Introduction
Mast cells (MCs) are local tissue-resident cells,
which are frequently located within the skin, respiratory tract and
gastrointestinal mucosa (1).
Increasing evidence indicates that MCs serve a crucial role in
allergic diseases, including bronchial asthma, allergic rhinitis,
atopic dermatitis and hypersensitivity (2–5). MCs are
activated when antigen cross-linking of immunoglobulin E (IgE)
binds to the high-affinity receptor (FcεRI), which results in the
phosphorylation of Syk tyrosine kinase, calcium (Ca2+)
influx, and the activation of protein kinase C, mitogen-activated
protein kinases and nuclear factor (NF)-κB (6). Following MC degranulation, inflammatory
mediators, including proteases, β-hexosaminidase, histamine and
inflammatory cytokines, such as tumor necrosis factor-α and
interleukin-6 are released and extensively associated with the
pathogenesis of allergic diseases (7). There is a positive correlation between
disease severity and the number of MCs (8), therefore regulation of MC activation is
considered as a promising and alternative treatment option for
MC-associated diseases.
MicroRNAs (miRs) are a group of small, non-coding,
single-stranded RNAs, which regulate gene expression at a
post-transcriptional level by targeting the 3′-untranslated region
(3′-UTR) of specific mRNAs for degradation or translational
repression (9). A previous study
indicated that miR-126 was overexpressed in a murine model of
asthma, which was induced by house dust mites (10). In addition, the overexpression of
miR-126 in bone marrow-derived MCs may promote FcεRI-mediated
cytokine production (11). These
findings suggested a possible association between miR-126
overexpression and the activation of MC degranulation. However, the
role of miR-126 in MC degranulation and the underlying molecular
mechanisms are required further clarification.
The present study aimed to investigate the effect of
miR-126 on MC activation triggered by IgE and explored the
underlying molecular mechanisms. The results revealed that miR-126
accelerated IgE-mediated MC degranulation, which was associated
with the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)
signaling pathway and enhanced Ca2+ influx.
Materials and methods
Animals
A total of 12 male 8-week-old Sprague-Dawley rats
(weight, 200.2±15.4 g) were purchased from the Hubei Research
Center of Laboratory Animals (Wuhan, China) and housed in specific
pathogen-free conditions. The rats were divided into three groups
as follows: i) Normal (n=4); ii) allergic contact dermatitis (ACD)
model (n=4); and iii) isolation of rat peritoneal mast cells
(RPMCs; n=4). The rats were housed 4 per cage and maintained at a
temperature of 22±1°C in a relative humidity of 55±10%, under a 12
h light/dark cycle for 1 week prior to the start of the
experiments. Water and a standard diet were provided ad
libitum throughout the study. The study protocol was approved
by the Ethics Committee of the Animal Care and Use Committee of the
Central Hospital of Wuhan (approval no. SCXK2015-0018; Wuhan,
China).
Establishing 2,4-dinitrofluorobenzene
(DNFB)-induced ACD
Briefly, the rats were sensitized by topically
applying 25 µl 0.5% DNFB (acetone:olive oil, 4:1; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) on the shaved hind flank on days 1
and 2. Following 5 days the rats were challenged with 10 µl 0.5%
DNFB on the left ear to induce ACD.
Isolation of RPMCs
At the end of the experiment, on day 8, rats in the
RPMC group were anesthetized with 7% intraperitoneal chloral
hydrate (350 mg/kg; Sigma-Aldrich; Merck KGaA). Following its
administration, no symptoms of chemical peritonitis were observed.
Dulbecco's minimal essential medium (DMEM; Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) with 10% fetal bovine
serum (Invitrogen; Thermo Fisher Scientific, Inc.) containing
penicillin (100 U/ml), streptomycin (100 µg/ml) and heparin (5
U/ml) (Gibco; Thermo Fisher Scientific, Inc.) was injected into the
peritoneal cavity. Once the lavage was completed, the rats
(252.1±10.6 g) were sacrificed via CO2 inhalation (the
flow rate of CO2 displaced >30% of the chamber
volume/minute) and cervical dislocation was used to sacrifice the
animals. Cells of the peritoneal lavage fluid were collected by
centrifugation at 400 × g for 15 min at room temperature and
resuspended in 1 ml serum-free DMEM. The macrophages were separated
from the MCs by differential centrifugation using a Percoll
solution (Sigma-Aldrich; Merck KGaA) as previously described
(12).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the RPMCs using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). RT was
conducted using a TaqMan MicroRNA Reverse Transcription kit (Thermo
Fisher Scientific, Inc.) at 42°C for 30 min followed by 75°C for 5
min. qPCR was performed using a TaqMan MicroRNA assay (Thermo
Fisher Scientific, Inc.). The following primer sequences were used
for the PCR: miR-126, forward 5′-CGTACCGTGAGTAATAATG-3′ and reverse
5′-AACTGGTGTCGTGGAG-3′; and U6, forward 5′-CAAGGATGACACGCAAAT-3′
and reverse 5′-TGGTGTCGTGGAGTCG-3′. The PCR protocol was 95°C for
10 min, followed by 40 cycles of 95°C for 10 sec, 60°C for 30 sec
and 72°C for 30 sec followed by final extension at 72°C for 5 min
and storage at 4°C. Each sample was analyzed in duplicate. The
expression of U6 was used as the endogenous control for miRNAs
analysis. The data were quantified using the 2−ΔΔCq
method (13).
Transfection
RPMCs were transfected with miR-126 mimic
(5′-UCGUACCGUGAGUAAUAAUCCG-3′), negative control
(5′-UUCUCCGAACGUGUCACGUTT-3′), miR-126 inhibitor
(5′-CAUUAUUACUUUUGGUACGCG-3′) and its control
(5′-CAGUACUUUUGUGUAGUACAA-3′) all supplied by Shanghai GenePharma
Co., Ltd. (Shanghai, China) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The final concentration of
oligonucleotides was 50 nM. Following 24 h incubation at 37°C, the
cells were processed for further experiments.
Analysis of β-hexosaminidase
release
RPMCs were activated for a degranulation assay.
Following challenge with dinitrophenyl (DNP)-human serum albumin
(HSA) (100 ng/ml) (Sigma-Aldrich; Merck KGaA) to sensitized cells,
the cell supernatant was mixed with an equal volume of substrate
solution (1 mM 4-nitrophenyl-N-acetyl-β-D-glucosaminide in 0.1 M
citrate buffer, pH 4.5) for 1 h at 37°C. The reaction was stopped
with carbonate buffer (0.1 M
Na2CO3/NaHCO3). The absorbance was
measured with a microplate reader at 405 nm.
Histamine release assay
To determine MC degranulation, the levels of
histamine in homogenates from the left ear of ACD rats and the
culture medium of RPMCs were measured. Histamine concentrations
were detected using an ELISA kit (KT60094; Kamiya Biomedical
Company, Seattle, WA, USA) according to the manufacturer's
protocol.
LY294002 assay
RPMCs were transfected with miR-126 mimics or its NC
and the cells were sensitized overnight at 37°C with anti-DNP IgE
(0.5 µg/ml; Sigma-Aldrich; Merck KGaA). Cells were subsequently
treated with DNP-HSA (100 ng/ml) at 37°C for 4 h in the presence or
absence of LY294002 (10 µM; Sigma-Aldrich; Merck KGaA). Following,
β-hexosaminidase and histamine release were detected.
Western blot analysis
RPMCs were obtained and lysed with a lysis buffer
(20 mM Tris-HCl, pH 7.5; 150 mM NaCl; 1 mM Na2EDTA; 1 mM
EGTA; 1% Triton; 2.5 mM sodium pyrophosphate; 1 mM
β-glycerophosphate; 1 mM Na3VO4 and 1 µg/ml
leupeptin) containing protease and phosphatase inhibitors (no.
9803; Cell Signaling Technology, Inc., Danvers, MA, USA).
Supernatants were analyzed for protein content using a BCA Protein
Assay kit (no. 7780; Cell Signaling Technology, Inc.). Proteins (20
µg) were separated by 12% SDS-PAGE gels and transferred onto
polyvinylidene difluoride membranes. Following blocking overnight
at 4°C with 5% skimmed milk, the membranes were incubated with
rabbit anti-rat primary antibodies against Akt (cat. no. SC-8312;
1:200), phosphorylated (p)-Akt(S473) (cat. no. SC-135651; 1:100)
and GAPDH (cat. no. SC-25778; 1:1,000; all Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). Following 3 washes with
Tris-buffered saline with Tween-20 buffer, the membranes were
incubated at room temperature for 2 h with the appropriate
horseradish-peroxidase-conjugated goat anti-rabbit secondary
antibodies (cat. no. SC-2004; 1:5,000; Santa Cruz Biotechnology,
Inc.). The proteins were visualized using an enhanced
chemiluminescence detection reagent (Pierce; Thermo Fisher
Scientific, Inc.).
Cytosolic Ca2+
measurement
Following treatment with anti-DNP IgE (0.5 µg/ml;
Sigma-Aldrich) at 37°C for 24 h, cells were washed three times in
Tyrode's buffer (135 mM NaCl; 5 mM KCl; 1.8 mM CaCl2;
1.0 mM MgCl2; 5.6 mM glucose; 20 mM HEPES; and 1 mg/ml
bovine serum albumin at pH 7.4) and co-incubated with 2 µM
fura-2/AM (Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for
30 min. The fura-2/AM was discarded and the cells were resuspended
in Tyrode's buffer and stimulated with 100 ng/ml DNP-HSA at 37°C
for 4 h. Measurements of Ca2+ influx were made using a
Leica DMI 6000B fluorescence microscope (Leica Microsystems GmbH,
Wetzlar, Germany) controlled with SlideBook software (Intelligent
Imaging Innovations, Inc.; Denver, CO, USA). Fluorescence emission
at 505 nm was monitored while alternating excitation between
340–380 nm at a frequency of 0.5 Hz. Ca2+ influx is
presented as 340/380 nm ratio.
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analyses were conducted using SPSS 16.0 software (SPSS,
Inc., Chicago, IL, USA). The results were analyzed using one-way
analysis of variance and Duncan's post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-126 is increased in IgE-stimulated
RPMCs
To investigate the effect of miR-126 on MC
degranulation, miR-126 expression was analyzed in a rat model of
hypersensitivity in vivo and in isolated RPMCs in
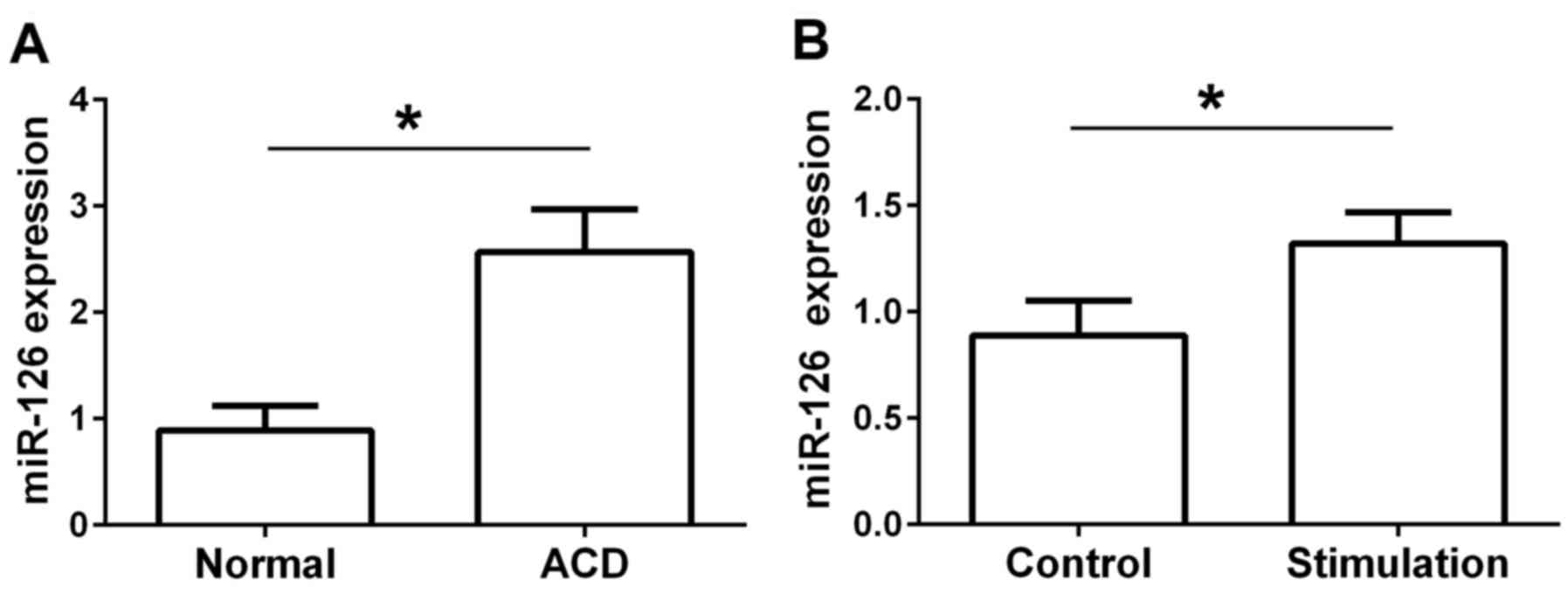
vitro. The results revealed that the expression of miR-126 was
significantly increased in rats with DNFB-induced ACD compared with
the normal group (Fig. 1A). In
comparison with the control group, the expression of miR-126 was
also significantly increased in the IgE-stimulated RPMCs (Fig. 1B).
miR-126 is overexpressed in RPMCs
transfected with miR-126 mimics
To assess the effect of miR-126 on MC degranulation,
RPMCs were transfected with miR-126 mimics to construct a model of
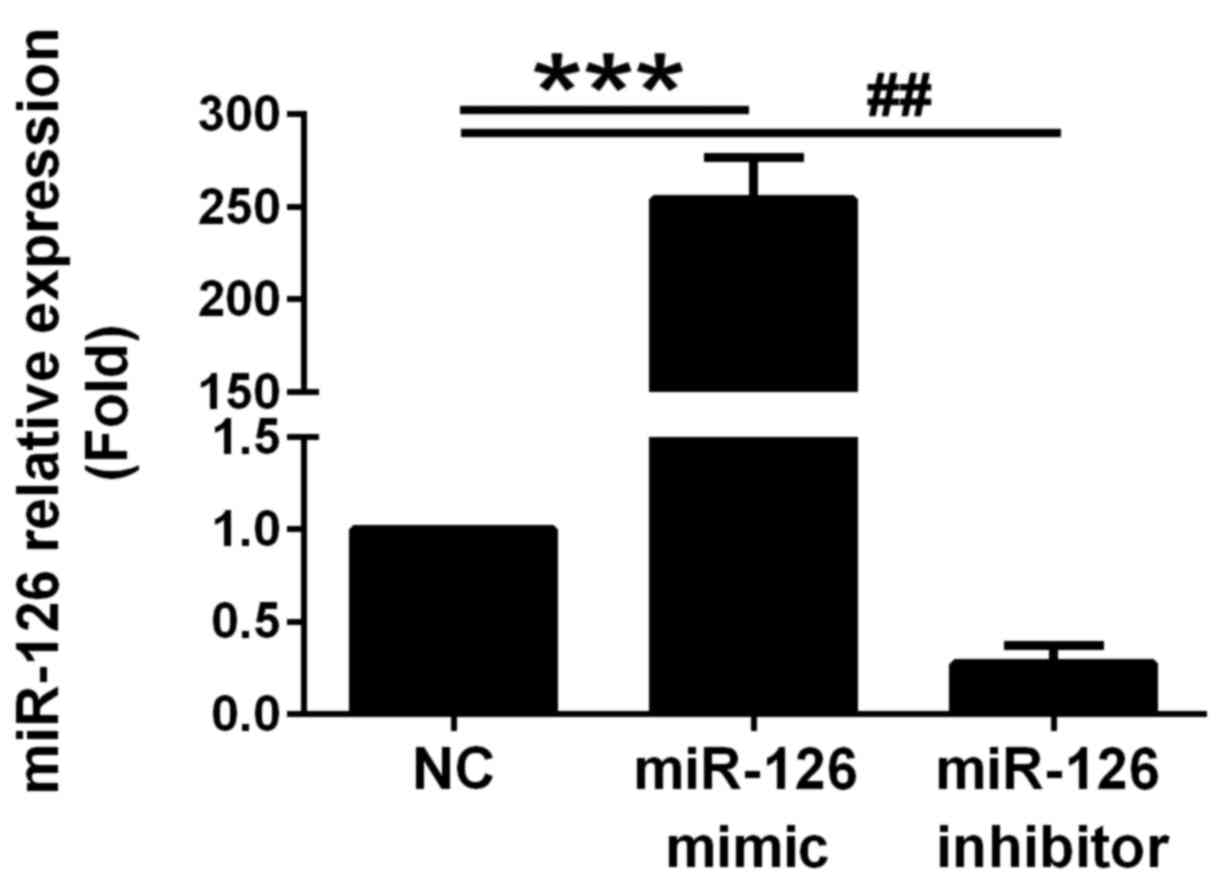
miR-126 overexpression. miR-126 expression was significantly
increased at 24 h following transfection with the miR-126 mimics
(Fig. 2). Conversely, miR-126
expression was clearly decreased following transfection with the
miR-126 inhibitor (Fig. 2).
Effect of miR-126 on MC
degranulation
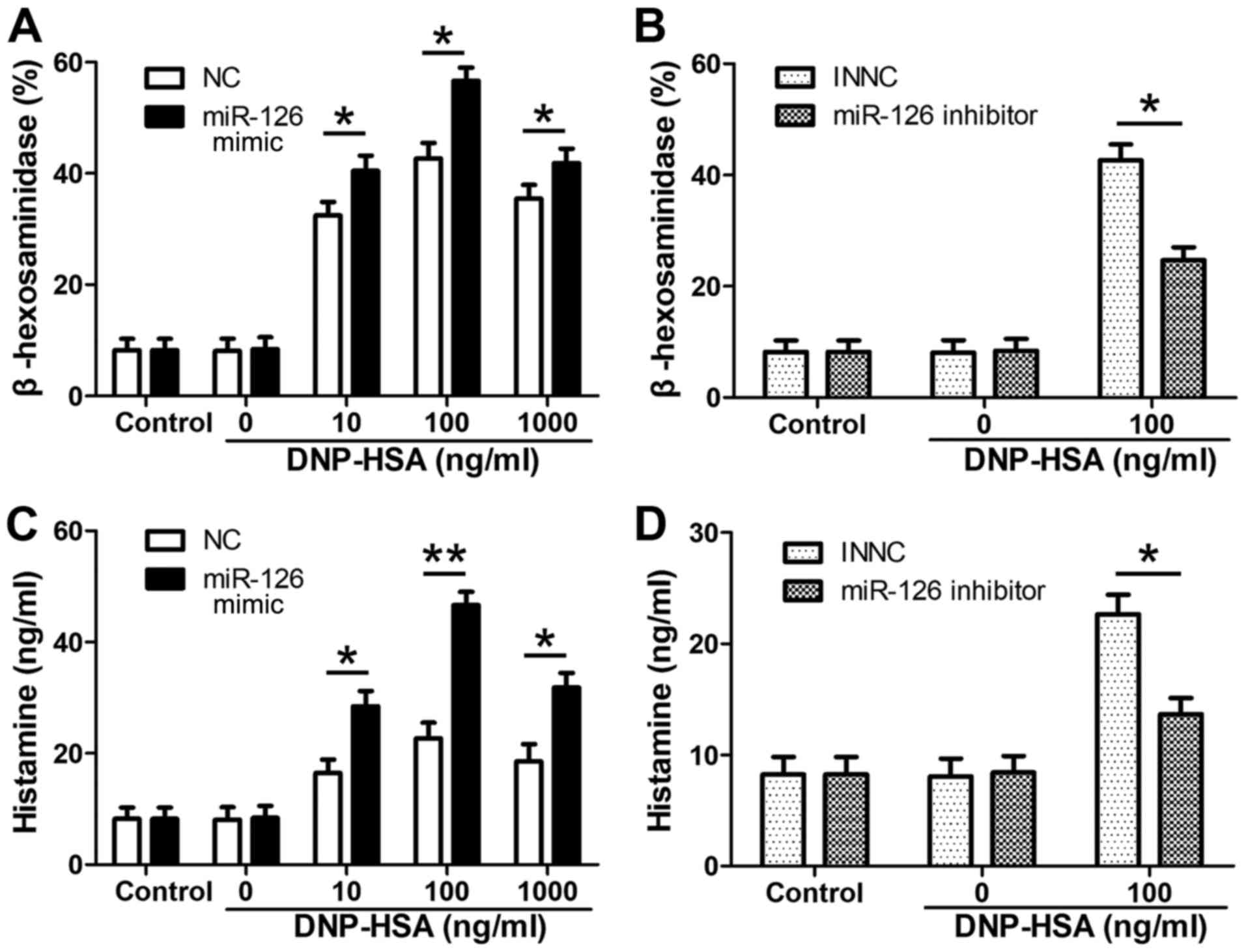
β-hexosaminidase and histamine are of MC
degranulation (14); their release
was detected to evaluate the level of MC degranulation. RPMCs were
transfected with miR-126 mimics, miR-126 inhibitor and NCs.
Following sensitization with anti-DNP IgE, cells were treated with
the indicated concentrations of DNP-HSA for 4 h. The release of
β-hexosaminidase was detected (Fig.
3). β-hexosaminidase release was significantly upregulated in
the miR-126 mimic group compared with the negative control group
(Fig. 3A), where as it was
significantly downregulated in the miR-126 inhibitor group
(Fig. 3B). Similar results were
observed for histamine release in the miR-126 mimic group (Fig. 3C) and the miR-126 inhibitor group
(Fig. 3D).
miR-126 enhances PI3K/Akt signal
activation in IgE-mediated MCs
To clarify if the PI3K/Akt signaling pathway was
associated with the effect of miR-126 on MC degranulation, RPMCs
were transfected with miR-126 mimics and the negative control and
the cells were either stimulated with DNP IgE and DNP-HSA or not
stimulated. The protein expression of Akt and p-Akt was then
determined by western blot analysis. Compared with the negative
control group, the phosphorylation of Akt was significantly
increased in the miR-126 mimic group in the stimulated cells
(Fig. 4). However, there were no
significant differences observed between the two groups of
non-stimulated cells.
Effect of the PI3K-inhibitor on
miR-126-enhanced MC degranulation
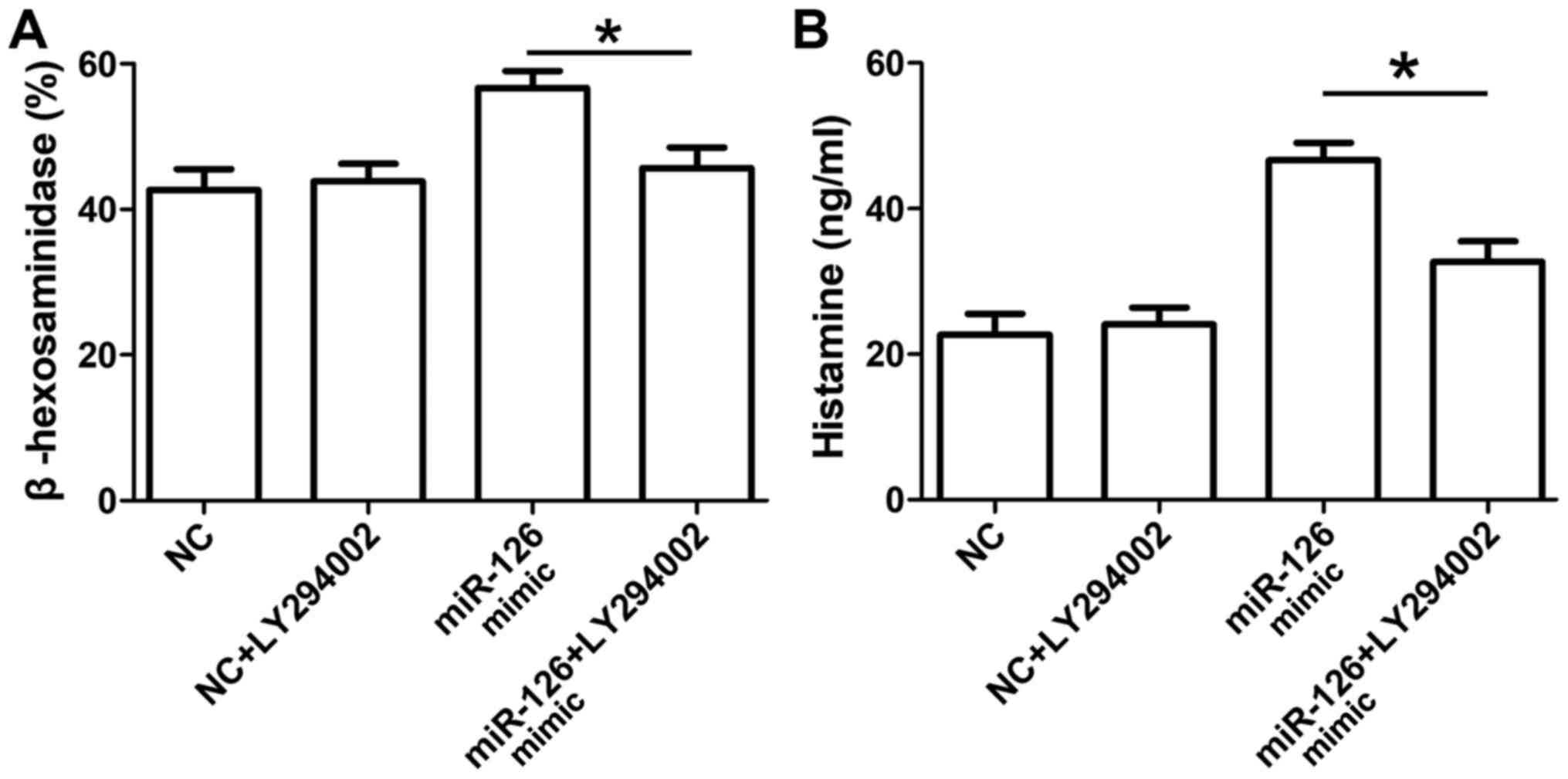
A specific PI3K-inhibitor (LY294002) was used to
confirm the role of the PI3K/Akt signaling pathway in
miR-126-enhanced MC degranulation. Compared with the miR-126 mimic
group, the release of β-hexosaminidase (Fig. 5A) and histamine (Fig. 5B) were significantly reduced in the
miR-126 mimic + LY294002 groups.
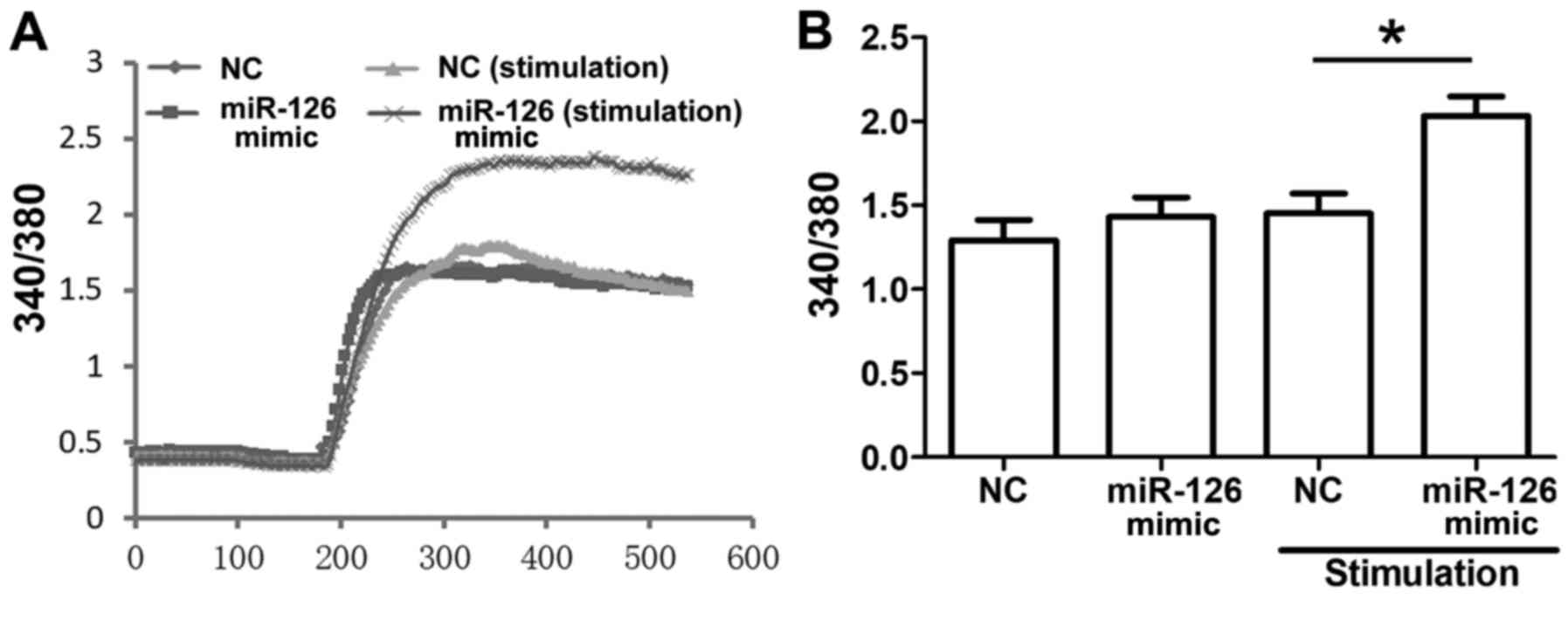
Ca2+ influx participates in
the regulation of miR-126 on MC degranulation
Ca2+ mobilization was indicated as
associated with MC degranulation, which responded to the
crosslinking of IgE binding to its receptors. Changes in cytosolic
Ca2+ were examined using a fura-2 assay. Ca2+
influx was significantly enhanced in the miR-126 mimic group
compared with the NC group when the cells were stimulated (Fig. 6).
Discussion
The association between miRNAs and mast cell
activation involved in allergic inflammation attracts increasing
attention. A previous study reported a significant upregulation of
miR-21, miR-142-3p, miR-142-5p and miR-223 in the skin of mice with
allergic contact dermatitis and also in humans sensitized with
diphenylcyclopropenone (15). The
present study observed that miR-126 was significantly upregulated
in ear samples from DNFB-treated rats and IgE-stimulated peritoneal
MCs. However, there were certain differences between the two
studies, which may attribute to the difference of miRs expression.
Rats were used for the ACD model in the present study as opposed to
mice and the time of repeated-DNFB application was different to the
above study by ~3 weeks. In addition, another previous study
detailed that miR-126 was significantly downregulated following the
extension of culture time in bone marrow-derived MCs, which
suggests that cell type and culture time may affect miR-126
expression (11). miR-126 can be
isolated from vascular endothelial cells and serves an important
role in maintaining endothelial cell proliferation and migration
(16). It may further influence
other cell processes, including angiogenesis and apoptosis
(17–19). miR-126 positively regulates MC
proliferation and cytokine production through suppression of
Sprouty-related Ena/VASP homology-1 domain-containing protein
(Spred1) (11). In the present
study, the model of miR-126 overexpression in RPMCs was established
following miR-126 mimic transfection, which was associated with the
activation of MC degranulation and the increased release of
β-hexosaminidase and histamine.
The PI3K/Akt signaling pathway is important for cell
growth, differentiation, metabolism, survival and apoptosis
(20). As the PI3K conformation
changes, Ser473 and Thr308 phosphorylation is required for Akt
activation (21). A number of
previous studies have demonstrated that the PI3K/Akt signaling
pathway participated in the regulation of miRNAs on MC
degranulation (22–24). miR-155 controlled MC activation by
modulating the PI3Kγ signaling pathway and anaphylaxis in mice with
cutaneous anaphylaxis (22).
Downregulation of miR-223 promoted MC degranulation via the
PI3K/Akt pathway by targeting insulin-like growth factor 1 receptor
(23). Lentiviral short hairpin RNA
against KCa3.1 inhibited the allergic response in allergic rhinitis
and suppressed MCs activity via the PI3K/AKT signaling pathway
(24). In the present study, the
phosphorylation of Akt was increased, accompanied by miR-126
overexpression, which was reversed by LY294002, the specific PI3K
inhibitor. These findings suggest that PI3K/Akt signaling was
associated with the regulation of miR-126 on MCs.
Ca2+ participates in intra- and
extracellular signaling pathways and serves a key role in cell
destiny (25). Crosslinking of FcεRI
complexes at the MC surface initiates cytoplasmic Ca2+
(Cai2+) mobilization and stimulates
Ca2+ influx, which is important for MC degranulation
(26). In a previous study, miR-214
knockout mice exhibited more severe cardiac injuries, including
increased cardiomyocyte loss and larger fibrotic regions, which
were associated with high Cai2+ levels and
Cai2+ overload (27). In addition, the overexpression of
miR-25 protected cardiomyocytes against oxidative damage by
inactivating the mitochondrial apoptosis pathway, which targets the
mitochondrial Ca2+ uniporter and reduces
H2O2-induced elevation of mitochondrial
Ca2+ concentrations (28). In line with these previous reports,
the results of the present study demonstrated that transfection
with miR-126 mimics promoted Ca2+ influx in
IgE-stimulated MCs. The present authors speculate that the
exogenous or endogenous stimulation induced the overexpression of
miR-126 on MCs, thereby enhancing the Ca2+ influx. The
decreased Ca2+ in the cytoplasm triggered the PI3K/Akt
signaling pathway, which contributed to the acceleration of
IgE-mediated MC degranulation.
In the present study, the effect of miR-126 on MC
degranulation was investigated based on the intrinsic association
between PI3K/Akt signaling and Ca2+ influx. The results
provide additional evidence supporting the possible molecular
mechanism of miR-126 on allergic skin inflammation. However, there
were certain limitations in the present study that should be
mentioned: i) Bone marrow-derived MCs were not used as a rat model
was selected. In the majority of cases, bone marrow-derived MCs
were isolated from mice and induced differentiation following
cytokine stimulation in vitro for 4–6 weeks. Due to time and
cost considerations, peritoneal MCs were used in the present study,
which was suitable for the experimental design; although bone
marrow-derived MCs may offer more comprehensive support of the
hypothesis. ii) If other signaling pathways or transcription
factors, including NF-κB and AP-1 were associated the biological
effect of miR-126, further studies are required to investigate
their underlying mechanisms and cascade reactions. iii) Although
certain targets of miR-126 were reported in different cell types
and disease models, including EGFL7 (29), pik3r2 (30), ROCK1 (31), IRS-1 and GOLPH3 (32), to the best of our knowledge, Spred1
was reported as a direct target of miR-126 and was associated with
MC activation (11). The present
study focused on the direct effect of miR-126 on MC degranulation
and its preliminary molecular mechanisms. However, it is possible
that the mRNA 3′-UTR may be degraded by miR-126 at a
post-transcriptional level and that proteins may regulate the
PI3K/Akt signaling pathway and Ca2+ influx. Further
studies are required to clarify the underlying molecular
mechanisms.
In conclusion, miR-126 expression was increased in
ACD rats and rat peritoneal MCs. Overexpression of miR-126
inhibited the release of β-hexosaminidase and histamine, attenuated
the phosphorylation of Akt and enhanced Ca2+ influx.
This suggests that miR-126 accelerated IgE-mediated MC
degranulation associated with the PI3K/Akt signaling pathway via
promoting Ca2+ influx. These findings provide an insight
into the potential role of miR-126 in association with the
treatment of allergic skin diseases.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YB, SW, YY and JL designed the experiments. YB and
SW performed the study and wrote the manuscript. YG and WZ
participated in establishing the animal model. HJ was involved in
analyzing the data. YY and JY revised the manuscript. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of the Animal Care and Use Committee of the Central
Hospital of Wuhan (approval no. SCXK2015-0018).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Reber LL, Sibilano R, Mukai K and Galli
SJ: Potential effector and immunoregulatory functions of mast cells
in mucosal immunity. Mucosal Immunol. 8:444–463. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Andersson C, Tufvesson E, Diamant Z and
Bjermer L: Revisiting the role of the mast cell in asthma. Curr
Opin Pulm Med. 22:10–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Le DD, Schmit D, Heck S, Omlor AJ, Sester
M, Herr C, Schick B, Daubeuf F, Fähndrich S, Bals R, et al:
Increase of mast cell-nerve association and neuropeptide receptor
expression on mast cells in perennial allergic rhinitis.
Neuroimmunomodulation. 23:261–270. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu FT, Goodarzi H and Chen HY: IgE, mast
cells, and eosinophils in atopic dermatitis. Clin Rev Allergy
Immunol. 41:298–310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gaudenzio N, Marichal T, Galli SJ and
Reber LL: Genetic and imaging approaches reveal pro-inflammatory
and immunoregulatory roles of mast cells in contact
hypersensitivity. Front Immunol. 9:12752018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morita H, Saito H, Matsumoto K and Nakae
S: Regulatory roles of mast cells in immune responses. Semin
Immunopathol. 38:623–629. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Otsuka A, Nonomura Y and Kabashima K:
Roles of basophils and mast cells in cutaneous inflammation. Semin
Immunopathol. 38:563–570. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saarinen JV, Harvima RJ, Naukkarinen A,
Horsmanheimo M and Harvima IT: Interleukin-4-positive mast cells
are highly associated with the extent of immediate allergic wheal
reaction in the skin. Allergy. 56:58–64. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dissanayake E and Inoue Y: MicroRNAs in
allergic disease. Curr Allergy Asthma Rep. 16:672016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mattes J, Collison A, Plank M, Phipps S
and Foster PS: Antagonism of microRNA-126 suppresses the effector
function of TH2 cells and the development of allergic airways
disease. Proc Natl Acad Sci USA. 106:18704–18709. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ishizaki T, Tamiya T, Taniguchi K, Morita
R, Kato R, Okamoto F, Saeki K, Nomura M, Nojima Y and Yoshimura A:
miR126 positively regulates mast cell proliferation and cytokine
production through suppressing Spred1. Genes Cells. 16:803–814.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Muñoz-Cruz S, Mendoza-Rodríguez Y,
Nava-Castro KE, Yepez-Mulia L and Morales-Montor J: Gender-related
effects of sex steroids on histamine release and FcεRI expression
in rat peritoneal mast cells. J Immunol Res. 2015:3518292015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Carlos D, Sá-Nunes A, de Paula L,
Matias-Peres C, Jamur MC, Oliver C, Serra MF, Martins MA and
Faccioli LH: Histamine modulates mast cell degranulation through an
indirect mechanism in a model IgE-mediated reaction. Eur J Immunol.
36:1494–1503. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vennegaard MT, Bonefeld CM, Hagedorn PH,
Bangsgaard N, Løvendorf MB, Odum N, Woetmann A, Geisler C and Skov
L: Allergic contact dermatitis induces upregulation of identical
microRNAs in humans and mice. Contact Dermatitis. 67:298–305. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Harris TA, Yamakuchi M, Ferlito M, Mendell
JT and Lowenstein CJ: MicroRNA-126 regulates endothelial expression
of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA.
105:1516–1521. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chistiakov DA, Orekhov AN and Bobryshev
YV: The role of miR-126 in embryonic angiogenesis, adult vascular
homeostasis, and vascular repair and its alterations in
atherosclerotic disease. J Mol Cell Cardiol. 97:47–55. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goerke SM, Kiefer LS, Stark GB, Simunovic
F and Finkenzeller G: miR-126 modulates angiogenic growth
parameters of peripheral blood endothelial progenitor cells. Biol
Chem. 396:245–252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kong F, Zhou J, Zhou W, Guo Y, Li G and
Yang L: Protective role of microRNA-126 in intracerebral
hemorrhage. Mol Med Rep. 15:1419–1425. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vara Fresno JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu JS and Cui W: Proliferation, survival
and metabolism: The role of PI3K/AKT/mTOR signalling in
pluripotency and cell fate determination. Development.
143:3050–3060. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Biethahn K, Orinska Z, Vigorito E,
Goyeneche-Patino DA, Mirghomizadeh F, Föger N and Bulfone-Paus S:
miRNA-155 controls mast cell activation by regulating the PI3Kγ
pathway and anaphylaxis in a mouse model. Allergy. 69:752–762.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao H, Deng H, Xu H, Yang Q, Zhou Y, Zhang
J, Zhao D and Liu F: MicroRNA-223 promotes mast cell apoptosis by
targeting the insulin-like growth factor 1 receptor. Exp Ther Med.
11:2171–2176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin H, Zheng C, Li J, Yang C and Hu L:
Lentiviral shRNA against KCa3.1 inhibits allergic response in
allergic rhinitis and suppresses mast cell activity via PI3K/AKT
signaling pathway. Sci Rep. 5:131272015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chorev E, Manor Y and Yarom Y: Density is
destiny-on [corrected] the relation between quantity of T-type Ca2+
channels and neuronal electrical behavior. CNS Neurol Disord Drug
Targets. 5:655–662. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Holowka D, Wilkes M, Stefan C and Baird B:
Roles for Ca2+ mobilization and its regulation in mast cell
functions: Recent progress. Biochem Soc Trans. 44:505–509. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aurora AB, Mahmoud AI, Luo X, Johnson BA,
van Rooij E, Matsuzaki S, Humphries KM, Hill JA, Bassel-Duby R,
Sadek HA and Olson EN: MicroRNA-214 protects the mouse heart from
ischemic injury by controlling Ca2+ overload and cell
death. J Clin Invest. 122:1222–1232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wahlquist C, Jeong D, Rojas-Muñoz A, Kho
C, Lee A, Mitsuyama S, van Mil A, Park WJ, Sluijter JP, Doevendans
PA, et al: Inhibition of miR-25 improves cardiac contractility in
the failing heart. Nature. 508:531–535. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Andersen M, Trapani D, Ravn J, Sørensen
JB, Andersen CB, Grauslund M and Santoni-Rugiu E:
Methylation-associated silencing of microRNA-126 and its host gene
EGFL7 in malignant pleural mesothelioma. Anticancer Res.
35:6223–6229. 2015.PubMed/NCBI
|
|
30
|
Song L, Xie X, Yu S, Peng F and Peng L:
MicroRNA-126 inhibits proliferation and metastasis by targeting
pik3r2 in prostate cancer. Mol Med Rep. 13:1204–1210. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang GM, Luo L, Ding XM, Dong DH, Li B,
Ma XC and Sun LJ: MicroRNA-126 inhibits tumor cell invasion and
metastasis by downregulating ROCK1 in renal cell carcinoma. Mol Med
Rep. 13:5029–5036. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li H, Meng F, Ma J, Yu Y, Hua X, Qin J and
Li Y: Insulin receptor substrate-1 and Golgi phosphoprotein 3 are
downstream targets of miR-126 in esophageal squamous cell
carcinoma. Oncol Rep. 32:1225–1233. 2014. View Article : Google Scholar : PubMed/NCBI
|