Introduction
Rheumatoid arthritis (RA) is a systemic and
autoimmune disease, which is caused by massive autoimmune reactive
cell populations and cytokines (1,2). The
therapeutic goals are to achieve clinical remission of disease
activity and reduce long-term joint destruction (3). It has been reported that the combined
use of biological and conventional synthetic disease-modifying
anti-rheumatic drugs (DMARDs) was more effective than monotherapy
in the treatment of RA (4). However,
an increasing number of studies have indicated that patients failed
to respond or tolerate conventional synthetic or biological DMARDs
following decades of treatment. Thus, targeted synthetic DMARDs
(4), oral low-molecular-weight
drugs, which target kinase proteins, including Janus kinases
(JAKs), have been developed to treat RA.
The JAK family, including JAK1, JAK2, JAK3 and
tyrosine kinase 2 (Tyk2), has critical roles in certain signaling
pathways through activating cytokine-induced phosphorylation of
signal transducer and activators of transcription (STAT), which is
then directly transported to the nucleus to regulate the
transcription of its target genes (5,6).
According to Fridman et al (7) and Shi et al (8), baricitinib is an novel, oral
low-molecular- weight JAK inhibitor with good selectivity for JAK1
[concentration leading to 50% inhibition (IC50)=5.7 nM]
and JAK2 (IC50=5.9 nM), and less selectivity for JAK3
(IC50>400 nM) or Tyk2 (IC50=53 nM), and
the kidneys are considered to be the principal organ to eliminate
baricitinib. Shi et al (8),
Kubo et al (9) and Emery
et al (10) reported that
baricitinib inhibits the phosphorylation of STATs induced by
various cytokines in the whole blood, and results in a transient
change in neutrophil and lymphocyte counts. A previous
meta-analysis suggested that the use of the JAK inhibitor
tofacitinib is associated with manageable safety and increased
clinical efficacy in the short-term compared with placebo (11). Tofacitinib has similar inhibitory
activity on JAK1 and JAK3, but less activity against JAK2 (12). To date, the clinical efficacy of JAK
inhibitors (preferential JAK1 and JAK2 inhibitors) has remained to
be accurately defined by the results of independent clinical
trials. The objective of the present meta-analysis was to determine
the clinical efficacy and safety of baricitinib administered at the
dosage of 4 mg once daily for patients with an inadequate response
to conventional synthetic or biological DMARDs.
Materials and methods
Inclusion and exclusion criteria
Randomized controlled trials (RCTs) were eligible if
they met the following inclusion criteria: i) Patients aged >18
years with an inadequate response or intolerance to conventional
synthetic or biological DMARDs; ii) the studies compared the use of
baricitinib with that of a placebo in the treatment of RA; and iii)
the studies provided data for evaluating the clinical efficacy and
safety of baricitinib. The exclusion criteria were as follows: i)
The studies included duplicate and incomplete data; and ii) the
entries retrieved were for unsuitable publications, including
conference abstracts or review articles.
Information sources and search
strategy
The present meta-analysis adhered to the guidelines
of the Preferred Reporting Items for Systematic Reviews and
Meta-Analyses statement (13). The
data was extracted independently by two investigators (ZPW and PZ)
and was rechecked following the first extraction. Two investigators
(JSH and JCW) discussed disagreements arising from the extraction
until a consensus was reached. Electronic databases, including
Medline (https://www.nlm.nih.gov/), Embase
(https://www.elsevier.com/), Science
Direct (https://www.sciencedirect.com/), Web of Science
(http://login.webofknowledge.com/) and
the Cochrane library (http://www.cochranelibrary.com/), were searched to
retrieve relevant studies published until July 3, 2017. The
publications were not restricted with regard to publication status
or language. The following data were extracted from the RCTs: Name
of first author, year of publication, country, duration of
follow-up, primary outcomes for efficacy and safety, and mean and
standard deviation of changes, including laboratory outcomes
regarding hemoglobin, alanine transaminase (ALT), neutrophil,
lymphocyte, creatinine, low density lipoprotein (LDL) and high
density lipoprotein (HDL) from baseline. The data, including the
American College of Rheumatology 20% response (ACR20) (14) and Simplified Disease Activity Index
(SDAI) ≤3.3 (15), were extracted
from the published figures using the ‘Get Data Graph Digitizer’
software (v2.24; http://getdata-graph-digitizer.com/). The risk of bias
of the eligible studies was assessed by the Cochrane
collaboration's tool (http://community.cochrane.org/help/tools-and-software/revman-5/).
Outcome measures
Efficacy measures were as follows: i) The proportion
of patients achieving an ACR20; ii) the SDAI ≤3.3; iii)
patient-reported outcomes (PROs), including the Patient's Global
Assessment of Disease Activity (PtGA) (16) and Scores on the Health Assessment
Questionnaire-Disability Index (HAQ-DI) (17). The safety outcomes included the
following: i) Adverse events (AEs), ii) discontinuation due to AEs,
iii) infections, and iv) serious infections, including pneumonia
and cellulitis. Serious infections were defined as those requiring
hospitalization and/or parenteral antibiotics or otherwise meeting
serious adverse events, including fatal or life-threatening,
requiring hospitalization or extension of existing hospitalization,
resulting in persistent or significant disability/incapacity or
congenital abnormality/birth defect or considered to be an
important medical event. All clinical laboratory outcomes are
reported as the least-squares mean change from baseline, which
included the following: i) Hemoglobin; ii) neutrophils; iii)
lymphocytes; iv) ALT; v) creatinine; vi) HDL; and vii) LDL.
Quality assessment
Two investigators (ZPW and PZ) independently
assessed the quality of the RCTs according to the method in the
Cochrane Reviewer's Handbook 5.1.0 (The Cochrane Collaboration,
London, UK) (18). The risk of bias
of individual studies was assessed according to the Cochrane risk
assessment scale and included the following: Details of the methods
of random sequence generation, allocation concealment, blinding,
incomplete outcome data, selective outcome reporting and other
sources of bias. Any disagreements were resolved by a third
reviewer (JSH).
Statistical and sensitivity
analysis
Statistical heterogeneity of data was evaluated by
using Cochran's Q statistics. If the Q statistics indicated
significant heterogeneity among the studies (P<0.10), a
random-effects model was employed for the meta-analysis, and
otherwise, a fixed-effects model was used. The results for
continuous data (HAQ-DI, PtGA, neutrophils, lymphocytes,
creatinine, hemoglobin LDL and HDL) are presented as the mean
difference (MD) with 95% confidence interval (CI). For ALT, the
standardized mean difference (SMD) was calculated using the inverse
variance method. For dichotomous data, the risk ratio (RR) was
calculated using the Mantel-Haenszel method. The mean difference
and standardized mean difference were considered statistically
significant at the P<0.05 level. Data analysis was performed by
using Review Manager 5.3 (The Cochrane Collaboration). Sensitivity
analysis was performed to assess the results through exclusion of
one eligible study at a time.
Results
Study selection
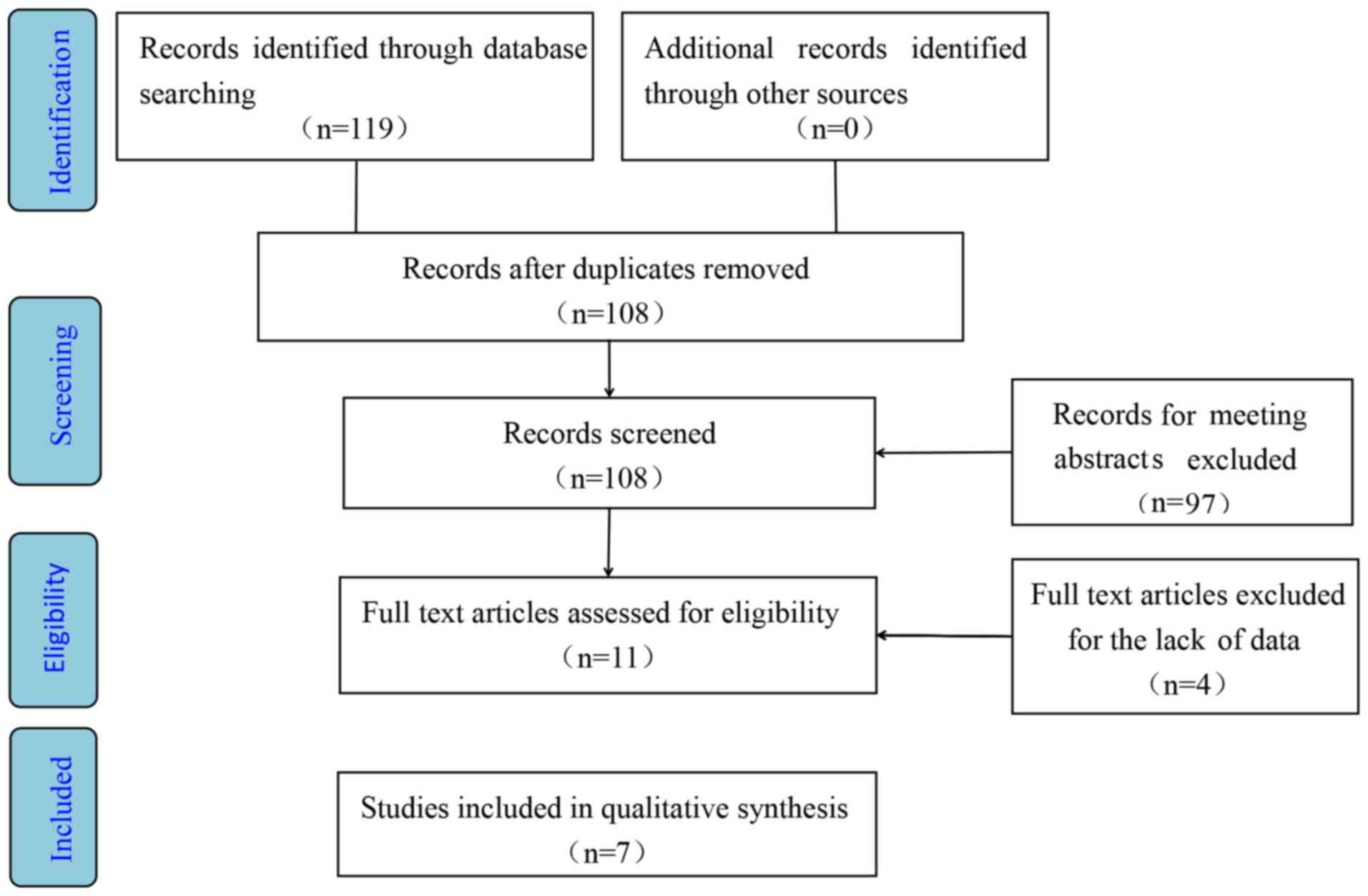
A total of 7 RCTs compared the use of baricitinib
with that of a placebo in patients with active RA with an
inadequate response or intolerance to conventional synthetic or
biological DMARDs (Fig. 1). The
study by Fleischmann et al (19) was ignored due to the combined use of
baricitinib and methotrexate (MTX). A total of 4 studies were
eliminated due to the lack of available data for analysis (20–23).
Finally, 7 RCTs were selected, including 2 phase-II (24,25)
trials and 5 phase-III trials (3,26–29). The
dosage of baricitinib ranged from 1 to 8 mg (1, 2, 4 and 8 mg) once
daily. A total of 4,173 patients were included in this
meta-analysis. 1,371 patients were included in the 4 mg baricitinib
group and 1,443 patients were included in placebo group. The mean
age of the patients ranged from 51.7 to 57.5 years in the 4 mg
group and 49 to 56 years in the placebo group. A summary of the
RCTs is presented in Table I.
 | Table I.Characteristics of eligible studies
included in the meta-analysis. |
Table I.
Characteristics of eligible studies
included in the meta-analysis.
| Author, year | Country |
Intervention/control group (n) | Females/males
(n) | Mean age
(years) | Control group
(n) | Intervention
group | Medical
history | Trial phase | Follow-up
(weeks) | (Refs.) |
|---|
| Dougados et
al, | France,
Netherlands, | 456/228 | Placebo,
189/39; | Placebo,
51±13; | 228 | Arm 1, baricitinib
2 mg | csDMARDs | III | 24 | (3) |
| 2017 | Taiwan and USA |
| arm 1, 184/45; | arm 1, 52±12; |
| once daily,
n=229; |
|
|
|
|
|
|
|
| arm 2, 187/40 | arm 2, 52±12 |
| arm 2, baricitinib
4 mg |
|
|
|
|
|
|
|
|
|
|
| once daily,
n=227 |
|
|
|
|
| Emery et
al, | UK, Spain,
Argentina, | 456/228 | Placebo,
189/39; | Placebo,
51±13; | 228 | Arm 1, baricitinib
2 mg | csDMARDs | III | 24 | (26) |
| 2017 | Taiwan and USA |
| arm 1, 184/45; | arm 1, 52±12; |
| once daily,
n=229; |
|
|
|
|
|
|
|
| arm 2, 187/40 | arm 2, 52±12 |
| arm 2, baricitinib
4 mg |
|
|
|
|
|
|
|
|
|
|
| once daily,
n=227 |
|
|
|
|
| Genovese et
al, | USA, Austria | 351/176 | Placebo,
145/31; | Placebo,
56±11; | 176 | Arm 1, baricitinib
2 mg |
TNF-inhibitors, | III | 24 | (24) |
| 2016 | and Poland |
| arm 1, 137/37; | arm 1, 55±11; |
| once daily,
n=174; | csDMARDs |
|
|
|
|
|
|
| arm 2, 149/28 | arm 2, 56±11 |
| arm 2, baricitinib
4 mg |
|
|
|
|
|
|
|
|
|
|
| once daily,
n=177 |
|
|
|
|
| Keystone et
al, | USA and Mexico | 203/98 | Placebo,
85/13; | Placebo,
49±12; | 98 | Arm 1,
baricitinib | csDMARDs, | IIb | 24 | (25) |
| 2015 |
|
| arm 1, 42/7; | arm 1, 53±11; |
| 1 mg once daily,
n=49; | MTX |
|
|
|
|
|
|
| arm 2, 44/8; | arm 2, 51±13; |
| arm 2, baricitinib
2 mg |
|
|
|
|
|
|
|
| arm 3, 37/15; | arm 3, 53±10; |
| once daily, n=52;
arm 3, |
|
|
|
|
|
|
|
| arm 4, 41/9 | arm 4, 53±11 |
| baricitinib 4 mg
once daily, |
|
|
|
|
|
|
|
|
|
|
| n=52; arm 4,
baricitinib |
|
|
|
|
|
|
|
|
|
|
| 8 mg once daily,
n=50 |
|
|
|
|
| Smolen et
al, | Austria, USA, | 351/176 | – | – | 176 | Arm 1, baricitinib
2 mg |
TNF-inhibitors, | III | 24 | (29) |
| 2017 | Spain and
France |
|
|
|
| once daily,
n=174; | csDMARDs |
|
|
|
|
|
|
|
|
|
| arm 2 baricitinib 4
mg |
|
|
|
|
|
|
|
|
|
|
| once daily,
n=177 |
|
|
|
|
| Tanaka et
al, | Japan | 96/49 | Placebo,
39/10; | Placebo,
51±12; | 49 | Arm 1, baricitinib
1 mg | csDMARDs, | IIb | 12 | (28) |
| 2016 |
|
| arm 1, 22/2; | arm 1, 53±13; |
| once daily,
n=24; | MTX |
|
|
|
|
|
|
| arm 2, 21/3; | arm 2, 56±12; |
| arm 2, baricitinib
2 mg |
|
|
|
|
|
|
|
| arm 3, 19/5; | arm 3, 58±10; |
| once daily,
n=24; |
|
|
|
|
|
|
|
| arm 4, 17/7 | arm 4, 54±11 |
| arm 3, baricitinib
4 mg |
|
|
|
|
|
|
|
|
|
|
| once daily,
n=24; |
|
|
|
|
|
|
|
|
|
|
| arm 4 baricitinib 8
mg |
|
|
|
|
|
|
|
|
|
|
| once daily,
n=24 |
|
|
|
|
| Taylor et
al, | USA, UK, | 817/488 | Placebo,
382/106; | Placebo, 53±2; | 24 weeks, | 24 weeks: | csDMARDs, | III | 52 | (27) |
| 2017 | Canada and
Japan |
| arm 1,
375/112; | arm 1, 54±2; | n=488; | Arm 1, baricitinib
4 mg | MTX |
|
|
|
|
|
|
| arm 2, 251/79 | arm 2, 53±12 | following, | once daily,
n=487; |
|
|
|
|
|
|
|
|
|
| 52 weeks | arm 2, adalimumab
40 mg |
|
|
|
|
|
|
|
|
|
| n=0 | once daily,
n=330; |
|
|
|
|
|
|
|
|
|
|
| following 52
weeks: |
|
|
|
|
|
|
|
|
|
|
| arm 1, baricitinib
4 mg |
|
|
|
|
|
|
|
|
|
|
| once daily,
n=487; |
|
|
|
|
|
|
|
|
|
|
| arm 2, adalimumab
40 mg |
|
|
|
|
|
|
|
|
|
|
| once daily,
n=330 |
|
|
|
|
Risk-of-bias assessment
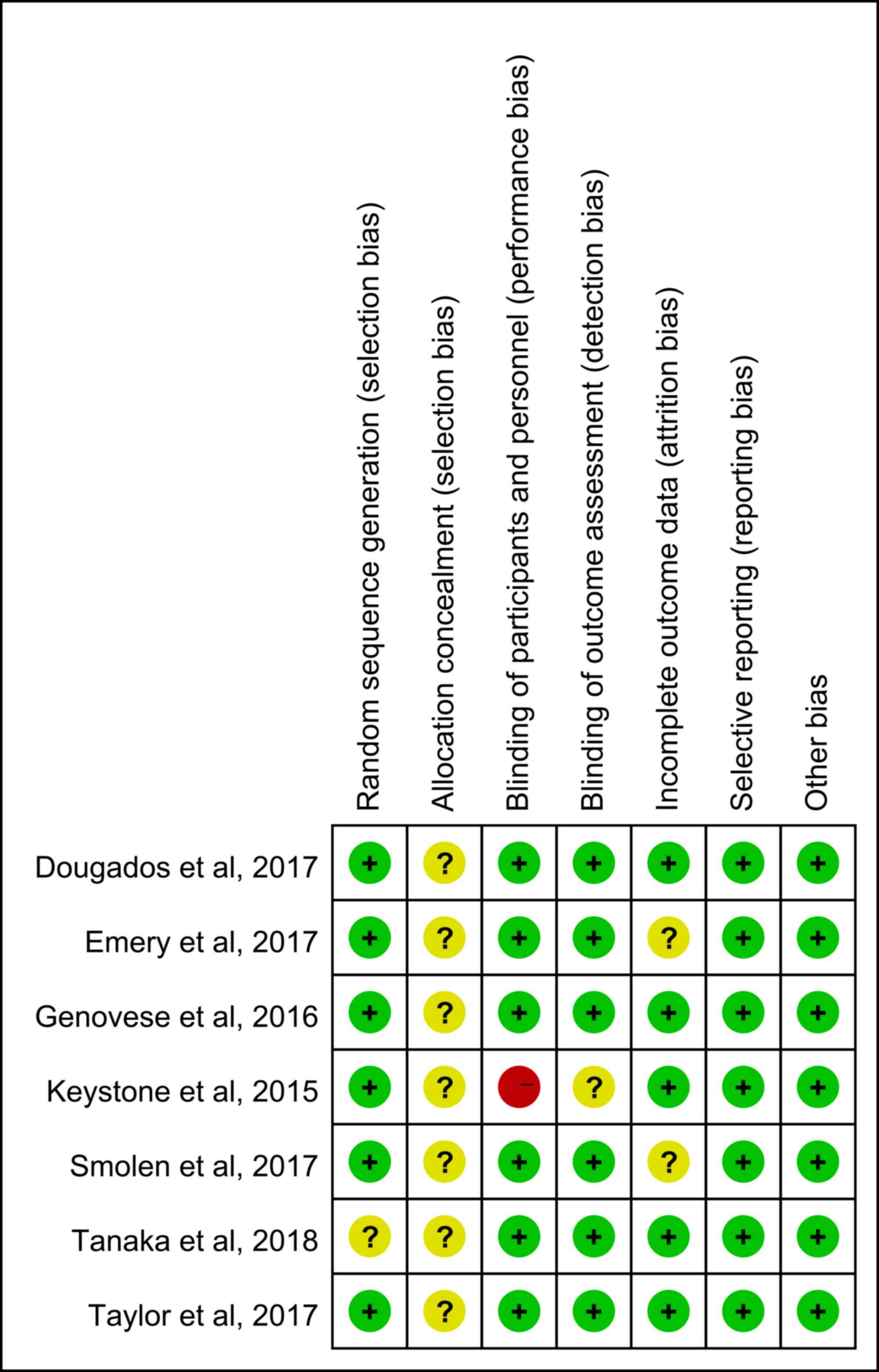
The results of the Cochrane risk of bias assessment
are presented in Fig. 2. None of the
studies included described the method of allocation concealment.
The study by Keystone et al (25) had a high risk of bias regarding the
blinding of participants and personnel and an unclear risk of bias
regarding the blinding during the outcome assessment. Two studies
presented an unclear risk of attrition bias (26,29).
Tanaka et al (28) did not
describe the method of random sequence generation. Sensitivity
analysis was performed by excluding one study at a time and in each
case all of the results remained stable. The efficacy and safety of
baricitinib was assessed at the dosage of 4 mg, as this dose was
considered to be most effective in all RCTs included. Furthermore,
the eligible studies did not provide sufficient data to evaluate
the efficacy and safety of baricitinib at any of the other
doses.
Efficacy of baricitinib (4 mg once daily)
at 12 and 24 weeks
ACR20 response rate
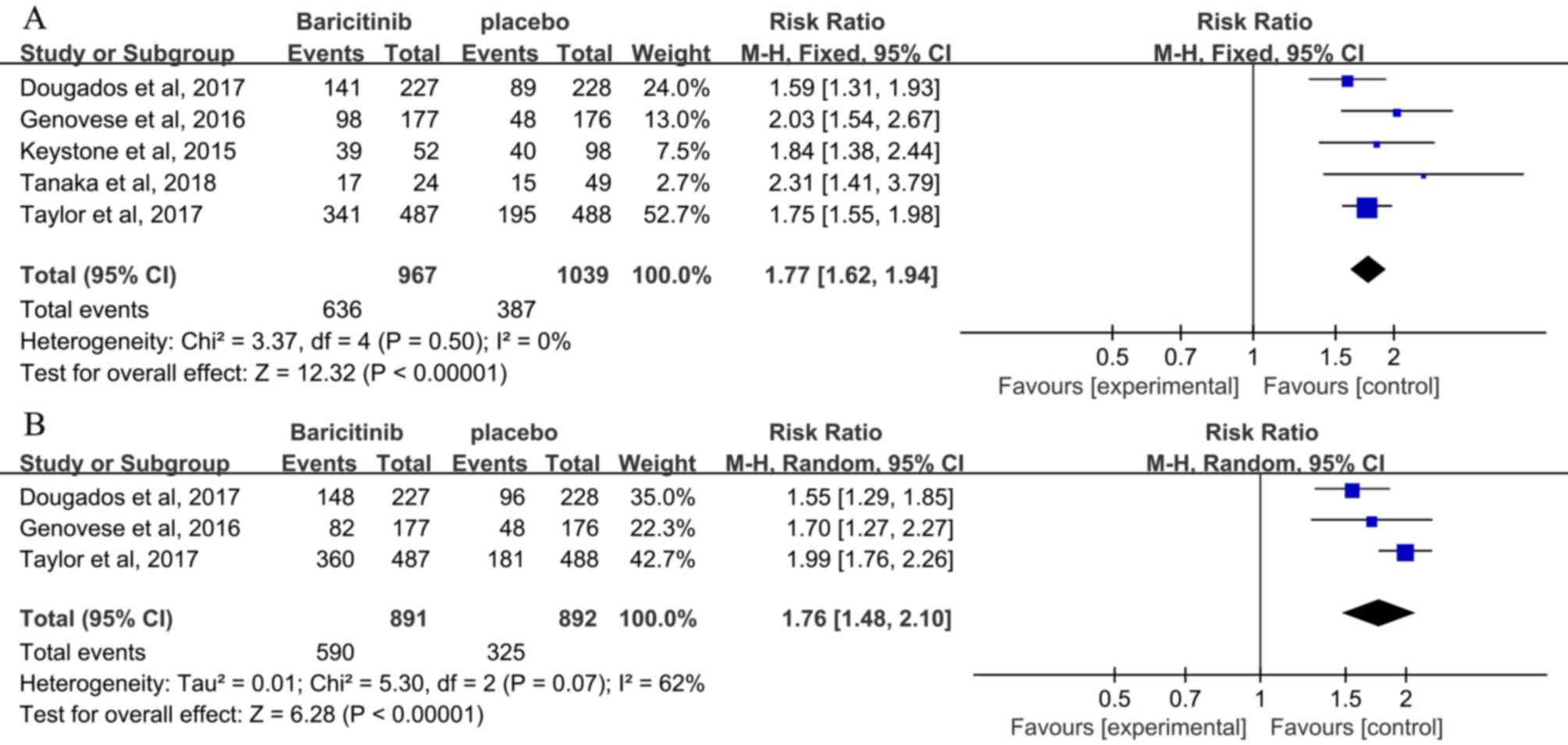
The ACR20 response rate at 12 weeks was extracted
from 5 studies (3,24,25,27,28), and
a low heterogeneity was observed (I2=0%). The efficacy
outcomes are presented in Table II.
At 12 weeks, the ACR20 response rate in the baricitinib group was
significantly higher than that in the placebo group [RR, 1.77; 95%
CI, 1.62–1.94; P<0.00001; Fig.
3A). At 24 weeks, the ACR20 response rate in the baricitinib-4
mg group, which was extracted from 3 studies (3,24,27), was
significantly higher than that in the placebo group (RR, 1.76; 95%
CI, 1.48–2.10; P<0.00001; Fig.
3B). A higher heterogeneity was seen when compared with that at
12 weeks (I2=62%).
 | Table II.Efficacy of baricitinib in patients
with active rheumatoid arthritis. |
Table II.
Efficacy of baricitinib in patients
with active rheumatoid arthritis.
| A, Baricitinib, 4
mg, 12 weeks |
|---|
|
|---|
|
|
| Meta-analysis | Test of
heterogeneity |
|---|
|
|
|
|
|
|---|
| Studies (n) | Outcome | RR | 95% CI | P-value | Model | P-value | I2
(%) |
|---|
| 2 | HAQ-DI | −0.22a | −0.30 to −0.14 | <0.00001 | F | 0.63 | 0 |
| 2 | PtGA | −10.99a | −14.55 to
−7.44 | <0.00001 | F | 0.17 | 47 |
|
| B, Baricitinib,
4 mg, 24 weeks |
|
|
|
|
Meta-analysis | Test of
heterogeneity |
|
|
|
|
|
| Studies
(n) | Outcome | RR | 95% CI | P-value | Model | P-value | I2
(%) |
|
| 2 | HAQ-DI | −0.26a | −0.34 to −0.17 | <0.00001 | F | 0.65 | 0 |
| 2 | PtGA | −12.4a | −16.02 to
−8.77 | <0.00001 | F | 0.14 | 55 |
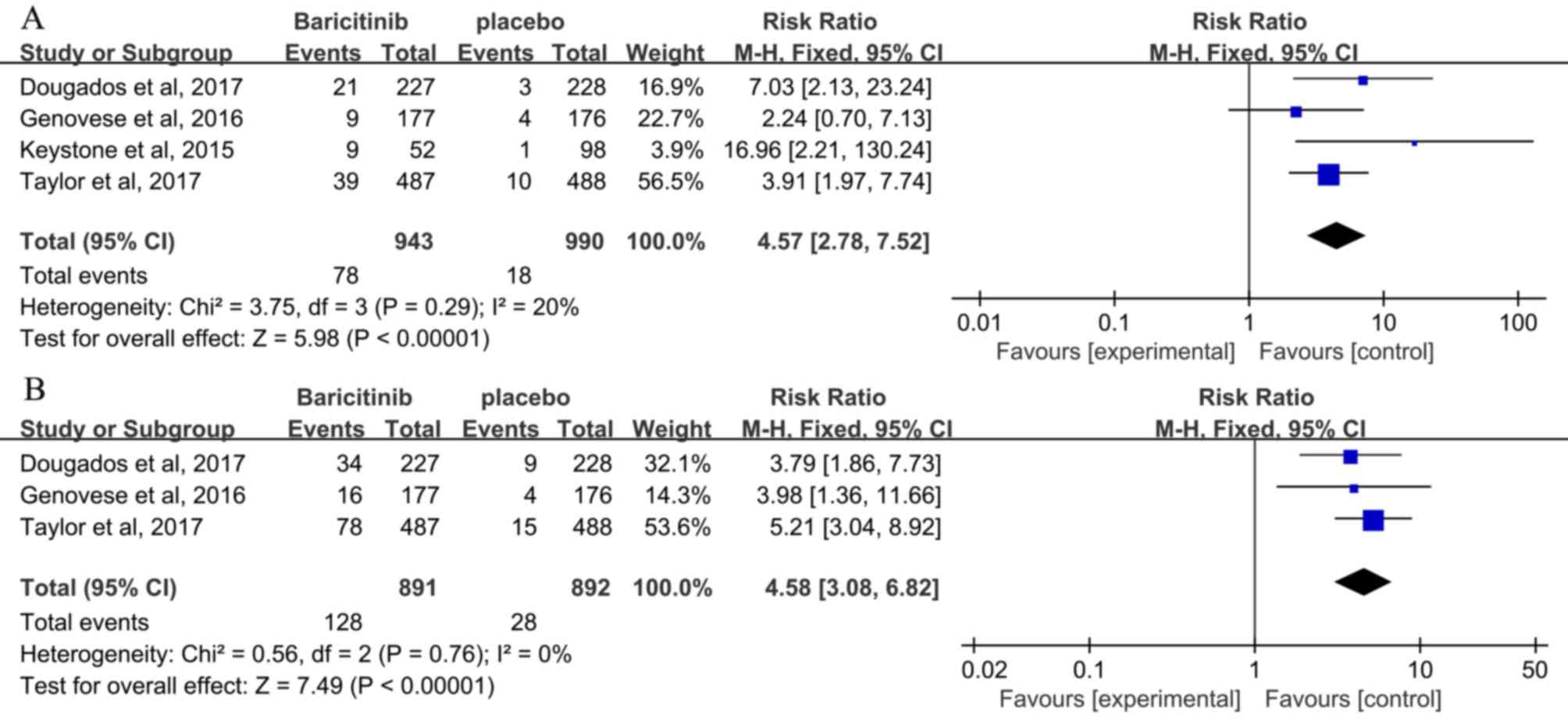
SDAI ≤3.3
The ratio of patients who achieved SDAI ≤3.3 at 12
weeks was reported in 4 studies (3,24,25,27)
and a low heterogeneity was observed (I2=20%). A
statistically significant improvement was observed in the
baricitinib once daily groups compared with that in the placebo
group (RR, 4.57; 95% CI, 2.78–7.52; P<0.00001; Fig. 4A). Patients receiving baricitinib (3
studies) (3,24,27) had
a significant improvement compared with those receiving placebo at
24 weeks (RR, 4.58; 95% CI, 3.08–6.82; P<0.00001; Fig. 4B).
HAQ-DI and PtGA
All parameters exhibited low heterogeneity and a
fixed effect model was applied. The HAQ-DI and PtGA were extracted
from 2 studies (26,29). Baricitinib was associated with a
significant reduction in the HAQ-DI score compared with that in the
placebo group at 12 weeks [MD, −0.22; 95% CI, -(0.30–0.14);
P<0.00001] (26,29) and 24 weeks [MD, −0.26; 95% CI,
-(0.34–0.17) (26,29); P<0.00001; Table II]. While the other studies did not
provide sufficient data for analysis, the results of all eligible
studies confirmed a statistically significant improvement in the
HAQ-DI in the baricitinib compared with that in the placebo group
in the short-term (24 weeks). Similarity, patients receiving
baricitinib had lower PtGA scores compared with those in the group
receiving placebo at 12 weeks (MD, −10.99; 95% CI, -(14.55–7.44);
P<0.00001] (26,29) and 24 weeks [MD, −12.4; 95% CI,
-(16.02–8.77); P<0.00001; Table
II) (26,29).
Safety of baricitinib (4 mg once
daily) at 12 and 24 weeks
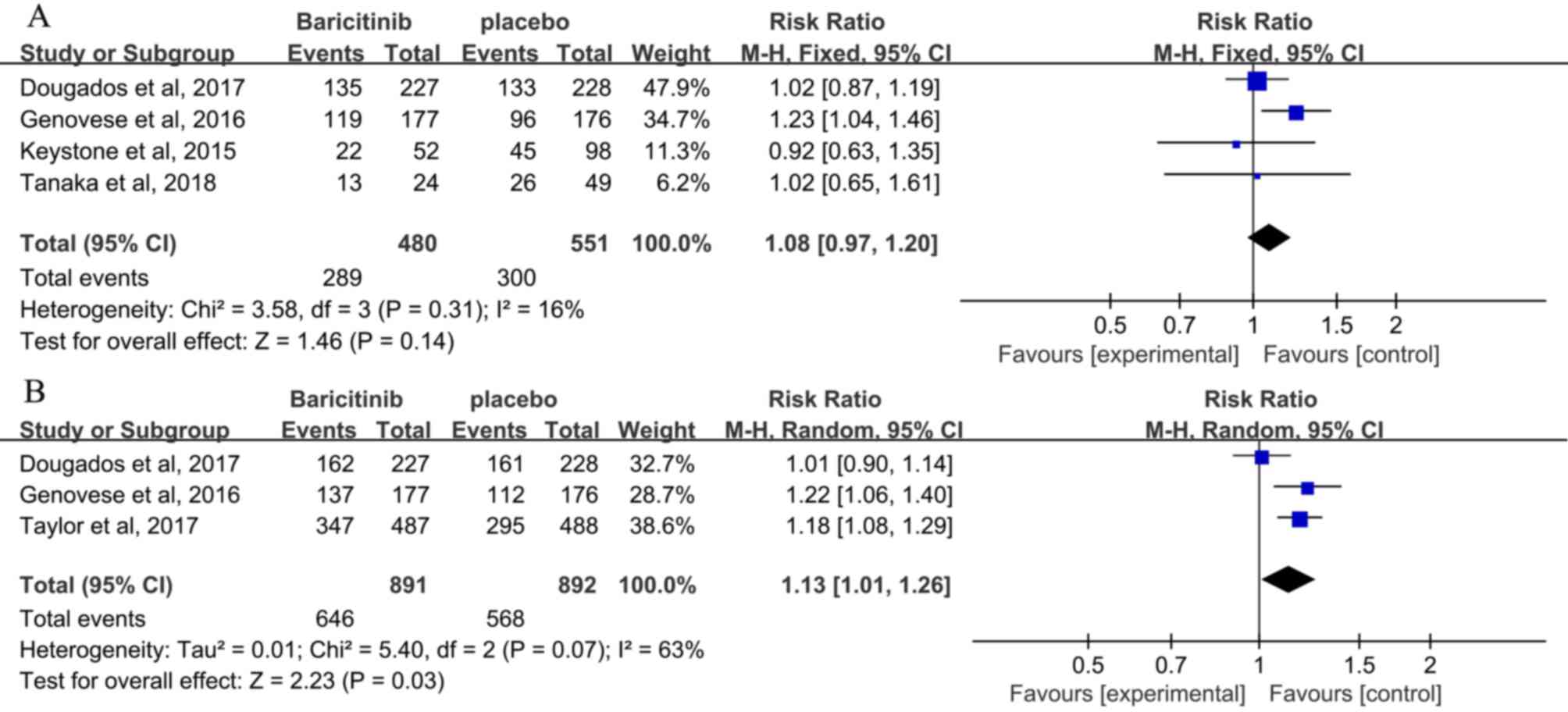
The safety outcomes are presented in Table III. A total of 4 studies reported
on AEs at 12 weeks (3,25,27,28), and
no significant difference in AEs was seen between patients
receiving baricitinib and those receiving placebo (RR, 1.08; 95%
CI, 0.97–1.20; P=0.14; Fig. 5A).
Furthermore, 3 studies reported on AEs at 24 weeks (3,24,27), and
an increased incidence of AEs was observed in patients receiving
baricitinib compared with that in patients receiving placebo (RR,
1.13; 95% CI, 1.01–1.26; P=0.03; Fig.
5B). Incidence of discontinuation between the baricitinib and
placebo groups at 12 weeks (4 studies; RR, 1.15; 95% CI, 0.60–2.22;
P=0.67) (3,24,25,28) and
24 weeks (3 studies; RR, 1.38; 95% CI, 0.90–2.13; P=0.14; Table III) did not differ (3,24,27). The
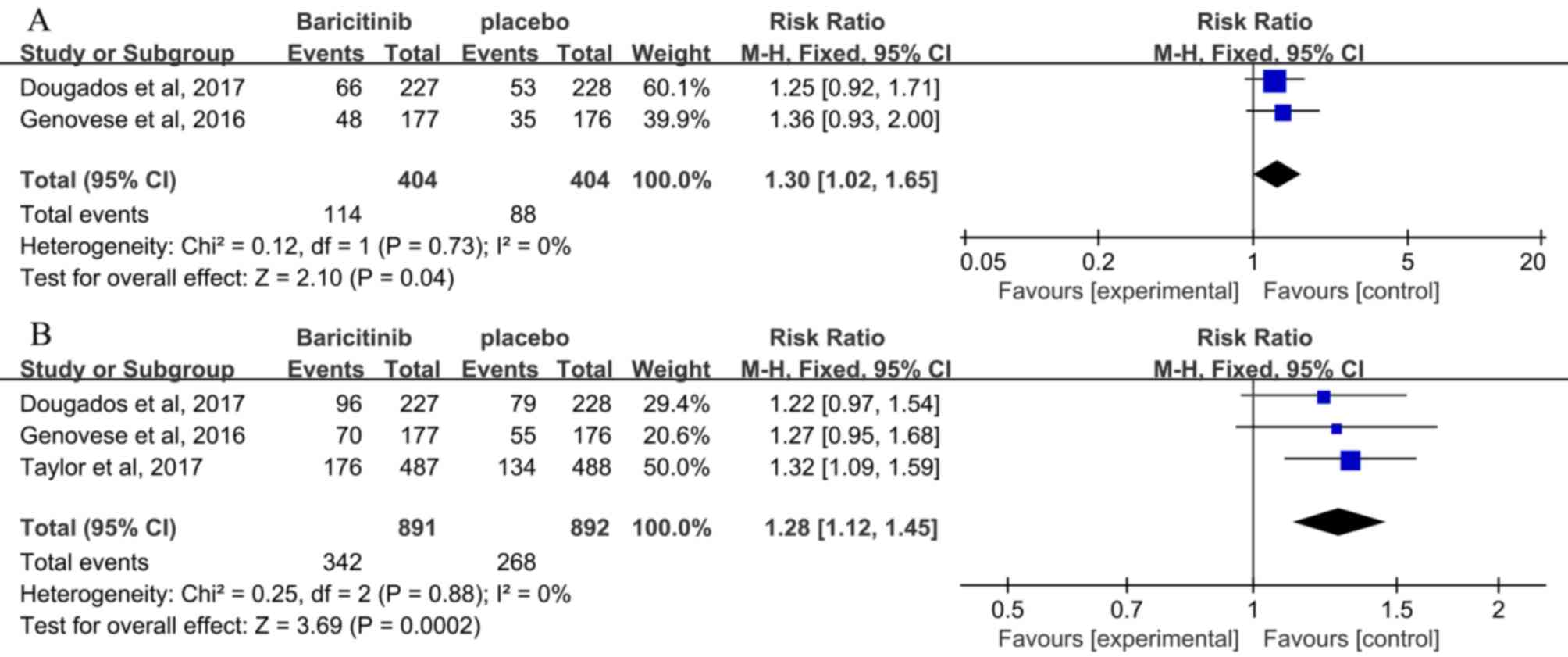
incidence of infection exhibited a significant improvement in the
baricitinib compared with that in the placebo group at 12 weeks (2
studies; RR, 1.30; 95% CI, 1.02–1.65; P=0.04) (3,24) and 24
weeks (3 studies; RR, 1.28; 95% CI, 1.12–1.45; P=0.0002; Fig. 6) (3,24,27).
However, the incidence of serious infection did not differ between
the baricitinib and placebo groups at 12 weeks (3 studies; RR,
0.83; 95% CI, 0.26–2.71; P=0.76) (3,24,25) and
24 weeks (3 studies; RR, 0.94; 95% CI, 0.47–1.88; P=0.86; Table III) (3,24,27).
 | Table III.Safety and laboratory outcomes of
baricitinib in patients with active rheumatoid arthritis. |
Table III.
Safety and laboratory outcomes of
baricitinib in patients with active rheumatoid arthritis.
| A, Baricitinib, 4
mg, 12 weeks |
|
|
|
| Meta-analysis | Test of
heterogeneity |
|---|
|
|
|
|
|
|---|
| Studies (n) | Outcome | RR | 95% CI | P-value | Model | P-value | I2
(%) |
|---|
| 4 | Discontinuation due
to AEs | 1.15 | 0.60 to 2.22 | 0.67 | F | 0.47 | 0 |
| 3 | Serious
infection | 0.83 | 0.26 to 2.71 | 0.76 | F | 0.75 | 0 |
| 3 | ALT | 0.33a | 0.20 to 0.46 | <0.01 | F | 0.51 | 0 |
| 3 | Neutrophils | −0.64b | −0.87 to −0.41 | <0.01 | F | 0.22 | 33 |
| 3 | Lymphocytes | 0.07b | −0.08 to 0.21 | 0.35 | R | 0.05 | 66 |
| 3 | Creatinine | 0.05b | 0.03 to 0.06 | <0.01 | F | 0.49 | 0 |
| 3 | HDL | 7.87b | 6.43 to 9.30 | <0.01 | F | 0.73 | 0 |
|
| B, Baricitinib,
4 mg, 24 weeks |
|
|
|
|
Meta-analysis | Test of
heterogeneity |
|
|
|
|
|
| Studies
(n) | Outcome | RR | 95% CI | P-value | Model | P-value | I2
(%) |
|
| 3 | Discontinuation due
to AEs | 1.38 | 0.90 to 2.13 | 0.14 | F | 0.91 | 0 |
| 3 | Serious
infection | 0.94 | 0.47 to 1.88 | 0.86 | F | 0.82 | 0 |
| 3 | ALT | 0.23a | 0.04 to 0.42 | 0.02 | R | 0.02 | 73 |
| 3 | Neutrophils | −0.67b | −0.87 to −0.47 | <0.01 | F | 0.49 | 0 |
| 3 | Lymphocytes | −0.02b | −0.08 to 0.05 | 0.61 | F | 0.30 | 17 |
| 3 | Creatinine | 0.05b | 0.03 to 0.06 | <0.01 | R | 0.10 | 57 |
| 3 | HDL | 8.69b | 7.46 to 9.91 | <0.01 | F | 0.26 | 26 |
Clinical laboratory measures
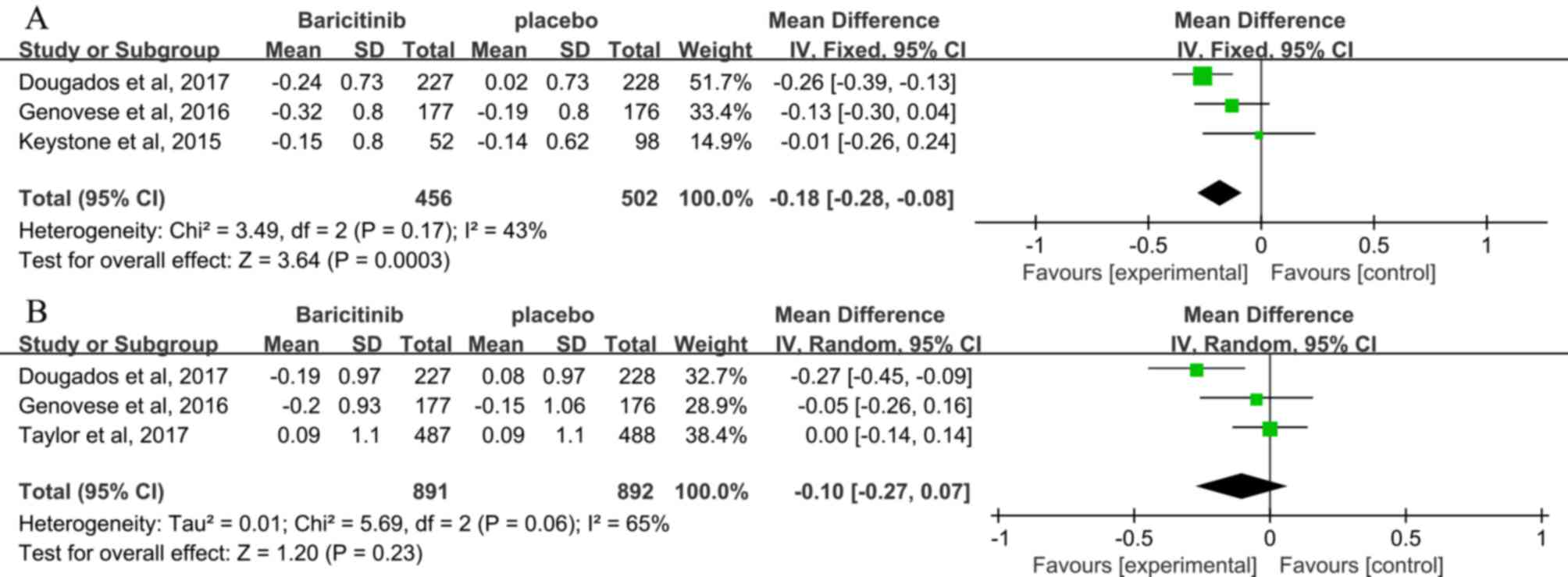
Hemoglobin levels
The clinical laboratory values are presented in
Table III. Hemoglobin levels at 12
and 24 weeks were reported by 4 studies (3,24,25,27),
and low heterogeneity was observed (I2=43%). At 12
weeks, significant reduction in hemoglobin levels was determined in
the baricitinib compared with that in the placebo group (MD, −0.18;
95% CI, -(0.28–0.08); P=0.0003; Fig.
7A) (3,24,25).
However, at 24 weeks, no difference in hemoglobin levels was
observed between patients receiving baricitinib and those receiving
placebo (3,24,27) (MD,
−0.10; 95% CI, −0.27–0.07; P=0.23; Fig.
7B).
Neutrophil, lymphocyte, ALT and
creatinine levels
Baricitinib treatment was associated with a
significantly lower neutrophil count than placebo treatment at 12
weeks [3 studies; MD, −0.64; 95% CI, -(0.87–0.41); P<0.00001]
(3,24,25) and
24 weeks [3 studies; MD, −0.67; 95% CI, -(0.87–0.47); P<0.00001;
Table III] (3,24,27). No
significant difference was observed in lymphocyte counts between
patients receiving baricitinib and those receiving placebo at 12
weeks (3 studies; MD, 0.07; 95% CI, −0.08–0.21; P=0.35) (3,24,25) and
24 weeks (3 studies; MD, −0.02; 95% CI, −0.08–0.05; P=0.61;
Table III) (3,24,27).
Patients receiving baricitinib had significantly higher ALT levels
compared with those receiving placebo at 12 weeks (3 studies; SMD,
0.33; 95% CI, 0.20–0.46; P<0.00001) (3,24,25) and
24 weeks (3 studies; SMD, 0.23; 95% CI, 0.04–0.42; P=0.02; Table III) (3,24,27).
Creatinine levels were significantly higher in the baricitinib
group compared with those in the placebo group at 12 weeks (3
studies; MD, 0.05; 95% CI, 0.03–0.06; P<0.00001) (3,24,25) and
24 weeks (3 studies; MD, 0.05; 95% CI, 0.03–0.06; P<0.00001;
Table III) (3,24,27).
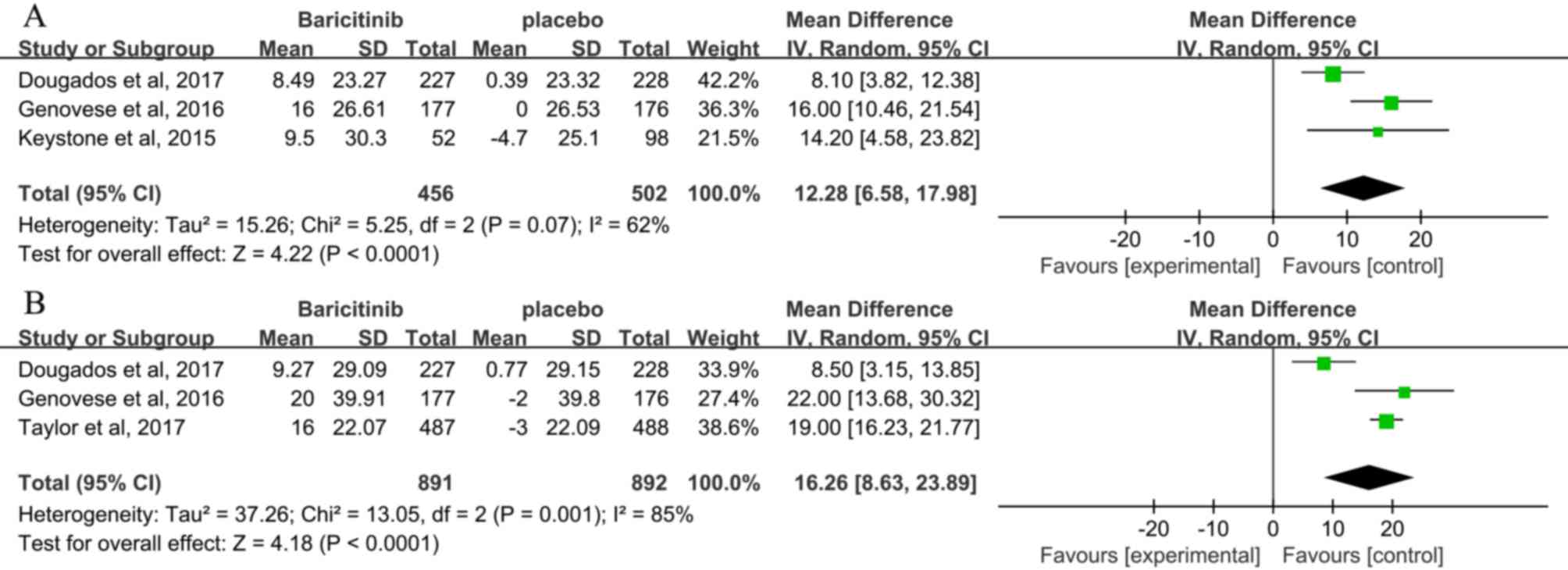
LDL and HDL levels
Baricitinib was associated with significantly higher
LDL levels than placebo at 12 weeks (3 studies; MD, 12.28; 95% CI,
6.58–17.98; P<0.00001) (3,24,25)
and 24 weeks (3 studies; MD, 16.26; 95% CI, 8.63–23.89;
P<0.00001; Fig. 8) (3,24,27).
Similarity, patients receiving baricitinib had significantly higher
HDL levels compared with those receiving placebo at 12 weeks (3
studies; MD, 7.87; 95% CI, 6.43–9.30; P<0.00001) (3,24,25) and
24 weeks (3 studies; MD, 8.69; 95% CI, 7.46–9.91; P<0.00001;
Table III) (3,24,27).
Discussion
In the present meta-analysis, clinical data of 2
phase-II and 5 phase-III trials using baricitinib at dose of 4 mg
once daily for a short term (24 weeks) were pooled. The studies
indicated that 4 mg baricitinib was the most effective dose.
Baricitinib at a dose of 2 and 4 mg provided significant clinical
improvements compared with the placebo at 12 and 24 weeks. However,
clinical benefits were larger in the 4 mg baricitinib group
compared with those in the 2 mg baricitinib group. Baricitinib at a
dose of 4 and 8 mg had similar effective outcomes, but 8 mg
baricitinib was associated with a higher incidence rate of adverse
events and abnormalities in laboratory parameters (25,28).
The present meta-analysis focused on the treatment
with baricitinib at 4 mg once a day for 12 and 24 weeks. The ACR20
response rate was the primary end-point to assess the efficacy of
baricitinib (14). According to the
present meta-analysis, the ACR20 response rate was significantly
higher in the baricitinib group compared with that in the placebo
group at 12 and 24 weeks, a significant clinical benefit of the
administration of baricitinib (4 mg) was revealed. As effective
measures, other PROs were also associated with a significant
improvement in the baricitinib group compared with that in the
placebo group at 12 and 24 weeks, and the improvement of PROs
occurred rapidly and at a high magnitude (3,24,26,29).
The baricitinib group had a significantly lower
neutrophil count compared with that in the placebo group at 12 and
24 weeks. Clinical laboratory measures potentially allow for the
evaluation of the safety of baricitinib, which inhibits the
JAK/STAT pathway and finally changes the whole blood cell counts.
JAK3 has a crucial role in the growth and maturation of
lymphocytes, which may be induced by interleukin (IL) −2, −4, −7,
−9, −15 and −21 (30). Compared with
other JAKs, baricitinib has a relatively low inhibitory effect on
JAK3, and thus, no significant difference was observed in the
lymphocyte count between the baricitinib and placebo groups. The
baricitinib group had a significantly lower neutrophil count
compared with that in the placebo group, and the inhibition of
cytokine-induced STAT3 phosphorylation may have been partly
accountable for this. Hemoglobin was significantly reduced in the
baricitinib group compared with that in the placebo groups at 12
weeks, but no significant difference was observed at 24 weeks. A
decrease in hemoglobin may be due to the inhibition of JAK2
phosphorylation, which has an important role in signal transduction
of erythropoietin (4,31). Regarding other safety outcomes (ALT,
creatinine, HDL and LDL), they were significantly higher in the
baricitinib group compared with those in the placebo group over a
short-term period (24 weeks). The mechanisms of these changes
remain elusive, but the increase in creatinine may be caused by
inhibition of tubular secretion. The increases in HDL and LDL with
treatment of baricitinib were similar to those observed with other
therapies that inhibit JAK and IL-6 activity (4,32,33).
However, most changes in laboratory parameters were minor and
transient, and the clinical significance of these changes remains
elusive (3,24,27).
Further studies with larger numbers of different populations and
long-term exposure are required for safety evaluation.
Baricitinib had a similar clinical efficacy, but
nearly all of the laboratory outcomes were identified to be
significantly changed. These changes were transient and generally
within normal ranges. Only two eligible studies included in the
present meta-analysis reported a statistically significant
reduction in radiographic progression, which delayed joint damage
in the baricitinib groups compared with that in the placebo groups
in the short term (24 weeks) (3,27).
Similarly, radiographic progression exhibited a significant
reduction in the baricitinib + MTX group compared with that in the
MTX monotherapy group from 24 to 52 weeks (19). Long-term results on radiographic
progression are required for evaluating the efficacy of baricitinib
in reducing joint damage.
Several limitations of the present meta-analysis
study should be considered: i) The reliability of the results of
the present study is limited due to a lack of associated studies
and the small sample of the included RCTs; ii) the follow-up was
short, and thus, the long-term efficacy and safety of baricitinib
were not determined; iii) radiographic progression is vital for
assessing joint damage; however, this was not included in the
present meta-analysis due to a lack of data; iv) the results on the
ACR20 and SDAI ≤3.3 rates may have been influenced by partial data
collection from published figures due to data extraction using ‘Get
Data Graph Digitizer’ software.
In conclusion, the present meta-analysis
demonstrated that selective inhibition of the JAK/STAT signaling
pathway with baricitinib produced a clinical improvement in the
treatment of RA within a short-term treatment period. Baricitinib
(4 mg once daily) was the most effective dosage in patients with an
inadequate response to conventional synthetic or biological DMARDs.
Nearly all of the laboratory outcomes exhibited significant changes
in the baricitinib group compared with the placebo group, but the
clinical significance of these changes remains elusive.
High-quality RCTs with long-term exposure and different populations
are required to determine the efficacy and safety of
baricitinib.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Hospital
Level Project of Subei People's Hospital (grant no.
yzucms201623).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
ZPW and PZ performed the data selection. JZB and YL
performed the data analysis. Sensitivity analyses were performed by
JCW. Data disagreements were resolved by JSH. The final version of
the manuscript has been read and approved by all authors, and each
author believes that the manuscript represents honest work.
Ethical approval and consent to
participate
This study was approved by the Ethics Committee of
The Second Xiangya Hospital of Central South University (Changsha,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
RA
|
rheumatoid arthritis
|
|
DMARD
|
disease-modifying anti-rheumatic
drug
|
|
RCT
|
randomized controlled trial
|
|
ACR20
|
American College of Rheumatology 20%
response rate
|
|
HAQ-DI
|
Health Assessment
Questionnaire-Disability Index score
|
|
SDAI
|
Simplified Disease Activity Index
score
|
|
PtGA
|
Patient's Global Assessment of Disease
Activity
|
|
AE
|
adverse event
|
|
ALT
|
alanine transaminase
|
|
LDL
|
low-density lipoprotein
|
|
HDL
|
high-density lipoprotein
|
|
RR
|
relative risk
|
|
MD
|
mean difference
|
|
SMD
|
standardized mean difference
|
|
CI
|
confidence interval
|
References
|
1
|
Carr A, Hewlett S, Hughes R, Mitchell H,
Ryan S, Carr M and Kirwan J: Rheumatology outcomes: The patient's
perspective. J Rheumatol. 30:880–883. 2003.PubMed/NCBI
|
|
2
|
Furst DE and Emer P: Rheumatoid arthritis
pathophysiology: Update on emerging cytokine and
cytokine-associated cell targets. Rheumatology. 53:1560–1569. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dougados M, van der Heijde D, Chen YC,
Greenwald M, Drescher E, Liu J, Beattie S, Witt S, de la Torre I,
Gaich C, et al: Baricitinib in patients with inadequate response or
intolerance to conventional synthetic DMARDs: Results from the
RA-BUILD study. Ann Rheum Dis. 76:88–95. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kubo S, Nakayamada S and Tanaka Y:
Baricitinib for the treatment of rheumatoid arthritis. Expert Rev
Clin Immunol. 12:911–919. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iwata S and Tanaka Y: Progress in
understanding the safety and efficacy of Janus kinase inhibitors
for treatment of rheumatoid arthritis. Expert Rev Clin Immunol.
12:1047–1057. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Darnell JE Jr, Kerr IM and Stark GR:
Jak-STAT pathways and transcriptional activation in response to
IFNs and other extracellular signaling proteins. Science.
264:1415–1421. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fridman JS, Scherle PA, Collins R, Burn
TC, Li Y, Li J, Covington MB, Thomas B, Collier P, Favata MF, et
al: Selective inhibition of JAK1 and JAK2 is efficacious in rodent
models of arthritis: Preclinical characterization of INCB028050. J
Immunol. 184:5298–5307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi JG, Chen X, Lee F, Emm T, Scherle PA,
Lo Y, Punwani N, Williams WV and Yeleswaram S: The
pharmacokinetics, pharmacodynamics, and safety of baricitinib, an
oral JAK 1/2 inhibitor, in healthy volunteers. J Clin Pharmacol.
54:1354–1361. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kubo S, Nakayamada S, Nakano K and Tanaka
Y: THU0203 baricitinib targets the type I IFN/STAT-medicated
activities of human T cells and dendritic cells. Ann Rheum Dis. 75
Suppl 2:S2602016. View Article : Google Scholar
|
|
10
|
Emery P, Mcinnes I, Genovese MC, Smolen
JS, Kremer J, Dougados M, Schlichting DE, Rooney T, Issa M, Bono
Sd, et al: A7.16 Characterisation of changes in lymphocyte subsets
in baricitinib-treated patients with rheumatoid arthritis in two
phase 3 studies. Ann Rheum Dis. 75 Suppl 1:A622016. View Article : Google Scholar
|
|
11
|
Song GG, Bae SC and Lee YH: Efficacy and
safety of tofacitinib for active rheumatoid arthritis with an
inadequate response to methotrexate or disease-modifying
antirheumatic drugs: A meta-analysis of randomized controlled
trials. Korean J Intern Med. 29:656–663. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meyer DM, Jesson MI, Li X, Elrick MM,
Funckes-Shippy CL, Warner JD, Gross CJ, Dowty ME, Ramaiah SK,
Hirsch JL, et al: Anti-inflammatory activity and neutrophil
reductions mediated by the JAK1/JAK3 inhibitor, CP-690,550, in rat
adjuvant-induced arthritis. J Inflamm (Lond). 7:412010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moher D, Liberati A, Tetzlaff J and Altman
DG; PRISMA Group, : Preferred reporting items for systematic
reviews and meta-analyses: The PRISMA statement. PLoS Med.
6:e10000972009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Felson DT, Anderson JJ, Boers M,
Bombardier C, Furst D, Goldsmith C, Katz LM, Lightfoot R Jr, Paulus
H, Strand V, et al: American College of Rheumatology. Preliminary
definition of improvement in rheumatoid arthritis. Arthritis Rheum.
38:727–735. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aletaha D and Smolen J: The Simplified
Disease Activity Index (SDAI) and the Clinical Disease Activity
Index (CDAI): A review of their usefulness and validity in
rheumatoid arthritis. Clin Exp Rheumatol. 23 5 Suppl 39:S100–S108.
2005.PubMed/NCBI
|
|
16
|
van Tuyl LH and Boers M: Patient's global
assessment of disease activity: What are we measuring? Arthritis
Rheum. 64:2811–2813. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bruce B and Fries JF: The Health
Assessment Questionnaire (HAQ). Clin Exp Rheumatol. 23 Suppl
39:S14–S18. 2005.PubMed/NCBI
|
|
18
|
Higgins JP and Green S: Cochrane handbook
for systematic reviews of interventions version 5.1.0.
Naunyn-Schmiedebergs Archiv Für Exp Pathol und Pharmakol.
5:S382011.
|
|
19
|
Fleischmann R, Schiff M, van der Heijde D,
Ramos-Remus C, Spindler A, Stanislav M, Zerbini CA, Gurbuz S,
Dickson C, de Bono S, et al: Baricitinib, methotrexate, or
combination in patients with rheumatoid arthritis and no or limited
prior disease-modifying antirheumatic drug treatment. Arthritis
Rheumatol. 69:506–517. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanaka Y, Ishii T, Cai Z, Schlichting D,
Rooney T and Macias W: Efficacy and safety of baricitinib in
Japanese patients with active rheumatoid arthritis: A 52-week,
randomized, single-blind, extension study. Mod Rheumatol. 28:20–29.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takeuchi T, Genovese M, Xie L, Issa M,
Pinto Correia AL, Rooney T, Emoto K and Smolen J: OP0228
baricitinib dose step-down following disease control in patients
with rheumatoid arthritis. Ann Rheumat Dis:. 75 Suppl 2:144.1–144.
2016. View Article : Google Scholar
|
|
22
|
Keystone EC, Genovese MC, Schlichting DE,
de la Torre I, Beattie SD, Rooney TP and Taylor PC: Safety and
efficacy of baricitinib through 128 weeks in an open-label,
longterm extension study in patients with rheumatoid arthritis. J
Rheumatol. 45:14–21. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kremer JM, Genovese MC, Keystone E, Taylor
PC, Zuckerman SH, Ruotolo G, Schlichting DE, Crotzer VL, Nantz E,
Beattie SD and Macias WL: Effects of baricitinib on lipid,
apolipoprotein, and lipoprotein particle profiles in a phase iib
study of patients with active rheumatoid arthritis. Arthritis
Rheumatol. 69:943–952. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Genovese MC, Kremer J, Zamani O, Ludivico
C, Krogulec M, Xie L, Beattie SD, Koch AE, Cardillo TE, Rooney TP,
et al: Baricitinib in patients with refractory rheumatoid
arthritis. N Engl J Med. 374:1243–1252. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Keystone EC, Taylor PC, Drescher E,
Schlichting DE, Beattie SD, Berclaz PY, Lee CH, Fidelus-Gort RK,
Luchi ME, Rooney TP, et al: Safety and efficacy of baricitinib at
24 weeks in patients with rheumatoid arthritis who have had an
inadequate response to methotrexate. Ann Rheum Dis. 74:333–340.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Emery P, Blanco R, Maldonado Cocco J, Chen
YC, Gaich CL, DeLozier AM, de Bono S, Liu J, Rooney T, Chang CH and
Dougados M: Patient-reported outcomes from a phase III study of
baricitinib in patients with conventional synthetic
DMARD-refractory rheumatoid arthritis. RMD Open. 3:e0004102017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Taylor PC, Keystone EC, van der Heijde D,
Weinblatt ME, Del Carmen Morales L, Reyes Gonzaga J, Yakushin S,
Ishii T, Emoto K, Beattie S, et al: Baricitinib versus placebo or
adalimumab in rheumatoid arthritis. N Engl J Med. 376:652–662.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tanaka Y, Emoto K, Cai Z, Aoki T,
Schlichting D, Rooney T and Macias W: Efficacy and safety of
baricitinib in Japanese patients with active rheumatoid arthritis
receiving background methotrexate therapy: A 12-week, double-blind,
randomized placebo-controlled study. J Rheumatol. 43:504–511. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Smolen JS, Kremer JM, Gaich CL, DeLozier
AM, Schlichting DE, Xie L, Stoykov I, Rooney T, Bird P, Sánchez
Bursón JM, et al: Patient-reported outcomes from a randomised phase
III study of baricitinib in patients with rheumatoid arthritis and
an inadequate response to biological agents (RA-BEACON). Ann Rheum
Dis. 76:694–700. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leonard WJ and O'Shea JJ: Jaks and STATs:
Biological implications. Annu Rev Immunol. 16:293–322. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pelletier S, Gingras S, Funakoshi-Tago M,
Howell S and Ihle JN: Two domains of the erythropoietin receptor
are sufficient for Jak2 binding/activation and function. Mol Cell
Biol. 26:8527–8538. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kawashiri SY, Kawakami A, Yamasaki S,
Imazato T, Iwamoto N, Fujikawa K, Aramaki T, Tamai M, Nakamura H,
Ida H, et al: Effects of the anti-interleukin-6 receptor antibody,
tocilizumab, on serum lipid levels in patients with rheumatoid
arthritis. Rheumatol Int. 31:451–456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Souto A, Salgado E, Maneiro JR, Mera A,
Carmona L and Gómez-Reino JJ: Lipid profile changes in patients
with chronic inflammatory arthritis treated with biologic agents
and tofacitinib in randomized clinical trials: A systematic review
and meta-analysis. Arthritis Rheumatol. 67:117–127. 2015.
View Article : Google Scholar : PubMed/NCBI
|