Introduction
Lung cancer is one of the leading factors of
cancer-associated fatality around the world (1). Every year, >300,000 new patients are
diagnosed with lung cancer in the United States (2). A complexity of factors and signaling
pathways contribute to the development and progression of lung
cancer, including environmental pollution (3). Among all lung cancer cases, non-small
cell lung cancer (NSCLC) accounts for ~85% (4). Although advances have been made on lung
cancer intervention, the outcomes of patients remain very poor. The
five-year survival rate of patients with NSCLC remains low
(5). Therefore, to improve the
outcome of NSCLC the identification of novel biomarkers for NSCLC
diagnosis and prognosis and the development of effective
therapeutic targets are urgently required.
MicroRNAs (miRs) are a class of endogenous noncoding
RNAs with a length of 19–22 nucleotides that are widely expressed
in almost all cell types (6). miRs
are able to regulate gene expression by binding to the
complementary sequence of the 3′-untranslated region (3′-UTR) of
target mRNAs (7). An increasing
number of studies indicate that miRs are critical for the
regulation of nearly all physiological processes, including the
cell cycle, proliferation, migration, invasion and differentiation
of cells (8). Therefore, abnormal
expression of miRs typically results in occurrence of human cancer,
including NSCLC (5). For example,
upregulation of miR-383 has been demonstrated to inhibit the
proliferation, migration and invasion of colon cancer cells
(9). A previous study also revealed
that miR-185 was downregulated and suppressed pancreatic cell
proliferation by targeting transcriptional coactivator with
PDZ-binding motif in pancreatic cancer (10). Furthermore, several miRs are reported
to be promising predictors for cancer diagnosis and prognosis
(11–13). Thus, investigation into the roles and
functional mechanisms of miRs is crucial to NSCLC therapy.
miR-936 is located at chromosome 10q25.1 and has a
length of 22 nucleotides (14). A
previous study indicated that miR-936 inhibits glioma cell
proliferation by targeting CKS1 (15). However, the function of miR-936 in
other cancer types remains unclear.
The present study investigated the expression of
miR-936 in NSCLC tissues and cell lines compared with normal
tissues or cells. Furthermore, the associated mechanism of miR-936
in NSCLC progression was assessed and its effect on the
proliferation, cell cycle and invasion of NSCLC cells was
determined.
Materials and methods
Clinical specimens
The procedures in the present study were approved by
the Ethics Committee on Human Experimentation of The Second
Hospital of Anhui Medical University (Hefei, China) and written
informed consent was also obtained from each patient. Samples of
primary cancer tissues (n=35; male:female ratio, 26:9; age range,
39–68 years; median age, 55 years) and adjacent normal tissues
(n=13) were obtained from patients who had undergone surgery at The
Second Hospital of Anhui Medical University from January 2014 to
December 2016. The patients who received chemotherapy and
radiotherapy were excluded. The clinical pathological parameters
are listed in Table I. All samples
were rapidly placed in liquid nitrogen and stored at −80°C until
use.
 | Table I.Association of the expression of
miR-936 with clinicopathological features. |
Table I.
Association of the expression of
miR-936 with clinicopathological features.
| Clinicopathological
parameters | miR-936 high
(n=17) | miR-936 low
(n=18) | P-valuea |
|---|
| Age (years) |
|
| 0.443 |
| ≤50 | 12 | 15 |
|
|
>50 | 5 | 3 |
|
| Tumor stage |
|
| 0.044 |
| I/II | 11 | 5 |
|
|
III/IV | 6 | 13 |
|
| Tumor size (cm) |
|
| 0.035 |
| ≤4 | 14 | 8 |
|
|
>4 | 3 | 10 |
|
| Lymph node
metastasis |
|
| 0.028 |
|
Negative | 8 | 2 |
|
|
Positive | 9 | 16 |
|
Cell culture and transfection
Lung adenocarcinoma cell lines (A549, SPC-A1, H1299,
and H23) and the bronchial epithelial cell line (16HBE) were
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA) and were cultured in Dulbecco's modified Eagle
medium (DMEM, Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) with 10% fetal bovine serum (FBS, Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in an atmosphere containing 5%
CO2. miR-936 mimic (5′-ACAGUAGAGGGAGGAAUCGCAG-3′) and
scramble mimic (5′-ACAUCUGCGUAAGAUUCGAGUCUA-3′) were synthesized by
the Shanghai GenePharma (Shanghai, China). An E2F2 coding sequence
was constructed into a pcDNA3 vector (Addgene, Inc., Cambridge, MA,
USA) to overexpress E2F2. miR-936 mimics (100 nM), scramble (100
nM) and pcDNA3-E2F2 vectors were transfected into A549 cells using
the Lipofectamine 2000 Kit (Invitrogen, Thermo Fisher Scientific,
Inc.) according to the manufacturer's information. A549 cells were
collected 48 h following transfection.
Cell proliferation assay
Cell proliferation was measured using the Cell
Counting Kit-8 (CCK-8, Beyotime Institute of Biotechnology,
Jiangsu, China) according to the manufacturer's instructions. A549
cells were seeded into 96-well plates at a density of 200
cells/well. At A549 cells were cultured at 37°C for 0, 24, 48, or
72 h, following which, 10 µl of CCK-8 reagent was added to each
well and the samples were incubated at 25°C for 1 h further. The
absorbance of each well was measured at 450 nm using a microplate
reader (Thermo Fisher Scientific, Inc.).
Transwell invasion assay
The invasion ability of A549 cells transfected with
miR-936 mimics and NC was analyzed using Transwell chambers with an
8-µm pore polycarbonate membrane (EMD Millipore, Billerica, MA,
USA). The Transwell chambers were pre-coated with Matrigel (BD
Biosciences, San Jose, CA, USA). A total of 2×104
transfected cells in 100 µl serum-free DMEM medium were placed into
the upper chambers. A volume of 500 µl DMEM medium supplemented
with 20% FBS was added into the lower chambers. Subsequent to
incubation at 37°C for 24 h, cells that did not invade through the
pores were carefully wiped away with cotton wool. Subsequently, the
inserts were fixed with 100% methanol for 10 min, and stained with
0.5% crystal violet (Beyotime Institute of Biotechnology) for 30
min at 25°C and imaged with an inverted light microscope (IX71;
Olympus Corporation, Tokyo, Japan) at a magnification of ×200.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cultured cell lines or
NSCLC tissues and clinical specimens using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). cDNA was synthesized
from isolated RNA using a TaqMan MicroRNA Reverse Transcription Kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.). qPCR was
performed with a Taqman MicroRNA Assay Kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.) on an ABI7500 PCR detection system.
U6 small nuclear RNA was used as an internal control. The
thermocycling conditions were as follows: Denaturation at 95°C for
10 min, followed by 40 cycles of denaturation at 95°C for 15 sec
and elongation at 60°C for 1 min. All reactions were run in
triplicates and the relative expression of miR-936 to U6 was
calculated. The fold change of miR-936 in NSCLC relative to the
adjacent noncancerous lung tissues was determined by the
2−ΔΔCq method (16). The
primer sequences were as follows: miR-936 forward,
5′-AACGAGACGACGACAGAC-3′ and reverse, 5′-ACAGTAGAGGGAGGAATCGCAG-3′;
U6 forward, 5′-AACGAGACGACGACAGAC-3′ and reverse,
5′-GCAAATTCGTGAAGCGTTCCATA-3′; E2F2 forward,
5′-GTCTCCGCCGAGCTTGAGG-3′ and reverse, 5′-GAGCAGAGAGCAGCGCTTAG-3′;
GAPDH forward, 5′-ATGTTGCAACCGGGAAGGAA-3′ and reverse,
5′-AGGAAAAGCATCACCCGGAG-3′.
Bioinformatics analysis
The Target Scan tool (http://www.targetscan.org/index.html) was used to
predict the potential targets of miR-936.
Western blot analysis
A549 cells were lyzed using radio
immunoprecipitation buffer (Thermo Fisher Scientific, Inc.) and
total protein lysates were obtained. Protein concentration was
determined using a BCA assay. Total protein (20 µg) was separated
in 4–20% SDS-PAGE gels and transferred to polyvinylidene fluoride
membranes at 4°C. Membranes were then blocked at 25°C for 1 h with
5–10% milk/Tris-buffered saline with Tween-20 and were incubated
with primary antibodies at 4°C overnight. These antibodies
included: E2F2 (1:1,000; cat. no. sc-9967), PCNA (1:2,000; cat. no.
sc-56), Cyclin D1 (1:1,000; cat. no. sc-4074) and GAPDH (1:2,000;
cat. no. sc-365062). All antibodies were sourced from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Membranes were incubated
with the goat anti-mouse horseradish perxoidase-conjugated
secondary antibody (1:2,000; cat. no. sc-2005; Santa Cruz
Biotechnology, Inc.). Protein signals on membranes were detected
using ECL reagents (GE Healthcare, Chicago, IL, USA).
Cell cycle analysis
Cells were harvested, washed twice with ice-cold PBS
and fixed in 70% ethanol for 24 h at 4°C. Cells were then washed
three times with ice cold PBS and incubated with 1 mg/ml RNase A
(R6148; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 30 min
at 37°C. Subsequently, cells were stained at 25°C for 10 min with
50 µg/ml propidium iodide (BD Bioscience, Franklin Lakes, NJ, USA)
in 0.5% Tween-20 with PBS and subjected to analysis of cell cycle
distribution using a BD FACScan flow cytometer (Becton Dickinson)
coupled with Cell Quest acquisition and analysis programs (version
2; BD Bioscience).
Luciferase reporter assay
The 3′-UTR region of E2F2 containing the binding
site of miR-936 was constructed into the pGL3 vector (Promega
Corporation, Madison, WI, USA). For the luciferase reporter assay,
A549 cells were plated in a 96-well plate and incubated at 37°C for
24 h. Subsequently, the cells were transfected with miR-936 mimics
or Scramble, and pGL3-E2F2-3′-UTR or pGL3-E2F2-3′-UTR-MUT plasmids
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. A total of 24 h
later, luciferase activity was measured using the Dual-Luciferase
Reporter Assay System (Promega Corporation). Luciferase activity
was normalized to Renilla luciferase activity.
Statistical analysis
All statistical analyses were performed using SPSS
20.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism (version 6;
GraphPad Software, Inc., La Jolla, CA, USA). The Student's t-test
and one-way analysis of variance followed by Tukey's post hoc test
were used to analyze two or multiple groups, respectively, for
statistical significance. Pearson correlation coefficient analysis
was used to determine the correlations. The association between the
expression of miR-936 and clinicopathological features was analyzed
using χ2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
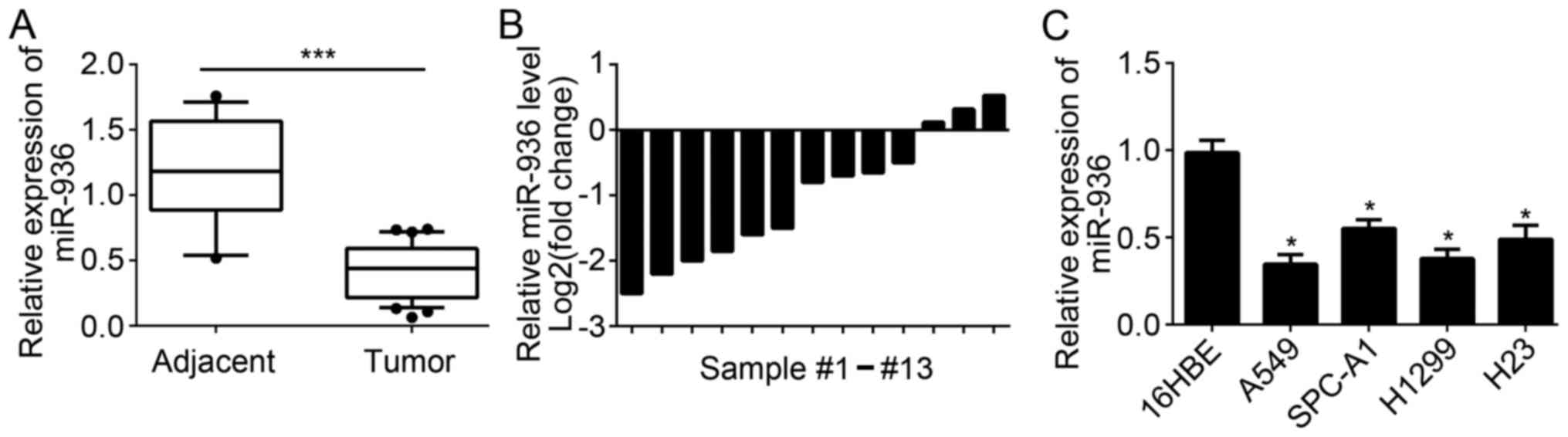
miR-936 expression is downregulated in
NSCLC tissues
A total of 35 NSCLC tissues and 13 adjacent normal
tissues were collected. RT-qPCR was used to measure the expression
levels of miR-936 in these tissues. Results indicated that miR-936
expression was significantly downregulated in 35 NSCLC tissues
compared with 13 normal tissues (Fig.
1A). Furthermore, the expression of miR-936 in 13 pairs of
NSCLC tissues and matched adjacent normal tissues was analyzed. The
results indicated that miR-936 expression was also downregulated in
the majority of the 13 NSCLC tissues compared to matched normal
tissues (Fig. 1B). Consistently,
significant downregulation of miR-936 expression was also observed
in NSCLC cell lines compared with 16HBE cells (Fig. 1C). These data demonstrated that
miR-936 was downregulated in NSCLC tissues and cells, which implied
miR-936 may be involved in the progression of NSCLC.
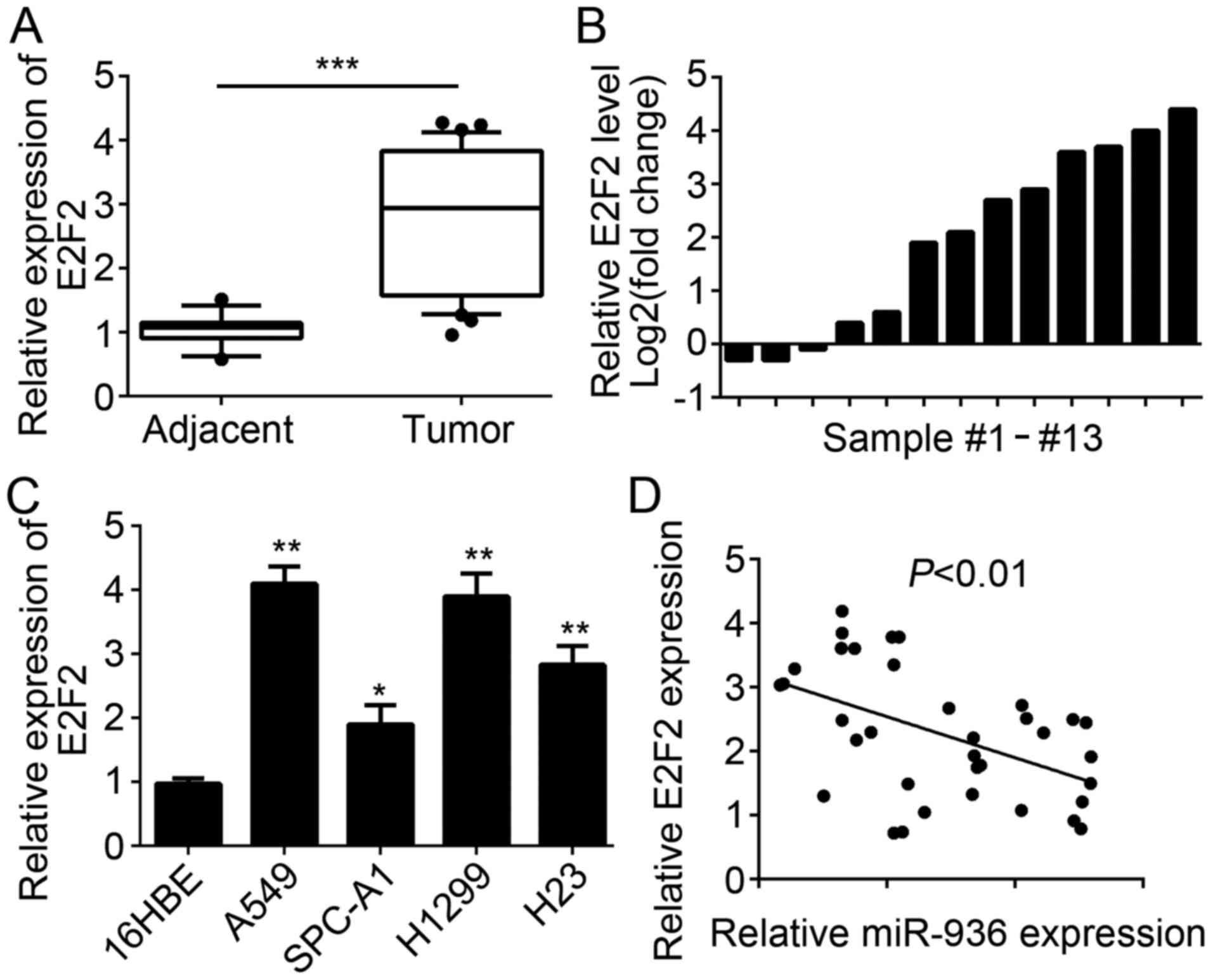
E2F2 expression is upregulated in
NSCLC tissues
The E2F2 expression level in NSCLC tissues and cell
lines was also examined. RT-qPCR analysis results indicated that
E2F2 was significantly upregulated in NSCLC tissues compared with
the adjacent normal tissues (Fig.
2A). Similarly, E2F2 expression was upregulated in the majority
of paired NSCLC tissues compared with 13 matched normal tissues
(Fig. 2B). Furthermore, the
expression patterns of E2F2 in NSCLC cell lines were determined and
the results indicated that E2F2 was significantly upregulated in
NSCLC cell lines compared with 16HBE cells (Fig. 2C). Additionally, miR-936 expression
level was inversely correlated with that of E2F2 in NSCLC tissues
(Fig. 2D).
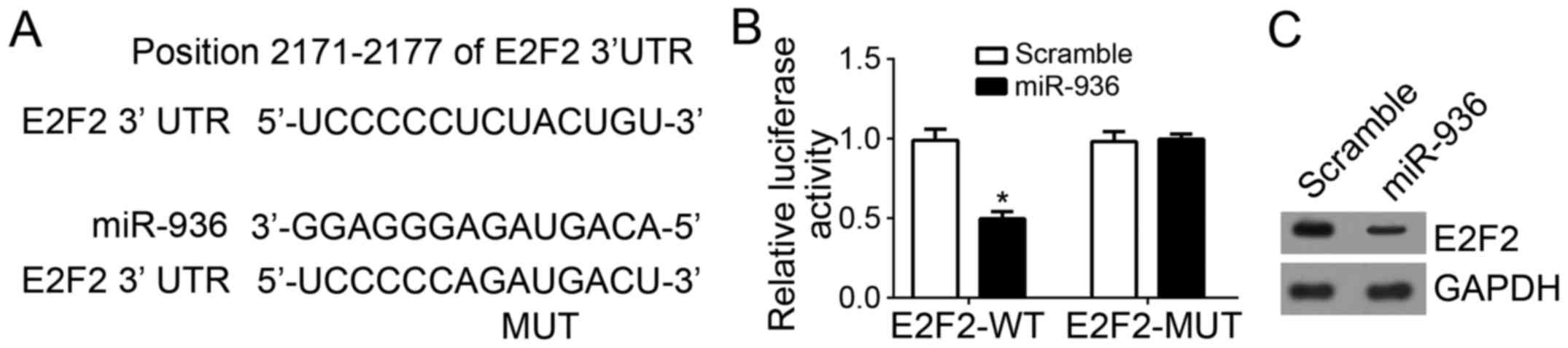
E2F2 is a direct target gene of
miR-936 in NSCLC cells
The binding site between miR-936 and E2F2 3′-UTR was
identified using Target Scan (http://www.targetscan.org/index.html) (Fig. 3A). To further confirm their
interaction, luciferase reporter assays were performed, which
revealed that overexpression of miR-936 significantly inhibited the
luciferase activity in A549 cells (Fig.
3B). Furthermore, the protein expression level of E2F2 in A549
cells was also downregulated after transfection with miR-936 mimics
compared with scramble control (Fig.
3C).
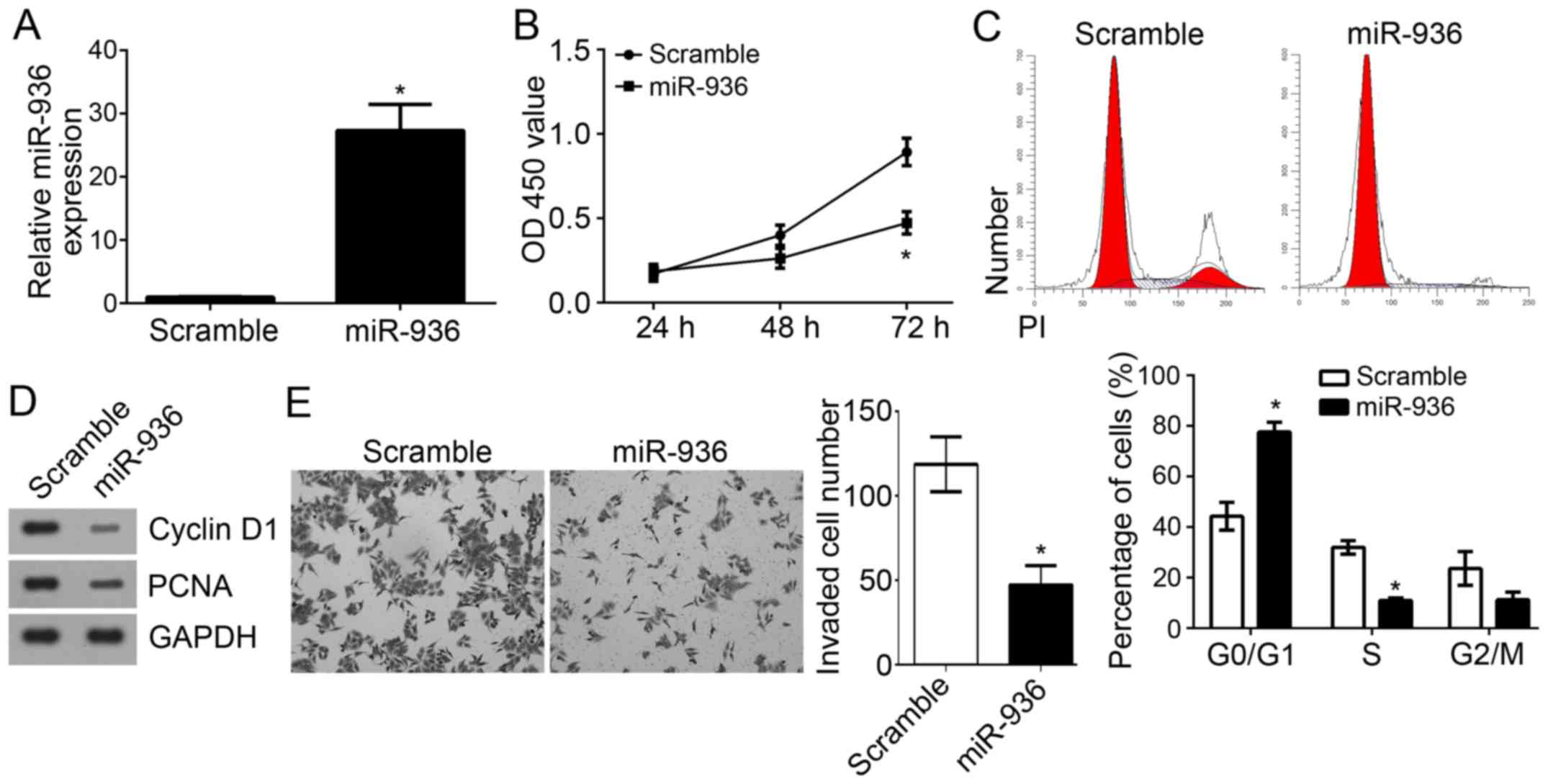
miR-936 overexpression suppresses the
proliferation, cell cycle and invasion of NSCLC cells
To further explore the functions of miR-936 in
NSCLC, miR-936 was overexpressed by transfection with miR-936
mimics in A549 cells. RT-qPCR analysis indicated that miR-936 was
effectively and significantly upregulated in A549 cells transfected
with miR-936 mimics (Fig. 4A). CCK8
assays were used to determine the proliferation of A549 cells.
Notably, overexpression of miR-936 significantly inhibited the
proliferation of A549 cells (Fig.
4B). As the cell cycle is directly linked to proliferation
(17), the effect of miR-936 on the
cell cycle was analyzed. Results indicated that overexpression of
miR-936 significantly reduced the cell percentage in S phase but
increased the cells in G0/G1 phase (Fig.
4C). Furthermore, lower protein expression levels of
proliferating cell nuclear antigen (PCNA) and Cyclin D1 were
observed in A549 cells transfected with miR-936 mimics compared
with the scramble control group (Fig.
4D). Notably, Transwell invasion assays revealed that
overexpression of miR-936 significantly suppressed the invaded A549
cell number (Fig. 4E). Taken
together, these findings demonstrated that miR-936 may serve as a
tumor suppressor by inhibiting cell proliferation, the cell cycle
and invasion.
miR-936 suppresses NSCLC cell
proliferation, the cell cycle and invasion by regulating E2F2
expression
E2F2 was identified as a target gene of miR-936 in
NSCLC and E2F2 expression was indicated to be upregulated in NSCLC
tissues in previous results. To investigate whether miR-936
regulates NSCLC progression by suppression of E2F2 expression, the
protein expression level of E2F2 was restored in A549 cells
transfected with miR-936 mimics. Western blot analysis indicated
that E2F2 expression was markedly upregulated in A549 cells
(Fig. 5A). CCK8 assays were
performed to evaluate cell proliferation. The results indicated
that miR-936 overexpression significantly suppressed the
proliferation of A549 cells, whereas restoration of E2F2 inhibited
this effect (Fig. 5B). Furthermore,
overexpression of miR-936 significantly arrested the cell cycle,
whereas overexpression of E2F2 in miR-936-overexpressing A549 cells
reversed this effect (Fig. 5C).
Transwell assays were performed to assess cell invasion. The
results revealed that overexpression of miR-936 significantly
suppressed cell invasion, whereas restoration of E2F2 expression
enhanced the invasion of A549 cells transfected with miR-936 mimics
(Fig. 5D). Taken together, these
data suggested that miR-936 suppressed the proliferation, cell
cycle and invasion of NSCLC cells by directly regulating E2F2
expression.
Discussion
NSCLC is a common malignant human cancer that is
associated with a large number of cancer-associated fatalities
globally (1). However, the
underlying molecular mechanism of NSCLC occurrence and progression
remains largely unknown. Thus, there is an urgent requirement to
identify the key molecules and signaling pathways involved in
NSCLC. In the present study, the expression of miR-936 was
significantly downregulated in NSCLC tissues compared with adjacent
normal tissues. Consistently, the expression of miR-936 was also
significantly downregulated in NSCLC cell lines compared with 16HBE
cells. Functional experiments suggested that overexpression of
miR-936 significantly inhibited the proliferation, cell cycle and
invasion of A549 cells. Mechanistically, the present study also
indicated that E2F2 was a direct target of miR-936 in NSCLC cells
and that overexpression of miR-936 significantly inhibited the
protein expression of E2F2 in A549 cells. Notably, a reverse
correlation between the expression of miR-936 and E2F2 in NSCLC
tissues was also demonstrated. Furthermore, E2F2 was significantly
upregulated in the NSCLC tissues and cell lines compared with
normal tissues or the 16HBE cell line. In addition, the restoration
of E2F2 in miR-936-overexpressing A549 cells enhanced the
proliferation, restored the cell cycle and promoted cell invasion.
Taken together, the present study demonstrated that miR-936 acted
as a tumor suppressor in NSCLC through targeting E2F2.
Accumulating studies have indicated that
dysregulated expression of miRs is a characteristic of nearly all
types of human malignancy, including stomach adenocarcinoma
(18), gastric cancer (19), esophageal squamous cell carcinoma
(20), head and neck squamous cell
carcinoma (21), cervical cancer
(22), breast cancer (23), liver cancer (24) and NSCLC (25). miRs have been reported to serve as
oncogenes or tumor suppressors to regulate cancer cell
proliferation and migration. For instance, Zhang et al
(26) reported that miR-630 promotes
cell proliferation and inhibits apoptosis in the HCT116 human
colorectal cancer cell line. Furthermore, Liu et al
(27) revealed that miR-1297
contributes to tumor growth of human breast cancer by targeting
PTEN/phosphoinositide 3-kinase/AKT signaling. Previous evidence has
also indicated that miR-936 were downregulated in glioma specimens
and that cell cycle arrest was induced, which targeted CKS1
(15). However, the functions of
miR-936 in other cancer types, including NSCLC, have not been
investigated. In the present study, it was demonstrated that
miR-936 was significantly downregulated in NSCLC tissues and cell
lines, which indicated that miR-936 may be involved in the
development and progression of NSCLC.
Identification of miR-936 target genes is crucial
for understanding its molecular mechanism in NSCLC carcinogenesis
and for the development of effective therapeutic targets. Through
bioinformatics analysis, it was identified that E2F2 was a target
gene of miR-936 in NSCLC cells. Notably, E2F2 is a E2F member,
which represent a family of transcription factors regulating a
myriad of biological processes (28). Previous findings have indicated that
E2F2 is an oncogene in some types of cancer, including lung cancer
(28), osteosarcoma (29), melanoma (30), ovarian cancer (31) and glioma (32). In addition, Wang et al
(33) reported that E2F2 was
inhibited by miR-31 and contributed to the malignance of gastric
cancer. However, how E2F2 expression is regulated in NSCLC requires
further investigation. In the present study, it was demonstrated
that miR-936 targeted E2F2 and suppressed the expression of E2F2 in
NSCLC cells. Furthermore, E2F2 was upregulated in NSCLC tissues and
restoration of E2F2 restored the proliferation, cell cycle and
invasion of miR-936-overexpressing A549 cells. These data indicated
that E2F2 was downregulated by miR-936 and acted as an oncogene in
NSCLC.
In conclusion, the present study demonstrated the
key role of miR-936 in NSCLC cells. It was also demonstrated that
miR-936 suppressed the proliferation, cell cycle and invasion of
NSCLC cells by targeting E2F2. The findings suggested that the
miR-936/E2F2 axis may serve as a potential therapeutic target for
NSCLC treatment.
Acknowledgements
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XZ and HT initiated, designed the present study,
analyzed, interpreted the results and wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
For the use of human samples, the protocol for the
present study was approved by the Institutional Ethics Committee of
The Second Hospital of Anhui Medical University and all enrolled
patients signed a written informed consent document.
Patient consent for publication
All patients within this study provide consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang X, Chen BB, Zhang MH and Wang XR:
MicroRNA-126 inhibits the proliferation of lung cancer cell line
A549. Asian Pac J Trop Med. 8:239–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peters S, Adjei AA, Gridelli C, Reck M,
Kerr K and Felip E: ESMO Guidelines Working Group: Metastatic
non-small-cell lung cancer (NSCLC): ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 23
Suppl 7:vii56–vii64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang S, Zhang Y, Zhao X, Wang J and Shang
J: microRNA-361 targets Wilms' tumor 1 to inhibit the growth,
migration and invasion of non-small-cell lung cancer cells. Mol Med
Rep. 14:5415–5421. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen C, Zhao Z, Liu Y and Mu D:
microRNA-99a is downregulated and promotes proliferation, migration
and invasion in non-small cell lung cancer A549 and H1299 cells.
Oncol Lett. 9:1128–1134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang H, Zhang H, Hu X and Li W: Knockdown
of long non-coding RNA XIST inhibits cell viability and invasion by
regulating miR-137/PXN axis in non-small cell lung cancer. Int J
Biol Macromol. 111:623–631. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lei T, Zhu Y, Jiang C, Wang Y, Fu J, Fan Z
and Qin H: MicroRNA-320 was downregulated in non-small cell lung
cancer and inhibited cell proliferation, migration and invasion by
targeting fatty acid synthase. Mol Med Rep. 14:1255–1262. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cui Y, Chen LG, Yao HB, Zhang J and Ding
KF: Upregulation of microRNA-383 inhibits the proliferation,
migration and invasion of colon cancer cells. Oncol Lett.
15:1184–1190. 2018.PubMed/NCBI
|
|
10
|
Xia D, Li X, Niu Q, Liu X, Xu W, Ma C, Gu
H, Liu Z, Shi L, Tian X, et al: MicroRNA-185 suppresses pancreatic
cell proliferation by targeting transcriptional coactivator with
PDZ-binding motif in pancreatic cancer. Exp Ther Med. 15:657–666.
2018.PubMed/NCBI
|
|
11
|
Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant
KC, Allen A, et al: Circulating microRNAs as stable blood-based
markers for cancer detection. Proc Natl Acad Sci USA.
105:10513–10518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K,
Guo J, Zhang Y, Chen J, Guo X, et al: Characterization of microRNAs
in serum: A novel class of biomarkers for diagnosis of cancer and
other diseases. Cell Res. 18:997–1006. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anninos P, Chatzimichael A, Adamopoulos A,
Kotini A and Tsagas N: A combined study of MEG and pico-Tesla TMS
on children with autism disorder. J Integra Neurosci. 15:497–513.
2016. View Article : Google Scholar
|
|
14
|
Olbromski M, Grzegrzolka J,
Jankowska-Konsur A, Witkiewicz W, Podhorska-Okolow M and Dziegiel
P: MicroRNAs modulate the expression of the SOX18 transcript in
lung squamous cell carcinoma. Oncol Rep. 36:2884–2892. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang D, Zhi T, Xu X, Bao Z, Fan L, Li Z,
Ji J and Liu N: MicroRNA-936 induces cell cycle arrest and inhibits
glioma cell proliferation by targeting CKS1. Am J Cancer Res.
7:2131–2143. 2017.PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang D, Cao Q, Qu M, Xiao Z, Zhang M and
Di S: MicroRNA-616 promotes the growth and metastasis of non-small
cell lung cancer by targeting SOX7. Oncol Rep. 38:2078–2086. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu GW, Qin ZM and Shen QH: An ensemble
method integrated with miRNA expression data for predicting miRNA
targets in stomach adenocarcinoma. Cancer Biomark. 20:617–625.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu S, Suo J, Wang C, Sun X, Wang D, He L,
Zhang Y and Li W: Prognostic significance of low miR-144 expression
in gastric cancer. Cancer Biomark. 20:547–552. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mei LL, Qiu YT, Huang MB, Wang WJ, Bai J
and Shi ZZ: miR-99a suppresses proliferation, migration and
invasion of esophageal squamous cell carcinoma cells through
inhibiting the IGF1R signaling pathway. Cancer Biomark. 20:527–537.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krishnan AR, Zheng H, Kwok JG, Qu Y, Zou
AE, Korrapati A, Li PX, Califano JA, Hovell MF, Wang-Rodriguez J
and Ongkeko WM: A comprehensive study of smoking-specific microRNA
alterations in head and neck squamous cell carcinoma. Oral Oncol.
72:56–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ou L, Wang D, Zhang H, Yu Q and Hua F:
Decreased expression of miR-138-5p by LncRNA H19 in cervical cancer
promotes tumor proliferation. Oncol Res. Aug 10–2017.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong Y, Liu Y, Jiang A, Li R, Yin M and
Wang Y: MicroRNA-335 suppresses the proliferation, migration, and
invasion of breast cancer cells by targeting EphA4. Mol Cell
Biochem. 439:95–104. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang L, Peng F, Qin J, Zhou H and Wang B:
Downregulation of microRNA-196a inhibits human liver cancer cell
proliferation and invasion by targeting FOXO1. Oncol Rep.
38:2148–2154. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ling DJ, Chen ZS, Zhang YD, Liao QD, Feng
JX, Zhang XY and Shi TS: MicroRNA-145 inhibits lung cancer cell
metastasis. Mol Med Rep. 11:3108–3114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang L, Feng G, Zhang X, Ding Y and Wang
X: microRNA630 promotes cell proliferation and inhibits apoptosis
in the HCT116 human colorectal cancer cell line. Mol Med Rep.
16:4843–4848. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu C, Liu Z, Li X, Tang X, He J and Lu S:
MicroRNA-1297 contributes to tumor growth of human breast cancer by
targeting PTEN/PI3K/AKT signaling. Oncol Rep. 38:2435–2443. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Feliciano A, Garcia-Mayea Y, Jubierre L,
Mir C, Hummel M, Castellvi J, Hernández-Losa J, Paciucci R, Sansano
I, Sun Y, et al: miR-99a reveals two novel oncogenic proteins E2F2
and EMR2 and represses stemness in lung cancer. Cell Death Dis.
8:e31412017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tao T, Shen Q, Luo J, Xu Y and Liang W:
MicroRNA-125a Regulates cell proliferation via directly targeting
E2F2 in osteosarcoma. Cell Physiol Biochem. 43:768–774. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao H, Tang W, Chen X, Wang S, Wang X, Xu
H and Li L: The NAMPT/E2F2/SIRT1 axis promotes proliferation and
inhibits p53-dependent apoptosis in human melanoma cells. Biochem
Biophys Res Commun. 493:77–84. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xie L, Li T and Yang LH: E2F2 induces
MCM4, CCNE2 and WHSC1 upregulation in ovarian cancer and predicts
poor overall survival. Eur Rev Med Pharmacol Sci. 21:2150–2156.
2017.PubMed/NCBI
|
|
32
|
Song H, Zhang Y, Liu N, Zhang D, Wan C,
Zhao S, Kong Y and Yuan L: Let-7b inhibits the malignant behavior
of glioma cells and glioma stem-like cells via downregulation of
E2F2. J Physiol Biochem. 72:733–744. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang H, Zhang X, Liu Y, Ni Z, Lin Y, Duan
Z, Shi Y, Wang G and Li F: Downregulated miR-31 level associates
with poor prognosis of gastric cancer and its restoration
suppresses tumor cell malignant phenotypes by inhibiting E2F2.
Oncotarget. 7:36577–36589. 2016.PubMed/NCBI
|