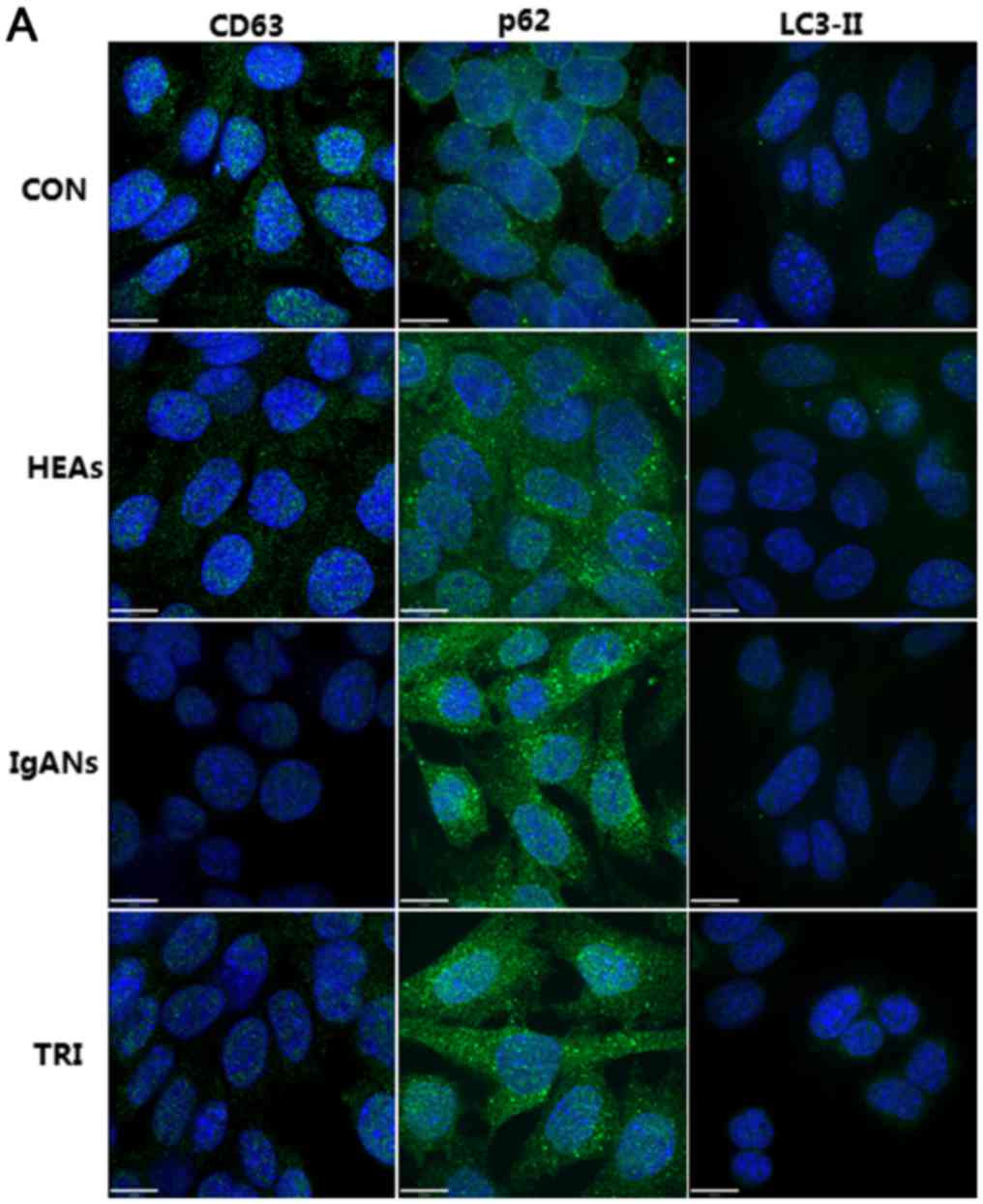
Podocytes exposed to the media of
mesangial cells treated with aIgA1 from IgAN patients showed
attenuated autophagy
We measured the level of autophagy-related proteins
in MPC5 cells cultured with the supernatant from MSC1097 cells
treated with aIgA1 from IgAN patients. The average optical density
of CD63 in the IgANs group was clearly decreased compared to the
CON and the HEAs (Fig. 1,
P<0.05). LC3-II binds to the autophagosomal membrane and
generates puncta when the autophagosome is produced. In this study,
bright puncta were clearly visible in the cytoplasm of CON group
MPC5 cells, but were rare in IgAN group cells (Fig. 1). Simultaneously, p62 accumulation
was observed in IgANs group podocytes. To confirm these
immunofluorescence results, we evaluated LC3-II and p62 protein
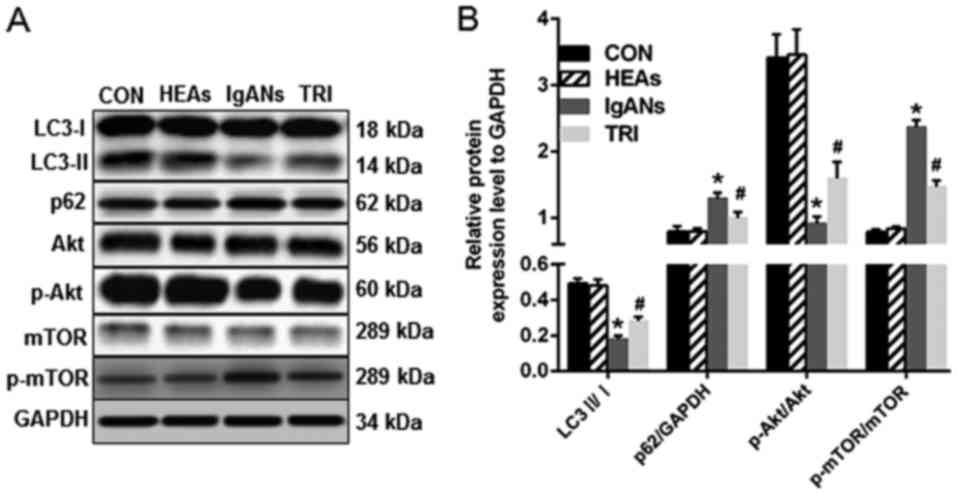
levels by western blotting (Fig. 2).
The LC3-II/I ratio of MPC5 cells in the IgANs group decreased
significantly and was only about one-third of the CON group
(Fig. 2, P<0.05). In contrast,
MPC5 cells in the IgANs group showed increased expression of p62
(Fig. 2, P<0.05 vs. the CON).
These western blotting results are in accordance with the
immunofluorescence, and revealed attenuated autophagy in the IgANs
group.
Triptolide induced podocyte autophagy
potentially through p-mTOR/mTOR
We inferred that the PI3K/Akt pathway was triggered
in IgANs group MPC5 cells with impaired autophagy. In agreement
with our hypothesis, the ratio of p-mTOR/mTOR was at basal levels
in the groups of CON and the HEAs, but was markedly elevated in the
IgANs group (Fig. 2, P<0.001),
and this was attenuated in the TRI group (Fig. 2, P<0.001). Interestingly, the
p-Akt/Akt ratio presented an opposite situation, where the
expression of Akt was first inhibited in the IgANs group (Fig. 2, P<0.001) and then activated in
the TRI group (Fig. 2, P<0.001).
A possible explanation is that autophagy was not mediated by the
classical mTOR signalling pathway or that p-Akt levels were
modulated as a negative feedback response of p-mTOR modulation.
Triptolide induces podocyte autophagy
and alleviates podocytes apoptosis
After co-culture with triptolide and the conditioned
media of MPC1097 cells treated with IgAN-patient derived aIgA1,
podocytes presented a notable induction of autophagy, which was
characterized by higher expression of CD63 (P=0.039 vs. the IgANs
group, Fig. 1), obviously elevated
LC3-II (P=0.013 vs. the IgANs group, Fig. 1), and attenuated expression of p62
(P<0.01 vs. the IgANs group). These results were also supported
by western blotting, which showed a higher LC3-II/LC3-I ratio and a
lower level of p62 in the TRI group (Fig. 2, P<0.01 vs. the IgANs group). We
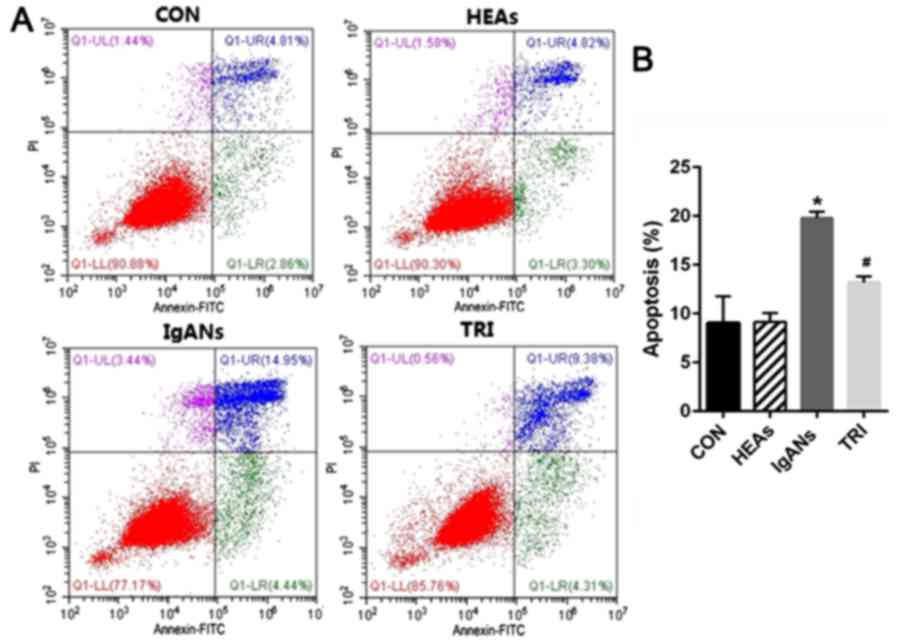
also examined the apoptosis rate of podocytes in the different
groups. Fig. 3 demonstrates that the
percentage of apoptotic cells increased from 9.05% in the control
to 19.8% in IgANs group (Fig. 3,
P<0.01). As expected, when compared with the IgANs group, the
apoptosis rate of podocytes in TRI group exhibited obvious
improvement from 19.8 to 13.2% (Fig.
3, P=0.001).
Discussion
A recent study indicated that the number of patients
with chronic kidney disease (CKD) in China is approximately 119
million (14), which creates a great
societal burden. Glomerular disease continues to be the primary
cause of CKD in China. Nowadays, IgAN is considered as the most
common immune disorder related to glomerulonephritis pathogenesis
(15). The single diagnostic
characteristic of IgAN is the observation of aggregated IgA immune
deposits in the glomerular mesangium in renal biopsy (16). Therefore, cultured mouse mesangial
cells were treated with aIgA1 in this study. Moreover,
aIgA1-MSC1097-conditioned medium was harvested to treat podocytes,
and podocyte autophagy was subsequently evaluated in the presence
or absence of triptolide.
Triptolide is among the most powerful and broadly
active natural products known today (17). It is a product of the epoxidation of
two terpene lactone compounds extracted from the traditional
Chinese herb Tripterygium wilfordii Hook F (TWHF).
Triptolide has been used in the treatment of glomerulonephritis for
more than 30 years in China because of its favourable cost-benefit
ratio and potent therapeutic immunosuppressive and
anti-inflammatory effects (18).
Having demonstrated dramatically attenuated albuminuria and renal
lesions in patients with multiple kidney diseases, triptolide could
be a comprehensive protective drug for delaying CKD progression.
Recently, an animal study (19)
demonstrated that triptolide alleviated mesangial proliferation,
mesangial expansion, and glomerular IgA deposition in an IgAN rat
model via anti-inflammatory effects. Triptolide can reduce
proteinuria and effectively protect podocytes in rats from
puromycin-induced nephropathy (20).
This protective effect may be associated with the upregulation and
distribution of nephrin and podocin (10). It was found that Tripterygium
preparations, which are mainly composed of triptolide, could
ameliorate proteinuria due to the enhanced expression of nephrin
(21). Triptolide has been reported
to markedly attenuate albuminuria and improve podocyte injury in a
rat model of diabetic nephropathy, possibly due to its inhibitory
effects on inflammation and macrophage infiltration in the kidney
(22). In addition, triptolide may
attenuate podocyte injury in rats with adriamycin-induced
nephropathy by regulating expression of miRNA-344b-3p and
miRNA-30b-3p (23). These data
showed that triptolide can protect podocytes from from different
kinds of injury. Autophagy is very important for the maintenance of
end-differentiated cells such as podocytes. Therefore, we inferred
that the protective effects of triptolide on podocytes can be
correlated to autophagy and conducted this study to observe whether
triptolide can maintain podocyte autophagy capability.
It has been reported that podocyte autophagy is
involved in the development of IgAN. Two types of autophagy were
found (24) in podocytes obtained
from renal biopsies of 16 children. Type I rarely transformed to
autophagic vacuoles and did not dissolve, while Type II frequently
transformed to autophagosomes and autophagic vacuoles, thus
facilitating protein and lipid clearance. Therefore, it was
concluded that type I autophagy was correlated with
histopathologically more progressive disease, possibly reflecting a
tendency towards poorer prognosis. We have previously reported
(25) that podocytes from the renal
biopsies of IgAN patients possessed more autophagosomes than
healthy subjects did. In this study, we found that autophagy was
reduced in podocytes exposed to IgAN-derived aIgA1-conditioned
medium compared to the CON group. This autophagy dysfunction was
characterized by decreased LC3-II and increased p62 levels.
Collectively, this evidence indicated that podocyte autophagy was
suppressed during the progression of IgAN. In this study, we found
that autophagy inhibition was recovered when podocytes were exposed
to triptolide. The results indicated that triptolide can induce
podocyte autophagy, promote the conversion of LC3-I to LC3-II, as
well as the elimination of p62. Similar phenomena have been
reported in other cell types. In an animal study it was found that
the administration of triptolide 12 h prior to middle cerebral
artery occlusion reduced infarction area through the activation of
autophagy (26). Triptolide could
induce autophagy and promote the clearance of both WT and
A53Tα-synuclein in neurons (27).
Another finding of this study was that podocyte
autophagy in IgAN was suppressed or induced by triptolide through
p-mTOR/mTOR. It is well known that the PI3K/Akt pathway is one of
the most important signalling pathways regulating autophagy.
Phosphorylated Akt can promote the formation of p-mTOR, an
inhibitor of cell autophagy. The activation of the PI3K/Akt pathway
was the main cause of impaired autophagy in ELK3-knockdown
MDA-MB-231 cells (28).
Surprisingly, we found increased p-mTOR and decreased p-Akt in the
IgANs group podocytes, whereas we detected decreased p-mTOR and
elevated p-Akt in the TRI group podocytes. An alternative
hypothesis may be that other signalling pathways are involved.
Another possible reason could be that the modulation of p-Akt was a
response to the modulation of p-mTOR.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Project of
Scientific research Foundation of Chinese Medicine (grant no.
2014ZB002).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL and QH designed the concept and performed the
experiments. JJ and XS performed the experiments. SL and XJ
analysed and interpreted the data. YL was involved in analyzing
data and drafting the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee. The study has
obtained human research ethics approval from the local ethics
committee and all subjects provided informed consent (approval no.
KY2014011).
Patient consent for publication
Informed consent was obtained from all individual
participants included in the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Berger J and Hinglais N: Intercapillary
deposits of IgA-IgG. J Urol Nephrol (Paris). 74:694–695. 1968.(In
French). PubMed/NCBI
|
|
2
|
Gharavi AG, Kiryluk K, Choi M, Li Y, Hou
P, Xie J, Sanna-Cherchi S, Men CJ, Julian BA, Wyatt RJ, et al:
Genome-wide association study identifies susceptibility loci for
IgA nephropathy. Nat Genet. 43:321–327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang C, Tang Y, Peng H, Ye ZC, Chen ZJ, Yu
XQ and Lou TQ: Supernatant of cultured mesangial cells with IgA1
from IgA nephropathy induces apoptosis of podocyte. Chin J Nephrol.
24:387–391. 2008.
|
|
4
|
Wang C, Ye Z, Peng H, Tang H, Liu X, Chen
Z, Yu X and Lou T: Effect of aggregated immunoglobulin A1 from
immunoglobulin A nephropathy patients on nephrin expression in
podocytes. Nephrology (Carlton). 14:213–218. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu L, Zhang Q, Shi S, Liu L, Lv J and
Zhang H: Synergistic effect of mesangial cell-induced CXCL1 and
TGF-β1 in promoting podocyte loss in IgA nephropathy. PLoS One.
8:e734252013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tewari R, Nada R, Rayat CS, Boruah D,
Dudeja P, Joshi K and Sakhuja V: Correlation of proteinuria with
podocyte foot process effacement in IgA nephropathy: An
ultrastructural study. Ultrastruct Pathol. 39:147–151. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen P, Shen J, Li W and He L: Urinary
podocyte can be an indicator for the pathogenetic condition of
patients with IgA nephropathy. Clin Lab. 60:1709–1715. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yasuda-Yamahara M, Kume S, Tagawa A,
Maegawa H and Uzu T: Emerging role of podocyte autophagy in the
progression of diabetic nephropathy. Autophagy. 11:2385–2386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hartleben B, Gödel M, Meyer-Schwesinger C,
Liu S, Ulrich T, Köbler S, Wiech T, Grahammer F, Arnold SJ,
Lindenmeyer MT, et al: Autophagy influences glomerular disease
susceptibility and maintains podocyte homeostasis in aging mice. J
Clin Invest. 120:1084–1096. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li XJ, Jiang ZZ and Zhang LY: Triptolide:
Progress on research in pharmacodynamics and toxicology. J
Ethnopharmacol. 155:67–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang S, Jin J, Lin B, Gong J, Li Y and He
Q: Rapamycin induces autophagy and reduces the apoptosis of
podocytes under a stimulated condition of immunoglobulin A
nephropathy. Kidney Blood Press Res. 42:177–187. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dai Q, Liu J, Du YL, Hao X, Ying J, Tan Y,
He LQ, Wang WM and Chen N: Histone deacetylase inhibitors attenuate
P-aIgA1-induced cell proliferation and extracellular matrix
synthesis in human renal mesangial cells in vitro. Acta Pharmacol
Sin. 37:228–234. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang Q, Sun M, Chen Y, Lu Y, Ye Y, Song H,
Xu X, Shi S and Wang J: Triptolide protects podocytes from
TGF-β-induced injury by preventing miR-30 downregulation. Am J
Transl Res. 9:5150–5159. 2017.PubMed/NCBI
|
|
14
|
Liu ZH: Nephrology in China. Nat Rev
Nephrol. 9:523–528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wyatt RJ and Julian BA: IgA nephropathy. N
Engl J Med. 368:2402–2414. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin M, Du L, Brandtzaeg P and
Pan-Hammarström Q: IgA subclass switch recombination in human
mucosal and systemic immune compartments. Mucosal Immunol.
7:511–520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Graziose R, Lila MA and Raskin I: Merging
traditional Chinese medicine with modern drug discovery
technologies to find novel drugs and functional foods. Curr Drug
Discov Technol. 7:2–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Q: Triptolide and its expanding
multiple pharmacological functions. Int Immunopharmacol.
11:377–383. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He L, Peng X, Liu G, Tang C, Liu H, Liu F,
Zhou H and Peng Y: Anti-inflammatory effects of triptolide on IgA
nephropathy in rats. Immunopharmacol Immunotoxicol. 37:421–427.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng CX, Chen ZH, Zeng CH, Qin WS, Li LS
and Liu ZH: Triptolide protects podocytes from puromycin
aminonucleoside induced injury in vivo and in vitro. Kidney Int.
74:596–612. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang YG, Sun W and Zhen YJ: Preventive
effect of multi-glycoside of Tripterygium wilfordii Hook. f. on
proteinuria and mesangial injury in experimental mesangial
proliferative glomerulonephritis. Zhongguo Zhong Xi Yi Jie He Za
Zhi. 25:817–821. 2005.(In Chinese).
|
|
22
|
Ma R, Liu L, Liu X, Wang Y, Jiang W and Xu
L: Triptolide markedly attenuates albuminuria and podocyte injury
in an animal model of diabetic nephropathy. Exp Ther Med.
6:649–656. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang CB, Wei MG, Tu Y, Zhu H, Li CQ, Jing
WM and Sun W: Triptolide attenuates podocyte injury by regulating
expression of miRNA-344b-3p and miRNA-30b-3p in rats with
adriamycin-induced nephropathy. Evid Based Complement Alternat Med.
2015:1078142015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sato S, Yanagihara T, Ghazizadeh M,
Ishizaki M, Adachi A, Sasaki Y, Igarashi T and Fukunaga Y:
Correlation of autophagy type in podocytes with histopathological
diagnosis of IgA nephropathy. Pathobiology. 76:221–226. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang S, Jin J, Gong J, Lin B, Li Y and He
Q: How many podocyte autophagosomes are there in immunoglobulin A
nephropathy and idiopathic membranous nephropathy? Int Urol
Nephrol. 48:2109–2114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang Y, Gao K, Hu Z, Li W, Davies H, Ling
S, Rudd JA and Fang M: Autophagy upregulation and apoptosis
downregulation in DAHP and triptolide treated cerebral ischemia.
Mediators Inflamm 2015. 1201982015.
|
|
27
|
Hu G, Gong X, Wang L, Liu M, Liu Y, Fu X,
Wang W, Zhang T and Wang X: Triptolide promotes the clearance of
α-synuclein by enhancing autophagy in neuronal cells. Mol
Neurobiol. 54:2361–2372. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park JH, Kim KP, Ko JJ and Park KS:
PI3K/Akt/mTOR activation by suppression of ELK3 mediates
chemosensitivity of MDA-MB-231 cells to doxorubicin by inhibiting
autophagy. Biochem Biophys Res Commun. 477:277–282. 2016.
View Article : Google Scholar : PubMed/NCBI
|